Abstract
The peptide hormone ghrelin regulates a variety of eating behaviors. Not only does it potently increase intake of freely-available food, but it also shifts food preference towards diets rich in fat, enhances operant responding for food rewards, and induces conditioned place preference for food rewards. Here, we postulated that ghrelin also enables cue-potentiated feeding, in which eating is enhanced upon presentation of a food-conditioned stimulus. To test this hypothesis, a novel cue-potentiated feeding protocol adapted for use in mice was designed and validated, and then the effects of pharmacologic ghrelin receptor (GHSR) antagonism and GHSR transcriptional blockade (as occurs in GHSR-null mice) were assessed. Sated C57BL/6J mice indeed demonstrated cue-potentiated intake of grain-based pellets specifically upon presentation of a positive conditioned stimulus (CS+) but not a negative conditioned stimulus (CS-). Treatment with a GHSR antagonist blocked potentiated feeding in sated C57BL/6J mice in response to the CS+. In contrast, while GHSR-null mice also lacked a potentiation of feeding specifically in response to the CS+, they displayed an enhanced intake of pellets in response to both the positive and negative conditioned stimuli. The pattern of immediate early gene expression within the basolateral amygdala -- a brain region previously linked to cue-potentiated feeding -- paralleled the observed behavior of these mice, suggesting uncharacteristic activation of the amygdala in response to negative conditioned stimuli in GHSR-null mice as compared to wild-type littermates. Thus, although the observed disruptions in cue-potentiated feeding are different depending upon whether GHSR activity or GHSR expression is blocked, a key role for GHSRs in establishing a specific positive cue-food association has now been established.
Keywords: ghrelin, GHSR, amygdala, feeding, cue
1. Introduction
The rates of obesity have been steadily and dramatically increasing [1]. Understanding the pathways that regulate complex eating behaviors and ultimately disturb homeostatic control of food intake is crucial for the development of effective obesity treatments. While genetic factors undoubtedly contribute to obesity, an individual's environment and upbringing are also likely involved [2–4]. The human environment is replete with visual, auditory, and olfactory cues which, via associative learning and Pavlovian conditioning, can become intimately linked to food, resulting in the induction and maintenance of eating [5]. Prime examples include logos of commercial enterprises that sell food [6]. With continued exposure, these cues can form such a strong association with eating that they may override satiety signals that otherwise would normally lead to eating cessation [5]. Recurrent exposure to these cues potentially can lead to an overabundance of food intake resulting in an increased risk for obesity. Of note, the motivational salience of food cues as measured by visual attention is greater in obese individuals than in lean subjects, suggesting that higher sensitivity to cues associated with food may contribute to their lack of control over food intake [7].
The cue-potentiated feeding paradigm models habitual eating that occurs with strong cue associations linked to food. Several studies have found that food-sated rats increase food consumption after presentation of a conditioned stimulus previously paired with food during a period of caloric restriction [8–9]. These elegant studies were performed with bland pellets similar to regular chow, signifying the strength of a conditioned cue's ability to enhance feeding behavior even without savory taste as a rewarding component. The amygdala and prefrontal cortex play a major role in this behavior as lesions of the basolateral amygdala (BLA) or medial prefrontal cortex (mPFC) in rats abolish the cue-induced potentiation of eating [9–12] and as connections from the BLA/basomedial amygdala and mPFC to the lateral hypothalamic area in rats are strongly activated by the positive conditioned stimulus [13]. While these studies using rats have determined some of the neural pathways and regional networks involved in cue-potentiated feeding, to our knowledge, this behavioral model has never been performed in mice using a non-savory food, which is an important distinction since a tasty or rewarding food adds another dimension to the learning aspect of conditioning. The use of mice in place of rats in this paradigm will facilitate studies that aim to identify the molecular mediators involved in shaping and activating these neural networks, as mice can be more easily genetically manipulated.
One potential mediator in the development of cue-potentiated feeding is the gastrointestinally-derived peptide hormone ghrelin [14]. Ghrelin potently induces intake of freely-available food upon binding to its receptor, the growth hormone secretagogue receptor (GHSR), in regions including the hypothalamus and brainstem, and it is through these pathways that endogenous ghrelin is thought to affect body weight homeostasis [15–17]. GHSR localization to the ventral tegmental area (VTA), hippocampus, and amygdala provides evidence that ghrelin also may mediate more complex eating behaviors that involve different aspects of learning, memory, and reward [18–20]. Indeed, several studies have investigated a role for ghrelin in complex eating behaviors. Ghrelin helps to define food preference -- shifting consumption towards sweet diets and those high in fat, and ghrelin also enhances operant responding for sweet and fatty food rewards [21–26]. Furthermore, ghrelin enables acquisition of conditioned place preference for food rewards upon its pharmacologic administration or upon its natural elevation as induced by caloric restriction or psychosocial stress [21, 27–28]. Several studies have indicated that blockade of ghrelin action, by pharmacologic blockade of or genetic deletion of GHSRs, blocks many of these same complex eating behaviors [21–22, 28–30]. To our knowledge, only one study, using a Pavlovian-to-instrumental transfer protocol to study motivational incentive learning, has reported an enhancement in reward behavior upon blockade of ghrelin action [31]. The ability of ghrelin to enhance performance in tests of behavioral memory also may be relevant to the pathways required for cue-potentiated feeding [32–33]. Here, we test the hypothesis that in addition to its previously-reported effects on homeostatic eating, food preference, and reward-based eating, ghrelin also participates in the development and expression of cue-potentiated feeding as well as the regulation of BLA activity in response to conditioned cues.
2. Materials and methods
2.1. Animals
C57BL/6J mice (Charles River, Wilmington, MA) were used in Experiments 1 and 2. GHSR-null and wild-type littermates, used in Experiments 3 and 4, were generated by breeding mice heterozygous for the GHSR-null allele, obtained after more than 10 generation backcrossing onto a C57BL/6J genetic background [34]. All studies were approved by the UTSW Institutional Animal Care and Use Committee.
2.2. Primary cue-potentiated feeding paradigm
Conditioning
This protocol (Figure 1A, used for Experiments 1, 3, and 4) was modeled after reported cue-potentiated protocols designed for use in rats with standard chow [9, 11–13]. Two-month-old mice, housed 2–3 per cage, were placed on a restricted feeding schedule which provided access to standard chow (Teklad Global Diet #2016 Madison, WI, which provides 3.0 kcal/g of energy and contains 16.4 g% protein, 4.0 g% fat, and 48.5 g% carbohydrates) for 3 ½ hr per day. Such was maintained during a run-in period (Days 1–5) and throughout a “simple” conditioning phase (Days 6–12) and a subsequent “discrimination” conditioning phase (Days 13–26).
Figure 1.
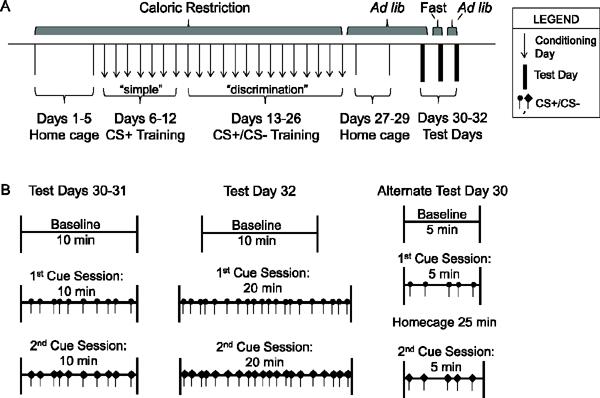
Schematic timeline of the primary cue-potentiated feeding protocol adapted for use in mice. A) This protocol consists of a 5 day run-in period during which mice are permitted 3 ½-hr daily access to food in their home cages. While still on this home cage caloric restriction protocol, the mice are exposed to 1 wk of daily “simple” conditioning sessions followed by 2 wks of daily “discrimination” conditioning sessions. For the next 3 days, mice are kept in their home cages with ad lib access to food, after which cue-potentiated feeding responses are tested on Day 30 (Experiments 1 and 4) or Days 30–32 (Experiment 3). B) Test days consist of 3 Test Sessions (Baseline, 1st Cue, and 2nd Cue) in the conditioning apparatus, as indicated.
Conditioning sessions were performed by placing individual mice into conditioning chambers (Model ENV307A, Med Associates, Inc., St. Albans, VT) just before the 3 ½– hr period of food availability. During the first, “simple” conditioning phase (Days 6–12), daily conditioning sessions were performed by pairing a light cue, which would become the conditioned positive stimulus (CS+), with delivery of a single 14-mg grain-based Dustless Precision Pellet (BioServ, Frenchtown, NJ, which provides 3.6 kcal/g of energy and contains 18.7% protein, 5.6% fat, and 59.1% carbohydrates). The CS+ was assigned to each mouse in a counterbalanced fashion as either the main “house” light (affixed near the ceiling) of the chamber or its central “nose-poke” light (affixed to the lower wall area of the chamber). Cues lasting 2-sec in duration were given at random intervals every 30 – 90 sec. A single food pellet was dispensed immediately after each CS+ into a food hopper using a programmed automatic pellet dispenser. Thirty cues were delivered per each 30 min-long simple conditioning session. During the second, “discrimination” conditioning phase (Days 13–26), daily conditioning sessions were performed using both positive light cues [conditioned positive stimuli (CS+)] and negative light cues [conditioned negative stimuli (CS−)]. A single food pellet was dispensed upon presentation of the CS+; no food pellet was dispensed upon presentation of the CS−. The CS− was assigned as whichever light cue was not used as the CS+. Twenty CS+ and 20 CS− cues of 2-sec duration each were delivered in random order and at random intervals every 30 – 90 sec during each 40 min-long discrimination conditioning session. When not in the conditioning chambers, mice were housed in their home cages.
Test sessions
During the first three days following completion of the conditioning (Days 27–29), mice were kept in their home cages with ad lib-access to standard chow. For Experiment 1, acquisition of cue-potentiated feeding was assessed on Day 30 by placing mice in the conditioning chambers for three 10-min test sessions: a baseline session where no cue was presented (Session 1), a session where only the CS+ was presented (Session 2 or 3), and a session where only the CS- was presented (Session 2 or 3). Ten cues of 2-sec duration were delivered at random intervals every 30 – 90 sec during Sessions 2 and 3. The orders of the CS+ test session and CS- test session were counterbalanced between animals. During these three test sessions, mice had free access to 30 food pellets within the food hopper. Between sessions, mice were placed into their home cages briefly while the pellets remaining were counted.
2.3. Cue-potentiated feeding with ghrelin receptor antagonist
For Experiment 2, the above protocol was modified slightly to allow more time for the mice to adapt to receiving an oral gavage of either a ghrelin receptor antagonist or its vehicle prior to each conditioning session (Figure 2). As such, the “simple” conditioning phase was extended to two weeks rather than one (Days 6–19), while the “discrimination” conditioning remained two weeks in length (Days 20–33). Also, for this modified protocol, mice were allowed free access to 20-mg grain-based Dustless Precision Pellets (BioServ, which provides 3.35 kcal/g of energy and contains 21.3 g% protein, 2.8 g% fat, and 54 g% carbohydrates) instead of standard chow during the 3 ½ hr-long daily feeding periods provided in the home cages after each conditioning session. These grain-based pellets were provided ad lib in home cages in the days following the conditioning phases. Neither antagonist nor vehicle was administered during the 5 day run-in period or the 3 days following conditioning.
Figure 2.
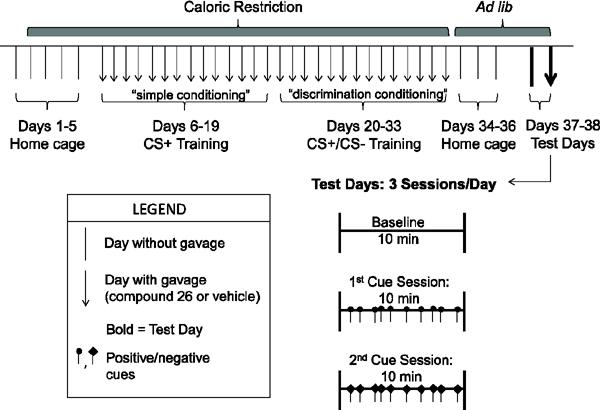
Schematic timeline of cue-potentiated feeding protocol modified for use in mice administered GHSR antagonist. Modifications of the primary protocol include the following: a prolonged simple conditioning period (2 wks), use of 20 mg grain-based pellets instead of standard chow during the 3 ½ hour-long daily home-cage feeding period, and 2 test days (1st test day - no gavage, 2nd test day - gavage 1 hr before test).
Ghrelin receptor antagonism was achieved by administering Compound 26 [also known as LXG-9342, and by its chemical name, (6-(4-Fluorophenoxy)-3-{[(3S)-1-isopropylpiperidin-3-yl]methyl}-2-methylquinazolin-4(3H)-one], which was synthesized by the UTSW Synthetic Chemistry Core, based on published protocols [35–36]. One hr prior to each conditioning session (Days 6–33), freshly-prepared Compound 26 suspended in PEG400:water (80:20), at a concentration of 30 μg/10 μL was orally administrated via gavage to provide 30 μg per gram body weight; vehicle-treated control mice were similarly treated with 10 μL PEG400:water (80:20) solution per gram body weight. The initial descriptions of Compound 26 demonstrated a relatively potent affinity to GHSR, high bioavailability, moderate clearance, and a very high volume of distribution including high brain penetrance [35–36]. Previously, Compound 26 at a gavage dose of 10 μg/g body weight was shown to reduce glucose excursion in a rat i.p. glucose tolerance test by 20% and to reduce body weights of diet-induced obese C57BL/6J mice by about 3–4% over a 9-day period [35]. Compound 26 at a gavage dose of 30 μg/g body weight previously was shown to significantly reduce food intake in C57BL/6J mice subjected to a fasting-refeed protocol, but not in similarly-treated GHSR-null mice, suggesting its specificity for GHSR [21, 35–36]. This same 30 μg/g body weight gavage dose of Compound 26 also previously was shown to block the acquisition of conditioned place preference for high-fat diet in chronically calorie-restricted C57BL/6J mice, but not the compensatory hyperphagia associated with chronic calorie restriction (greater than 1 wk restricted, 4-hr-long daily access to standard chow) [21].
The first test day (Day 37) was performed in the same manner as in Experiment 1, without Compound 26 or vehicle. A second test day (Day 38) involved administration of Compound 26 (30 μg/g body weight) or vehicle 1 hr prior to the baseline session. The length and order of test sessions were the same as described for Experiment 1.
2.4. Cue-potentiated feeding in GHSR-null mice
Experiment 3 involved the use of GHSR-null mice and wild-type littermates. The protocol was identical to that used in Experiment 1 (Figure 1A), except two additional test days were performed on Days 31 and 32 (Figure 1B). On Day 31, the same three 10-min sessions from Day 30 were performed on the mice following a 16-hr overnight fast imposed in their home cages. On Day 32, after the mice had again been provided ad lib access to standard chow, cue-potentiated feeding was again assessed, only this time, Sessions 2 and 3 lasted 20 min each.
2.5. Cue-potentiated feeding paradigm for gene expression study
For Experiment 4, the above protocol was adjusted for a separate cohort of GHSR-null and wild-type littermates to determine whether the conditioned stimuli acutely activate BLA neurons (Figure 1B). A single, alternate test day was performed on Day 30 consisting of three 5-min sessions. In between Sessions 2 and 3, the mice were placed back in their home cages for 25 min in order to match the timing of specific immediate early gene peak expression levels to neuronal activation. Tissue collections occurred directly after Session 3. Control mice consisting of both GHSR-null and wild-type littermates (“no cue” group) underwent Days 1–29 of the protocol, as usual, but did not undergo Day 30 testing; tissue collections were done on Day 30.
2.6. Sample collection and quantitative real-time PCR (qPCR)
Mice were euthanized by live decapitation. Brains were extracted, placed in cold diethylpyrocarbonate-PBS, and then sectioned into 1 mm-thick coronal slices by use of a stainless steel mouse brain matrix and standard razor blades. Bilateral tissue punches corresponding to the locations of the BLA, mPFC, and VTA were frozen with liquid nitrogen after being excised using a 15-g needle. Quantitative PCR was performed on total RNA that was extracted and reverse transcribed, as described previously [28, 37]. Arc, Homer1a, c-Fos, GHSR, and cyclophilin primers (Table 1) were previously used and/or validated for the appropriate specificity and efficiency using template titration and dissociation curves [28, 38–39] and were designed to span exon-exon junctions. Cyclophilin expression levels were used for normalization, and relative levels were calculated by the comparative threshold cycle (ΔΔCt) method, with comparison of the wild-type control (no-cue) group as the baseline.
Table 1.
Primers utilized for ghrehn receptor expression and immediate early gene expression.
| Gene | Primer | |
|---|---|---|
| Arc | Forward | 5'-AATGCAGCTGAAGCAGCAGACCTG-3' |
| Reverse | 5'-TCTCAGCAGCCTTGAGACCTGGTGT-3' | |
|
| ||
| Homer1a | Forward | 5'-CAAACACTGTTTATGGACTG-3' |
| Reverse | 5'-TGCTGAATTGAATGTGTACC-3' | |
|
| ||
| c-Fos | Forward | 5'-CTACTACCATTCCCCAGCCG-3' |
| Reverse | 5'-GTTGGCACTAGAGACGGACAGA-3' | |
|
| ||
| GHSR | Forward | 5'-CACAGTGAGGCAGAAGACCG-3' |
| Reverse | 5'-ACCGTGATGGTATGGGTGTCG-3' | |
|
| ||
| cyclophilin | Forward | 5'-TGGAGAGCACCAAGACAGACA-3' |
| Reverse | 5'-TGCCGGAGTCGACAATGAT-3' | |
2.7. Statistical Analyses
A one way ANOVA was performed when analyzing the effect of cue on pellet consumption (Experiment 1). Repeated measures two way ANOVA was performed when analyzing the effects of administered compound (Experiment 2) or genotype (Experiment 3) and cue on pellet consumption. Two way ANOVAs were performed when analyzing the effects of genotype and cue presentation on relative immediate early gene mRNA expression (Experiment 4). Bonferroni post-hoc analysis was used for all comparisons with significant P values. Linear regression analysis was performed on correlation plots for gene expression and pellet consumption, allowing us to determine coefficients of determination (R2) for the goodness of fit; slopes that were statistically significantly different from a slope of zero were considered as indicating significant correlations (Experiment 4). Data are expressed as mean ± SEM, with P < 0.05 considered statistically significant. Analyses were performed using GraphPad Prism 5.0.
3. Results
3.1. Experiment 1: Cue-potentiated feeding of grain-based pellets in mice
A cue-potentiated feeding protocol (Figure 1) was adapted for use in mice in order to recapitulate the behavior observed by others in rats. These adaptations mostly reflect the slower learning curve of mice and were done to ensure adequate conditioning. Male C57BL/6J mice were first subjected to a 5-day run-in period during which home cage access to standard chow was made available for 3 ½ hrs per day. Next, while still on this caloric restriction regimen, mice were subjected to a “simple” conditioning session on 7 successive days followed by a “discrimination” conditioning session on 14 successive days. Conditioning sessions were performed in special chambers just prior to the 3 ½ hrs of daily food availability in the home cages. During these conditioning sessions, a single14-g grain-based pellet was dispensed into a food hopper upon the randomly-timed presentation of each positive conditioned stimulus (CS+), whereas no pellet was dispensed upon the randomly-timed presentation of negative conditioned stimuli (CS−). The stimuli consisted of either the chamber's ceilinged house light or its mural central nose-poke light. As described more fully in Materials and Methods, the “simple” conditioning sessions included CS+ cues whereas the “discrimination” conditioning sessions included a mixture of CS+ and CS− cues. Following the conditioning period, mice were given ad lib access to standard chow in their home cages and subsequently were tested for the acquisition of cue-potentiated feeding.
Caloric restriction reduced body weights by 18.8 ± 0.5% by Day 6, which persisted throughout the conditioning period (Figure 3A). A similar, 4-hr per day restricted food availability protocol previously was shown to stimulate a two-fold elevation in circulating acyl-ghrelin [21]. By Day 3, the mice had learned to eat all of the pellets dispensed into the food hopper (Figure 3B). After completing both conditioning phases, the mice regained their original weights after only two days of ad lib feeding, signifying a presumed sated state (Figure 3A).
Figure 3.
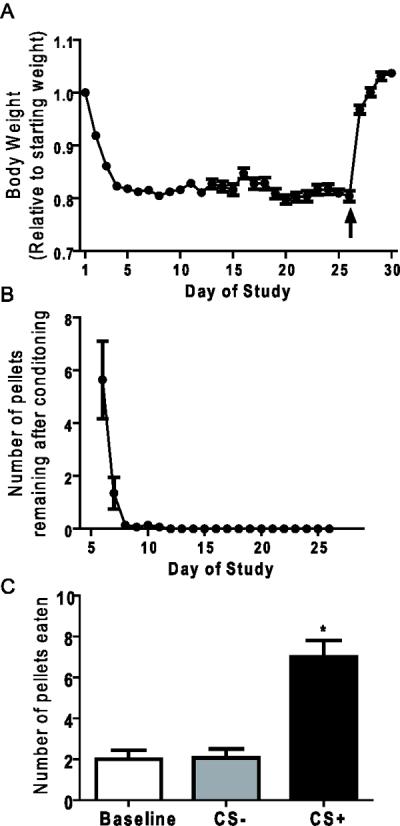
Responses of C57BL/6J mice to the cue-potentiated feeding protocol (Experiment 1). A) Body weights of mice throughout the time course of the cue-potentiated feeding protocol (arrow indicates return to ad-lib feeding). B) The number of pellets remaining after each conditioning session. C) Test Day 30 cue-potentiated feeding responses. [n=14; * represents significant difference from other test sessions (*P <0.05)].
The primary outcome of the study was assessing the acquisition of cue-potentiated feeding. Indeed, on Day 30, the CS+ induced a specific potentiation of feeding as compared to baseline and the CS−, as indicated by the 2-fold increase in number of pellets consumed (Figure 3C). Thus, even without a savory component to the food, mice are capable of forming strong specific cue-food associations that manifest in the sated state.
3.2. Experiment 2: Effects of ghrelin receptor antagonism on cue-potentiated feeding
To assess the role of ghrelin in mediating the development of the cue-food associations, we performed the cue-potentiated feeding experiment using a GHSR antagonist, Compound 26 [21, 35–36]. Caloric restriction resulted in reductions of 16.6 ± 0.5% (vehicle-treated group) and 17.2 ± 0.4% (Compound 26-treated group), with no statistically-significant differences between the groups (Figure 4A). Although Compound 26 initially was associated with an increased number of pellets leftover after conditioning sessions as compared to vehicle during the majority of the “simple” conditioning phase, throughout the “discrimination” conditioning phase, both groups were eating all of the pellets presented during the conditioning sessions (Figure 4B).
Figure 4.
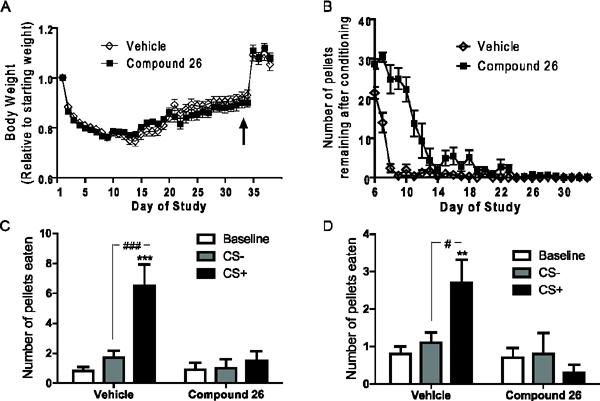
Cue-potentiated feeding responses of wild-type mice receiving Compound 26 or vehicle (Experiment 2). A) Body weights of mice throughout the time course of the cue-potentiated feeding protocol, during which either Compound 26 or vehicle was provided 1 hr prior to each conditioning session (arrow indicates return to ad-lib feeding). B) Number of pellets remaining after each conditioning session. C) Test Day 37 feeding responses to positive and negative cues by mice. D) Test Day 38 feeding responses to positive and negative cues by mice given oral gavage of Compound 26 or vehicle 1 hr before testing. [n=10/group; **, *** represent cue sessions significantly different from baseline (**P <0.01, ***P <0.001); #, ### represent cue sessions significantly different from each other (#=P <0.05, ###P <0.001)].
As expected based on the Experiment 1 results, mice receiving vehicle displayed a significant enhancement of food intake in response to the CS+ as compared to baseline and the CS−. In contrast, Compound 26 given during the conditioning period only but not on the test day completely blocked the acquisition of cue-potentiated feeding, as the number of pellets eaten was not enhanced by the positive cue (Figure 4C). The same was true when Compound 26 was also given on the test day (Figure 4D).
3.3. Experiment 3: Effects of deleted GHSR expression on cue-potentiated feeding
In an attempt to corroborate the findings with Compound 26, we next utilized GHSR-null mice, which lack ghrelin receptor expression, in the cue-potentiated feeding paradigm [34]. GHSR-null and wild-type littermates were calorically restricted and conditioned for cue-potentiated feeding. Mice of both genotypes lost 16.4 ± 0.2% of their original body weights upon caloric restriction (Figure 5A). In contrast to Experiment 2, mice of both genotypes quickly learned to eat all the pellets dispensed during the conditioning sessions, with no difference between genotypes (data not shown).
Figure 5.
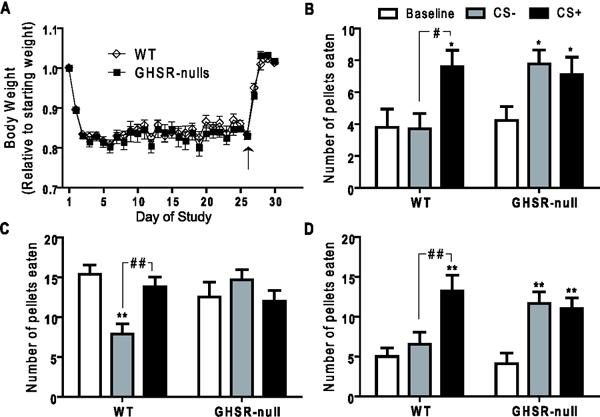
Responses of GHSR-null and wild-type littermates to the cue-potentiated feeding protocol (Experiment 3). A) Body weights of mice throughout the time course of the cue-potentiated feeding protocol (arrow indicates return to ad-lib feeding). B) Test Day 30 feeding responses to positive and negative cues by ad lib-fed mice. C) Test Day 31 feeding responses to positive and negative cues by overnight-fasted mice. D) Test Day 32 feeding responses to positive and negative cues by ad lib-fed mice when permitted extended access to the pellets. Legend in Panel B also applies to panels C–D. [n=10/group; *, ** represent cue sessions significantly different from baseline (*P <0.05, **P <0.01); #, ## represent cue sessions significantly different from each other (#=P <0.05, ##P <0.01)].
Similar to previous experiments, wild-type mice ate significantly more during the CS+ session than during the CS− or baseline sessions (Figure 5B). GHSR-null mice instead increased their intake of pellets with both the CS+ and the CS− cues (Figure 5B). Slightly modified tests of cue-potentiated feeding were performed over the next two days. On Day 31, the mice were assessed after first having been fasted in their home cages overnight. Among many other changes, such overnight fasting is known to raise plasma acyl-ghrelin levels [21] although it is presumed that only wild-type mice with intact GHSR expression, and not GHSR-null mice, can respond to this elevated acyl-ghrelin. Both wild-type and GHSR-null littermates ate more pellets during the baseline session (in the absence of either the CS+ or CS−), as compared to during the previous Day 30 baseline session, as expected since this was their first exposure to food since the overnight fast began 16 h earlier (Figure 5C). Furthermore, wild-type mice maintained an increase of food intake with presentation of the CS+ as compared to the CS−, although not compared to baseline (Figure 5C). GHSR-null mice again displayed no significant difference in the amount eaten between the CS+ and CS− test sessions. Upon prolongation of the test sessions to 20 min each (Day 32) using mice under ad lib-fed conditions, wild-type mice again ate significantly more pellets during the CS+ session than the CS− session. GHSR-null mice showed an elevated intake of pellets during both cue sessions as compared to baseline, with no significant difference in pellets eaten between the two cue sessions (Figure 5D).
3.4. Experiment 4: Effect of deleted GHSR expression on BLA activity in response to cues
A directed examination of GHSR gene expression within the mPFC and BLA was performed given their known roles in cue-potentiated feeding [9, 11, 13]. GHSR levels in BLA brain punches were on par with those in the VTA, which is a region well-characterized for GHSR expression, while the mPFC displayed a lack of significant GHSR expression, confirming previous reports [18, 28, 30] (Figure 6A).
Figure 6.
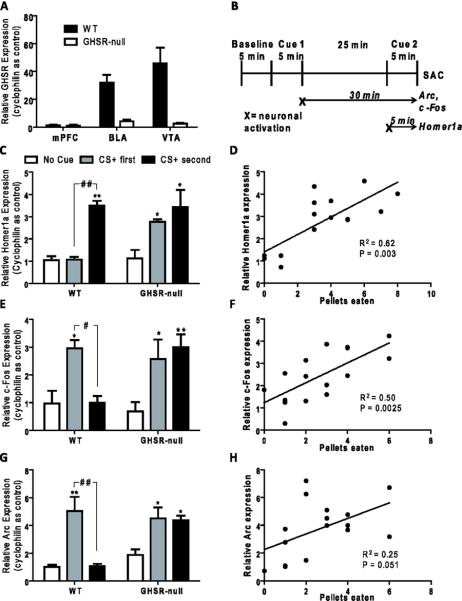
GHSR and immediate early gene expression in the basolateral amygdala. A) Expression levels of GHSR mRNA in selected brain regions of wild-type and GHSR-null littermates, as determined by qPCR. (n=7/group). B) Schematic illustrating the timing of BLA neuronal activation based on qPCR detection of peak expression levels of mRNA encoding Arc, c-Fos and Homer1a (peak c-Fos and Arc mRNA levels occur at time of sacrifice if neurons activate during first cue test; peak Homer1a mRNA levels occur at time of sacrifice if neurons activate during second cue test). C,E,F) Immediate early gene expression levels, as determined by qPCR, in BLA tissue punches of mice sacrificed immediately following the second cue test. Legend in Panel C also applies to panels E,F. [n=3–4/group; *, ** represent groups significantly different from “no cue” control group (*P <0.05, **P <0.01); #, ## represent groups significantly different from each other (#=P <0.05, ##P <0.01)]. D,F,G) Correlation plots showing relative gene expression as compared to number of pellets eaten for representative test session [n=16]. Each point represents a single mouse including both GHSR-null and wild-type littermates. Correlation coefficients of determination (R2) are displayed along with the P-values.
Given the observations that GHSR-null mice responded with a non-specific increase of feeding in response to both positive and negative cues, we next examined the activity of the BLA in response to the cues. In order determine whether BLA neuronal activation was specific to the CS+, expression of a set of immediate early genes was examined using a protocol previously used in rats [13]. We employed an alternate version of the Day 30 Test Sessions, with the timing of the sessions designed such that the immediate early gene qPCR data would inform the timing of neuronal activation. In particular, this method relies on differential, unique peak mRNA expression signatures for the tested immediate early genes in relation to neuronal activation: c-Fos and Arc are not expressed at high levels until about 30 min after neuronal activation, while Homer1a is expressed highly at about 5 min following neuronal activation but is degraded by 30 min [40–43]. By spacing the CS+ test and CS− test 25 min apart from each other (with the order still counterbalanced) and obtaining BLA brain punches from mice directly following Test Session 3, it is possible to determine whether neurons are activated by the Session 2 cue or by the Session 3 cue by assessing mRNA levels for these immediate early genes (Figure 6B). As such, elevated Homer1a levels (as compared to control mice given no cues) indicate neuronal activation by the Session 3 cue (second cue); elevated Arc and c-Fos levels indicate neuronal activation by the Session 2 cue (first cue).
In wild-type mice, elevated BLA Homer 1a expression did not occur when the CS+ was exhibited during Session 2 (CS+ first) but was observed when the CS+ was exhibited during Session 3 (CS+ second), suggesting specific BLA neuronal activation in response to the CS+ but not the CS− (Figure 6C). Supporting these data, in wild-type mice, c-Fos and Arc levels were both increased when the CS+ was tested first but not when the CS− was tested first (Figures 6E,G). In contrast, GHSR-null mice demonstrated elevated Homer1a regardless of which cue was first. Similarly, in GHSR-null mice, both c-Fos and Arc levels were elevated when either the CS+ or CS− was tested first. These data suggest BLA neuronal activation by both cues in GHSR-null mice.
Immediate early gene expression within the BLA of GHSR-null and wild-type littermates was also compared to the number of pellets eaten by these animals during Test Sessions 2 and 3. Relative expression of Homer1a positively correlated with the number of pellets eaten by the mice during Session 3, while the relative expression of c-Fos positively correlated with the number of pellets eaten during Session 2 (Figures 6D, F). No statistically significant correlation between the relative expression of Arc and the number of pellets eaten during Session 2 was observed.
4. Discussion
In the present study, we describe the design and validation of a cue-potentiated feeding protocol for enhanced consumption of a non-savory food in mice. Previous studies using the cue-potentiated feeding paradigm in rats, on which the current mouse protocol was modeled, have already provided some key insights into the neural pathways regulating this habitual eating behavior. A related behavioral model, Pavlovian-Instrumental Transfer, which assesses the willingness to lever press in response to a cue previously paired with a rewarding food substance, has been reported for mice in addition to rats [31, 44–45]. The new mouse protocol described here reduces the complexity of these previous Pavlovian Instrumental Transfer studies to focus primarily on the importance of developing a cue-food association rather than motivation. This protocol also successfully employs the use of non-savory, bland food pellets for the cue-potentiated feeding design, as in the classic studies with rats, so as to avoid adding the additional component of a food reward that has a naturally high hedonic value. Thus, the success of this design permits future opportunities to tease apart any distinctions between molecular mediators involved in the simple cue-induced potentiation of food intake as opposed to potentiated intake of food rewards with a high hedonic value.
After validating the appropriate cue-potentiated feeding response for grain-based pellets in C57BL/6J mice, we assessed the involvement of ghrelin signaling in this behavior due to ghrelin's numerous roles in other feeding behaviors. Given ghrelin's previously described roles in stimulating intake of freely-available food and multiple reward-based eating behaviors – we had predicted that neither the CS+ nor the CS− would potentiate feeding. As predicted, upon administration of a GHSR antagonist before each conditioning session, mice no longer displayed a potentiation of food intake in response to cues, regardless of whether the antagonist was additionally given prior to the test sessions or not. Such suggests the requirement for intact ghrelin signaling in the acquisition of the cue potentiated feeding behavior.
A potential caveat in the interpretation of the GHSR antagonist studies is that during the initial “simple” conditioning phase, mice receiving Compound 26 seemed slower to adapt to the conditioning and failed to consume all of the pellets provided with the CS+. That said, by the “discrimination” phase of training, mice in both the vehicle-treated and Compound 26-treated groups were eating all or nearly all of the pellets provided during each conditioning session. Therefore, we believe that both groups consumed enough pellets during the “discrimination” sessions to have received appropriate conditioning to the cues.
As a means of corroborating the effects of pharmacologic blockade of ghrelin action on cue-potentiated feeding, we next compared the performance of mice lacking GHSRs with that of wild-type littermates in the cue-potentiated feeding protocol. Utilizing GHSR-null mice and wild-type littermates, it was determined that lack of GHSRs disrupts the specific potentiation of eating in response to a CS+ that is otherwise exhibited by wild-type mice. However, while we had expected a similar response in mice lacking GHSRs to the mice receiving Compound 26 (no potentiation of feeding with either the CS+ or the CS− cues), instead both the CS+ and the CS− potentiated feeding. While the reasons for this observed behavior are not yet clear, it does seem that GHSR-null mice form an abnormal association with the negative cue, resulting in enhanced food intake. It could be that GHSR-null mice have a harder time learning the specific association of a single cue with receiving food and automatically associate any cue in that particular environmental context with the receipt of food. Therefore, the GHSR-null mice may simply respond to any change in the environment, such as the short presentations of light, with an increase in food intake. Otherwise stated, the GHSR-null mice may have difficulties discriminating the two discrete cues, and therefore, cannot distinguish between the two as separate prompts.
Failure of GHSR-null mice to discriminate the CS+ and CS− cues makes sense from the standpoint of most of the reported effects of ghrelin on learning and memory. Learning deficits might also have contributed to the prolonged number of conditioning sessions taken by Compound 26-treated C57BL/6J mice to finish all of the pellets provided upon presentation of the CS+ during conditioning. .Previously, ghrelin-KO mice were shown to perform poorly in tests of behavioral memory such as novel object recognition, while ghrelin administration reverses these deficits [32]. Upon chronic caloric restriction, administration of ghrelin to supraphysiological levels improves novel object recognition in wild-type mice [46]. Direct microinjection of ghrelin into the hippocampus, which is a well-known learning and memory regulatory region, as well as into the amygdala, dose-dependently increase memory retention in wild-type mice [33]. Also, GHSR-KO mice have deficits in contextual fear conditioning, which is mediated by the amygdala [47–48]. Performance of GHSR-KO mice in the Morris water maze has been mixed, with one study showing reduced and another showing improved spatial learning [47, 49]. Regarding the hippocampus, GHSRs are found throughout all its regions, peripherally-administered radiolabeled ghrelin is taken up by the hippocampus, and ghrelin can increase hippocampal spine synapse density [32]. These findings are thought may be relevant to cue-potentiated feeding since a strong learning component is involved in the development of specific cue-food associations and reduced learning capacity may cause problems with cue discrimination.
Notwithstanding the above-described GHSR expression in and ghrelin action at the hippocampus, previous lesioning studies in rats have demonstrated that the BLA and mPFC are required for the cue-potentiated feeding response [11–12]. Thus the effects of GHSR deletion on cue-potentiated feeding likely also reflect direct and/or indirect effects of ghrelin at the BLA and/or mPFC. Any direct effects of ghrelin would necessitate GHSR expression at those sites. Here, GHSR expression was indeed localized to the BLA but not to the mPFC, using qPCR methodology. Of note, our previous in situ hybridization histochemistry characterization study of GHSR expression in the rat and mouse brains did not reveal amygdala expression [18], although this likely underrepresents the actual amygdala GHSR expression and is in contrast to several subsequent studies: GHSR expression has been localized to the rat amygdala by qPCR [50]; GHSR-IRES-tauGFP and GHSR-eGFP reporter mice display fluorescent signal in the amygdala [19, 51]; β-galactosidase expression occurs in the anterior cortical amygdala in GHSR-KO mice in which LacZ-reporter gene expression marks the sites of usual GHSR expression [52]. Importantly, ghrelin injection into the BLA increases memory retention in rats, and in humans, ghrelin modulates the amygdala in response to food cues [53–54].
While GHSR localization to the BLA does not prove that direct ghrelin action at the amygdala mediates ghrelin's effects on cue-potentiated feeding, we hypothesized that the abnormal behavioral responses of GHSR-null mice upon testing in the cuepotentiated feeding paradigm would manifest in the BLA. More specifically, we questioned whether BLA neuronal activation in response to the cues would parallel the cue-potentiated feeding behavior observed in wild-type and GHSR-null mice. Similar to previous studies with rats which demonstrated specific activation of BLA/basomedial amygdala neurons in response to a CS+ [13], here, elevations in Homer1a, Arc, and c-Fos mRNA levels in wild-type mice correlated with the timing of presumed neuronal activation during the CS+ test session and not the CS− session. In GHSR-null mice, the pattern of immediate early gene mRNA elevations correlated with BLA neuronal activation following presentation of both the CS+ and CS− cues. This suggests a non-discriminatory activation of the BLA in GHSR-null mice in response to cues, supporting the notion that GHSR deletion prevents formation of a specific association with the CS+, as otherwise occurs in wild-type mice. Importantly, the relative levels of Homer1a and c-Fos expression correlated with the number of pellets eaten in GHSR-null and wild-type mice, verifying the importance of the BLA in the expression of cue-potentiated feeding. Future studies should include those that can definitively confirm the requirement and sufficiency for GHSR expression in the BLA and/or other sites, such as the hippocampus or VTA, for ghrelin's effects on cue-potentiated feeding. .
While it is surprising that genetic GHSR deletion resulted in a potentiation of feeding with both cues rather than the complete absence of potentiated feeding as in mice receiving Compound 26, such may provide insight as to differences between life-long absence of GHSR expression and pharmacologic competitive GHSR antagonism in adults. A caveat of the GHSR-null mouse model is that these mice have developed without the presence of GHSR expression from inception. Such may impact neurodevelopment including that of compensatory pathways, which in turn could influence the cue-potentiated feeding behavior investigated here [55]. Fundamental differences between the two methods employed here to alter ghrelin/GHSR signaling may also be a result of differential effects of these methods on GHSR interactions with other cell surface receptors. As a prime example, recent developments into GHSR function have revealed the formation of heteromers between GHSR and subtype-2 dopamine receptors, and it was found that the presence of GHSRs influence D2 receptors independently of actual GHSR activity [56].
5. Conclusions
The multitude of food-related cues to which we are exposed may very well contribute to the obesity epidemic. The cue-potentiated feeding model is a useful tool for studying the neuroanatomical circuitry and molecular mechanisms that contribute to habitual eating behaviors. Blockade of ghrelin action by either GHSR antagonist administration or genetic GHSR deletion both disrupt the development of the usual cue-potentiated feeding response. However, while GHSR antagonist blocks potentiated feeding specifically in response to a positive conditioned stimulus, life-long GHSR deletion results in non-specific cue-food associations, as evidenced by potentiated feeding in response to both positive and negative conditioned stimuli. Although the sources of the observed discrepancies are unclear, these studies nonetheless demonstrate the importance of intact ghrelin signaling in the development of cue-potentiated feeding. Further studies are needed to better understand the degree to which and the mechanisms by which ghrelin influences this important feeding behavioral response.
Highlights
A novel cue-potentiated feeding protocol has been adapted for use in mice.
We examine the role of the peptide hormone ghrelin in cue-potentiated feeding.
Ghrelin receptor antagonist blocks feeding potentiated by a positive conditioned stimulus.
Ghrelin receptor-deficient mice eat in response to both negative and positive conditioned cues.
A role for ghrelin in establishing a specific positive cue-food association has been established.
Acknowledgements
The authors would like to acknowledge Sherri Osborne-Lawrence and Chelsea Migura for help with animal maintenance and Dr. Shari Birnbaum for helpful discussions regarding experimental design. This work was supported by funding from the NIH [T32DA7290 (to A.K.W.) and 1R01DA024680 (to J.M.Z.)] and a UTSW Medical Scientist Training Program Summer Undergraduate Research Fellowship (to I.E.I).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflict of interest.
References
- [1].Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- [2].Zimmerman FJ, Bell JF. Associations of television content type and obesity in children. Am J Public Health. 2010;100:334–40. doi: 10.2105/AJPH.2008.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harris JL, Pomeranz JL, Lobstein T, Brownell KD. A crisis in the marketplace: how food marketing contributes to childhood obesity and what can be done. Annu Rev Public Health. 2009;30:211–25. doi: 10.1146/annurev.publhealth.031308.100304. [DOI] [PubMed] [Google Scholar]
- [4].Saelens BE, Sallis JF, Frank LD, Couch SC, Zhou C, Colburn T, et al. Obesogenic neighborhood environments, child and parent obesity: the Neighborhood Impact on Kids study. Am J Prev Med. 2012;42:e57–64. doi: 10.1016/j.amepre.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol Behav. 2005;86:747–61. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cohen DA. Obesity and the built environment: changes in environmental cues cause energy imbalances. Int J Obes (Lond) 2008;32(Suppl 7):S137–42. doi: 10.1038/ijo.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, et al. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes (Lond) 2009;33:1063–73. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- [8].Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220:431–3. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- [9].Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur J Neurosci. 2003;17:1680–94. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- [10].Petrovich GD, Gallagher M. Control of food consumption by learned cues: a forebrain-hypothalamic network. Physiol Behav. 2007;91:397–403. doi: 10.1016/j.physbeh.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol Behav. 2002;76:117–29. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- [12].Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. J Neurosci. 2007;27:6436–41. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci. 2005;25:8295–302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- [15].Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- [16].Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–5. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- [17].Perello M, Scott MM, Sakata I, Lee CE, Chuang JC, Osborne-Lawrence S, et al. Functional implications of limited leptin receptor and ghrelin receptor coexpression in the brain. J Comp Neurol. 2012;520:281–94. doi: 10.1002/cne.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–48. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jiang H, Betancourt L, Smith RG. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol. 2006;20:1772–85. doi: 10.1210/me.2005-0084. [DOI] [PubMed] [Google Scholar]
- [20].Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–9. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- [21].Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry. 2010;67:880–6. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Skibicka KP, Hansson C, Egecioglu E, Dickson SL. Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addict Biol. 2012;17:95–107. doi: 10.1111/j.1369-1600.2010.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–37. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- [24].Disse E, Bussier AL, Veyrat-Durebex C, Deblon N, Pfluger PT, Tschop MH, et al. Peripheral ghrelin enhances sweet taste food consumption and preference, regardless of its caloric content. Physiol Behav. 2010;101:277–81. doi: 10.1016/j.physbeh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- [25].Shimbara T, Mondal MS, Kawagoe T, Toshinai K, Koda S, Yamaguchi H, et al. Central administration of ghrelin preferentially enhances fat ingestion. Neurosci Lett. 2004;369:75–9. doi: 10.1016/j.neulet.2004.07.060. [DOI] [PubMed] [Google Scholar]
- [26].Landgren S, Simms JA, Thelle DS, Strandhagen E, Bartlett SE, Engel JA, et al. The ghrelin signalling system is involved in the consumption of sweets. PLoS One. 2011;6:e18170. doi: 10.1371/journal.pone.0018170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Disse E, Bussier AL, Deblon N, Pfluger PT, Tschop MH, Laville M, et al. Systemic ghrelin and reward: effect of cholinergic blockade. Physiol Behav. 2011;102:481–4. doi: 10.1016/j.physbeh.2010.12.006. [DOI] [PubMed] [Google Scholar]
- [28].Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, et al. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121:2684–92. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D, Bohlooly YM, et al. Ghrelin increases intake of rewarding food in rodents. Addict Biol. 2010;15:304–11. doi: 10.1111/j.1369-1600.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–39. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Johnson AW, Canter R, Gallagher M, Holland PC. Assessing the role of the growth hormone secretagogue receptor in motivational learning and food intake. Behav Neurosci. 2009;123:1058–65. doi: 10.1037/a0016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–8. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- [33].Carlini VP, Varas MM, Cragnolini AB, Schioth HB, Scimonelli TN, de Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun. 2004;313:635–41. doi: 10.1016/j.bbrc.2003.11.150. [DOI] [PubMed] [Google Scholar]
- [34].Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–72. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rudolph J, Esler WP, O'Connor S, Coish PD, Wickens PL, Brands M, et al. Quinazolinone derivatives as orally available ghrelin receptor antagonists for the treatment of diabetes and obesity. J Med Chem. 2007;50:5202–16. doi: 10.1021/jm070071+. [DOI] [PubMed] [Google Scholar]
- [36].Esler WP, Rudolph J, Claus TH, Tang W, Barucci N, Brown SE, et al. Small-molecule ghrelin receptor antagonists improve glucose tolerance, suppress appetite, and promote weight loss. Endocrinology. 2007;148:5175–85. doi: 10.1210/en.2007-0239. [DOI] [PubMed] [Google Scholar]
- [37].Kurrasch DM, Huang J, Wilkie TM, Repa JJ. Quantitative real-time polymerase chain reaction measurement of regulators of G-protein signaling mRNA levels in mouse tissues. Methods Enzymol. 2004;389:3–15. doi: 10.1016/S0076-6879(04)89001-3. [DOI] [PubMed] [Google Scholar]
- [38].Besnard A, Bouveyron N, Kappes V, Pascoli V, Pages C, Heck N, et al. Alterations of molecular and behavioral responses to cocaine by selective inhibition of Elk-1 phosphorylation. J Neurosci. 2011;31:14296–307. doi: 10.1523/JNEUROSCI.2890-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Roloff AM, Anderson GR, Martemyanov KA, Thayer SA. Homer 1a gates the induction mechanism for endocannabinoid-mediated synaptic plasticity. J Neurosci. 2010;30:3072–81. doi: 10.1523/JNEUROSCI.4603-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kelly MP, Deadwyler SA. Experience-dependent regulation of the immediate-early gene arc differs across brain regions. J Neurosci. 2003;23:6443–51. doi: 10.1523/JNEUROSCI.23-16-06443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Knapska E, Radwanska K, Werka T, Kaczmarek L. Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol Rev. 2007;87:1113–73. doi: 10.1152/physrev.00037.2006. [DOI] [PubMed] [Google Scholar]
- [42].Vazdarjanova A, McNaughton BL, Barnes CA, Worley PF, Guzowski JF. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J Neurosci. 2002;22:10067–71. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Day HE, Kryskow EM, Nyhuis TJ, Herlihy L, Campeau S. Conditioned fear inhibits c-fos mRNA expression in the central extended amygdala. Brain Res. 2008;1229:137–46. doi: 10.1016/j.brainres.2008.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lederle L, Weber S, Wright T, Feyder M, Brigman JL, Crombag HS, et al. Reward-related behavioral paradigms for addiction research in the mouse: performance of common inbred strains. PLoS One. 2011;6:e15536. doi: 10.1371/journal.pone.0015536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].O'Connor EC, Stephens DN, Crombag HS. Modeling appetitive Pavlovian-instrumental interactions in mice. Curr Protoc Neurosci. 2010;Chapter 8(Unit 8):25. doi: 10.1002/0471142301.ns0825s53. [DOI] [PubMed] [Google Scholar]
- [46].Carlini VP, Martini AC, Schioth HB, Ruiz RD, Fiol de Cuneo M, de Barioglio SR. Decreased memory for novel object recognition in chronically food-restricted mice is reversed by acute ghrelin administration. Neuroscience. 2008;153:929–34. doi: 10.1016/j.neuroscience.2008.03.015. [DOI] [PubMed] [Google Scholar]
- [47].Albarran-Zeckler RG, Brantley AF, Smith RG. Growth hormone secretagogue receptor (GHS-R1a) knockout mice exhibit improved spatial memory and deficits in contextual memory. Behav Brain Res. 2012;232:13–9. doi: 10.1016/j.bbr.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem. 2001;8:148–55. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Davis JF, Choi DL, Clegg DJ, Benoit SC. Signaling through the ghrelin receptor modulates hippocampal function and meal anticipation in mice. Physiol Behav. 2011;103:39–43. doi: 10.1016/j.physbeh.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Landgren S, Engel JA, Hyytia P, Zetterberg H, Blennow K, Jerlhag E. Expression of the gene encoding the ghrelin receptor in rats selected for differential alcohol preference. Behav Brain Res. 2011;221:182–8. doi: 10.1016/j.bbr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- [51].Spencer SJ, Xu L, Clarke MA, Lemus M, Reichenbach A, Geenen B, et al. Ghrelin Regulates the Hypothalamic-Pituitary-Adrenal Axis and Restricts Anxiety After Acute Stress. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.03.010. [DOI] [PubMed] [Google Scholar]
- [52].Tong J, Mannea E, Aime P, Pfluger PT, Yi CX, Castaneda TR, et al. Ghrelin enhances olfactory sensitivity and exploratory sniffing in rodents and humans. J Neurosci. 2011;31:5841–6. doi: 10.1523/JNEUROSCI.5680-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–9. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- [54].Goshadrou F, Ronaghi A. Attenuating the effect of Ghrelin on memory storage via bilateral reversible inactivation of the basolateral amygdale. Behav Brain Res. 2012;232:391–4. doi: 10.1016/j.bbr.2012.03.035. [DOI] [PubMed] [Google Scholar]
- [55].Steculorum SM, Bouret SG. Developmental effects of ghrelin. Peptides. 2011;32:2362–6. doi: 10.1016/j.peptides.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73:317–32. doi: 10.1016/j.neuron.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]