Summary
Membrane-associated guanylate kinases (MAGUKs) are the major family of scaffolding proteins at the postsynaptic density. The PSD-MAGUK subfamily, which includes PSD-95, PSD-93, SAP97 and SAP102, is well accepted to be primarily involved in the synaptic anchoring of numerous proteins, including N-methyl-D-aspartate receptors (NMDARs). Notably, the synaptic targeting of NMDARs depends on the binding of the PDZ ligand on the GluN2B subunit to MAGUK PDZ domains as disruption of this interaction dramatically decreases NMDAR surface and synaptic expression. We recently reported a secondary interaction between SAP102 and GluN2B, in addition to the PDZ interaction. Here, we identify two critical residues on GluN2B responsible for the non-PDZ binding to SAP102. Strikingly, either mutation of these critical residues or knock-down of endogenous SAP102 can rescue the defective surface expression and synaptic localization of PDZ binding-deficient GluN2B. These data reveal an unexpected, non-scaffolding role for SAP102 in the synaptic clearance of GluN2B-containing NMDARs.
Introduction
NMDARs are ionotropic glutamate receptors that play important roles in excitatory neurotransmission, synaptic plasticity and neuronal development (Lau and Zukin, 2007). Precise regulation of NMDAR trafficking and synaptic localization is essential for these functions. NMDARs are localized at the postsynaptic membrane, and are stabilized through interactions with membrane-associated guanylate kinases (MAGUKs)(Wenthold et al., 2003). PSD-93, PSD-95, SAP97 and SAP102 are collectively known as PSD-MAGUKs and possess three PDZ domains, a Src homology 3 (SH3) domain and an inactive guanylate kinase (GK) domain (Elias and Nicoll, 2007). The PDZ domains bind to the C-termini of NMDARs, whereas the SH3 and GK domains interact with cytoskeletal proteins and intracellular signaling complexes. Although PSD-MAGUKs share a common modular structure, each family member possesses a distinct N-terminal domain. The N-termini of PSD-95, PSD-93 and SAP97 contain either a pair of palmitoylated cysteines that stabilize them at synapses or an L27 domain capable of multimerization (Schluter et al., 2006). The N-terminus of SAP102, however, is not palmitoylated and does not have an L27 domain and thus has an unknown function. Recently, we found that the N-terminus of SAP102 contains a GluN2B specific NMDAR binding site (Chen et al., 2011).
Functional NMDARs are heterotetramers assembled with two GluN1 subunits and two GluN2 (GluN2A-GluN2D) and/or GluN3 (GluN3A-GluN3B) subunits. The GluN2 subunits have distinct expression patterns with GluN2A and GluN2B being the major GluN2 subunits in the forebrain. The GluN2 content of NMDARs determines their channel properties, as well as their coupling to distinct intracellular signaling cascades (Cull-Candy and Leszkiewicz, 2004). During development, GluN2B is predominantly expressed in immature neurons and the expression of GluN2A gradually increases, leading to a synaptic switch from GluN2B- to primarily GluN2A-containing NMDARs. In mature neurons, GluN2A-containing NMDARs are primarily localized at synapses, whereas GluN2B-containing receptors are still present at synapses, but also enriched at extrasynaptic sites (Li et al., 2002; Stocca and Vicini, 1998; Tovar and Westbrook, 1999). In addition, GluN2B-containing NMDARs undergo more robust endocytosis (Lavezzari et al., 2004; Roche et al., 2001) and have higher surface mobility than GluN2A-containing receptors (Groc et al., 2006). Biochemical studies have shown that GluN2A preferentially binds to PSD-95 and GluN2B preferentially binds to SAP102 (Sans et al., 2000; van Zundert et al., 2004; although see Al-Hallaq et al., 2007), and it has been proposed that PSD-95 and SAP102 play a role in the subunit-specific regulation of receptor trafficking and localization (van Zundert et al., 2004). Interestingly, SAP102 is not palmitoylated and is highly mobile at the postsynaptic density, similar to GluN2B (Zheng et al., 2010).
GluN2A and GluN2B share an identical PDZ ligand. However, we have recently identified a secondary non-PDZ GluN2B binding site in the N-terminal domain of SAP102, which might allow for preferential binding of SAP102 and GluN2B (Chen et al., 2011). In the present study, we investigated the role of the PDZ-independent interaction of GluN2B with SAP102 in NMDAR trafficking. We identify two amino acids within the GluN2B C terminus (D1391; D1392) that are critical for binding to the SAP102 N-terminal domain.
Mutating the PDZ ligand on GluN2B profoundly reduces surface expression of NMDARs (Chung et al., 2004; Prybylowski et al., 2005) and the activity-dependent phosphorylation of GluN2B within the PDZ ligand by casein kinase 2 (CK2) drives the removal of GluN2B from the synapse (Sanz-Clemente et al., 2010). We now show that disruption of the secondary SAP102 binding site on GluN2B unexpectedly and dramatically rescues the surface and synaptic expression of PDZ binding-deficient GluN2B. Furthermore, RNAi knock-down of SAP102 also rescued the surface and synaptic expression defect of the GluN2B PDZ ligand mutant. Together, our findings reveal an unexpected role for the PDZ-independent interaction between SAP102 and GluN2B in mediating the synaptic clearance of GluN2B-containing NMDARs.
Results
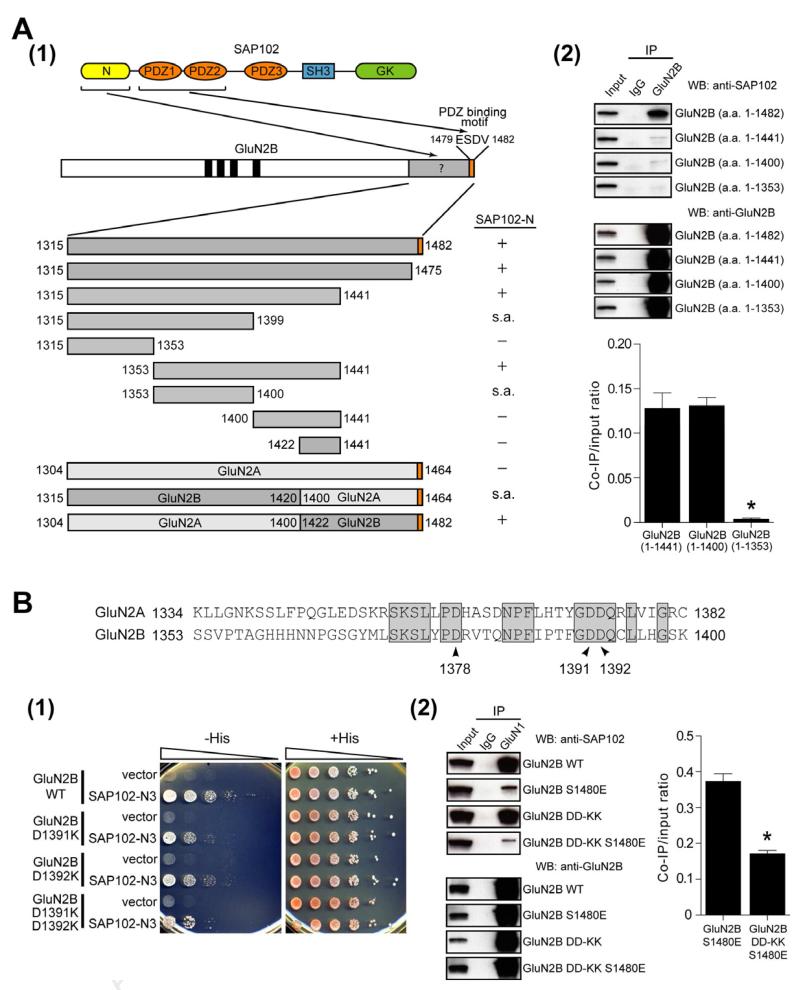
To identify the PDZ-independent SAP102 binding site in GluN2B, we analyzed a series of GluN2B truncations using a yeast two hybrid assay. We found a region of the GluN2B C-terminus (amino acids 1353-1441) that is required for the non-PDZ interaction (Figure 1A), but attempts to delineate this region further were limited by self-activation of the yeast two-hybrid system. We next generated GluN2A-GluN2B chimeras and found that the GluN2A (1304-1400) - GluN2B (1422-1482) chimera (Figure 1A) interacted with the SAP102 N-terminal domain (Figure 1A), suggesting a short region in GluN2B (1422-1441) is critical for the interaction. Surprisingly, however, this region alone did not interact with the N-terminus of SAP102. We therefore postulated that the adjacent region of GluN2B (1353-1400) is a key molecular determinant for binding to the SAP102 N-terminal domain, whereas 1422-1441 is simply permissive but cannot interact independently. Consistently, we found that GluN2B (1-1441) and GluN2B (1-1400), but not GluN2B (1-1353), co-immunoprecipitated with SAP102 (Figures 1A and S1). Based on the chimeras, we hypothesized that the critical residues in the GluN2B (1353-1400) segment must be conserved between GluN2A and GluN2B, but that these residues only interact with the SAP102 N-terminus when the GluN2B (1422-1441) region is also present. To test this possibility, we made specific amino acid substitutions within GluN2B (1353-1400), which are identical in the analogous region of GluN2A (1304-1400) (Figure 1B). We first targeted several charged residues and found that the GluN2B D1391K and D1392K mutations (Figure 1B), but not D1378K (Figure S1), disrupted the PDZ-independent binding to SAP102. Moreover, the GluN2B D1391K and D1392K (GluN2B DD-KK) double mutant further reduced the interaction (Figure 1B). We also examined the PDZ-independent interaction using a coimmunoprecipitation assay in HEK-293 cells expressing GluN1, GluN2B DD-KK or GluN2B DD-KK S1480E and SAP102. We found that GluN2B DD-KK S1480E showed a 54% reduction in SAP102 binding compared with GluN2B S1480E (Figure 1B), demonstrating that GluN2B D1391 and D1392 are involved in the interaction of GluN2B with the SAP102 N-terminus.
Figure 1. Two critical residues in GluN2B regulate the PDZ-independent interaction with SAP102.
(A) (1) Schematics of PDZ-dependent and PDZ-independent interactions between GluN2B and SAP102. Various constructs are shown aligned under full-length GluN2B. The interaction of GluN2B constructs with the N-terminal domain of SAP102 are shown, as measured by the yeast two-hybrid binding assay. s.a. indicates there was self activation of the expression construct so the interaction assay could not be performed. (2) HEK-293 cells were transfected with GFP-GluN2B (a.a. 1-1482), GFP-GluN2B (a.a. 1-1441), GFP-GluN2B (a.a. 1-1400) or GFP-GluN2B (a.a. 1-1353) and SAP102. Receptors were immunoprecipitated from cell lysates with anti-GluN2B antibodies or IgG antibodies as a negative control. Immunoprecipitates were resolved by SDS-PAGE and immunoblotted with anti-SAP102 or anti-GluN2B antibodies. Input = 10 % of total cell lysate. The data were quantified by measuring co-IP/input SAP102 band intensity ratios using ImageJ software. Data represent means ± SEM (N = 3 independent experiments). See also Figure S1. (B) An alignment of GluN2A (1334-1382) and GluN2B (1353-1400) are shown. GluN2B D1378, D1391 and D1392 are indicated with arrowheads. The N3 domain of SAP102 (101-148) is the minimum region required for the non-PDZ interaction (Chen et al., 2011) (1) Yeast were co-transformed with LexA-GluN2B, LexA-GluN2B D1391K, LexA-GluN2B D1392K, or LexA-GluN2B D1391K D1392K and Gal4 vector or Gal4-SAP102-N3, and growth was evaluated on appropriate yeast selection medium. Results shown are 10-fold serial dilutions of yeast cells. (2) HEK-293 cells were transfected with GluN1, SAP102 and GluN2B constructs. Receptors were immunoprecipitated from cell lysates with anti-GluN1 antibodies or IgG antibodies as a negative control. Immunoprecipitates were immunoblotted with anti-SAP102 or anti-GluN2B antibodies. Input = 5 % of total cell lysate. The data were quantified by measuring Co-IP/input SAP102 band intensity ratios using ImageJ software. Data represent means ± SEM (N = 3 independent experiments; *p<0.01). See also Figure S1.
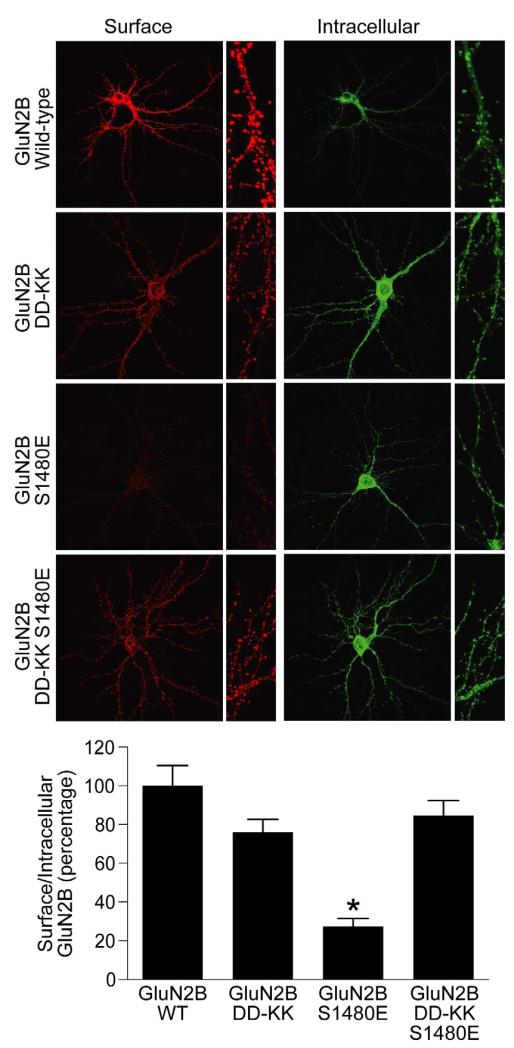
Phosphorylation of Ser1480 within the GluN2B PDZ-binding motif by CK2 disrupts the interaction of GluN2B with PSD-95 and SAP102 and decreases surface expression of GluN2B (Chung et al., 2004). However, the role of the PDZ-independent interaction of GluN2B with SAP102 in NMDAR trafficking is not known. Therefore, we used the GluN2B DD-KK double mutant to study whether the PDZ-independent interaction with SAP102 regulates surface expression of GluN2B. We expressed GFP-GluN2B WT or GFP-GluN2B DD-KK in hippocampal neurons and visualized surface-expressed receptors with an anti-GFP antibody. Surface expression of GFP-GluN2B DD-KK was similar to that of GFP-GluN2B WT (Figure 2). We then investigated whether disruption of both the PDZ and PDZ-independent interactions could affect NMDAR trafficking. To this end, we mutated Ser1480 of GluN2B to glutamate to mimic phosphorylation of Ser1480, which disrupts the PDZ binding of GluN2B and examined the surface expression of GFP-GluN2B S1480E and a combined GFP-GluN2B DD-KK S1480E mutant. Consistent with previous reports (Chung et al., 2004), surface expression of GFP-GluN2B S1480E was dramatically reduced compared to WT (Figure 2). Strikingly, surface expression was recovered with GFP-GluN2B DD-KK S1480E and was similar to wild-type GluN2B (Figure 2), demonstrating that DD-KK mutations, which disrupt binding to the SAP102 N-terminus, rescue the surface expression defect of GFP-GluN2B S1480E.
Figure 2. DD-KK mutations rescue the surface expression of GluN2B S1480E in neurons.
Hippocampal neurons were transfected with GluN2B constructs containing an extracellular GFP tag. Surface staining was performed with anti-GFP and Alexa 568-conjugated (red) anti-rabbit secondary antibody, followed by fixation and permeabilization, and the internal pool of receptors was labeled with anti-GFP and Alexa 488-conjugated (green) anti-rabbit secondary antibody. Data represent means ± SEM. (n=27; *p<0.01) (N = 3 independent experiments). See also Figure S2.
Disruption of an endocytic motif (YEKL) near the C-terminus of GluN2B that binds to the clathrin adaptor protein complex AP-2 also restores the surface expression of GluN2B that lacks PDZ-binding (Prybylowski et al., 2005; Sanz-Clemente et al., 2010) Thus, to determine if the surface expression rescue seen with the GluN2B DD-KK S1480E mutant (Figure 2) is due to decreased AP-2 binding, we used a yeast two-hybrid binding assay to examine the interaction of GluN2B with μ2, the medium chain of AP-2 that binds to the GluN2B YEKL motif (Lavezzari et al., 2003). However, the DD-KK mutations had no effect on GluN2B binding to μ2 (Figure S2), showing that the rescue of the surface expression of GluN2B S1480E is not mediated by the disruption of AP-2 binding.
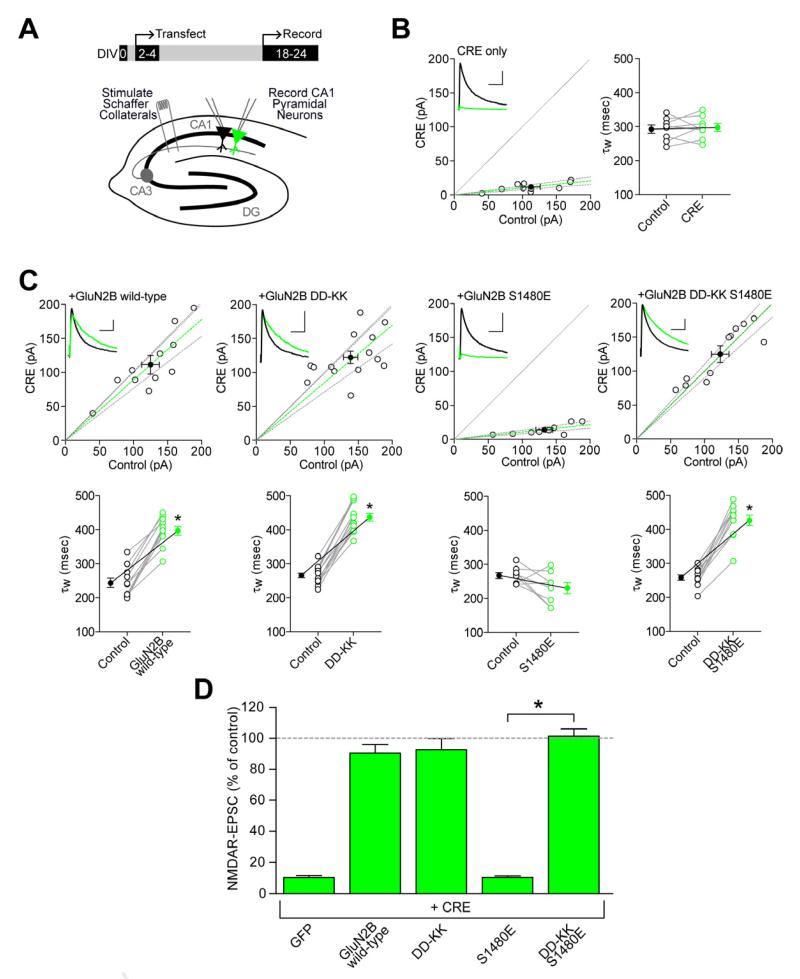
To physiologically assess the effects of GluN2B mutations on the synaptic localization of NMDARs, we developed a genetic molecular replacement strategy in organotypic hippocampal slice cultures prepared from mice with conditional knock-out alleles for both GluN2A and GluN2B (Grin2afl/flGrin2bfl/fl)(Granger et al., 2011). We have previously shown that neonatal injection of a Cre-expressing virus into the hippocampus of Grin2afl/flGrin2bfl/fl mice completely eliminates synaptic NMDAR responses in CA1 pyramidal neurons by postnatal day 14, suggesting that GluN2A and GluN2B account for all synaptic NMDARs in these cells (Gray et al., 2011). Here, hippocampal slice cultures prepared from the Grin2afl/flGrin2bfl/fl mice were biolistically transfected with Cre on DIV2-4, and paired whole-cell recordings were obtained from a Cre-expressing and a neighboring control cell at DIV18-24 (Figure 3A). Simultaneous paired dual-cell recordings allow for a rigorous, quantitative study of the postsynaptic effects of the genetic manipulation while controlling for presynaptic inputs. As shown in Figure 3B, two weeks of Cre expression reduced the NMDAR-mediated excitatory postsynaptic current (EPSC) by approximately 90% of control cell levels. Longer incubation prior to recording would likely reduce the EPSC further, though slice health becomes limiting. Importantly, the duration of NMDAR-EPSC decays (τW) of the small remaining currents were not different from control (Figure 3B), suggesting that this small population of NMDARs is similar in composition to control cells. When Cre was co-expressed with a wild-type GluN2B construct, there was nearly complete recovery (90%) of the NMDAR-EPSC, with the expected lengthening of the decay kinetics from an introduced population of purely GluN2B-containing NMDARs (Figure 3C,D).
Figure 3. Disruption of non-PDZ SAP102 binding rescues the synaptic targeting of PDZ-deficient GluN2B.
(A) Organotypic hippocampal slice cultures were made from P7 Grin2afl/flGrin2bfl/fl mice, biolistically transfected on DIV2-4, and paired whole-cell recordings were obtained from Cre-expressing and neighboring CA1 pyramidal neurons on DIV18-24. (B-C) Scatter plots of peak amplitudes of NMDAR-EPSCs from single pairs (open circles) and mean ± SEM (filled circles) from transfected and control cells. Dashed lines represent linear regression and 95% confidence interval. Sample traces are as follows: control cell, black; transfected cell, green; scale bars represent 100 msec and 40 pA. NMDAR-EPSC decay times expressed in msec as a weighted tau (τw) from paired transfected and control cells. (B) Transfection with Cre alone. (C) Co-transfection of Cre with wild-type GluN2B, GluN2B DD-KK, GluN2B S1480E or the double GluN2B mutant, DD-KK S1480E. Decay kinetics were analyzed by a paired Student’s t-test, *p<0.0001. (D) Summary graph of data. Bars represent the mean ± SEM of the ratios of transfected to control cells from each pair, expressed as percentages. Data were analyzed by the Mann-Whitney U test, *p<0.0001 compared with GluN2B-S1480E. Actual values for all data can be found in Supplemental Table S1.
To examine the role of the PDZ-independent interaction of GluN2B with SAP102 in the synaptic localization of NMDARs, we co-expressed Cre with the GluN2B DD-KK mutant. Consistent with the surface expression studies in dispersed neuronal cultures, the DD-KK mutant had synaptic NMDAR currents similar to wild-type, whereas GluN2B S1480E did not increase synaptic current over Cre-alone (Figure 3C,D), suggesting a complete exclusion of GluN2B S1480E from synapses. Remarkably, adding the DD-KK mutation to the S1480E mutant completely rescued the synaptic localization of NMDARs (Figure 3C,D). Taken together, these results show that the secondary, PDZ-independent, SAP102 binding site on GluN2B precisely regulates the synaptic localization of NMDARs.
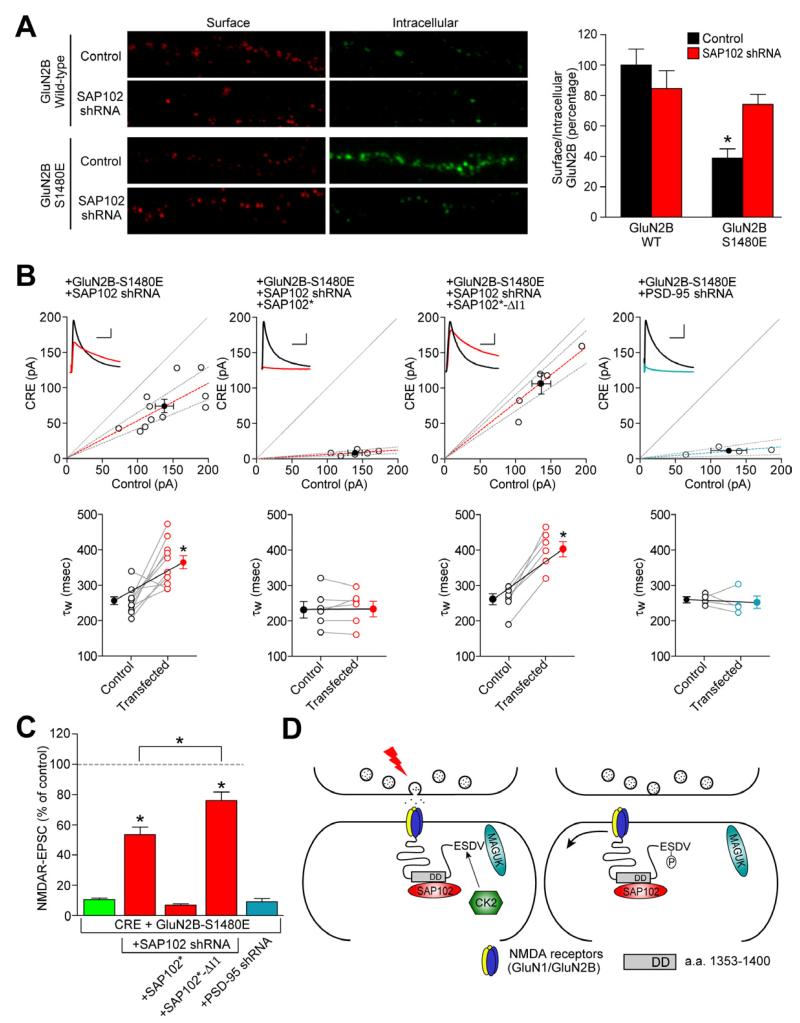
We next investigated if SAP102 knock-down can also rescue the surface expression defect of GluN2B S1480E. We generated an shRNA that efficiently reduced expression of SAP102 in dissociated cortical neurons (Figure S3) and transduced neurons with lentivirus expressing the SAP102 shRNA at DIV5 and expressed FLAG-GluN2B (WT or S1480E) at DIV10. We observed reduced surface expression for the GluN2B S1480E mutant (Figure 4A). Notably, however, surface expression of GluN2B S1480E was markedly increased upon SAP102 knock-down, whereas there was no significant change in GluN2B WT surface expression (Figure 4A). Using an shRNA against mouse SAP102 (Figure S3), we knocked-down SAP102 in the mouse hippocampal slice culture system. Co-expressing mouse SAP102 shRNA with the GluN2B S1480E mutant rescued approximately 50% of the synaptic NMDAR-EPSC (Figure 4B,C). This rescue was completely reversed by co-expression of an shRNA-proofed full-length SAP102, but not a SAP102 splice variant lacking the I1 cassette in the N-terminal domain (Figure 4B,C) (Chen et al., 2011). Importantly, knockdown of PSD-95 did not rescue the synaptic expression of GluN2B S1480E (Figures 4B,C and S3). Taken together, these results convincingly demonstrate a critical role for the PDZ-independent SAP102 binding site on GluN2B in the synaptic removal of PDZ binding-deficient GluN2B.
Figure 4. SAP102 controls GluN2B synaptic expression.
(A) SAP102 was knocked-down in hippocampal cultures by the lentiviral induction of a specific shRNA at DIV5 and Flag-tagged GluN2B constructs (wt or S1480E) were transfected at DIV10. Surface staining was performed with anti-Flag and Alexa-568 secondary antibodies (red) and, after permeabilization, and the intracellular pool was labeled with anti-Flag and Alexa-663 secondary antibodies (green). n for GluN2B wt (+/− shRNA) = 26, 26; n for S1480E (+/− shRNA)= 35, 32 (N = 4 independent experiments). Data represent means ± SEM. *p<0.001. See also Figure S3. (B) Co-transfection of Cre with GluN2B S1480E, shRNA against mouse SAP102, shRNA-proof SAP102 variants (SAP102*), or shRNA against mouse PSD-95 in hippocampal slice cultures from P7 Grin2afl/flGrin2bfl/fl mice. Top, scatter plot of peak amplitudes of NMDAR-EPSCs from single pairs (open circles) and mean ± SEM (filled circles) from transfected and control cells. Dashed lines represent linear regression and 95% confidence interval. Sample traces are as follows: control cell, black; transfected cell, red; scale bars represent 100 msec and 40 pA. Bottom, NMDAR-EPSC decay times expressed as a weighted tau (τw) from paired transfected and control cells. Decay kinetics were analyzed by a paired Student’s t-test. See also Figure S3. (C) Bar graph represents mean ± SEM of the ratios of transfected to control cells from each pair, expressed as percentages (GluN2B S1480E is from Figure 3). Data were analyzed by the Mann-Whitney U test, *p<0.0001 compared with GluN2B-S1480E or as indicated. Actual values for all data can be found in Supplemental Table S1. (D) Model for the role of SAP102 in regulating GluN2B-containing NMDARs at the synapse. GluN2B-containing NMDARs are stabilized on synaptic membranes through the PDZ interactions with PSD-MAGUKs (PSD-95, PSD-93, SAP97 or SAP102) and non-PDZ interactions with SAP102. Synaptic activity induces GluN2B phosphorylation on S1480 by CK2, which disrupts the PDZ interaction between synaptic NMDARs and MAGUKS at the post-synaptic density. The secondary interaction of GluN2B with the N-terminus of SAP102 then facilitates the lateral diffusion of NMDARs to perisynaptic endocytic zones.
Discussion
In this study, we have revealed a novel mechanism for the subunit-specific regulation of NMDARs. First, we identify two critical residues in the C-terminus of GluN2B (DD-KK mutation) responsible for the GluN2B interaction with the SAP102 N-terminus. Second, we show that mutation of these two critical residues rescues the surface expression defect of PDZ binding-deficient GluN2B. Third, we find that knocking down SAP102 also rescues the surface expression defect of PDZ binding-deficient GluN2B, providing powerful evidence that the DD-KK mutation on the GluN2B C-termini regulates surface expression via specific binding to SAP102. Thus, the PDZ-independent interaction between GluN2B and SAP102 plays an important role in the trafficking and synaptic localization of the GluN2B-containing NMDARs. These findings support a model in which SAP102 acts as an adaptor to regulate the lateral movement of GluN2B-containing NMDARs from the synaptic to extrasynaptic membrane (Figure 4D).
During development, the subunit composition of synaptic NMDARs changes from mainly GluN2B-containing receptors to primarily GluN2A-containing receptors. This subunit switch is activity-dependent and underlies changes in the functional properties of NMDARs including acceleration of the kinetics of NMDAR-mediated EPSCs and increases in the channel open probabilities (Barria and Malinow, 2002; Bellone and Nicoll, 2007; Gray et al., 2011; Philpot et al., 2001; Sanz-Clemente et al., 2010). In addition, NMDARs are rapidly exchanged between synaptic and extrasynaptic sites through lateral diffusion in immature neurons (Tovar and Westbrook, 2002). It is clear that the subunit composition of synaptic NMDARs can undergo rapid and bidirectional switching depending on the pattern of NMDAR activation at neonatal synapses (Bellone and Nicoll, 2007; Matta et al., 2011). However, the molecular mechanisms underlying the differential NMDAR trafficking during development and synaptic plasticity are unclear. It is clear that both phosphorylation, and protein-protein interactions regulate subunit-specific NMDAR trafficking (Chen and Roche, 2007). In this study, we have uncovered a specific mechanism, which regulates the trafficking and synaptic targeting of GluN2B-containing NMDARs.
PSD-MAGUKs act as scaffolding proteins at the postsynaptic density and organize various signal transduction cascades. Each family member has unique properties, but they can functionally compensate for each other (Elias and Nicoll, 2007). The expression of PSD-MAGUKs is differentially regulated during development. For example, SAP102 is expressed early and is dominant for trafficking and anchoring NMDARs at immature synapses (Sans et al., 2003; Washbourne et al., 2004), whereas PSD-95 is expressed later and is involved in maturation and stabilization of excitatory synapses (El-Husseini et al., 2000). Although the synaptic function of PSD-95 has been extensively studied, there are relatively few studies on SAP102. Mutations in the human gene encoding SAP102 have been reported to cause mental retardation (Tarpey et al., 2004; Zanni et al., 2010), indicating that SAP102 plays a crucial role in synaptic function. Indeed, mice lacking SAP102 display specific impairments in synaptic plasticity and show cognitive deficits (Cuthbert et al., 2007). However, how SAP102 regulates synaptic function remains elusive. Using fluorescence recovery after photobleaching (FRAP), studies have shown that the majority of SAP102 is highly mobile in dendritic spines and the mobility is rapidly regulated by synaptic activity (Zheng et al., 2010). In contrast, only about a third of PSD-95 is mobile and the mobility is not affected by synaptic activity. Interestingly, studies using single molecule/particle approaches have provided evidence that surface GluN2A-containing NMDARs are much less mobile compared to surface GluN2B-containing receptors (Groc et al., 2006). These results are consistent with earlier studies showing preferential binding between GluN2B and SAP102 (Sans et al., 2000). We have shown that the SAP102 N-terminus contains an NMDAR binding site specific for GluN2B (Chen et al., 2011). We now have been able to disrupt this non-PDZ binding by mutating two critical residues in the GluN2B C-terminus (DD-KK), although it remains unclear if these residues represent a direct binding site or if the mutations result in disruptive conformational changes. Furthermore, we demonstrate that the specific interaction between GluN2B and SAP102 is important for trafficking of GluN2B-containing NMDARs out of the synapse. Based on our findings, it is likely that the high surface mobility of GluN2B is due to the preferential binding to SAP102, which is extremely mobile in spines.
Glutamate receptor trafficking plays a major role in controlling the number and type of functional receptors during synaptic plasticity. Although endocytosis and exocytosis play a critical role in trafficking of glutamate receptors and synaptic plasticity, lateral diffusion is also important (Cognet et al., 2006; Kennedy and Ehlers, 2006). Endocytic proteins, such as clathrin, AP-2 and dynamin, are localized lateral to the PSD, forming “endocytic zones” allowing efficient endocytosis of receptors that diffuse away from synapses (Racz et al., 2004). Therefore, it has been suggested that removal of synaptic receptors is a two-step process: uncoupling of receptors from the PSD, followed by lateral movement to an endocytic zone (Racz et al., 2004). Our studies identify a function of SAP102 in regulating internalization of GluN2B-containing receptors. Our model is consistent with the two-step process of internalization (Figure 4D). First, phosphorylation of GluN2B on S1480 within the PDZ ligand by CK2 disrupts the interaction between synaptic NMDARs and synaptic MAGUKs, such as PSD-95. Second, GluN2B bound to SAP102 through the non-PDZ binding is laterally diffused to the endocytic zone. Extrasynaptic GluN2B is dephosphorylated on Y1472, associates with the AP-2-clathrin endocytic complex, and undergoes endocytosis. Our findings on the subunit-specific interactions between GluN2B and SAP102 provide insight into MAGUK-specific roles in regulating synaptic NMDARs, which were previously unappreciated.
Experimental Procedures
Yeast two-hybrid assays were performed using L40 yeast strain co-transformed with pBHA and pGAD10 constructs. Immunoprecipitation (IP) was performed at 24 h after transfection with indicated antibodies. Immunocytochemistry assays were performed in 14 DIV dissociated hippocampal cultures 2 days after transfection with GluN2B constructs. Hippocampi were dissected from P7 Grin2afl/flGrin2bfl/fl mice and co-transfected after 2-4 days in culture with pFUGW-Cre:mCherry and either pCAG-GFP or pCAG-GluN2B-IRES-GFP or mutants. Slices were cultured for an additional 14-20 days and dual whole-cell patch-clamp recordings were performed from neighboring CA1 pyramidal cells. NMDAR-EPSCs were recorded at +40mV in the presence of 10 μM NBQX. Further details available in the Supplemental Experimental Procedures.
Supplementary Material
Acknowledgements
We thank the NINDS sequencing facility and light imaging facility. The research was supported by the NINDS Intramural Research Program (B.-S.C., A.S.-C., E.V.T. and K.W.R.), a NINDS Career Transition Award (E.V.T., Z.W. and B.-S.C.), grants from the NIMH (J.A.G. and R.A.N.). J.A.G. is funded by a NARSAD Young Investigator Award and is the NARSAD Hammerschlag Family Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci. 2007;27:8334–8343. doi: 10.1523/JNEUROSCI.2155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55:779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BS, Thomas EV, Sanz-Clemente A, Roche KW. NMDA receptor-dependent regulation of dendritic spine morphology by SAP102 splice variants. J Neurosci. 2011;31:89–96. doi: 10.1523/JNEUROSCI.1034-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Huang YH, Lau LF, Huganir RL. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci. 2004;24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognet L, Groc L, Lounis B, Choquet D. Multiple routes for glutamate receptor trafficking: surface diffusion and membrane traffic cooperate to bring receptors to synapses. Sci STKE. 2006;2006:pe13. doi: 10.1126/stke.3272006pe13. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Cuthbert PC, Stanford LE, Coba MP, Ainge JA, Fink AE, Opazo P, Delgado JY, Komiyama NH, O’Dell TJ, Grant SG. Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies. J Neurosci. 2007;27:2673–2682. doi: 10.1523/JNEUROSCI.4457-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17:343–352. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Granger AJ, Gray JA, Lu W, Nicoll RA. Genetic analysis of neuronal ionotropic glutamate receptor subunits. J Physiol. 2011;589:4095–4101. doi: 10.1113/jphysiol.2011.213033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll RA. Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron. 2011;71:1085–1101. doi: 10.1016/j.neuron.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103:18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci. 2006;29:325–362. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Lee R, Roche KW. Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharmacology. 2003;45:729–737. doi: 10.1016/s0028-3908(03)00308-3. [DOI] [PubMed] [Google Scholar]
- Li B, Chen N, Luo T, Otsu Y, Murphy TH, Raymond LA. Differential regulation of synaptic and extra-synaptic NMDA receptors. Nat Neurosci. 2002;5:833–834. doi: 10.1038/nn912. [DOI] [PubMed] [Google Scholar]
- Matta JA, Ashby MC, Sanz-Clemente A, Roche KW, Isaac JT. mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron. 2011;70:339–351. doi: 10.1016/j.neuron.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz B, Blanpied TA, Ehlers MD, Weinberg RJ. Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci. 2004;7:917–918. doi: 10.1038/nn1303. [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Prybylowski K, Petralia RS, Chang K, Wang YX, Racca C, Vicini S, Wenthold RJ. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat Cell Biol. 2003;5:520–530. doi: 10.1038/ncb990. [DOI] [PubMed] [Google Scholar]
- Sanz-Clemente A, Matta JA, Isaac JT, Roche KW. Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron. 2010;67:984–996. doi: 10.1016/j.neuron.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter OM, Xu W, Malenka RC. Alternative N-terminal domains of PSD-95 and SAP97 govern activity-dependent regulation of synaptic AMPA receptor function. Neuron. 2006;51:99–111. doi: 10.1016/j.neuron.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol. 1998;507(Pt 1):13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpey P, Parnau J, Blow M, Woffendin H, Bignell G, Cox C, Cox J, Davies H, Edkins S, Holden S, et al. Mutations in the DLG3 gene cause nonsyndromic X-linked mental retardation. Am J Hum Genet. 2004;75:318–324. doi: 10.1086/422703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- van Zundert B, Yoshii A, Constantine-Paton M. Receptor compartmentalization and trafficking at glutamate synapses: a developmental proposal. Trends Neurosci. 2004;27:428–437. doi: 10.1016/j.tins.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Liu XB, Jones EG, McAllister AK. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J Neurosci. 2004;24:8253–8264. doi: 10.1523/JNEUROSCI.2555-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003;43:335–358. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- Zanni G, van Esch H, Bensalem A, Saillour Y, Poirier K, Castelnau L, Ropers HH, de Brouwer AP, Laumonnier F, Fryns JP, Chelly J. A novel mutation in the DLG3 gene encoding the synapse-associated protein 102 (SAP102) causes non-syndromic mental retardation. Neurogenetics. 2010;11:251–255. doi: 10.1007/s10048-009-0224-y. [DOI] [PubMed] [Google Scholar]
- Zheng CY, Petralia RS, Wang YX, Kachar B, Wenthold RJ. SAP102 is a highly mobile MAGUK in spines. J Neurosci. 2010;30:4757–4766. doi: 10.1523/JNEUROSCI.6108-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.