Abstract
Context:
Obesity is associated with reduced GH secretion and increased cardiovascular disease risk.
Objective:
We performed this study to determine the effects of augmenting endogenous GH secretion on body composition and cardiovascular disease risk indices in obese subjects with reduced GH secretion.
Design, Patients and Methods:
A randomized, double-blind, placebo-controlled study was performed involving 60 abdominally obese subjects with reduced GH secretion. Subjects received tesamorelin, a GHRH1–44 analog, 2 mg once daily, or placebo for 12 months. Abdominal visceral adipose tissue (VAT) was assessed by abdominal computed tomography scan, and carotid intima-media thickness (cIMT) was assessed by ultrasound. Treatment effect was determined by longitudinal linear mixed-effects modeling.
Results:
VAT [−16 ± 9 vs.19 ± 9 cm2, tesamorelin vs. placebo; treatment effect (95% confidence interval): −35 (−58, −12) cm2; P = 0.003], cIMT (−0.03 ± 0.01 vs. 0.01 ± 0.01 mm; −0.04 (−0.07, −0.01) mm; P = 0.02), log C-reactive protein (−0.17 ± 0.04 vs. −0.03 ± 0.05 mg/liter; −0.15 (−0.30, −0.01) mg/liter, P = 0.04), and triglycerides (−26 ± 16 vs. 12 ± 8 mg/dl; −37 (−67, −7) mg/dl; P = 0.02) improved significantly in the tesamorelin group vs. placebo. No significant effects on abdominal sc adipose tissue (−6 ± 6 vs. 3 ± 11 cm2; −10 (−32, +13) cm2; P = 0.40) were seen. IGF-I increased (86 ± 21 vs. −6 ± 8 μg/liter; 92 (+52, +132) μg/liter; P < 0.0001). No changes in fasting, 2-h glucose, or glycated hemoglobin were seen. There were no serious adverse events or differences in adverse events between the groups.
Conclusion:
Among obese subjects with relative reductions in GH, tesamorelin selectively reduces VAT without significant effects on sc adipose tissue and improves triglycerides, C-reactive protein, and cIMT, without aggravating glucose.
Obesity is associated with increased abdominal sc adipose tissue (SAT) and visceral adipose tissue (VAT), and VAT appears to be strongly related to increased cardiometabolic complications of obesity (1–5). GH is an anabolic hormone with important biological function, particular on fat and glucose metabolism (6). Obesity, and more specifically abdominal or visceral obesity, is associated with reductions in both stimulated (7, 8) and endogenous pulsatile (9–11) GH secretion. Recent data suggest that reduced GH secretion in obesity is associated with increased cardiovascular disease (CVD) risk assessed by increased carotid intima-media thickness (cIMT) as well as an unfavorable lipid profile and increased inflammatory markers (12, 13).
GH replacement in patients with GH deficiency reduces VAT and improves the lipid profile and other cardiovascular risk factors (14, 15). Therefore, strategies to augment reduced GH levels in obesity may similarly have a beneficial effect on cardiovascular risk by reducing VAT and improving atherosclerotic and inflammatory indices. In this regard, exogenous GH may not be ideal, because it does not augment endogenous pulsatile GH secretion, shown to be abnormal in obesity, and has been associated with side effects including hyperglycemia.
In contrast, GHRH, first identified in 1982 (16–18), increases endogenous GH secretion in a pulsatile manner (19, 20). Tesamorelin (Theratechnologies, Inc., Montreal, Quebec, Canada) is a synthetic GH-releasing factor analog that increases pulsatile secretion of endogenous GH and has been shown to have minimal effects on insulin sensitivity in short-term studies (21). Tesamorelin was shown to reduce abdominal adiposity in viscerally obese subjects with HIV lipodystrophy (22). However, tesamorelin has not been studied in generalized abdominal obesity. In this study, we assessed the effects of tesamorelin in obese patients with reduced GH to determine the effects of augmentation of endogenous GH on body composition and CVD indices.
Subjects and Methods
Subjects
Subjects were recruited at the Massachusetts General Hospital between June 2008 and November 2010. Eligibility criteria were 1) men and women 18–55 yr old, 2) body mass index (BMI) 30 kg/m2 or higher, 3) waist circumference (WC) 102 cm or higher (men) and 88 cm or higher (women), 4) peak stimulated GH no higher than 9 μg/liter on a standardized GHRH-arginine stimulation test, and 5) hemoglobin over 12.0 g/dl, serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic acid pyruvic transaminase (SGPT) less than 2.5 times normal, and creatinine below 1.5 mg/dl. Exclusion criteria were 1) obesity due to a known secondary cause (Cushing's syndrome, hypothyroidism, etc.) or previous gastric bypass; 2) diabetes or use of any antidiabetic drugs; 3) use of weight-lowering drugs; 4) use of estrogen, hormone replacement therapy, oral contraceptives, testosterone, glucocorticoids, anabolic steroids, GHRH, GH, or IGF-I within 3 months of enrollment; 5) changes in lipid-lowering or antihypertensive regimen within 3 months of screening; 6) chronic illness including HIV, anemia, chronic kidney disease, and liver disease; 7) history of malignancy (except surgically cured basal or squamous cell skin cancers) or history of abnormalities on mammography, colonoscopy, fecal occult blood testing, prostate exam, or prostate-specific antigen over 5 ng/ml) 8) history of hypopituitarism, pituitary surgery, pituitary/brain radiation, or traumatic brain injury or other condition known to affect the GH axis; 9) history of recent cardiovascular event, unstable angina pectoris, or severe pulmonary disease; 10) recent substance abuse; 11) pregnant or lactating females; and 12) FSH over 20 IU/liter (women).
The study was approved by the Partners Institutional Review Board. All subjects provided written informed consent before participation in the study.
Study design and interventions
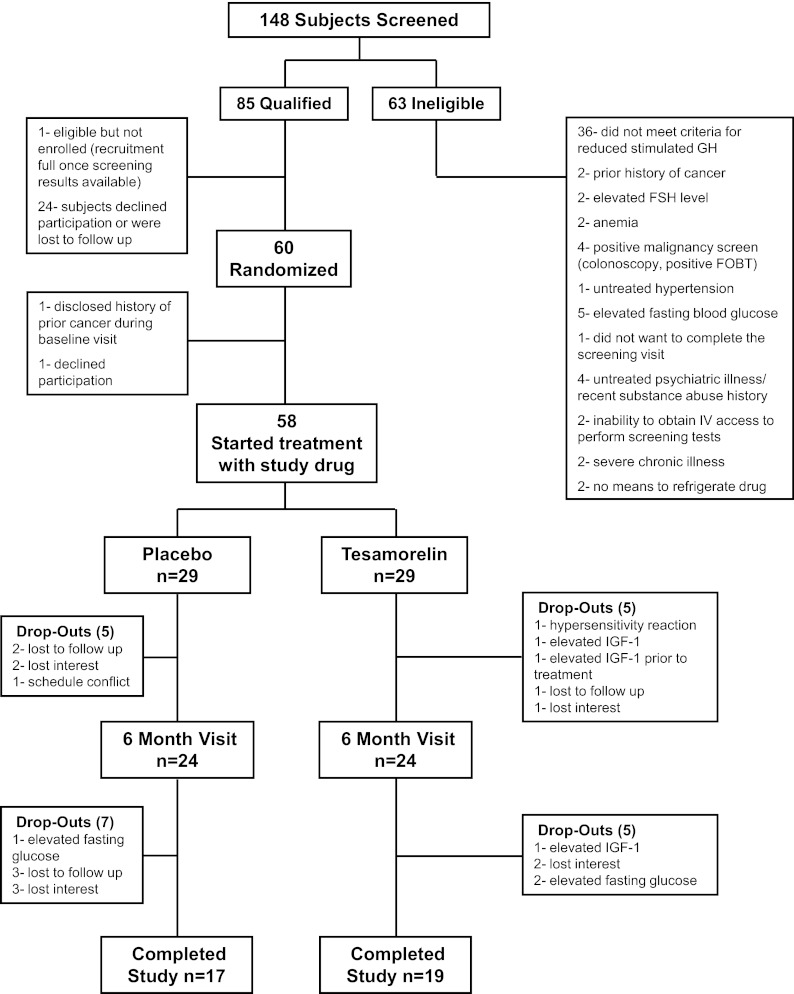
Subjects were randomized in a 1:1 fashion to receive 2 mg tesamorelin or matching placebo. Tesamorelin and matching placebo were supplied as a lyophilized powder, and subjects were trained to reconstitute and self-inject the medication sc daily for 1 yr. The randomization was stratified by gender. Investigators and subjects were blinded to the treatment randomization. Assessment for the primary endpoint of abdominal VAT and CVD risk factors were performed at baseline and 26 and 52 wk. Safety parameters including fasting glucose and IGF-I were also measured at 2, 4, 12, and 36 wk. Subjects with a fasting glucose greater than 125 mg/dl were discontinued from the study. IGF-I levels were monitored by a physician independent from the study team. A dose-reduction algorithm was incorporated into the protocol such that if a subject's IGF-I level increased above the normal range for the subject's age, then the subject (and a placebo-treated subject at a similar time point in the study) would be instructed to decrease the study drug dose to 1 mg once daily. The dummy change was made in the placebo-treated subject to ensure blinding of investigators to treatment status throughout the study. Subjects with a repeatedly elevated IGF-I for their age, despite dose adjustment, were discontinued from the study (Fig. 1). Tesamorelin was used under an investigator-initiated Investigational New Drug application (IND no. 73,329) to S.K.G.
Fig. 1.
Enrollment and outcomes. FOBT, Fecal occult blood test.
Assessments
GH stimulation test
Standardized GHRH-arginine stimulation testing was performed after an overnight fast (8, 13).
Body composition
Abdominal VAT and SAT were assessed using a single cross-sectional slice from noncontrast computed tomography at the L4 level (23). Lean mass, fat mass, and trunk fat were assessed by dual-energy x-ray absorptiometry (Discovery A, Hologic Inc., Waltham, MA). WC was measured in a standardized fashion in triplicate on each patient by a trained research dietician. The average of the three measurements was used in the analysis. The precision and reproducibility of the WC measurement were 0.06 and 99.9%, respectively.
Carotid intima-media thickness
cIMT was measured via ultrasound as previously described (13, 24). The average cIMT over the length of the measured segments on the right carotid artery is reported.
Laboratory assessments
GH was assessed using a chemiluminescent immunoassay (Beckman Coulter, Chaska, MN). IGF-I was measured using the Immulite 2000 assay (Siemens Diagnostics, Deerfield, IL); normal ranges are 182–780 μg/liter (18–24 yr), 114–492 μg/liter (25–39 yr), 90–360 μg/liter (40–54 yr), and 71–290 μg/liter (55 yr). In addition to reporting the absolute changes, change is reported as an sd score. IGF-I is also calculated as a percentage of upper limit of normal (ULN) for age. Lipids, glucose, and high-sensitivity C-reactive protein (CRP) were assessed at the Massachusetts General Hospital clinical laboratory.
Compliance
Adherence to the study drug was monitored via subject self-reported injection log as well as a vial count of returned vials at each visit.
Statistical methods
The primary endpoint in this study was change in VAT from baseline. Secondary endpoints included changes in cIMT, body composition, IGF-I, lipids, glucose, glycated hemoglobin (HbA1c) and other safety parameters. Assuming evaluable post-baseline data on 48 patients, the study was powered at 80% to detect a difference of 32 cm2 in VAT between the groups, with α = 0.05 and an estimated treatment sd of 38.6 cm2 from previous studies of tesamorelin (22).
Variables were compared by χ2 test for noncontinuous variables, t test for continuous variables that were normally distributed, and Wilcoxon rank sum test for continuous variables that were not normally distributed at baseline. Normality of the data was determined by the Wilk-Shapiro test. Efficacy endpoints were analyzed using longitudinal linear mixed-effects modeling with all available data and the last value carried forward for patients who discontinued and had missing data. The reported treatment effect represents estimated differences between tesamorelin and placebo treatment over 12 months in the model [mean, 95% confidence interval (CI)]. For those variables not normally distributed in Table 1, data were log transformed, and the model was rerun with log-transformed data in a sensitivity analysis. A secondary sensitivity analysis was performed using longitudinal linear mixed-effects modeling with all available data, without carry forward. Additional sensitivity analyses were performed excluding data from subjects with elevated IGF-I (n = 4). These analyses were conducted to explore whether the effect on VAT and other endpoints was seen if the analysis was limited to patients in whom IGF-I was within normal limits for the study duration. In addition, sensitivity analyses were performed including use of lipid-lowering and antihypertensive therapies in the mixed-effects modeling for cIMT and use of lipid-lowering therapy for triglyceride. The P value was determined from the time × randomization effect in the model. Statistical analyses were performed using SAS and JMP version 9.0 (SAS Institute, Cary, NC). All reported P values are two-sided.
Table 1.
Demographic and baseline clinical characteristics of the patients enrolled in the study stratified by treatment group
| Tesamorelin (n = 31) | Placebo (n = 29) | P value | |
|---|---|---|---|
| Demographics | |||
| Age (yr) | 42.5 ± 1.3 | 39.9 ± 1.8 | 0.25 |
| Gender [male/female (% male)] | 20/11 (65.5) | 19/10 (64.5) | 0.94 |
| Race [n (% Caucasian)] | 16 (51.6) | 19 (65.5) | 0.28 |
| Current or past tobacco use [n (%)] | 18 (58) | 13 (45) | 0.31 |
| Medication usea [n (%)] | |||
| HMG CoA reductase inhibitor | 3 (10) | 1 (3) | 0.33 |
| Lipid-lowering therapyb | 8 (26) | 12 (41) | 0.20 |
| Antihypertensive | 10 (32) | 5 (17) | 0.18 |
| Body composition | |||
| Weight (kg) | 115.7 ± 3.8 | 115.8 ± 3.3 | 0.99 |
| BMI (kg/m2) | 37.5 (34, 41.4) | 38.3 (34.4, 40.5) | 0.81 |
| WC (cm) | 121 ± 2 | 121 ± 2 | 0.94 |
| VAT (cm2) | 209 ± 13 | 193 ± 14 | 0.39 |
| GH parameters | |||
| Peak stimulated GH on GHRH-arginine stimulation test (μg/liter) | 4.22 ± 0.46 | 5.19 ± 0.47 | 0.15 |
| IGF-I (μg/liter) | 101 (79, 145) | 131 (107, 172) | 0.17 |
Results are presented as the mean ± sem for normally distributed data and analyzed by Student's t test. For data that is not normally distributed, results are presented as median with interquartile range (25, 75%) and analyzed using the Wilcoxon rank sum test. Noncontinuous variables are compared using χ2 test. One patient in the tesamorelin group discontinued the study before obtaining baseline body composition data. VAT was measured via abdominal computed tomography scan. HMG CoA, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase.
Medication use includes baseline data as well as initiation of new medications during the course of the study.
Lipid-lowering medication use includes subjects using HMG CoA reductase inhibitors, niacin, and fish oil.
Results
Subjects
Of 148 obese patients screened, 60 obese subjects with reduced GH secretion were enrolled and randomized (Fig. 1). Baseline demographics were similar between the groups including age, race, and gender (Table 1). No patient had a history of known coronary artery disease. No significant differences in VAT, other body composition parameters, cIMT, or IGF-I were noted between the tesamorelin and the placebo group. The two groups did not differ for use of lipid-lowering or antihypertensive medication use. The tesamorelin group had higher mean triglycerides at baseline (Table 2).
Table 2.
Effect of treatment with tesamorelin vs. placebo on body composition, biochemical indices, and cIMT
| Baseline |
6 months |
12 months |
Change |
Effect size for tesamorelin vs. placebo |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tesamorelin | Placebo | P value | Tesamorelin | Placebo | Tesamorelin | Placebo | Tesamorelin | Placebo | Effect size (95% CI) | P value | |
| Body composition | |||||||||||
| VAT (cm2) | 209 ± 13 | 193 ± 14 | 0.39 | 188 ± 14 | 205 ± 16 | 193 ± 15 | 212 ± 16 | −16 ± 9 | 19 ± 9 | −35 (−58, −12) | 0.003 |
| SAT (cm2) | 480 ± 26 | 535 ± 28 | 0.15 | 475 ± 26 | 547 ± 27 | 463 ± 25 | 538 ± 29 | −6 ± 6 | 3 ± 11 | −10 (−32, 13) | 0.40 |
| Weight (kg) | 115.7 ± 3.8 | 115.8 ± 3.3 | 0.99 | 116.0 ± 3.9 | 116.7 ± 3.5 | 116.5 ± 3.7 | 116.6 ± 3.4 | 0.1 ± 1.1 | 0.9 ± 1.1 | −0.8 (−3.2, 1.6) | 0.52 |
| BMI (kg/m2) | 38.2 ± 0.9 | 37.9 ± 0.7 | 0.81 | 38.4 ± 0.9 | 38.4 ± 0.7 | 38.2 ± 0.9 | 38.4 ± 0.8 | −0.1 ± 0.4 | 0.6 ± 0.4 | −0.6 (−1.4, 0.2) | 0.14 |
| WC (cm) | 121 ± 2 | 121 ± 2 | 0.94 | 120 ± 3 | 121 ± 2 | 120 ± 2 | 122 ± 2 | −2 ± 1 | 1 ± 1 | −3 (−5, −0.3) | 0.03 |
| Lean mass (kg) | 71.8 ± 2.5 | 70.7 ± 2.2 | 0.74 | 72.7 ± 2.6 | 70.6 ± 2.3 | 72.8 ± 2.6 | 70.3 ± 2.1 | 1.0 ± 0.5 | −0.4 ± 0.4 | 1.4 (0.2, 2.6) | 0.03 |
| Fat mass (kg) | 42.6 ± 1.9 | 44.0 ± 2.0 | 0.26 | 42.1 ± 2.0 | 44.8 ± 2.1 | 41.9 ± 2.0 | 45.0 ± 2.2 | −0.7 ± 0.7 | 1.0 ± 0.7 | −1.7 (−3.4, −0.1) | 0.04 |
| Trunk fat (kg) | 23.3 ± 1.3 | 23.3 ± 1.1 | 0.53 | 22.7 ± 1.3 | 23.8 ± 1.2 | 22.6 ± 1.3 | 24.0 ± 1.3 | −0.6 ± 0.4 | 0.7 ± 0.4 | −1.4 (−2.4, −0.3) | 0.01 |
| Biochemical | |||||||||||
| IGF-I (μg/liter) | 160 ± 43 | 142 ± 12 | 0.17 | 225 ± 42 | 134 ± 9 | 234 ± 41 | 139 ± 10 | 86 ± 21 | −6 ± 8 | 92 (52, 132) | <0.0001 |
| Total cholesterol (mg/dl) | 176 ± 5 | 168 ± 5 | 0.28 | 186 ± 5 | 175 ± 5 | 180 ± 6 | 174 ± 6 | 4 ± 4 | 7 ± 5 | −2 (−13, 9) | 0.69 |
| Triglycerides (mg/dl) | 196 ± 26 | 132 ± 11 | 0.03 | 166 ± 17 | 133 ± 10 | 170 ± 18 | 141 ± 13 | −26 ± 16 | 12 ± 8 | −37 (−67, −7) | 0.02 |
| HDL cholesterol (mg/dl) | 35 ± 1 | 36 ± 2 | 0.61 | 40 ± 2 | 40 ± 2 | 39 ± 2 | 39 ± 2 | 4 ± 1 | 3 ± 1 | 1 (−3, 5) | 0.66 |
| LDL cholesterol (mg/dl) | 114 ± 5 | 117 ± 6 | 0.72 | 125 ± 6 | 119 ± 6 | 118 ± 5 | 117 ± 6 | 4 ± 4 | 1 ± 4 | 3 (−8, 14) | 0.60 |
| Log CRPa | 0.51 ± 0.15 | 0.66 ± 0.09 | 0.89 | 0.42 ± 0.15 | 0.65 ± 0.08 | 0.35 ± 0.15 | 0.64 ± 0.08 | −0.17 ± 0.04 | −0.03 ± 0.05 | −0.15 (−0.30, −0.01) | 0.04 |
| cIMT (mm) | 0.68 ± 0.02 | 0.66 ± 0.04 | 0.18 | 0.68 ± 0.02 | 0.67 ± 0.04 | 0.66 ± 0.02 | 0.67 ± 0.04 | −0.03 ± 0.01 | 0.01 ± 0.01 | −0.04 (−0.07, −0.01) | 0.02 |
Results are presented as mean ± sem. P values from baseline data were obtained by Student's t test for normally distributed variables and Wilcoxon rank sum for nonnormally distributed samples. Effect size and P values were obtained by longitudinal linear mixed-effects modeling for each parameter over 12 months with last value carried forward. One patient in the tesamorelin group discontinued the study before obtaining baseline body composition and biochemical data.
For CRP, the final value in one patient was 5 sd above all other data points and over 100 points above all her previous data points. This patient had skin manifestations and swelling from multiple insect bites while camping immediately before the final visit. Therefore, this data point was excluded as an outlier. Instead, data were assessed from her 9-month safety visit, before the insect bites, and used in the analysis.
Two subjects completed the baseline visit but discontinued before initiation of the study drug, resulting in 58 subjects who initiated treatment [tesamorelin (n = 29) and placebo (n = 29)]. Five subjects in each arm discontinued before the 6-month visit for a retention rate of 83% among those who received at least one dose of study drug at 6 months in each arm. Seventeen subjects in the placebo group and 19 subjects in the tesamorelin group completed the 1-yr study, for a per-protocol retention rate among those who received at least one dose of study drug of 62%, which was not different between the groups (Fig. 1). Therefore, there was an equivalent retention rate across all time points in the study, and 83% of subjects contributed post-baseline data to the last observation carried forward analysis. Noncompleters were similar to completers at baseline (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Body composition
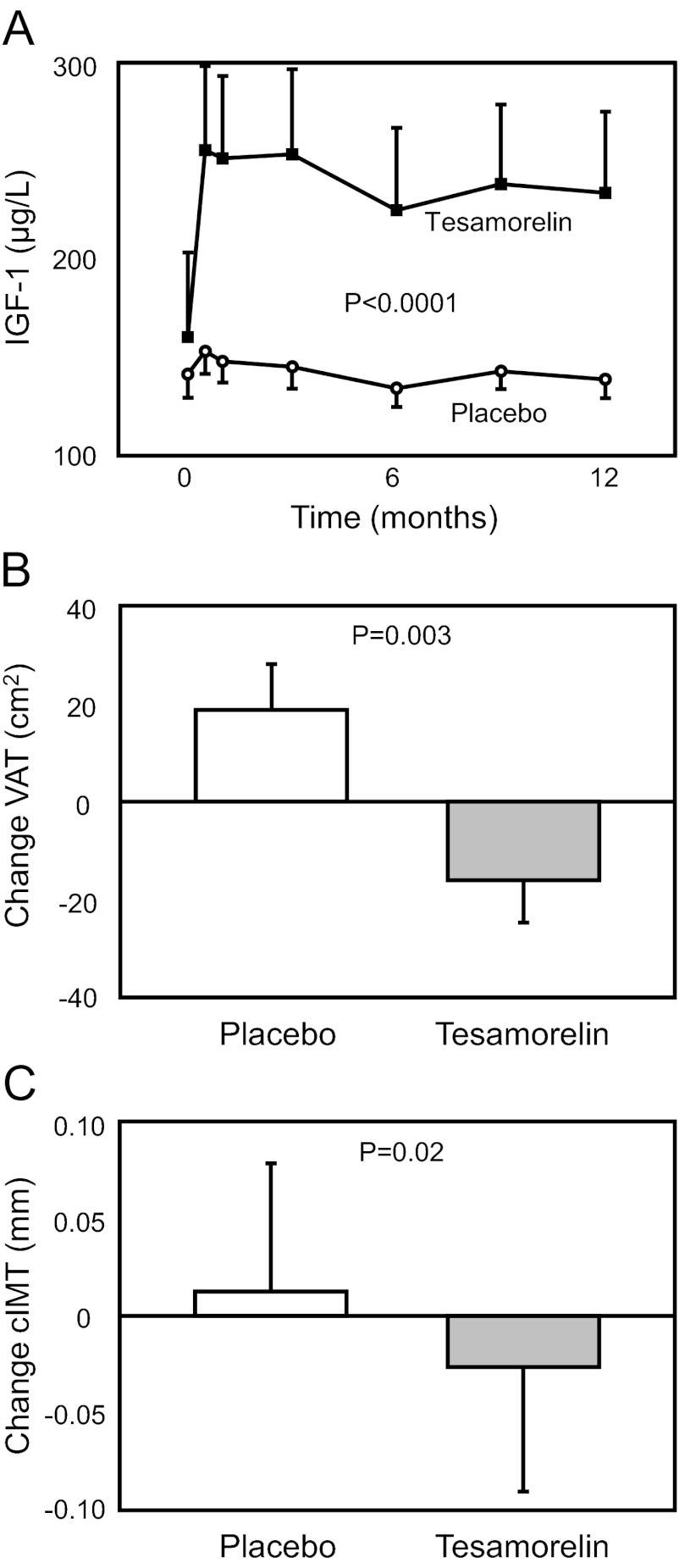
VAT [−16 ± 9 vs. 19 ± 9 cm2, tesamorelin vs. placebo; net treatment effect vs. placebo (95% CI): −35 (−58, −12) cm2; P = 0.003] (Table 2 and Fig. 2) improved significantly in the tesamorelin vs. placebo group. Other body composition parameters including WC [−2 ± 1 vs. 1 ± 1 cm; −3 (−5, −0.3) cm; P = 0.03], trunk fat [−0.6 ± 0.4 vs. 0.7 ± 0.4 kg; −1.4 (−2.4, −0.3) kg; P = 0.01], total fat [−0.7 ± 0.7 vs. 1.0 ± 0.7 kg; −1.7 (−3.4, −0.1) kg; P = 0.04], and lean body mass (1.0 ± 0.5 vs. −0.4 ± 0.4 kg; 1.4 (0.2, 2.6) kg; P = 0.03] improved in the tesamorelin vs. placebo groups, whereas no significant effects on abdominal SAT [−6 ± 6 vs. 3 ± 11 cm2; −10 (−32, 13) cm2; P = 0.40] or weight [0.1 ± 1.1 vs. 0.9 ± 1.1 kg; −0.8 (−3.2, 1.6) kg, P = 0.52] were seen. The changes in VAT amounted to a net treatment effect vs. placebo of −19%, comprised of a statistically significant 8% reduction within the tesamorelin group and a statistically significant 11% increase within the placebo group (P < 0.05 for both within-group comparisons), demonstrating significant between- and within-group effects of tesamorelin to reduce and prevent additional VAT increases. Results remained significant after adjusting for gender (P = 0.01) without any gender effect seen in the model. In addition, the change at 6 months in VAT was maintained and not significantly different from the change in VAT at 12 months (P = 0.26 for differences between changes at 6 and 12 months). Similar results were seen in a sensitivity analysis performed without carrying forward missing data (data not shown).
Fig. 2.
Effects of tesamorelin vs. placebo on IGF-I (A), abdominal VAT area (B), and cIMT (C). Panel A demonstrates the change in IGF-I from baseline over time in tesamorelin vs. placebo. Each point represents mean ± sem of IGF-I in each study group at baseline, 2 wk, and 1, 3, 6, 9, and 12 months. Although IGF-I levels at baseline were similar between the two groups (P = 0.17), IGF-I levels at all subsequent time points were significantly different between each group (P < 0.05 at each time point) with overall P < 0.0001 by longitudinal linear mixed-effects modeling. Panel B demonstrates the difference in VAT between treatment groups with tesamorelin demonstrating a net −19% improvement in VAT compared with placebo. Panel C demonstrates the differences in cIMT between treatment groups with tesamorelin demonstrating a net −6% improvement in cIMT compared with placebo. The T bars in B and C denote the se. Statistical significance was determined by longitudinal linear mixed-effects modeling with the last value carried forward for both B and C.
Carotid intima-media thickness
Treatment with tesamorelin for 12 months resulted in a significant reduction in cIMT compared with placebo [−0.03 ± 0.01 vs. 0.01 ± 0.01 mm; −0.04 (−0.07, −0.01) mm; P = 0.02] for a net difference in mean percentage change of −6% (Table 2 and Fig. 2). Results remained significant in sensitivity analyses performed without carrying forward missing data (data not shown).
Biochemical indices
Treatment with tesamorelin resulted in increased IGF-I [86 ± 21 vs. −6 ± 8 μg/liter; 92 (52, 132) μg/liter; P < 0.0001] (Fig. 2), decreased triglycerides [−26 ± 16 vs. 12 ± 8 mg/dl; −37 (−67, −7) mg/dl; P = 0.02], and decreased log CRP [−0.17 ± 0.04 vs. −0.03 ± 0.05 mg/liter; −0.15 (−0.30, −0.01) mg/liter, P = 0.04] compared with placebo. In terms of overall percent change, tesamorelin increased IGF-I by 90% and decreased triglycerides by 20% and log CRP by 24% compared with placebo. Tesamorelin did not affect total cholesterol, high-density lipoprotein (HDL), or low-density lipoprotein (LDL) (Table 2).
IGF-I, represented as a percentage of the age-specific ULN, was 30 ± 3 vs. 34 ± 4% at baseline (P = 0.34), 47 ± 4 vs. 32 ± 3% ULN at 6 months (P = 0.007), and 49 ± 4 vs. 33 ± 3% ULN at 12 months (P = 0.003), tesamorelin vs. placebo. Expressing the change as an sd score, the changes were 1.14 ± 0.25 (95% CI: 0.63, 1.65) vs. −0.12 ± 0.13 (95% CI: −0.39, 0.15) (P < 0.0001) at 6 months and 1.25 ± 0.27 (95% CI: 0.69, 1.81) vs. −0.05 ± 0.14 (95% CI: −0.34, 0.25) (P = 0.0001) at 12 months, tesamorelin vs. placebo.
Dietary and physical activity measurements
There were no differences in dietary intake or physical activity between groups (all P > 0.10) (data not shown).
Sensitivity analyses
Similar results were seen, with significant effects of tesamorelin compared with placebo on VAT [−32 (−56, −9) cm2; P = 0.008], cIMT [−0.05 (−0.08, −0.02) mm; P = 0.004], triglyceride [−36 (−68, −4) mg/dl; P = 0.03], log CRP [−0.15 (−0.30, 0) mg/liter; P = 0.05), and IGF-I [78 (44, 111) μg/liter; P < 0.0001] in the sensitivity analyses excluding patients with dose reductions and elevated IGF-I. The effects of tesamorelin remained significant for cIMT and triglyceride in longitudinal linear mixed-effect models including use of lipid-lowering and antihypertensive therapies. For nonnormally distributed baseline data in Table 1, the longitudinal linear-effects model with log-transformed data demonstrated similar results.
Safety parameters
One subject was discontinued from the study after his baseline IGF-I level, drawn before initiation of study drug, was found to be elevated. Three subjects had elevated IGF-I during the study requiring dose reductions, and two subjects were discontinued despite dose adjustment. Both subjects had normalization of IGF-I after discontinuation of tesamorelin.
There were no differences in fasting blood glucose, 2-h glucose, or HbA1c between tesamorelin and placebo treatment (Supplemental Table 2). In addition, there were no differences in glucose between the groups at early time points (Supplemental Table 3). Three subjects (one placebo and two tesamorelin) were discontinued due to a repeatedly elevated fasting glucose above 125 mg/dl (Fig. 1). There were no differences in blood pressure or liver transaminases between tesamorelin and placebo (Supplemental Table 2).
Adverse events
There were no serious adverse events during the study and no significant difference in the rates of adverse events between tesamorelin and placebo (Table 3).
Table 3.
Adverse events
| Event | Tesamorelin [n (%)] | Placebo [n (%)] | P value |
|---|---|---|---|
| Any adverse event | 28 (90.3) | 26 (89.7) | 0.93 |
| Related to treatment | 22 (75.9) | 21 (80.8) | 0.66 |
| Resulted in discontinuation from study | 4 (13.8) | 1 (3.9) | 0.20 |
| Serious adverse event | 0 (0) | 0 (0) | N/A |
| Discontinued before 6 months | 5 (17.2) | 5 (17.2) | 1.00 |
| Discontinued after 6 months | 5 (16.1) | 7 (24.1) | 0.44 |
| Dose adjustment before 6 months | 3 (9.7) | 0 (0) | N/A |
| Dose adjustment after 6 months | 0 (0) | 0 (0) | N/A |
| Adverse events potentially related to tesamorelin | |||
| Injection-site bruising | 17 (54.8) | 21 (72.4) | 0.16 |
| Hypertension | 8 (29.6) | 5 (17.2) | 0.27 |
| Injection-site bleeding | 4 (12.9) | 5 (17.2) | 0.64 |
| Injection-site pain | 3 (9.7) | 2 (6.9) | 0.70 |
| Hyperglycemia | 3 (9.7) | 2 (6.9) | 0.70 |
| Tingling/paresthesia | 3 (9.7) | 1 (3.5) | 0.33 |
| Peripheral edema | 3 (9.7) | 1 (3.5) | 0.33 |
| Injection-site pruritis | 2 (6.5) | 1 (3.5) | 0.59 |
| Injection-site reaction | 2 (6.5) | 0 (0) | 0.16 |
| Arthralgia | 2 (6.5) | 0 (0) | 0.16 |
| Injection-site erythema | 2 (6.5) | 0 (0) | 0.16 |
| Carpal tunnel syndrome | 1 (3.2) | 0 (0) | 0.33 |
| Hypersensitivity reaction | 1 (3.2) | 0 (0) | 0.33 |
| Malignancy | 0 (0) | 0 (0) | N/A |
| Adverse events occurring in >5% of subjects | |||
| Anxiety | 1 (3.2) | 3 (10.3) | 0.27 |
| Abdominal pain | 2 (6.5) | 3 (10.3) | 0.59 |
| Atypical chest pain | 2 (6.5) | 2 (6.9) | 0.95 |
| Back pain | 4 (12.9) | 4 (13.8) | 0.92 |
| Constipation | 2 (6.5) | 0 (0) | 0.16 |
| Depression | 0 (0) | 2 (6.9) | 0.14 |
| Dermatitis | 3 (9.7) | 1 (3.5) | 0.33 |
| Diarrhea | 3 (9.7) | 1 (3.5) | 0.33 |
| Fatigue | 2 (6.5) | 1 (3.5) | 0.59 |
| Fungal infection | 0 (0) | 3 (10.3) | 0.07 |
| Gastroenteritis | 2 (6.5) | 1 (3.5) | 0.59 |
| GERD | 0 (0) | 2 (6.9) | 0.14 |
| Headache | 2 (6.5) | 5 (17.2) | 0.19 |
| Infection | 0 (0) | 2 (6.9) | 0.14 |
| Mechanical injury | 0 (0) | 3 (10.3) | 0.07 |
| Musculoskeletal injury | 5 (16.1) | 5 (17.2) | 0.91 |
| Musculoskeletal pain | 4 (12.9) | 5 (17.2) | 0.64 |
| Myalgia | 2 (6.5) | 3 (10.3) | 0.59 |
| Nasopharyngitis | 0 (0) | 2 (6.9) | 0.14 |
| Nausea | 3 (9.7) | 0 (0) | 0.09 |
| Sinusitis | 2 (6.5) | 6 (20.7) | 0.11 |
| Tendonitis | 2 (6.5) | 0 (0) | 0.16 |
| Toothache | 2 (6.5) | 0 (0) | 0.16 |
| URI | 5 (16.1) | 7 (24.1) | 0.44 |
No serious adverse events were reported during the study. There were no significant differences in the rate of adverse events between study groups. GERD, Gastroesophageal reflux disease; URI, upper respiratory tract infection.
Compliance
Compliance was not different between the groups based on subject self-reported injection log (89 vs. 94%, P = 0.11) or vial count of returned vials at each visit (85 vs. 82%, P = 0.95, tesamorelin vs. placebo).
Discussion
In this study, we demonstrate for the first time an effect of a GHRH analog, tesamorelin, to reduce VAT and improve cIMT in obese subjects with relative reductions in GH secretory capacity. The reduction in cIMT in this study suggests a true cardiovascular benefit associated with reduction in VAT in this population.
Tesamorelin demonstrated a large net effect (−19%) to reduce VAT relative to placebo in the study. This was achieved as the result of significant reductions in VAT in tesamorelin-treated subjects and increases in VAT in placebo-treated subjects. Changes over 6 and 12 months were not significantly different, suggesting that these changes were maintained over 12 months. More studies are needed to determine whether these changes can be maintained over longer treatment periods.
The effect on VAT was selective, without significant effects on SAT. A selective effect of tesamorelin on VAT was seen in previous studies of subjects with HIV lipodystrophy (22). However, in contrast to lipodystrophy in which patients demonstrate an increase in VAT and loss in SAT, obese patents in the general population demonstrate an increase in both VAT and SAT. Moreover, recent evidence suggests that SAT is cardioprotective, whereas VAT increases CVD risk (25, 26), and relative preservation of SAT may be advantageous during treatments to reduce VAT in obese subjects. We are not aware of previous strategies to selectively reduce VAT in obesity.
In addition to reducing VAT, tesamorelin resulted in a significant reduction in WC. Moreover, the 3-cm reduction in WC was achieved based exclusively on reductions in VAT, because SAT was unchanged. The clinical impact of a VAT-based reduction in WC may be greater than a reduction in WC comprised of simultaneous VAT and SAT loss.
It is important to consider these effects on VAT were achieved despite a strict dose titration algorithm requiring IGF-I to be in the normal range for a subject's age. Expressing IGF-I as a percentage of the age-specific ULN demonstrates that the tesamorelin-treated patients were well within the normal range for age, and the IGF-I change reported as sd score also reflects this with the upper bound of the 95% CI below 2.0 sd. This is further reflected in the lack of GH-related adverse events among the tesamorelin- vs. placebo-treated subjects (Table 3). Moreover, only two subjects required discontinuation for persistently high IGF-I, and the results, including significant effect on VAT and cIMT were confirmed in a sensitivity analysis, limited to those without any dose titrations and thus normal IGF-I throughout the study. Thus, these results were achieved with entirely physiological increases in IGF-I.
Among obese subjects, previous studies have investigated strategies using GH (27). However, these studies have not used a strategy to increase endogenous GH with a GHRH analog. The mean IGF-I achieved in these previous GH studies was an increase of 171 μg/liter (27), approximately twice as large as in our study of 92 μg/liter, yet the net effect of tesamorelin on VAT in our study [−35 (95% CI: −58, −12) cm2], was greater than seen in these previous studies of GH [−23 (−40, −6) cm2). In contrast to these previous studies using GH, we also assessed for GH secretory capacity at baseline and did not treat those patients with a normal GH response to GHRH-arginine. Moreover, we used a specific GHRH analog to increase endogenous GH secretion. Previous studies have demonstrated the utility of an oral ghrelin mimetic to increase endogenous GH secretion. However, in contrast to tesamorelin, this agent was associated with weight gain and a decrease in insulin sensitivity, which would be counterproductive in obesity (28).
Although exogenous GH has been shown to improve cIMT in hypopituitary subjects with GH deficiency (15), no previous study has evaluated the effects of either GH or a GH-releasing factor on cIMT in obese subjects with reduced GH secretion. In contrast, other strategies investigated in obesity, including rimonabant, did not decrease cIMT (29). Studies employing dietary strategies have shown reduced cIMT in obesity, usually in relationship to the amount of weight lost. For example, the reduction in cIMT achieved in our study was very similar to that seen in obese patients with 8.4 kg of weight loss after 12 months of a dietary intervention but was obtained without an effect on weight per se in our study (30). Moreover, cIMT increases in the placebo group are on par with other studies (31, 32), and tesamorelin not only reduces cIMT but also prevents the expected increase over time in this variable. Additional studies are necessary to determine the clinical significance of the changes in cIMT seen in the current study in response to tesamorelin.
The potential mechanism by which cIMT decreases in the current study may relate to the selective loss of VAT, because increased VAT has been shown to be independently associated with increased cIMT among obese patients (33). The loss of VAT may result in reduced triglyceride and CRP, as seen in this study, which could contribute to improved cIMT. Alternatively, increasing GH among obese subjects with reduced GH may have direct effects to reduce de novo lipogenesis and triglyceride (34) and to reduce inflammation. No effects on LDL or HDL were seen in the current study.
Weight loss was not seen in the current study because lean body mass increased and fat mass decreased to commensurate degrees. It is interesting to consider this study in light of previous studies suggesting that massive reduction in SAT without reduction in VAT did not improve metabolic and CVD risk parameters (35), whereas small surgically induced selective reductions in VAT by omentectomy did improve a number of metabolic parameters in one study (36). The results of the current study extend the results of these previous studies and highlight the potential benefits of selective VAT reduction.
Tesamorelin, importantly, did not affect parameters of glucose homeostasis, either early or late in this study. This is consistent with a previous short-term physiology study demonstrating a complete absence of effect of tesamorelin on the gold standard, hyperinsulinemic-euglycemic clamp (21). Interestingly, this is contrary to the known effects of exogenous GH to worsen insulin resistance (27). However, in our study, no significant effects were noted on either fasting or 2-h glucose or HbA1c, suggesting divergent effects of strategies using exogenous GH and those designed to increase endogenous GH secretion using a GH-releasing factor and resulting in more physiological increases in IGF-I. Over time, the reduction in VAT, more physiological increases in pulsatile GH, and potential insulin-sensitizing effects of increased IGF-I per se may help to maintain glucose control in response to tesamorelin.
For the purposes of this study, we chose a GH cutoff of less than 9 μg/liter to enroll subjects with relative reductions in GH. The exact cutoff point to define this population has yet to be determined. Corneli et al. (37) and Biller et al. (38) had previously identified a cutoff point of 4.2 μg/liter as providing optimal sensitivity and specificity for the diagnosis of pathological GH deficiency in obese patients with pituitary disease. However, our intent with this study was not to identify obese subjects with pathological GH deficiency but rather to identify subjects with relative reductions in GH who may benefit from normalization of GH. To this end, Ghigo et al. (39) previously demonstrated the first centile limit of peak stimulated GH in otherwise healthy normal-weight subjects is 9 μg/liter, suggesting obtaining a peak stimulated GH of more than 9 μg/liter may be an appropriate goal. We therefore selected subjects with peak stimulated GH of less than 9 μg/liter and, thus, relative reductions in GH. Of note, our previous work (13) demonstrates obese subjects with peak GH below 4.2 or below 9 μg/liter may benefit from intervention to raise their GH levels because each cutoff was associated with increased CVD risk indices including cIMT. Thus, we used that cutoff in the current protocol to define functionally consequential, relative GH deficiency in obesity and indeed showed a decrease in cIMT with tesamorelin.
The current study is unique from and significantly advances the previous studies of tesamorelin in HIV patients with increased visceral adiposity, because we now investigate this strategy to augment endogenous GH in patients with generalized obesity and reduced GH, a much larger at-risk population with a different biology of fat gain, assessing for reduced GH at study entry, monitoring for physiological increases in IGF-I, and demonstrating for the first time an effect of this strategy to benefit cardiovascular indices, e.g. cIMT.
This study has some limitations. The dropout rates noted were on par with other, long-term placebo-controlled studies in obesity (40). Dropout rates were similar in placebo and treated groups, and noncompleters were similar to completers in terms of baseline characteristics. Moreover, our results were significant in analyses using all available data and carrying forward results using a conservative approach to account for missing data. The study was adequately powered to show effects on primary and secondary endpoints. No safety signal in glucose or other endpoints was seen, but larger studies will be needed to confirm safety in this population. Some portion of the increase in lean body mass using dual-energy x-ray absorptiometry might represent fluid and shifts in intracellular water, but there was no obvious excess in peripheral edema among the tesamorelin-treated subjects, and IGF-I levels were kept within the normal age range. We did not assess hepatic fat in the current study, and this will be important to assess in future studies, given the robust effects of tesamorelin on VAT. cIMT levels were lower than among obese patients reported in our previous study but are increased above the range seen in nonobese patients in that study (13). Direct comparisons cannot be made because subjects in the current study were younger and were chosen based on a GH cutoff of 9 μg/liter.
Tesamorelin, a GHRH analog, significantly reduces VAT and improves cIMT in obese patients with relative reductions in GH secretory capacity. Tesamorelin was well tolerated, without major side effects, and did not aggravate glucose. Together these data advance our understanding of the neuroendocrine physiology of obesity, suggesting there may be significant functional consequences of low GH in obesity and that augmentation of endogenous GH secretion with a specific GHRH analog may improve CVD risk in this population. In addition, this study suggests, more broadly, that strategies to selectively reduce VAT may improve CVD risk, even while SAT is preserved and BMI is unchanged.
Supplementary Material
Acknowledgments
We gratefully acknowledge the contributions of Pouneh Fazeli, M.D., who independently performed the dose adjustments based on IGF-I. We also acknowledge the nurses and staff at the Massachusetts General Hospital Clinical Research Center as well as the subjects who participated in the study.
This work was supported by National Institutes of Health Grant K23DK087857 to H.M.; Grants R01HL085268, P30DK040561, and K24DK064545 to S.K.G.; and Grants M01RR01066 and UL1RR025758 to the Harvard Clinical and Translational Science Center from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Partial research funding and study drug was provided by Theratechnologies, Inc.
Disclosure Summary: S.K.G. has served as a consultant to Theratechnologies, Inc.; Aileron Therapeutics, Inc.; Alize Pharma SAS; F Hoffmann-La Roche Ltd.; and EMD Serono Inc., all unrelated to this manuscript, and received investigator-initiated research funds from Theratechnologies, Inc. H.M. has received investigator-initiated research support from Pfizer, Inc., unrelated to the manuscript.
This study was registered at www.clinicaltrials.gov as NCT00675506.
Footnotes
- BMI
- Body mass index
- CI
- confidence interval
- cIMT
- carotid intima-media thickness
- CRP
- C-reactive protein
- CVD
- cardiovascular disease
- HbA1c
- glycated hemoglobin
- HDL
- high-density lipoprotein
- LDL
- low-density lipoprotein
- SAT
- sc adipose tissue
- ULN
- upper limit of normal
- VAT
- visceral adipose tissue
- WC
- waist circumference.
References
- 1. Després JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. 1990. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 10:497–511 [DOI] [PubMed] [Google Scholar]
- 2. Nguyen-Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. 2003. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab 284:E1065–E1071 [DOI] [PubMed] [Google Scholar]
- 3. Lemieux I, Pascot A, Prud'homme D, Alméras N, Bogaty P, Nadeau A, Bergeron J, Després JP. 2001. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol 21:961–967 [DOI] [PubMed] [Google Scholar]
- 4. Lear SA, Humphries KH, Kohli S, Frohlich JJ, Birmingham CL, Mancini GB. 2007. Visceral adipose tissue, a potential risk factor for carotid atherosclerosis: results of the Multicultural Community Health Assessment Trial (M-CHAT). Stroke 38:2422–2429 [DOI] [PubMed] [Google Scholar]
- 5. Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, Shofer JB, Wahl PW. 1999. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care 22:1808–1812 [DOI] [PubMed] [Google Scholar]
- 6. Møller N, Jørgensen JO. 2009. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 30:152–177 [DOI] [PubMed] [Google Scholar]
- 7. Bonert VS, Elashoff JD, Barnett P, Melmed S. 2004. Body mass index determines evoked growth hormone (GH) responsiveness in normal healthy male subjects: diagnostic caveat for adult GH deficiency. J Clin Endocrinol Metab 89:3397–3401 [DOI] [PubMed] [Google Scholar]
- 8. Makimura H, Stanley T, Mun D, You SM, Grinspoon S. 2008. The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J Clin Endocrinol Metab 93:4254–4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iranmanesh A, Lizarralde G, Veldhuis JD. 1991. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) ceretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab 73:1081–1088 [DOI] [PubMed] [Google Scholar]
- 10. Vahl N, Jørgensen JO, Skjaerbaek C, Veldhuis JD, Orskov H, Christiansen JS. 1997. Abdominal adiposity rather than age and sex predicts mass and regularity of GH secretion in healthy adults. Am J Physiol 272:E1108–E1116 [DOI] [PubMed] [Google Scholar]
- 11. Pijl H, Langendonk JG, Burggraaf J, Frölich M, Cohen AF, Veldhuis JD, Meinders AE. 2001. Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab 86:5509–5515 [DOI] [PubMed] [Google Scholar]
- 12. Utz AL, Yamamoto A, Hemphill L, Miller KK. 2008. Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J Clin Endocrinol Metab 93:2507–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Makimura H, Stanley T, Mun D, Chen C, Wei J, Connelly JM, Hemphill LC, Grinspoon SK. 2009. Reduced growth hormone secretion is associated with increased carotid intima-media thickness in obesity. J Clin Endocrinol Metab 94:5131–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bengtsson BA, Johannsson G. 1999. Effect of growth-hormone therapy on early atherosclerotic changes in GH-deficient adults. Lancet 353:1898–1899 [DOI] [PubMed] [Google Scholar]
- 15. Colao A, Di Somma C, Spiezia S, Savastano S, Rota F, Savanelli MC, Lombardi G. 2008. Growth hormone treatment on atherosclerosis: results of a 5-year open, prospective, controlled study in male patients with severe growth hormone deficiency. J Clin Endocrinol Metab 93:3416–3424 [DOI] [PubMed] [Google Scholar]
- 16. Thorner MO, Perryman RL, Cronin MJ, Rogol AD, Draznin M, Johanson A, Vale W, Horvath E, Kovacs K. 1982. Somatotroph hyperplasia. Successful treatment of acromegaly by removal of a pancreatic islet tumor secreting a growth hormone-releasing factor. J Clin Invest 70:965–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rivier J, Spiess J, Thorner M, Vale W. 1982. Characterization of a growth hormone-releasing factor from a human pancreatic islet tumour. Nature 300:276–278 [DOI] [PubMed] [Google Scholar]
- 18. Guillemin R, Brazeau P, Böhlen P, Esch F, Ling N, Wehrenberg WB. 1982. Growth hormone-releasing factor from a human pancreatic tumor that caused acromegaly. Science 218:585–587 [DOI] [PubMed] [Google Scholar]
- 19. Vance ML, Kaiser DL, Evans WS, Furlanetto R, Vale W, Rivier J, Thorner MO. 1985. Pulsatile growth hormone secretion in normal man during a continuous 24-hour infusion of human growth hormone releasing factor (1–40). Evidence for intermittent somatostatin secretion. J Clin Invest 75:1584–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Munafo A, Nguyen TX, Papasouliotis O, Lécuelle H, Priestley A, Thorner MO. 2005. Polyethylene glycol-conjugated growth hormone-releasing hormone is long acting and stimulates GH in healthy young and elderly subjects. Eur J Endocrinol 153:249–256 [DOI] [PubMed] [Google Scholar]
- 21. Stanley TL, Chen CY, Branch KL, Makimura H, Grinspoon SK. 2011. Effects of a growth hormone-releasing hormone analog on endogenous GH pulsatility and insulin sensitivity in healthy men. J Clin Endocrinol Metab 96:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Falutz J, Allas S, Blot K, Potvin D, Kotler D, Somero M, Berger D, Brown S, Richmond G, Fessel J, Turner R, Grinspoon S. 2007. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med 357:2359–2370 [DOI] [PubMed] [Google Scholar]
- 23. Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. 1982. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr 36:172–177 [DOI] [PubMed] [Google Scholar]
- 24. Chan R, Kaufhold J, Hemphill LC, Lees RS, Karl WC. 2000. Anisotropic edge-preserving smoothing in carotid B-mode ultrasound for improved segmentation and intima-media thickness (IMT) measurement. Comput Cardiol 27:37–40 [Google Scholar]
- 25. Yim JE, Heshka S, Albu JB, Heymsfield S, Gallagher D. 2008. Femoral-gluteal subcutaneous and intermuscular adipose tissues have independent and opposing relationships with CVD risk. J Appl Physiol 104:700–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McLaughlin T, Lamendola C, Liu A, Abbasi F. 2011. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab 96:E1756–E1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mekala KC, Tritos NA. 2009. Effects of recombinant human growth hormone therapy in obesity in adults: a meta analysis. J Clin Endocrinol Metab 94:130–137 [DOI] [PubMed] [Google Scholar]
- 28. Nass R, Pezzoli SS, Oliveri MC, Patrie JT, Harrell FE, Jr, Clasey JL, Heymsfield SB, Bach MA, Vance ML, Thorner MO. 2008. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med 149:601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Leary DH, Reuwer AQ, Nissen SE, Després JP, Deanfield JE, Brown MW, Zhou R, Zabbatino SM, Job B, Kastelein JJ, Visseren FL. 2011. Effect of rimonabant on carotid intima-media thickness (CIMT) progression in patients with abdominal obesity and metabolic syndrome: the AUDITOR Trial. Heart 97:1143–1150 [DOI] [PubMed] [Google Scholar]
- 30. de las Fuentes L, Waggoner AD, Mohammed BS, Stein RI, Miller BV, 3rd, Foster GD, Wyatt HR, Klein S, Davila-Roman VG. 2009. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol 54:2376–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crouse JR, 3rd, Tang R, Espeland MA, Terry JG, Morgan T, Mercuri M. 2002. Associations of extracranial carotid atherosclerosis progression with coronary status and risk factors in patients with and without coronary artery disease. Circulation 106:2061–2066 [DOI] [PubMed] [Google Scholar]
- 32. Chambless LE, Folsom AR, Davis V, Sharrett R, Heiss G, Sorlie P, Szklo M, Howard G, Evans GW. 2002. Risk factors for progression of common carotid atherosclerosis: the Atherosclerosis Risk in Communities Study, 1987–1998. Am J Epidemiol 155:38–47 [DOI] [PubMed] [Google Scholar]
- 33. Kim SK, Park SW, Kim SH, Cha BS, Lee HC, Cho YW. 2009. Visceral fat amount is associated with carotid atherosclerosis even in type 2 diabetic men with a normal waist circumference. Int J Obes (Lond) 33:131–135 [DOI] [PubMed] [Google Scholar]
- 34. Schwarz JM, Mulligan K, Lee J, Lo JC, Wen M, Noor MA, Grunfeld C, Schambelan M. 2002. Effects of recombinant human growth hormone on hepatic lipid and carbohydrate metabolism in HIV-infected patients with fat accumulation. J Clin Endocrinol Metab 87:942. [DOI] [PubMed] [Google Scholar]
- 35. Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. 2004. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 350:2549–2557 [DOI] [PubMed] [Google Scholar]
- 36. Thörne A, Lönnqvist F, Apelman J, Hellers G, Arner P. 2002. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord 26:193–199 [DOI] [PubMed] [Google Scholar]
- 37. Corneli G, Di Somma C, Baldelli R, Rovere S, Gasco V, Croce CG, Grottoli S, Maccario M, Colao A, Lombardi G, Ghigo E, Camanni F, Aimaretti G. 2005. The cut-off limits of the GH response to GH-releasing hormone-arginine test related to body mass index. Eur J Endocrinol 153:257–264 [DOI] [PubMed] [Google Scholar]
- 38. Biller BM, Samuels MH, Zagar A, Cook DM, Arafah BM, Bonert V, Stavrou S, Kleinberg DL, Chipman JJ, Hartman ML. 2002. Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab 87:2067–2079 [DOI] [PubMed] [Google Scholar]
- 39. Ghigo E, Aimaretti G, Arvat E, Camanni F. 2001. Growth hormone-releasing hormone combined with arginine or growth hormone secretagogues for the diagnosis of growth hormone deficiency in adults. Endocrine 15:29–38 [DOI] [PubMed] [Google Scholar]
- 40. Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S. 2005. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365:1389–1397 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.