Abstract
The physiological role of the TSH receptor (TSHR) as a major regulator of thyroid function is well understood, but TSHRs are also expressed in multiple normal extrathyroidal tissues, and the physiological roles of TSHRs in these tissues are unclear. Moreover, TSHRs play a major role in several pathological conditions including hyperthyroidism, hypothyroidism, and thyroid tumors. Small molecule, “drug-like” TSHR agonists, neutral antagonists, and inverse agonists may be useful as probes of TSHR function in extrathyroidal tissues and as leads to develop drugs for several diseases of the thyroid. In this Update, we review the most recent findings regarding the development and use of these small molecule TSHR ligands.
The physiological role of the TSH receptor (TSHR)1 as a major regulator of thyroid function by controlling the size and number of thyroid cells (thyrocytes) and their synthesis and secretion of thyroid hormones is well understood. Importantly, TSHRs are also expressed in multiple normal extrathyroidal tissues including fat, fibroblasts, bone, brain, kidney, testis, and cells of the immune system (1), but the physiological roles of TSHRs in these tissues are unclear. A role for TSHR in maintaining normal bone homeostasis has been proposed (2), but this conclusion has been challenged (3).
TSHRs play a major role in several pathological conditions, including hyperthyroidism, hypothyroidism, and thyroid tumors. With regard to the number of patients affected, the role of TSHRs in Graves' disease (GD), an autoimmune disease (prevalence is about 1% of the U.S. population), is most important (4). In GD, TSHRs on thyroid cells are continuously activated by circulating thyroid stimulatory antibodies (TSAbs) causing overproduction of thyroid hormones leading to hyperthyroidism (5). In addition, it is thought that TSHRs on preadipocytes (fibroblasts)/adipocytes in the orbital space of patients with GD are activated to cause cell proliferation and increased deposition of extracellular matrix leading to expansion of orbital tissue causing Graves' orbitopathy/ophthalmopathy (GO) (6). In a much less common form of nonautoimmune hyperthyroidism, TSHRs on thyroid cells are mutated receptors that are active in the absence of TSH or TSAb, and these mutated TSHRs signal constitutively (constantly) leading to hyperthyroidism (7). If these mutations are in germline cells, a symmetric goiter (as found in GD) is formed, but if the mutation occurred in a somatic cell, an adenoma forms.
A role for TSHR in some forms of hypothyroidism has also been established. Loss-of-function TSHR mutations in which the binding or signaling functions of the receptor are diminished will lead to a state in which the thyroid gland is underdeveloped and unable to produce sufficient thyroid hormone to maintain a euthyroid state (8). A similar deficiency of thyroid hormone production can be produced by a decrease in TSH production or production of a TSH that exhibits a deficiency in biological activity (9). In these conditions, TSHR still plays a central role.
Lastly, TSHR may play an important role in the pathogenesis of thyroid cancer. This idea is based on several findings, including that TSHR expression is required for thyroid tumor initiation in a mouse model (10) and correlation between higher serum concentrations of TSH and greater risk of the genesis of thyroid cancer in patients with nodular goiter (11). TSHR plays a central role in the follow-up of patients with thyroid cancer. Recombinant human TSH (rhTSH) has been used to great advantage in the follow-up of thyroid cancer patients (12, 13). And TSHR activation by rhTSH is being evaluated as an adjunct in the treatment of patients with thyroid cancer and nodular goiter (14, 15).
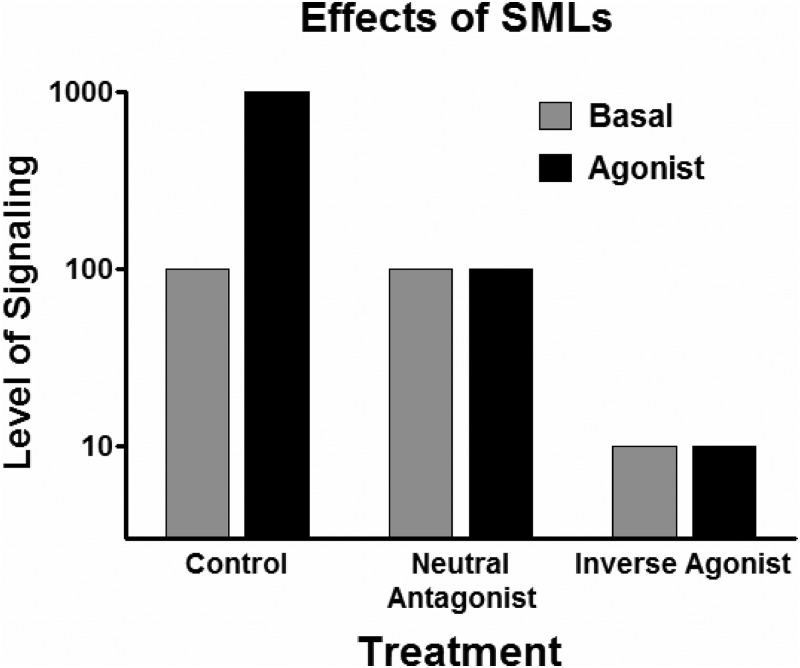
Small molecule, “drug-like” TSHR agonists (ligands that activate receptors), neutral antagonists (ligands that inhibit receptor activation by agonists), and inverse agonists (ligands that inhibit receptor activation by agonists and additionally inhibit agonist-independent, also termed basal or constitutive, signaling) (Fig. 1) may be useful as probes of TSHR function in extrathyroidal tissues and as leads to develop drugs for several thyroid diseases. With regard to clinical usefulness, agonists could be used to develop drugs that could be used in place of rhTSH in patients with thyroid cancer or benign multinodular nontoxic goiter. Neutral antagonists could be used as leads to develop drugs to antagonize TSAb activation of TSHR in patients with GO. Inverse agonists could be used as leads to develop drugs to inhibit constitutive TSHR signaling in patients with residual thyroid cancer and thereby treat them more effectively than by TSH suppression alone. Furthermore, patients with non-autoimmune hyperthyroidism caused by constitutively activating germline mutations could benefit from an inverse agonist. Indeed, antibodies with these various characteristics have been proposed as therapies for several of these diseases (16).
Fig. 1.
Effects on receptor signaling by agonists, neutral antagonists, and inverse agonists. Control illustrates signaling in the absence of neutral antagonists or inverse agonists. Basal signaling, which represents agonist-independent (or constitutive) signaling, is set as 100. (TSHR is a receptor that exhibits significant basal signaling activity.) Antagonist is a general term that includes neutral antagonists and inverse agonists, which have also been named negative antagonists.
Because a recent article has reviewed the topic of TSHR antibodies (16), we will limit this Update to small molecule, “drug-like” ligands (SMLs)2 of TSHR and to findings published during the last 2 yr.
SML Binding to TSHR
TSHR is a member of a small subfamily of related glycoprotein hormone receptors (GPHRs) that includes receptors for LH/chorionic gonadotropin (CG) and FSH, within class A of the large family of G protein-coupled (seven transmembrane-spanning) receptors (GPCRs). GPCRs comprise the single largest family of signaling molecules found in the human genome. GPHRs are different than other members of class A GPCRs by having a very large amino-terminal ectodomain (Fig. 2). It has been known for a number of years that TSH and TSAbs bind primarily to TSHR via interactions with regions within the large ectodomain (17). By contrast, we showed that SMLs bind in a pocket within the seven helical transmembrane bundle of TSHR (18, 19). For receptor functionality, this difference is important because TSHRs with mutations in their ectodomain that do not bind TSH or TSAbs, and thereby are not stimulated by them, can be activated by SMLs (see below). Moreover, because TSAbs bind to the extracellular domain, whereas SML antagonists bind within the transmembrane domain, the likelihood is high that signal transduction initiated by the majority of TSAbs can be blocked by SML antagonists (see below). Signal transduction is accomplished by interactions between the intracellular surface of the transmembrane helices and the carboxyl-terminal tail of TSHR with coupling proteins, such as G proteins and arrestins.
Fig. 2.
Domains of binding to TSHR of TSH, TSAbs, and SMLs. TSHR, like the receptors for FSH and LH, has a large amino-terminal ectodomain that protrudes from the surface of the cells. TSH and TSAbs bind primarily to the TSHR ectodomain. By contrast, SMLs bind to a pocket within the transmembrane domain, which contains the seven α-helical bundles that are characteristic of GPCRs. Ectodomain, Red. Ntt shows the amino terminus of TSHR. Transmembrane domain: Individual helices are ribbons in different colors. The terminal part of the intracellular carboxyl tail (Ctt) is not shown. This model was kindly provided by Gunnar Kleinau, Ph.D., Charité Campus Virchow Klinikum, Berlin, Germany.
SML TSHR Agonists
Although we reported on several TSHR agonists earlier (18, 20, 21), the first breakthrough was our report in 2009 describing a TSHR agonist (NCGC00161870) (Fig. 3) that exhibited high affinity, potency, and efficacy at TSHR in a model cell system in vitro, in primary cultures of normal thyrocytes in vitro, and in mice in vivo (22). Moreover, NCGC00161870 was active in mice after oral administration. NCGC00161870 is highly selective and does not activate the two closely related glycoprotein hormone receptors, LH receptor or FSH receptor. As stated above, NCGC00161870 binds to the transmembrane domain of TSHR, in contrast to TSH that binds to the TSHR ectodomain, and is therefore termed an allosteric ligand; the TSH binding site is termed the orthosteric site. To our knowledge, NCGC00161870 is the only effective SML TSHR agonist reported to date.
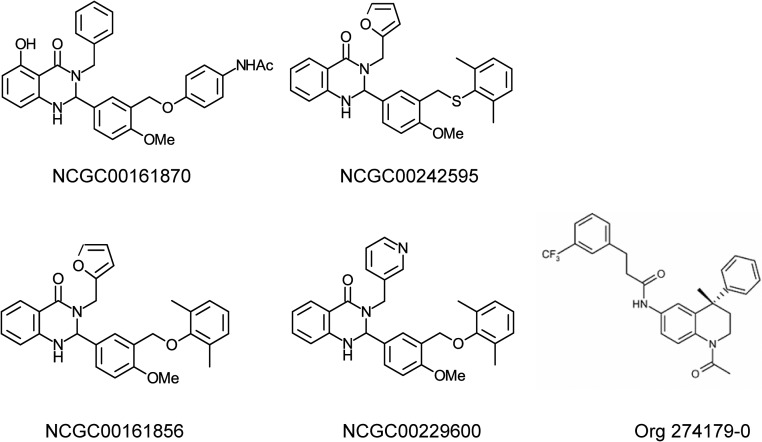
Fig. 3.
Structures of TSHR SMLs. TSHR agonist: NCGC00161870, N-(4-(5-(3-benzyl-5-hydroxy-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)-2-methoxybenzyloxy)-phenyl)acetamide. TSHR neutral antagonist: NCGC00242595, 2-(3-((2,6-dimethylphenylthio)methyl)-4-methoxyphenyl)-3-(furan-2-ylmethyl)-2,3-dihydroquinazolin-4(1H)-one. TSHR inverse agonists: NCGC00161856, 2-(3-((2,6-dimethylphenoxy)methyl)-4-methoxyphenyl)-3-(furan-2-ylmethyl)-2,3-dihydroquinazolin-4(1H)-one]; NCGC00229600, 2-(3-((2,6-dimethylphenoxy)methyl)-4-methoxyphenyl)-3-(pyridin-3-ylmethyl)-2,3-dihydroquinazolin-4(1H)-one; and Org 274179-0, (S)-N-(1-acetyl-4-methyl-4-phenyl-1,2,3,4-tetrahydro-quinolin-6-yl)-3-(3-trifluoromethyl-phenyl)-propionamide.
We have used NCGC00161870 to probe the domains within TSHR that are involved in receptor activation, i.e. the structure-activity relationship of a SML agonist with TSHR (23, 24). Specifically, we found that a high proportion of amino acid residues that comprise the binding pocket for NCGC00161870 within the transmembrane domain when mutated lead to constitutively active TSHRs. These findings identify a signaling-sensitive domain within TSHR that may have general applicability to other members of the GPCR family. Moreover, these findings may allow for the design of better TSHR agonists.
NCGC00161870 has been used to better understand the pathophysiology of an unusual form of hypothyroidism caused by TSHR mutations (25). These patients present with subclinical hypothyroidism, i.e. with normal circulating levels of thyroid hormones maintained by high levels of TSH, because they are usually heterozygotes with one normal TSHR allele and a mutant TSHR allele. Before our studies, it was postulated that the mutant TSHRs either had a mutation that makes the receptor unable to bind TSH with high affinity or a mutation that makes the mutant receptor incapable of signaling. In both cases, the patients would present with TSH-insensitive (TSH-resistant) hypothyroidism. We studied several mutant TSHRs identified as the cause of this type of hypothyroidism and found that NCGC00161870 could activate the two mutant receptors with mutations in the TSHR ectodomain but could not activate signaling from a receptor with a mutation in the transmembrane domain. We were thus able to demonstrate that some mutations (in the TSHR ectodomain) affected TSH binding but not signaling and others affected TSHR signaling. We suggested that patients with TSH-binding defective mutant receptors could be treated with a SML agonist but that this was not a practical therapeutic approach because these patients were readily treated with thyroid hormone replacement.
Currently, NCGC00161870 and several of its analogs are being further evaluated in preclinical studies for possible submission of an Investigational New Drug application to the Food and Drug Administration to allow testing in human subjects.
SML TSHR Antagonists
Although putative TSHR SML antagonists were reported earlier (see below), we reported the first well-characterized SML neutral antagonist (NIDDK-CEB-52) that selectively antagonizes TSHR in 2008 (19). NIDDK-CEB-52 was shown to inhibit activation of TSHR by TSH and TSAbs in a model cell system and inhibited up-regulation of thyroperoxidase expression by these agonists in primary cultures of human thyrocytes. We suggested that these findings represented proof of principle that SML antagonists could serve as drugs to treat patients with GD.
In 2007, a report appeared claiming that the insecticide 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (DDT) and Aroclor 1254 (a complex mixture of polychlorinated biphenyls) were inverse agonists at TSHR by showing that these compounds inhibited basal and TSH stimulation of cAMP production in a model cell system (26). In a follow-up study (27), these authors identified a number of structurally similar compounds and found that many of them inhibited TSH stimulation also. Moreover, these compounds were able to inhibit stimulation by LH of its receptor. The authors concluded that there may be an allosteric site on TSHR and LH receptor that mediates inhibition of receptor activation. However, in the original study, these authors found that DDT and Aroclor 1254 inhibited forskolin-stimulated cAMP accumulation also. In our opinion, because forskolin acts downstream of TSHR by activating adenylyl cyclase, the enzyme that synthesizes cAMP, one cannot conclude that these compounds are acting at TSHR.
During the last 2 yr, reports on different compounds discovered by two groups have added important findings to the development of TSHR antagonists. Two reports were published using a compound developed at Merck Sharp & Dohme Corporation (Org 274179-0) (28, 29). In the first report (28), the authors showed that Org 274179-0 (Fig. 2) was a TSHR inverse agonist that inhibited the basal, TSH-stimulated, and TSAb-stimulated signaling in a model system expressing TSHRs, basal signaling by constitutively active mutant TSHRs in a model system, and TSH- and TSAb-stimulated signaling in a rat thyroid cell line (FRTL-5). We had previously described two TSHR inverse agonists (see below). Org 274179·0 has the advantage of higher potency than the other reported TSHR antagonists (see below). However, it has a significant disadvantage in that it is a partial agonist at the LH receptor. In a follow-up report (29), the authors showed that Org 274179-0 inhibited TSH-, TSAb-, and M22- [a monoclonal antibody generated from the serum of a patient with GD (30)] stimulated cAMP production in orbital fibroblasts derived from patients with GO that had been made to differentiate into adipocytes in vitro. This is an important finding (see below) because TSHRs on Graves' orbital fibroblasts are thought to be a primary target for TSAbs in the pathogenesis of GO (6).
We have developed a series of TSHR antagonists by chemical modification of the scaffold of our agonist NCGC00161870 (22). Interestingly, all except one analog of the agonist NCGC00161870 were inverse agonists. Only NCGC00242595 (Fig. 3) was a neutral antagonist at TSHR (31). A neutral antagonist is a good tool to be able to distinguish between effects of TSH- or TSAb-stimulated and basal TSHR activation, and therefore it is useful as a probe of TSHR function in addition to its clinical potential. The first report describing this series of antagonists showed that NCGC00161856 (Fig. 3) was the first inverse agonist at TSHR to be described. NCGC00161856, like NCGC00161870, bound to TSHR in a transmembrane pocket and therefore is an allosteric ligand. NCGC00161856 inhibited basal and TSH-stimulated signaling by TSHR and basal signaling by constitutively active mutant TSHRs in a model cell system and lowered the basal levels of expression of four thyroid-specific genes—thyroglobulin, thyroperoxidase, sodium iodide symporter, and TSHR—in primary cultures of normal human thyrocytes. These data were proof of principle that a SML could act as an inverse agonist in normal human thyroid cells.
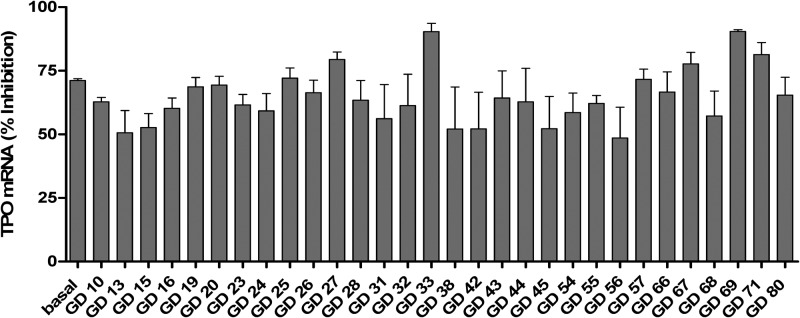
We developed a better TSHR inverse agonist by modifying NCGC00161856. The new ligand NCGC00229600 (Fig. 3) bound to the allosteric site within the transmembrane domain of TSHR also and inhibited basal and TSH-stimulated signaling (32). Because it would be of greater importance to show that an antagonist would inhibit activation of TSHR signaling stimulated by TSAbs from multiple patients with GD, we determined the effect of NCGC00229600 on signaling stimulated by sera from 30 patients with GD. NCGC00229600 inhibited stimulation of signaling by all 30 sera tested both in a model cell system and in primary human thyrocytes in culture. Perhaps more importantly, NCGC00229600 inhibited the up-regulation of thyroperoxidase caused by these TSAbs in human thyrocytes (Fig. 4). Thus, we suggested that an antagonist like NCGC00229600 would likely be an effective inhibitor of TSAbs from most, if not all, patients with GD and could be used to treat the hyperthyroidism of GD and perhaps GO (see below).
Fig. 4.
Inhibition of basal and TSAb-induced up-regulation of the expression of thyroperoxidase mRNA in primary cultures of human thyrocytes. NCGC00229600 inhibited the levels by 65 ± 2.0% (mean ± sem). Importantly, although the thyrocytes were exposed to NCGC00229600 for 48 h, there was no evidence of toxicity. [Reproduced from S. Neumann et al.: A new small-molecule antagonist inhibits Graves' disease antibody activation of the TSH receptor. J Clin Endocrinol Metab 96:548–554, 2011 (36), with permission. © The Endocrine Society.]
As stated above, TSHRs on orbital fibroblasts or adipocytes, or both, are thought to be a primary target for TSAbs in the pathogenesis of GO (6). It is thought that activation of TSHR by TSAbs leads to proliferation of preadipocyte fibroblasts or adipocytes, stimulation of extracellular matrix production, and perhaps differentiation of preadipocytes to adipocytes. Therefore, showing that TSHR antagonists inhibit activation of TSHRs on Graves' orbital fibroblasts (GOFs) would be a step in the development of a medical therapy for GO; there is not a good alternative to surgical decompression at the present time. We determined the effects of NCGC00229600 on undifferentiated GOFs and GOFs differentiated into adipocytes; the adipocytes displayed many lipid vesicles and high levels of the adipocyte-specific gene adiponectin (33). GOFs exhibited higher absolute levels of basal and forskolin-stimulated cAMP production than differentiated adipocytes. Consistent with previous findings, TSH stimulated cAMP production in the majority of differentiated adipocyte strains and less consistently in GOFs. Most importantly, NCGC00229600 reduced both TSH- and M22-stimulated signaling in both cell types. Moreover, NCGC00229600 was a selective TSHR antagonist in these orbital cells because it did not inhibit signaling by activation of prostaglandin D2 receptors, which use the same cAMP signaling pathway as TSHRs. We concluded, as did the authors using Org 274179-0 (29), that SML TSHR antagonists may have a role in the treatment of GO.
Conclusions
During the last several years, significant advances have been made in the development of SMLs for TSHR. These compounds can be used as probes to better delineate the extrathyroidal actions of TSHR and as lead compounds to develop drugs to treat several thyroid diseases. As described in this Update, some of these agonists, neutral antagonists, and inverse agonists are already being used as probes of TSHR function. And the agonist we have developed is undergoing preclinical studies to determine whether it could be entered into clinical trials for patients with thyroid cancer.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (Z01 DK011006, Z01 DK047044.
Disclosure Summary: The authors state that they have no conflicts of interest.
We use the abbreviation TSHR to represent the human TSHR and add the species if the receptor is other than human.
We define SMLs as nonpeptidic compounds of molecular weight less than 750 Da.
- CG
- Chorionic gonadotropin
- GD
- Graves' disease
- GO
- Graves' orbitopathy/ophthalmopathy
- GOF
- Graves' orbital fibroblast
- GPCR
- G protein-coupled receptor
- GPHR
- glycoprotein hormone receptor
- rhTSH
- recombinant human TSH
- SML
- small molecule ligand
- TSAb
- thyroid stimulatory antibody
- TSHR
- TSH receptor.
References
- 1. Bassett JH, Williams GR. 2008. Critical role of the hypothalamic-pituitary-thyroid axis in bone. Bone 43:418–426 [DOI] [PubMed] [Google Scholar]
- 2. Blair HC, Robinson LJ, Sun L, Isales C, Davies TF, Zaidi M. 2011. Skeletal receptors for steroid-family regulating glycoprotein hormones: a multilevel, integrated physiological control system. Ann NY Acad Sci 1240:26–31 [DOI] [PubMed] [Google Scholar]
- 3. Bassett JH, Williams AJ, Murphy E, Boyde A, Howell PG, Swinhoe R, Archanco M, Flamant F, Samarut J, Costagliola S, Vassart G, Weiss RE, Refetoff S, Williams GR. 2008. A lack of thyroid hormones rather than excess thyrotropin causes abnormal skeletal development in hypothyroidism. Mol Endocrinol 22:501–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davies TF, Ando T, Lin RY, Tomer Y, Latif R. 2005. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest 115:1972–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rapoport B, McLachlan SM. 2007. The thyrotropin receptor in Graves' disease. Thyroid 17:911–922 [DOI] [PubMed] [Google Scholar]
- 6. Bahn RS. 2010. Graves' ophthalmopathy. N Engl J Med 362:726–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duprez L, Parma J, Van Sande J, Rodien P, Dumont JE, Vassart G, Abramowicz M. 1998. TSH receptor mutations and thyroid disease. Trends Endocrinol Metab 9:133–140 [DOI] [PubMed] [Google Scholar]
- 8. Refetoff S. 2003. Resistance to thyrotropin. J Endocrinol Invest 26:770–779 [DOI] [PubMed] [Google Scholar]
- 9. Almandoz JP, Gharib H. 2012. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am 96:203–221 [DOI] [PubMed] [Google Scholar]
- 10. Franco AT, Malaguarnera R, Refetoff S, Liao XH, Lundsmith E, Kimura S, Pritchard C, Marais R, Davies TF, Weinstein LS, Chen M, Rosen N, Ghossein R, Knauf JA, Fagin JA. 2011. Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc Natl Acad Sci USA 108:1615–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, Chen H. 2008. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab 93:809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pacini F, Castagna MG. 2008. Diagnostic and therapeutic use of recombinant human TSH (rhTSH) in differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab 22:1009–1021 [DOI] [PubMed] [Google Scholar]
- 13. Duntas LH, Cooper DS. 2008. Review on the occasion of a decade of recombinant human TSH: prospects and novel uses. Thyroid 18:509–516 [DOI] [PubMed] [Google Scholar]
- 14. Woodmansee WW, Haugen BR. 2004. A review of the potential uses for recombinant human TSH in patients with thyroid cancer and nodular goiter. Clin Endocrinol (Oxf) 61:163–173 [DOI] [PubMed] [Google Scholar]
- 15. Fast S, Nielsen VE, Grupe P, Boel-Jørgensen H, Bastholt L, Andersen PB, Bonnema SJ, Hegedüs L. 2012. Prestimulation with recombinant human thyrotropin (rhTSH) improves the long-term outcome of radioiodine therapy for multinodular nontoxic goiter. J Clin Endocrinol Metab 97:2653–2660 [DOI] [PubMed] [Google Scholar]
- 16. Sanders J, Miguel RN, Furmaniak J, Rees Smith B. 2010. TSH receptor monoclonal antibodies with agonist, antagonist, and inverse agonist activities. Methods Enzymol 485:393–420 [DOI] [PubMed] [Google Scholar]
- 17. Vassart G, Pardo L, Costagliola S. 2004. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci 29:119–126 [DOI] [PubMed] [Google Scholar]
- 18. Jäschke H, Neumann S, Moore S, Thomas CJ, Colson AO, Costanzi S, Kleinau G, Jiang JK, Paschke R, Raaka BM, Krause G, Gershengorn MC. 2006. A low molecular weight agonist signals by binding to the transmembrane domain of thyroid-stimulating hormone receptor (TSHR) and luteinizing hormone/chorionic gonadotropin receptor (LHCGR). J Biol Chem 281:9841–9844 [DOI] [PubMed] [Google Scholar]
- 19. Neumann S, Kleinau G, Costanzi S, Moore S, Jiang JK, Raaka BM, Thomas CJ, Krause G, Gershengorn MC. 2008. A low-molecular-weight antagonist for the human thyrotropin receptor with therapeutic potential for hyperthyroidism. Endocrinology 149:5945–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore S, Jaeschke H, Kleinau G, Neumann S, Costanzi S, Jiang JK, Childress J, Raaka BM, Colson A, Paschke R, Krause G, Thomas CJ, Gershengorn MC. 2006. Evaluation of small-molecule modulators of the luteinizing hormone/choriogonadotropin and thyroid stimulating hormone receptors: structure-activity relationships and selective binding patterns. J Med Chem 49:3888–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Titus S, Neumann S, Zheng W, Southall N, Michael S, Klumpp C, Yasgar A, Shinn P, Thomas CJ, Inglese J, Gershengorn MC, Austin CP. 2008. Quantitative high-throughput screening using a live-cell cAMP assay identifies small-molecule agonists of the TSH receptor. J Biomol Screen 13:120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neumann S, Huang W, Titus S, Krause G, Kleinau G, Alberobello AT, Zheng W, Southall NT, Inglese J, Austin CP, Celi FS, Gavrilova O, Thomas CJ, Raaka BM, Gershengorn MC. 2009. Small molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice. Proc Natl Acad Sci USA 106:12471–12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kleinau G, Haas AK, Neumann S, Worth CL, Hoyer I, Furkert J, Rutz C, Gershengorn MC, Schülein R, Krause G. 2010. Signaling-sensitive amino acids surround the allosteric ligand binding site of the thyrotropin receptor. FASEB J 24:2347–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haas AK, Kleinau G, Hoyer I, Neumann S, Furkert J, Rutz C, Schülein R, Gershengorn MC, Krause G. 2011. Mutations that silence constitutive signaling activity in the allosteric ligand-binding site of the thyrotropin receptor. Cell Mol Life Sci 68:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allen MD, Neumann S, Gershengorn MC. 2011. Small-molecule thyrotropin receptor agonist activates naturally occurring thyrotropin-insensitive mutants and reveals their distinct cyclic adenosine monophosphate signal persistence. Thyroid 21:907–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rossi M, Dimida A, Dell'anno MT, Trincavelli ML, Agretti P, Giorgi F, Corsini GU, Pinchera A, Vitti P, Tonacchera M, Maggio R. 2007. The thyroid disruptor 1,1,1-trichloro-2,2-bis(p-chlorophenyl)-ethane appears to be an uncompetitive inverse agonist for the thyrotropin receptor. J Pharmacol Exp Ther 320:465–474 [DOI] [PubMed] [Google Scholar]
- 27. Rossi M, Dimida A, Ferrarini E, Silvano E, De Marco G, Agretti P, Aloisi G, Simoncini T, Di Bari L, Tonacchera M, Giorgi F, Maggio R. 2009. Presence of a putative steroidal allosteric site on glycoprotein hormone receptors. Eur J Pharmacol 623:155–159 [DOI] [PubMed] [Google Scholar]
- 28. van Koppen CJ, de Gooyer ME, Karstens WJ, Plate R, Conti PG, van Achterberg TA, van Amstel MG, Brands JH, Wat J, Berg RJ, Lane JR, Miltenburg AM, Timmers CM. 2012. Mechanism of action of a nanomolar potent, allosteric antagonist of the thyroid-stimulating hormone receptor. Br J Pharmacol 165:2314–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Zeijl CJ, van Koppen CJ, Surovtseva OV, de Gooyer ME, Plate R, Conti P, Karstens WJ, Timmers M, Saeed P, Wiersinga WM, Miltenburg AM, Fliers E, Boelen A. 2012. Complete inhibition of rhTSH-, Graves' disease IgG-, and M22-induced cAMP production in differentiated orbital fibroblasts by a low-molecular-weight TSHR antagonist. J Clin Endocrinol Metab 97:E781–E785 [DOI] [PubMed] [Google Scholar]
- 30. Sanders J, Evans M, Premawardhana LD, Depraetere H, Jeffreys J, Richards T, Furmaniak J, Rees Smith B. 2003. Human monoclonal thyroid stimulating autoantibody. Lancet 362:126–128 [DOI] [PubMed] [Google Scholar]
- 31. Boutin A, Allen MD, Geras-Raaka E, Huang W, Neumann S, Gershengorn MC. 2011. Thyrotropin receptor stimulates internalization-independent persistent phosphoinositide signaling. Mol Pharmacol 80:240–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Neumann S, Eliseeva E, McCoy JG, Napolitano G, Giuliani C, Monaco F, Huang W, Gershengorn MC. 2011. A new small-molecule antagonist inhibits Graves' disease antibody activation of the TSH receptor. J Clin Endocrinol Metab 96:548–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neumann S, Pope A, Geras-Raaka E, Raaka BM, Bahn RS, Gershengorn MC. 2012. A drug-like antagonist inhibits thyrotropin receptor-mediated stimulation of cAMP production in Graves' orbital fibroblasts. Thyroid 22:839–843 [DOI] [PMC free article] [PubMed] [Google Scholar]