Abstract
Background:
There is conflicting literature regarding the association of the BRAF V600E mutation and aggressive clinicopathological features of papillary thyroid cancer (PTC). Nevertheless, some propose that BRAF status be incorporated into the management of patients with PTC, specifically recommendations regarding lymph node dissection. We therefore performed a meta-analysis to examine the relationship between BRAF and clinicopathological features of PTC.
Methods:
A literature search was performed within PubMed and EMBASE databases using the following Medical Subject Headings (MeSH) and keywords: “braf,” “mutation,” “thyroid,” “neoplasm(s),” “tumor,” “cancer,” and “carcinoma.” Individual study-specific odds ratios and confidence intervals were calculated, as were Mantel-Haenszel pooled odds ratios for the combined studies.
Results:
Thirty-two studies including 6372 patients were reviewed. BRAF mutation was associated with lymph node metastases (LNM), advanced stage, extrathyroidal extension, tumor size, male gender, multifocality, absence of capsule, classic PTC, and tall-cell variant PTC. There was no association with age or vascular invasion. Only two studies were prospective; nine included consecutive patients, whereas one included randomly selected patients; and only two included patients who had undergone routine central lymph node dissection and were thus evaluable for the presence of LNM.
Conclusion:
Meta-analysis found that BRAF mutation is associated with LNM, stage, extrathyroidal extension, tumor size, male gender, multifocality, absence of capsule, classic PTC, and tall-cell variant PTC in PTC. However, almost all studies were retrospective and only two of 32 included patients who had undergone routine central lymph node dissection, emphasizing the need for well-designed studies to appropriately examine this association before making important clinical decisions.
The incidence of thyroid cancer has significantly increased over the past three decades with an annual increase of 6.4% from 1997–2008 and with 11.0 per 100,000 men and women now presenting annually (seer.cancer.gov). The most common form of thyroid cancer is papillary thyroid cancer (PTC), comprising 65–88% of all differentiated thyroid cancers (1). Although the overall 10-yr survival rate for patients with PTC is high (>90%), 5–10% of PTC patients will experience regional recurrences and 10–15% will experience distant metastases with an associated overall 10-yr survival rate of only 40% (2).
Although multiple prognostic staging systems exist to help stratify differentiated thyroid cancers, they generally use final histopathological parameters and therefore cannot be applied preoperatively to determine initial surgical management (3). Furthermore, and relevant to this, the extent of surgical management recommended for patients with less advanced disease remains controversial. Some suggest that the optimal surgical management of patients with PTC and no clinically evident lymph node metastases should include a total thyroidectomy with bilateral prophylactic level VI lymph node compartment dissection (4). Others, however, put forth the fact that no prospective randomized trial has been published that demonstrates the benefits of prophylactic central neck dissection in terms of local recurrence or survival rates (5). Because several features of PTC, including histology, grade, metastases, and completeness of surgery, used by the various prognostic systems [AGES (age, grade, extent, size); AMES (age, metastasis, extent, size); OHU (Ohio State University); and TNM (tumor, node, metastasis)] can only be determined postoperatively, the appropriate surgical management of patients with PTC continues to remain debatable (6). Therefore, there is a great need for more accurate preoperative risk stratification systems to inform the initial management of patients with PTC.
The BRAF V600E mutation composed of a T to A transversion, is found in up to 73.4% of PTCs (7) and results in the substitution of valine for glutamate at position 600 of the b-raf protein. The V600E mutant BRAF constitutively activates the MAPK pathway, thus stimulating tumorigenesis. Since its initial discovery, BRAF V600E has emerged as a promising diagnostic as well as prognostic indicator of PTC.
In 2007, Lee et al. (8) published a meta-analysis examining the clinicopathological significance of BRAF V600E in PTC. They found that BRAF mutation was associated with histological subtype, the presence of extrathyroidal extension (ETE), and higher clinical stage but not with age, sex, race, or tumor size. Twelve articles published before July 2006 were included in this study. Since July 2006, numerous additional studies have been performed to further investigate the prognostic value of BRAF mutation in PTC. However, many of these studies report contradictory results regarding the association of the BRAF V600E mutation status and prognosis. Although some studies report that BRAF mutation is associated with multiple aggressive clinicopathological features including higher rate of ETE (9–12) and lymph node metastases (10–13), other studies fail to report any significant association between the mutation and aggressive clinicopathological features (14, 15). Despite these discrepancies that exist in the literature, some have already proposed that BRAF V600E mutation detection be incorporated into the management algorithm of patients with PTC, specifically, that patients who have BRAF-positive tumors on fine-needle aspiration (FNA) undergo a prophylactic central lymph node dissection (LND) (16, 17). Because of these recent conflicting reports in the literature and the tremendous implications of basing the surgical management of patients with PTC on a molecular marker, we chose to reexamine the association between BRAF V600E mutation and clinicopathological features of PTC. To accomplish this, we conducted a meta-analysis of the literature that included a total of 32 articles published before September 2011. With regard to lymph node metastases, we also examined studies to determine whether patients underwent prophylactic or therapeutic LNDs, because the latter group would bias the results by including patients who underwent a LND only if they had advanced disease as measured by other preoperative or intraoperative parameters.
Materials and Methods
A systematic literature review was performed within PubMed and EMBASE databases using the following MeSH (Medical Subject Headings) terms and keywords: “braf,” “mutation,” “thyroid,” “neoplasms,” “tumor,” “cancer,” and “carcinoma.” Two reviewers (C.L. and K.L.) used the EndNote reference tool to independently screen and select articles for inclusion (Fig. 1). The review includes studies published before September 2011.
Fig. 1.
Article selection process.
Duplicate articles were removed, and only English full-text articles were included. Titles and abstracts were screened for the terms BRAF mutation and papillary thyroid cancer and any terms associated with disease prognosis: gender, sex, age, size, stage, capsule, multifocality, multicentricity, extrathyroidal extension, extracapsular invasion, lymph node metastasis, histological subtype, variant, and other clinicopathological features. Full-text articles were then reviewed in their entirety and selected if they studied the prognostic significance of BRAF mutation by examining the association between BRAF V600E mutation and any clinicopathological features of PTC. Specific features including patient age, gender, tumor size, multifocality, histological subtype, presence of capsule, ETE, lymph node metastasis, vascular invasion, and clinical stage were identified in the full-text articles. All review articles and single case reports were excluded. In instances where the same study cohort was used in multiple articles, either the most recent or the most appropriately informative single article was included. For example, Kim et al. 2005 (48) was used only for a subset analyses that included microcarcinomas and was not used in the overall analysis because it contained a cohort overlapping with Kim et al. 2006 (32), used in the overall PTC analysis.
The reviewers also independently assessed references of relevant articles and reviews to identify additional studies for inclusion. At each stage of the selection process, discrepancies in article selection between the two reviewers were discussed by study team members and resolved. Individual study-specific odds ratios (ORs) and confidence intervals were calculated, and Mantel-Haenszel pooled ORs for the combined studies were calculated using a fixed-effect model. Because variables used for model adjustment differed substantially across studies, the fixed-effect models were based upon actual counts of patient BRAF status and clinicopathologic features rather than combined adjusted ORs. This was done specifically to avoid any adjustment-based imbalance in the pooled results. Thus, unadjusted ORs were calculated for each study and actual count numbers were used to generate the pooled ORs reported in the results and forest plots.
As recommended by Stroup et al. (18), further analyses were performed to assign quality scores to each study. Studies were reviewed and assigned additional points in quality if they were prospective, selected patients consecutively or randomly, and were multi-institutional. For the outcome of lymph node metastasis, studies were also weighted more heavily if they performed routine central LNDs (CLNDs). The scoring system produced a quality rating between 0 and 5. A sensitivity analysis was performed where weights were applied to the original count data based upon the rigor of the study, as scored using the 0–5 rating system. The same unadjusted fixed-effect analyses were used to generate Mantel-Haenszel pooled ORs for the combined studies using weighted counts. Stata version 11.2 (StataCorp, College Station, TX) was used for all analyses.
Results
Thirty-two studies comprising 6372 patients were included in the meta-analysis, and 3244 (50.9%) of these patients had BRAF mutation-positive PTCs. The earliest study was published in September 2003, and the latest study was published in September 2011. The largest study by Basolo et al. (10) included 1060 patients, and the smallest study by Nakayama et al. (19) included 40 patients. Not all studies reported on all variables examined in the meta-analysis (Table 1); therefore, only studies that reported the variable of interest were analyzed for BRAF association with that variable. Only two of 32 studies were prospective (20, 21), whereas the rest were either retrospective or not specified. Nine of 33 studies comprised consecutive patients (9, 10, 13, 16, 20–24), whereas one examined randomly selected patients (12); the remainder did not specify.
Table 1.
A summary of the 32 studies included in the meta-analysis and the associated prognostic factors examined
| Study | Total number | BRAF mutation (%) | Gender | Age | Size | LNM | ETE | Clinical stage | Histological subtype | Multifocality | Absence of tumor capsule | Vascular invasion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adeniran et al., 2006 (9) | 96 | 41.7 | Y | N | N | Y | Y | Y | Y | N | N | N |
| Basolo et al., 2010 (10) | 1060 | 44.6 | Y | Y | Y | Y | Y | Y | N | Y | Y | N |
| Czarniecka et al., 2010 (27) | 88 | 43.2 | Y | N | N | Y | Y | N | N | Y | N | N |
| Elisei et al., 2008 (36) | 102 | 37.3 | Y | N | N | Y | Y | Y | N | Y | N | Y |
| Frasca et al., 2008 (11) | 323 | 38.7 | Y | N | N | Y | Y | Y | Y | Y | N | N |
| Fugazzola et al., 2004 (14) | 56 | 32.1 | Y | Y | N | Y | N | Y | N | Y | N | N |
| Goutas et al., 2008 (40) | 55 | 27.3 | Y | Y | N | Y | N | Y | N | N | N | N |
| Guan et al., 2009 (12) | 1032 | 61.9 | N | N | N | Y | Y | Y | N | N | N | N |
| Ito et al., 2009 (15) | 631 | 38.4 | Y | N | Y | Y | N | Y | Y | N | N | N |
| Jo et al., 2006 (28) | 161 | 63.4 | Y | N | N | Y | Y | Y | N | N | N | N |
| Jung et al., 2010 (29) | 210 | 77.1 | Y | Y | Y | Y | Y | N | Y | Y | N | N |
| Kim et al., 2005 (30) | 79 | 81.0 | Y | Y | N | Y | N | N | N | N | N | N |
| Kim et al., 2006 (13) | 103 | 33.0 | Y | N | N | Y | N | Y | N | N | N | N |
| Kim et al., 2006 (32) | 203 | 73.4 | Y | N | N | Y | Y | Y | N | Y | N | N |
| Kim et al., 2009 (31) | 101 | 87.1 | Y | N | N | N | N | N | N | N | N | N |
| Kwak et al., 2009 (22) | 339 | 62.8 | Y | N | N | Y | Y | Y | N | Y | N | N |
| Lee et al., 2009 (25) | 64 | 37.5 | Y | N | N | Y | Y | N | Y | N | Y | N |
| Lee et al., 2006 (33) | 100 | 58.0 | Y | N | N | Y | Y | Y | N | N | N | Y |
| Lin et al., 2010 (34) | 61 | 34.4 | Y | N | N | Y | Y | N | N | Y | N | N |
| Liu et al., 2005 (23) | 105 | 46.7 | Y | Y | N | Y | Y | Y | Y | Y | N | N |
| Nakayama et al., 2007 (19) | 40 | 65.0 | Y | N | N | Y | Y | Y | N | N | N | N |
| Namba et al., 2003 (7) | 126 | 30.2 | Y | Y | N | Y | Y | Y | N | N | N | N |
| O'Neill et al., 2010 (41) | 101 | 59.4 | Y | N | N | Y | Y | Y | Y | Y | Y | Y |
| Oler et al., 2009 (24) | 120 | 48.3 | Y | Y | N | Y | Y | N | N | Y | N | N |
| Pelizzo et al., 2011 (20) | 141 | 69.5 | Y | N | Y | Y | Y | Y | Y | Y | N | N |
| Riesco-Eizaguirre et al., 2006 (37) | 67 | 41.8 | Y | N | N | Y | Y | Y | Y | Y | N | N |
| Sapio et al., 2006 (38) | 43 | 44.2 | Y | N | N | Y | N | Y | Y | Y | N | N |
| So et al., 2011 (21) | 71 | 62.0 | N | N | N | Y | N | N | N | N | N | N |
| Sykorova et al., 2010 (39) | 242 | 33.5 | Y | Y | N | Y | N | Y | N | Y | Y | Y |
| Xing et al., 2009 (12) | 190 | 38.4 | Y | N | N | Y | Y | Y | N | Y | N | N |
| Xu et al., 2003 (42) | 56 | 37.5 | Y | N | N | Y | N | N | N | N | N | N |
| Yip et al., 2009 (16) | 206 | 51.5 | Y | N | N | Y | Y | N | Y | Y | N | N |
| Total (patients, prevalence, number of studies examined) | 6372 | 50.9 | 30 | 9 | 4 | 31 | 22 | 22 | 11 | 18 | 4 | 4 |
Y indicates that the study was evaluated for the corresponding prognostic factor; N indicates that the study was not evaluated for the corresponding prognostic factor.
Gender
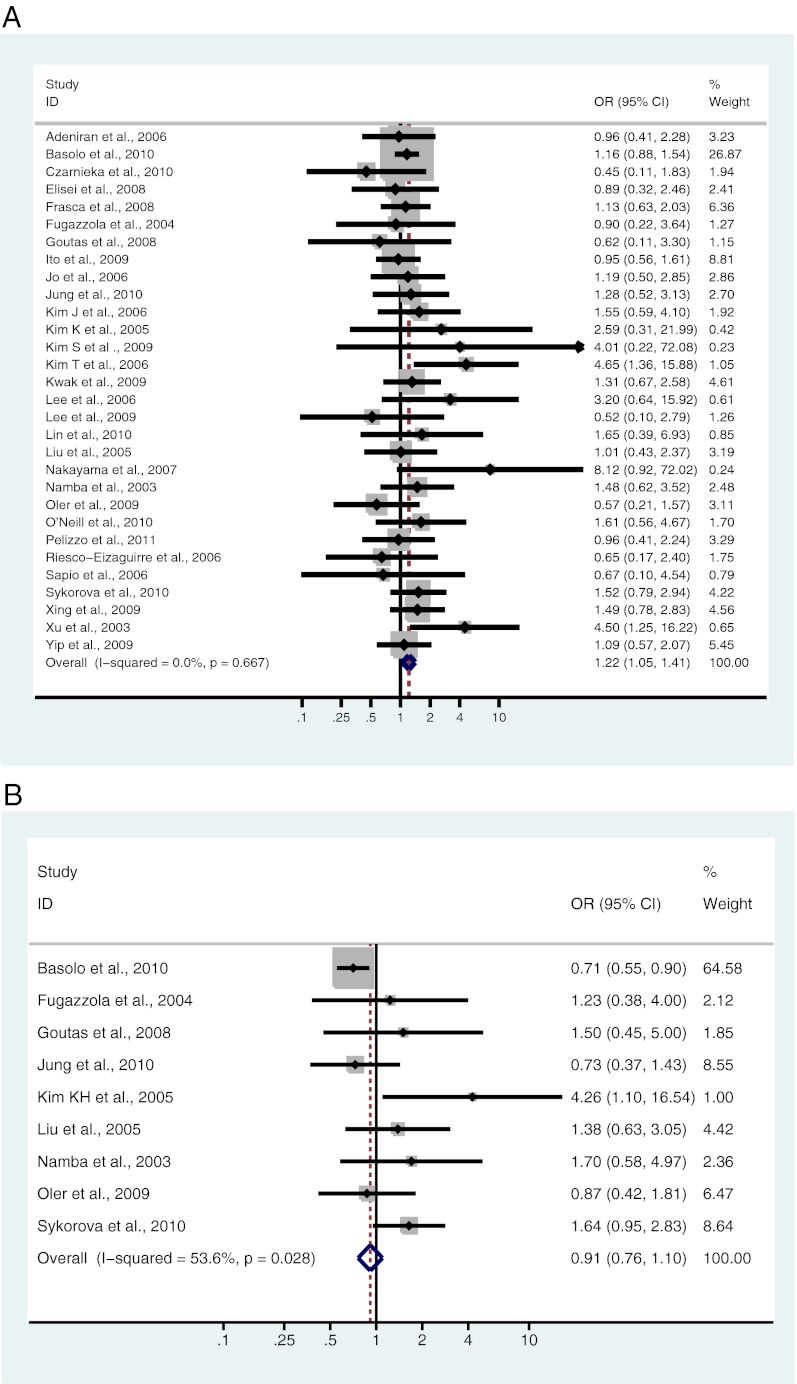
Thirty studies, including 5238 patients, were analyzed for the association between BRAF mutation and gender. Of 992 male patients, 512 (51.6%) were BRAF mutation positive, and 2046 (48.2%) of 4246 female patients were BRAF mutation positive. There was a significant association between BRAF mutation and male gender [OR = 1.22; 95% confidence interval (CI) = 1.05–1.41] (Fig. 2A and Table 2).
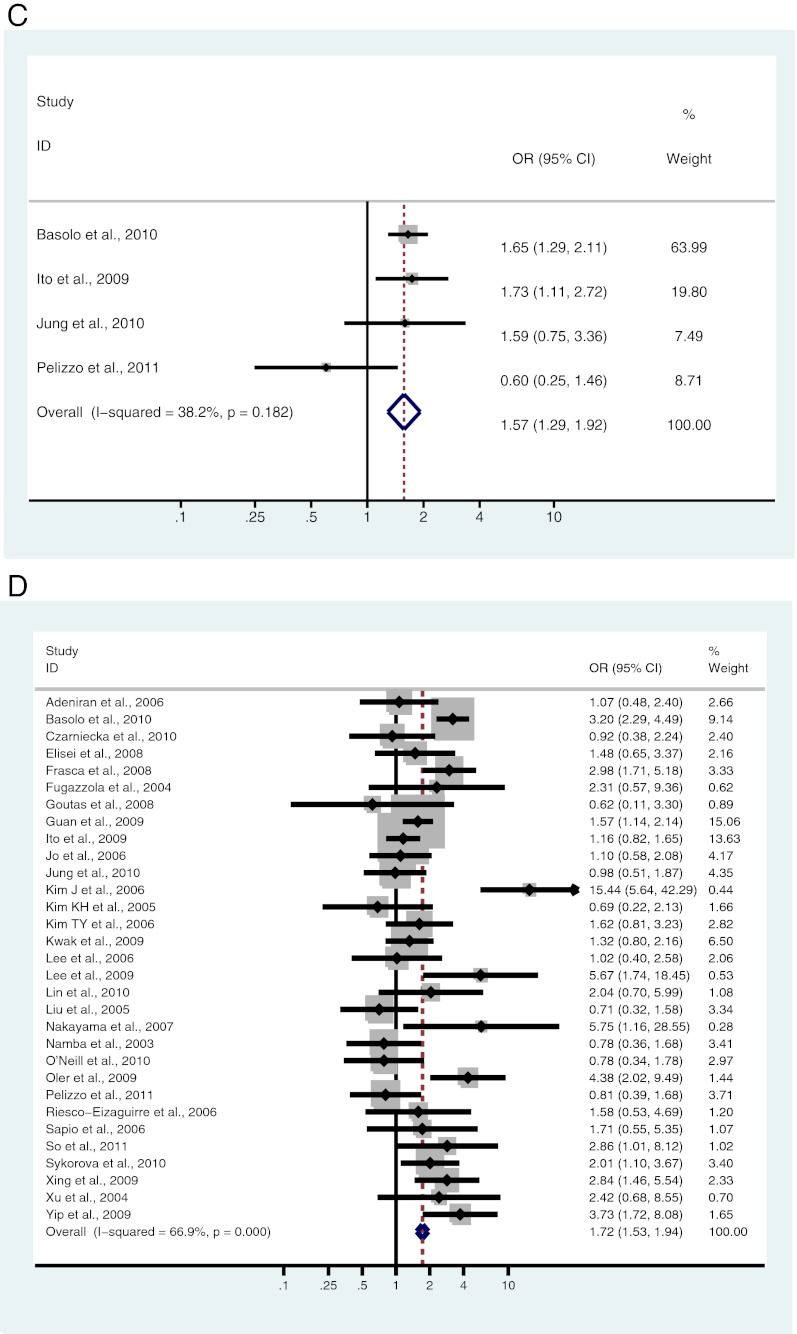
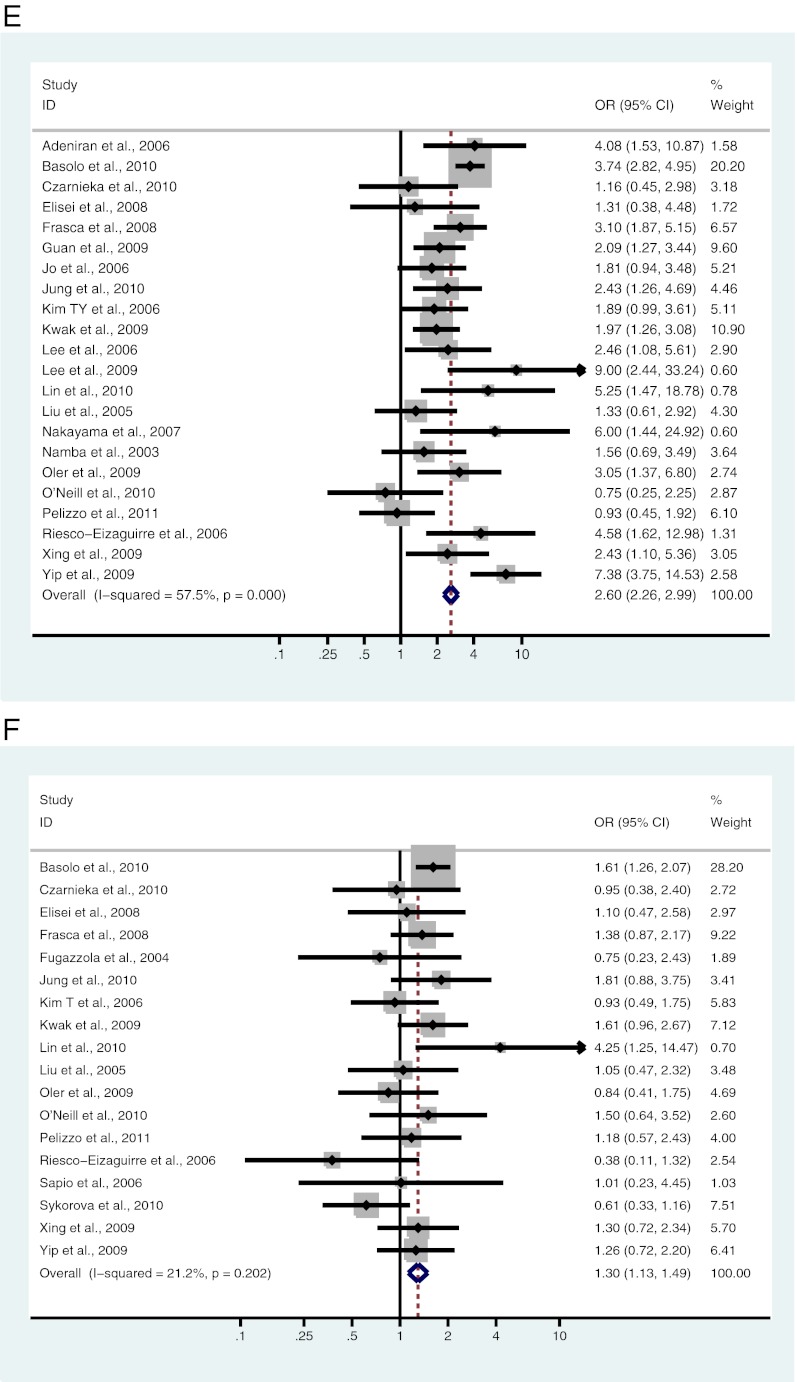
Fig. 2.
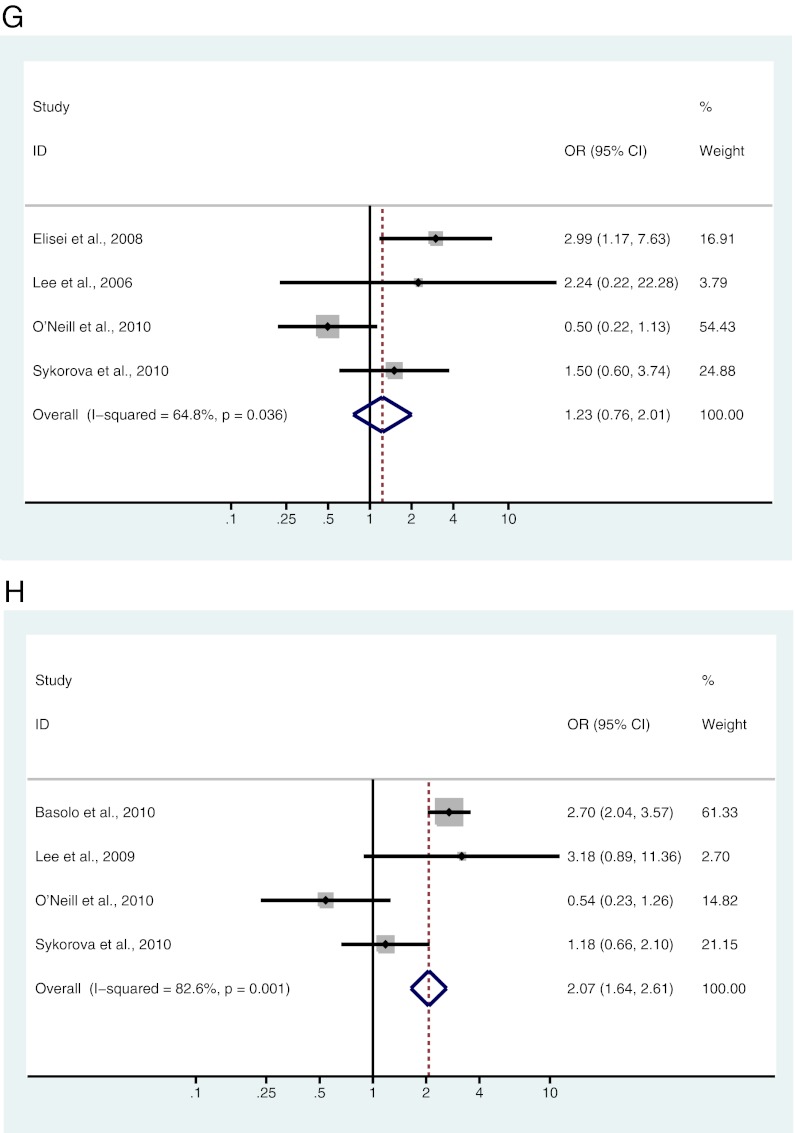
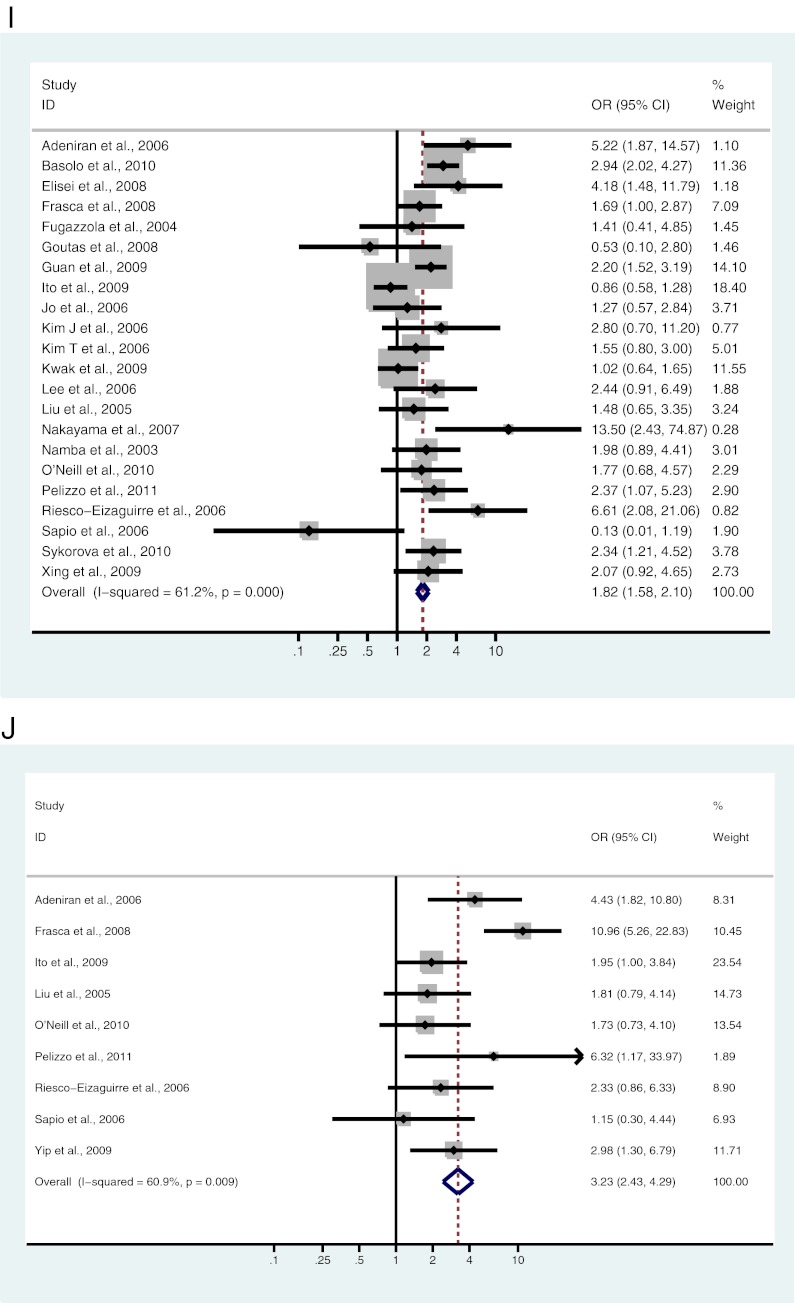
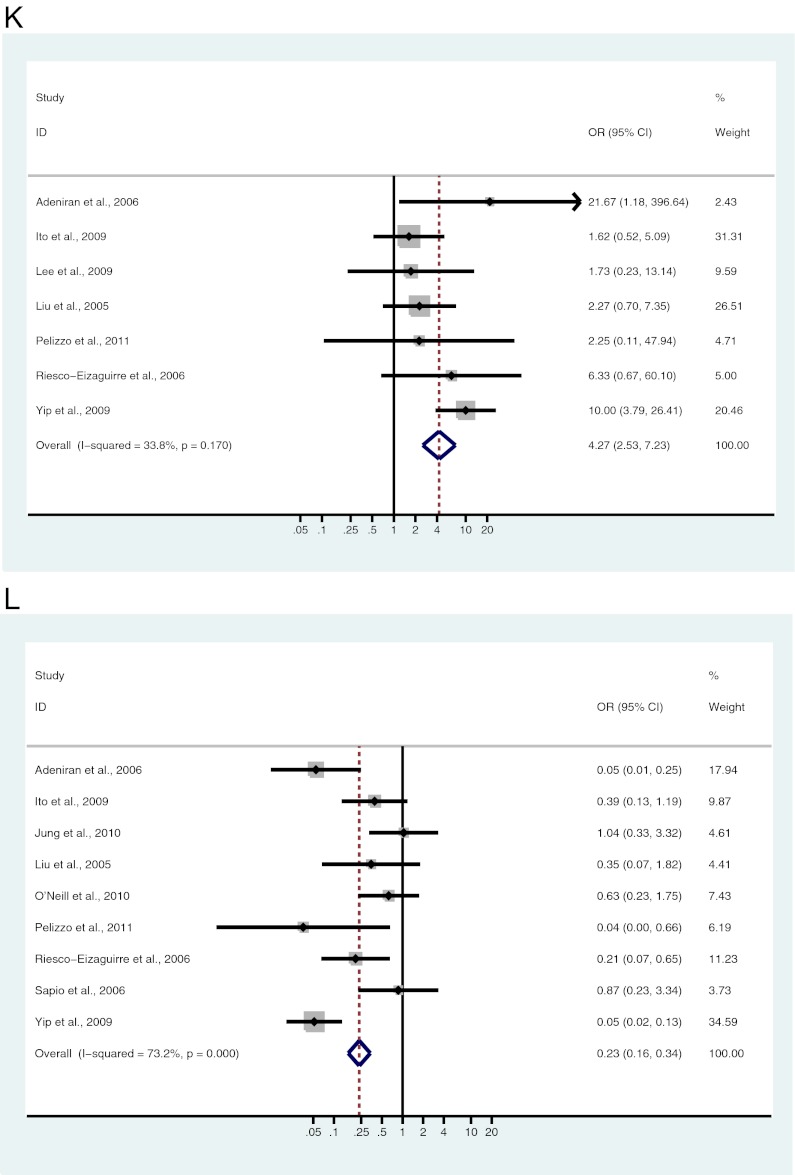
A–L, Study-specific and Mantel-Haenszel pooled OR and 95% CI for the association between BRAF mutation and gender (A), age (B), tumor size (C), lymph node metastases (D), ETE (E), multifocality (F), vascular invasion (G), absence of capsule (H), clinical stage (I), classical variant (J), tall cell variant (K), and follicular variant (L) in patients with PTC.
Table 2.
Association of BRAF mutation with clinicopathological features of all PTCs and of micro-PTCs
| Prognostic factor | OR | 95% CI |
|---|---|---|
| All PTCs | ||
| Male gendera | 1.22 | 1.05–1.41 |
| Age | 0.91 | 0.76–1.10 |
| Sizea | 1.57 | 1.29–1.92 |
| Lymph node metastasisa | 1.72 | 1.53–1.94 |
| ETEa | 2.60 | 2.27–2.99 |
| Multifocalitya | 1.30 | 1.13–1.49 |
| Vascular invasion | 1.23 | 0.76–2.01 |
| Absence of capsulea | 2.07 | 1.64–2.61 |
| Advanced clinical stagea | 1.82 | 1.58–2.10 |
| Microcarcinomas | ||
| Male gender | 1.1 | 0.80–1.50 |
| Age | 0.63 | 0.46–0.87 |
| Lymph node metastasisa | 1.54 | 1.14–2.10 |
| ETEa | 2.79 | 2.10–3.72 |
| Multifocalitya | 1.39 | 1.06–1.82 |
| Advanced clinical stagea | 1.72 | 1.21–2.42 |
Statistically significant for aggressive prognostic features.
Age
Nine studies, including 2015 patients, were analyzed for the association between BRAF mutation and age. Of 1045 patients 45 yr or older, 482 (46.1%) were BRAF mutation positive, and 472 (48.7%) of 970 patients younger than 45 yr old were BRAF mutation positive. No significant association was found between BRAF mutation and age (OR = 0.91; 95% CI = 0.76–1.10) (Fig. 2B and Table 2).
Tumor size
Four studies, including 2029 patients, analyzed the association between BRAF mutation and size of the PTC. Of 1159 tumors greater than 1.0 cm, 578 (49.9%) were positive for the BRAF mutation, and 397 (45.6%) of 870 tumors less than or equal to 1.0 cm in size were positive for the BRAF mutation. There was a significant association between BRAF mutation and tumor size greater than 1.0 cm (OR = 1.57; 95% CI = 1.29–1.92) (Fig. 2C and Table 2).
Lymph node metastases
Thirty-one studies, including 5895 patients, were analyzed for the association between BRAF mutation and lymph node metastasis. Of 2230 lymph node-positive patients, 1293 (58.9%) tested positive for the BRAF mutation, and 1688 (46.1%) of 3665 lymph node-negative patients tested positive for the BRAF mutation. A significant association was found between BRAF mutation and the presence of lymph node metastases (OR = 1.72; 95% CI = 1.53–1.94) (Fig. 2D and Table 2). Twenty-nine of 32 studies either did not specify the type of LND performed or stated that patients underwent varying extents of thyroidectomies and therapeutic LND. Kim et al. (13) included patients who received lymphatic mapping and sentinel LNDs. Only So et al. (21) and Lee et al. (25) used prospectively collected patients who underwent routine bilateral CLND.
Extrathyroidal extension
Twenty-two studies, including 4668 patients, were analyzed for the association between BRAF mutation and ETE. Of 1547 patients with ETE, 1064 (68.8%) were positive for the BRAF mutation, and 1414 (45.3%) of 3121 patients with no ETE were positive for the BRAF mutation. There was a significant association between BRAF mutation and ETE in patients with PTC (OR = 2.60; 95% CI = 2.27–2.99) (Fig. 2E and Table 2).
Multifocality
Eighteen studies, including 3585 patients, were analyzed for the association between BRAF mutation and multifocal PTC. Of 1378 patients with multifocal disease, 736 (53.4%) were BRAF mutation positive, and 1054 (47.8%) of 2207 patients with unifocal disease were BRAF mutation positive. A significant association exists between BRAF mutation and multifocality (OR = 1.30; 95% CI = 1.13–1.49) (Fig. 2F and Table 2).
Vascular invasion
Four studies, including 505 patients, were analyzed for the association between BRAF mutation and vascular invasion. Of 89 patients with vascular invasion, 45 (50.6%) were BRAF mutation positive, and 78 (43.3%) of 180 patients with no vascular invasion were BRAF mutation positive. There was no significant association found between BRAF mutation and vascular invasion (OR = 1.23; 95% CI = 0.76–2.01) (Fig. 2G and Table 2).
Absence of tumor capsule
Four studies, including 1457 patients, were analyzed for the association of BRAF mutation with absence of tumor capsule. Of 959 patients lacking a tumor capsule, 499 (52.0%) were BRAF mutation positive, and 166 (33.3%) of 498 patients with encapsulated PTC were BRAF mutation positive. There was a significant association between BRAF mutation and absence of tumor capsule in patients with PTC (OR = 2.07; 95% CI = 1.64–2.61) (Fig. 2H and Table 2).
Clinical stage
Twenty-two studies, including 5014 patients, were analyzed for the association between BRAF mutation and clinical stage. Of 3813 patients diagnosed with stage I or stage II disease, 1742 (45.7%) were BRAF mutation positive, and 731 (60.9%) of 1201 patients diagnosed with stage III or stage IV disease were BRAF mutation positive. There was a significant association between BRAF mutation and advanced clinical stage (OR = 1.82; 95% CI = 1.58–2.10) (Fig. 2I and Table 2).
Histological subtype
Nine studies, including 1638 patients, were analyzed for the association of BRAF mutation and classical variant of PTC (CPTC). Of 1226 CPTC patients, 604 (49.3%) were BRAF mutation positive, and 118 (28.6%) of 412 patients with other subtypes of PTC, including follicular, tall cell, warthin-like tumor, macrofollicular, diffuse sclerosing, cribriform-morular, columnar, oncocytic, micropapillary, and solid/trabecular variants, were BRAF mutation positive. A significant association exists between BRAF mutation and CPTC (OR = 3.23; 95% CI = 2.43–4.29) (Fig. 2J and Table 3).
Table 3.
Association of BRAF mutation with histological subtype of PTC
| OR | 95% CI | |
|---|---|---|
| Classical | 3.23 | 2.43–4.29 |
| Tall cell | 4.27 | 2.53–7.23 |
| Follicular | 0.23 | 0.16–0.34 |
Seven studies, including 1235 patients, were analyzed for the association of BRAF mutation and tall cell variant of PTC (TCVPTC). Of 79 TCVPTC, 59 (74.7%) patients were BRAF mutation positive, and 483 (41.8%) of 1156 with other subtypes of PTC were BRAF mutation positive. A significant association exists between BRAF mutation and TCVPTC (OR = 4.27; 95% CI = 2.53–7.23) (Fig. 2K and Table 3).
Nine studies, including 1525 patients, were analyzed for the association of BRAF mutation and follicular variant of PTC (FVPTC). Of 194 FVPTC, 48 (24.7%) patients were BRAF mutation positive, and 711 (53.4%) of 1331 non-FVPTC patients were BRAF mutation positive. A significant association exists between BRAF mutation and non-FVPTC (OR = 0.23; 95% CI = 0.16–0.34) (Fig. 2L and Table 3).
Microcarcinoma
Six studies presented clinicopathological data corresponding to papillary microcarcinoma (tumor size ≤ 1 cm). In six studies, including 1249 patients, BRAF mutation was significantly associated with lymph node metastases (OR = 1.54; 95% CI = 1.14–2.10). In five studies, including 1102 patients, BRAF mutation was significantly associated with ETE (OR = 2.79; 95% CI = 2.10–3.72). In three studies, including 975 patients, BRAF mutation was significantly associated with clinical stage (III/IV vs. I/II) (OR = 1.72; 95% CI = 1.21–2.42). In four studies, including 1038 patients, BRAF mutation was significantly associated with multifocal disease (OR = 1.39; 95% CI = 1.06–1.82). Association between BRAF mutation and patient age or gender was not significant (Table 2).
Additional analyses
To investigate the presence of publication bias, a funnel plot of effects calculated from individual studies examining the association between BRAF mutation and lymph node metastases was performed. Because small studies with negative results do indeed exist in the literature, there is no strong indication of publication bias among the set of studies included in this meta-analysis.
Weighting the studies by quality, as described above, did not significantly change our initial results.
Discussion
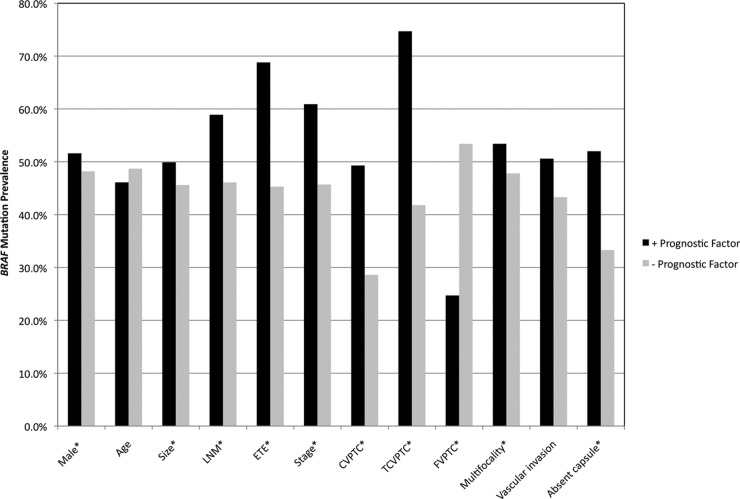
The majority of prognostic factors currently used to stage and manage patients with PTC depend on final histopathological evaluation that is available only postoperatively. BRAF V600E mutation has been proposed as a potential preoperative tool for risk stratification in patients with PTC, guiding whether the patient should or should not undergo prophylactic CLND (10, 16, 26). However, discrepancies exist among studies that have attempted to determine the association between BRAF mutation and poor prognosis. This meta-analysis, encompassing 6372 patients in total, found that BRAF V600E mutation is associated with several of the variables used in prognostic staging systems, including male gender, classical variant subtype, larger tumor size, multifocality, ETE, regional lymph node metastasis, absence of tumor capsule, and advanced clinical stage (III/IV vs. I/II) (6) (Fig. 3). BRAF V600E mutation, however, was not associated with advanced age (≥45 yr) or vascular invasion.
Fig. 3.
BRAF mutation prevalence among patients with and without specific prognostic factors. The term +prognostic factor indicates BRAF mutation prevalence among patients positive for the corresponding prognostic factor, and −prognostic factor indicates BRAF mutation prevalence among patients negative for the corresponding prognostic factor. *, Statistically significant association between BRAF mutation and aggressive prognostic features.
With respect to study design of the manuscripts included in the meta-analysis, however, there were several limitations. Only two of the 32 studies (20, 21) evaluated their cohorts prospectively, and only nine studies included consecutive patients (9, 10, 13, 16, 20–24), whereas Guan et al. (12) examined randomly selected patients. Because the majority of studies, including Basolo et al. (10), who studied 1060 patients, did not analyze consecutive patients, there may exist a possible bias toward larger tumors, because these would be more readily available for collection and genetic analysis. This limitation also allows for selection bias toward patients with better-documented disease. For example, patients with known PTC on FNA would more likely undergo LND than those in whom the diagnosis was unclear (e.g. FVPTC), and therefore would more likely have metastases identified. Indeed, a subset analysis of the two prospective studies found no significant association between BRAF mutation and lymph node metastases. Of the two studies, only So et al. (21) found a significant association between BRAF mutation and lymph node metastases.
Of 32 studies, So et al. (21) and Lee et al. (25) were the only ones to report the use of routine bilateral central neck dissections for their patient population. So et al. (21) demonstrated an association only in tumors 0.5–1 cm in size, and Lee et al. (25) in only microcarcinomas. The pathological data included in the other 30 studies to determine the association between LNM and BRAF mutation could only have been obtained from those patients who underwent CLND for a suspicious feature, seen on ultrasound for example or noted at the time of surgery, thus biasing the study toward the evaluation of only patients who had significant lymphadenopathy. Those patients who would have undergone neck dissections due to a suspicion on ultrasound, other imaging, or intraoperatively represent only a subset of the target population for the potential use of the BRAF biomarker. Were one to suggest using BRAF preoperatively, one would by definition be proposing that patients who were positive undergo CLND even without any evidence of significant nodal disease. Furthermore, and on a practical matter, proposing that patients who are BRAF positive should undergo routine CLND because 59% are likely to have metastases as opposed to 46% of those who are BRAF negative harbor metastases appears to be based upon a statistical result rather than what would be applicable clinically.
Differences in BRAF mutation detection methods were also used among the 32 studies analyzed and, given the discrepancies in their sensitivities, may contribute to the differing results. The majority of studies used direct sequencing (7, 11–15, 19–23, 27–35). Other methods of mutation detection included fluorescence melting-curve analysis (9, 16), single-strand conformation polymorphism (SSCP) (10, 24, 36–39), restriction fragment length polymorphism analysis (25, 40, 41), mutation allele-specific amplification (MASA) (38, 42), and shifted termination assay (STA) (17). With regard to the different methods, Sapio et al. (38) compared DNA sequencing, SSCP, and MASA to determine which detection method was most sensitive. Using a mixed sample of wild-type and BRAF mutant DNA, they found that SSCP and DNA sequencing were equally sensitive, able to detect BRAF V600E mutation at concentrations down to 60%. MASA was the most sensitive, able to detect BRAF mutation down to a concentration of 20%. Shackelford et al. (43) demonstrated that the STA was capable of detecting low-copy-number heterogeneous clinical samples with a higher sensitivity compared with single-base primer-extension methods. When STA was applied to the detection of the BRAF mutation in a clinical study by Shackelford et al. (43), the STA was able to correctly identify BRAF mutation status in all 90 samples, whereas PCR restriction enzyme analysis misclassified 10 wild-type samples as mutant and direct sequencing misclassified one mutant sample as wild type. Because direct sequencing methods will also read wild-type DNA mixed within the clinical sample, a misreading will occur any time there is greater than 80% wild-type DNA and less than 20% mutant DNA (43). Conversely, STA can detect mutant DNA in concentrations as low as 1% (43). Xing et al. (26) evaluated the utility of STA in the detection of BRAF mutation in FNA specimens and found that STA exhibited 100% sensitivity and specificity compared with direct DNA sequencing. Given the heterogeneous nature of FNA specimens, STA seems to be the most sensitive method for BRAF mutation detection at the preoperative level. The majority of past studies used direct sequencing, which can give false-negative readings if the mutant DNA concentrations fall below 20%.
Our meta-analysis showed that prevalence of BRAF mutation is highest in the TCVPTC (74.7%) and lowest in the FVPTC (24.7%), with an intermediate prevalence in the CPTC (49.3%). This is not surprising because TCVPTC is known to behave more aggressively compared with CPTC, with higher rates of ETE, older age at presentation, higher risk of locoregional and distant relapse, and decreased survival (44, 45). With other major prognostic factors controlled for, TCVPTC has been shown to be an independent prognostic factor for disease-specific death (44). Compared with CPTC, FVPTC exhibits less aggressive behavior, with lower rates of cervical lymph node metastases and ETE (46). With the exception of four studies (12, 14, 24, 32) that focused on only CPTC, the majority of studies included in this meta-analysis either did not specify or explicitly included a mixture of different histological subtypes of PTC. Because different subtypes have varying disease patterns and BRAF mutation prevalence, the results of these past studies may be skewed depending on the composition of the tumor collection analyzed. Furthermore, current criteria for diagnosing FVPTC are often not uniformly agreed upon as Elsheikh et al. (47) have demonstrated both significant interobserver and intraobserver variation in the diagnosis of FVPTC. Complete agreement among experts in diagnosing FVPTC was observed in only 13% of cases, and intraobserver agreement ranged from 17–100%. The inclusion of misdiagnosed follicular adenomas, which would exhibit extremely low rates of BRAF mutation, might also skew the results toward a stronger association between BRAF mutation and aggressive features. Therefore, a histological homogeneous population would be more informative.
In summary, meta-analysis of the literature shows BRAF V600E mutation correlates with poor prognostic features of PTC. Most studies, however, use disparate methods of BRAF mutation detection, do not evaluate only patients who have undergone routine CLND, and include a heterogeneous mix of PTC subtypes. Before one recommends that BRAF mutation be incorporated into the management algorithm of thyroid cancer, additional well-designed prospective trials that include only patients who have undergone routine CLND are needed to address these limitations, particularly with regard to recommending prophylactic LNDs in patients who have BRAF-positive tumors.
Acknowledgments
This work was supported by a National Institutes of Health Clinical and Translational Science Award (CTSA)-funded Predoctoral Clinical Research Training Grant and a Johns Hopkins University School of Medicine Deans Year of Research Fellowship.
Disclosure Summary: All authors have nothing to declare.
Footnotes
- CI
- Confidence interval
- CLND
- central LND
- CPTC
- classical variant of PTC
- ETE
- extrathyroidal extension
- FNA
- fine-needle aspiration
- FVPTC
- follicular variant of PTC
- MASA
- mutation allele-specific amplification
- OR
- odds ratio
- PTC
- papillary thyroid cancer
- SSCP
- single-strand conformation polymorphism
- STA
- shifted termination assay
- TCVPTC
- tall cell variant of PTC.
References
- 1. Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS. 2009. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev 18:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schlumberger MJ. 1998. Papillary and follicular thyroid carcinoma. N Engl J Med 338:297–306 [DOI] [PubMed] [Google Scholar]
- 3. Handkiewicz-Junak D, Czarniecka A, Jarzab B. 2010. Molecular prognostic markers in papillary and follicular thyroid cancer: current status and future directions. Mol Cell Endocrinol 322:8–28 [DOI] [PubMed] [Google Scholar]
- 4. Mazzaferri EL. 2009. What is the optimal initial treatment of low-risk papillary thyroid cancer (and why is it controversial)? Oncology (Williston Park) 23:579–588 [PubMed] [Google Scholar]
- 5. Randolph GW. 2010. Papillary cancer nodal surgery and the advisability of prophylactic central neck dissection: primum, non nocere. Surgery 148:1108–1112 [DOI] [PubMed] [Google Scholar]
- 6. Zeiger MA, Dackiw AP. 2005. Follicular thyroid lesions, elements that affect both diagnosis and prognosis. J Surg Oncol 89:108–113 [DOI] [PubMed] [Google Scholar]
- 7. Namba H, Nakashima M, Hayashi T, Hayashida N, Maeda S, Rogounovitch TI, Ohtsuru A, Saenko VA, Kanematsu T, Yamashita S. 2003. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab 88:4393–4397 [DOI] [PubMed] [Google Scholar]
- 8. Lee JH, Lee ES, Kim YS. 2007. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer 110:38–46 [DOI] [PubMed] [Google Scholar]
- 9. Adeniran AJ, Zhu Z, Gandhi M, Steward DL, Fidler JP, Giordano TJ, Biddinger PW, Nikiforov YE. 2006. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol 30:216–222 [DOI] [PubMed] [Google Scholar]
- 10. Basolo F, Torregrossa L, Giannini R, Miccoli M, Lupi C, Sensi E, Berti P, Elisei R, Vitti P, Baggiani A, Miccoli P. 2010. Correlation between the BRAF V600E mutation and tumor invasiveness in papillary thyroid carcinomas smaller than 20 millimeters: analysis of 1060 cases. J Clin Endocrinol Metab 95:4197–4205 [DOI] [PubMed] [Google Scholar]
- 11. Frasca F, Nucera C, Pellegriti G, Gangemi P, Attard M, Stella M, Loda M, Vella V, Giordano C, Trimarchi F, Mazzon E, Belfiore A, Vigneri R. 2008. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer 15:191–205 [DOI] [PubMed] [Google Scholar]
- 12. Guan H, Ji M, Bao R, Yu H, Wang Y, Hou P, Zhang Y, Shan Z, Teng W, Xing M. 2009. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab 94:1612–1617 [DOI] [PubMed] [Google Scholar]
- 13. Kim J, Giuliano AE, Turner RR, Gaffney RE, Umetani N, Kitago M, Elashoff D, Hoon DS. 2006. Lymphatic mapping establishes the role of BRAF gene mutation in papillary thyroid carcinoma. Ann Surg 244:799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fugazzola L, Mannavola D, Cirello V, Vannucchi G, Muzza M, Vicentini L, Beck-Peccoz P. 2004. BRAF mutations in an Italian cohort of thyroid cancers. Clin Endocrinol (Oxf) 61:239–243 [DOI] [PubMed] [Google Scholar]
- 15. Ito Y, Yoshida H, Maruo R, Morita S, Takano T, Hirokawa M, Yabuta T, Fukushima M, Inoue H, Tomoda C, Kihara M, Uruno T, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Miyauchi A. 2009. BRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr J 56:89–97 [DOI] [PubMed] [Google Scholar]
- 16. Yip L, Nikiforova MN, Carty SE, Yim JH, Stang MT, Tublin MJ, Lebeau SO, Hodak SP, Ogilvie JB, Nikiforov YE. 2009. Optimizing surgical treatment of papillary thyroid carcinoma associated with BRAF mutation. Surgery 146:1215–1223 [DOI] [PubMed] [Google Scholar]
- 17. Xing M, Clark D, Guan H, Ji M, Dackiw A, Carson KA, Kim M, Tufaro A, Ladenson P, Zeiger M, Tufano R. 2009. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J Clin Oncol 27:2977–2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. 2000. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 19. Nakayama H, Yoshida A, Nakamura Y, Hayashi H, Miyagi Y, Wada N, Rino Y, Masuda M, Imada T. 2007. Clinical significance of BRAF (V600E) mutation and Ki-67 labeling index in papillary thyroid carcinomas. Anticancer Res 27:3645–3649 [PubMed] [Google Scholar]
- 20. Pelizzo MR, Boschin IM, Barollo S, Pennelli G, Toniato A, Zambonin L, Vianello F, Piotto A, Ide EC, Pagetta C, Sorgato N, Torresan F, Girelli ME, Nacamulli D, Mantero F, Mian C. 2011. BRAF analysis by fine needle aspiration biopsy of thyroid nodules improves preoperative identification of papillary thyroid carcinoma and represents a prognostic factor. A mono-institutional experience. Clin Chem Lab Med 49:325–329 [DOI] [PubMed] [Google Scholar]
- 21. So YK, Son YI, Park JY, Baek CH, Jeong HS, Chung MK. 2011. Preoperative BRAF mutation has different predictive values for lymph node metastasis according to tumor size. Otolaryngol Head Neck Surg 145:422–427 [DOI] [PubMed] [Google Scholar]
- 22. Kwak JY, Kim EK, Chung WY, Moon HJ, Kim MJ, Choi JR. 2009. Association of BRAFV600E mutation with poor clinical prognostic factors and US features in Korean patients with papillary thyroid microcarcinoma. Radiology 253:854–860 [DOI] [PubMed] [Google Scholar]
- 23. Liu RT, Chen YJ, Chou FF, Li CL, Wu WL, Tsai PC, Huang CC, Cheng JT. 2005. No correlation between BRAFV600E mutation and clinicopathological features of papillary thyroid carcinomas in Taiwan. Clin Endocrinol (Oxf) 63:461–466 [DOI] [PubMed] [Google Scholar]
- 24. Oler G, Cerutti JM. 2009. High prevalence of BRAF mutation in a Brazilian cohort of patients with sporadic papillary thyroid carcinomas: correlation with more aggressive phenotype and decreased expression of iodide-metabolizing genes. Cancer 115:972–980 [DOI] [PubMed] [Google Scholar]
- 25. Lee X, Gao M, Ji Y, Yu Y, Feng Y, Li Y, Zhang Y, Cheng W, Zhao W. 2009. Analysis of differential BRAF(V600E) mutational status in high aggressive papillary thyroid microcarcinoma. Ann Surg Oncol 16:240–245 [DOI] [PubMed] [Google Scholar]
- 26. Xing M, Tufano RP, Tufaro AP, Basaria S, Ewertz M, Rosenbaum E, Byrne PJ, Wang J, Sidransky D, Ladenson PW. 2004. Detection of BRAF mutation on fine needle aspiration biopsy specimens: a new diagnostic tool for papillary thyroid cancer. J Clin Endocrinol Metab 89:2867–2872 [DOI] [PubMed] [Google Scholar]
- 27. Czarniecka A, Rusinek D, Stobiecka E, Krajewska J, Kowal M, Kropiñska A, Zebracka J, Kowalska M, Włoch J, Maciejewski A, Handkiewicz-Junak D. 2010. Occurrence of BRAF mutations in a Polish cohort of PTC patients: preliminary results. Endokrynol Pol 61:462–466 [PubMed] [Google Scholar]
- 28. Jo YS, Li S, Song JH, Kwon KH, Lee JC, Rha SY, Lee HJ, Sul JY, Kweon GR, Ro HK, Kim JM, Shong M. 2006. Influence of the BRAF V600E mutation on expression of vascular endothelial growth factor in papillary thyroid cancer. J Clin Endocrinol Metab 91:3667–3670 [DOI] [PubMed] [Google Scholar]
- 29. Jung CK, Kang YG, Bae JS, Lim DJ, Choi YJ, Lee KY. 2010. Unique patterns of tumor growth related with the risk of lymph node metastasis in papillary thyroid carcinoma. Mod Pathol 23:1201–1208 [DOI] [PubMed] [Google Scholar]
- 30. Kim KH, Suh KS, Kang DW, Kang DY. 2005. Mutations of the BRAF gene in papillary thyroid carcinoma and in Hashimoto's thyroiditis. Pathol Int 55:540–545 [DOI] [PubMed] [Google Scholar]
- 31. Kim SK, Song KH, Lim SD, Lim YC, Yoo YB, Kim JS, Hwang TS. 2009. Clinical and pathological features and the BRAF(V600E) mutation in patients with papillary thyroid carcinoma with and without concurrent Hashimoto thyroiditis. Thyroid 19:137–141 [DOI] [PubMed] [Google Scholar]
- 32. Kim TY, Kim WB, Rhee YS, Song JY, Kim JM, Gong G, Lee S, Kim SY, Kim SC, Hong SJ, Shong YK. 2006. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 65:364–368 [DOI] [PubMed] [Google Scholar]
- 33. Lee JH, Lee ES, Kim YS, Won NH, Chae YS. 2006. BRAF mutation and AKAP9 expression in sporadic papillary thyroid carcinomas. Pathology 38:201–204 [DOI] [PubMed] [Google Scholar]
- 34. Lin KL, Wang OC, Zhang XH, Dai XX, Hu XQ, Qu JM. 2010. The BRAF mutation is predictive of aggressive clinicopathological characteristics in papillary thyroid microcarcinoma. Ann Surg Oncol 17:3294–3300 [DOI] [PubMed] [Google Scholar]
- 35. Zuo H, Nakamura Y, Yasuoka H, Zhang P, Nakamura M, Mori I, Miyauchi A, Kakudo K. 2007. Lack of association between BRAF V600E mutation and mitogen-activated protein kinase activation in papillary thyroid carcinoma. Pathol Int 57:12–20 [DOI] [PubMed] [Google Scholar]
- 36. Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, Romei C, Miccoli P, Pinchera A, Basolo F. 2008. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 93:3943–3949 [DOI] [PubMed] [Google Scholar]
- 37. Riesco-Eizaguirre G, Gutiérrez-Martínez P, García-Cabezas MA, Nistal M, Santisteban P. 2006. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I− targeting to the membrane. Endocr Relat Cancer 13:257–269 [DOI] [PubMed] [Google Scholar]
- 38. Sapio MR, Posca D, Troncone G, Pettinato G, Palombini L, Rossi G, Fenzi G, Vitale M. 2006. Detection of BRAF mutation in thyroid papillary carcinomas by mutant allele-specific PCR amplification (MASA). Eur J Endocrinol 154:341–348 [DOI] [PubMed] [Google Scholar]
- 39. Sykorova V, Dvorakova S, Ryska A, Vcelak J, Vaclavikova E, Laco J, Kodetova D, Kodet R, Cibula A, Duskova J, Hlobilkova A, Astl J, Vesely D, Betka J, Hoch J, Smutny S, Cap J, Vlcek P, Novak Z, Bendlova B. 2010. BRAFV600E mutation in the pathogenesis of a large series of papillary thyroid carcinoma in Czech Republic. J Endocrinol Invest 33:318–324 [DOI] [PubMed] [Google Scholar]
- 40. Goutas N, Vlachodimitropoulos D, Bouka M, Lazaris AC, Nasioulas G, Gazouli M. 2008. BRAF and K-RAS mutation in a Greek papillary and medullary thyroid carcinoma cohort. Anticancer Res 28:305–308 [PubMed] [Google Scholar]
- 41. O'Neill CJ, Bullock M, Chou A, Sidhu SB, Delbridge LW, Robinson BG, Gill AJ, Learoyd DL, Clifton-Bligh R, Sywak MS. 2010. BRAF(V600E) mutation is associated with an increased risk of nodal recurrence requiring reoperative surgery in patients with papillary thyroid cancer. Surgery 148:1139–1145; discussion 1145–1136 [DOI] [PubMed] [Google Scholar]
- 42. Xu X, Quiros RM, Gattuso P, Ain KB, Prinz RA. 2003. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res 63:4561–4567 [PubMed] [Google Scholar]
- 43. Shackelford W, Deng S, Murayama K, Wang J. 2004. A new technology for mutation detection. Ann NY Acad Sci 1022:257–262 [DOI] [PubMed] [Google Scholar]
- 44. Morris LG, Shaha AR, Tuttle RM, Sikora AG, Ganly I. 2010. Tall-cell variant of papillary thyroid carcinoma: a matched-pair analysis of survival. Thyroid 20:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leung AK, Chow SM, Law SC. 2008. Clinical features and outcome of the tall cell variant of papillary thyroid carcinoma. Laryngoscope 118:32–38 [DOI] [PubMed] [Google Scholar]
- 46. Lang BH, Lo CY, Chan WF, Lam AK, Wan KY. 2006. Classical and follicular variant of papillary thyroid carcinoma: a comparative study on clinicopathologic features and long-term outcome. World J Surg 30:752–758 [DOI] [PubMed] [Google Scholar]
- 47. Elsheikh TM, Asa SL, Chan JK, DeLellis RA, Heffess CS, LiVolsi VA, Wenig BM. 2008. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol 130:736–744 [DOI] [PubMed] [Google Scholar]
- 48. Kim TY, Kim WB, Song JY, Rhee YS, Gong G, Cho YM, Kim SY, Kim SC, Hong SJ, Shong YK. 2005. The BRAF mutation is not associated with poor prognostic factors in Korean patients with conventional papillary thyroid microcarcinoma. Clin Endocrinol 63:588–593 [DOI] [PubMed] [Google Scholar]