Abstract
Context:
Thyroid cancer is a major component of Cowden syndrome (CS). CS patients with an underlying PTEN mutation (PTENmut+) have a 70-fold increased risk of developing epithelial thyroid cancer. In contrast, less than 1% of sporadic epithelial thyroid cancer patients carry a germline PTEN mutation. Cost-efficient markers capable of shortlisting thyroid cancers for CS genetic testing would be clinically useful.
Objective:
Our objective was to analyze the utility of patient blood phosphate and tensin homolog deleted on chromosome 10 (PTEN) protein levels in predicting germline PTEN mutations.
Design, Setting, and Patients:
We conducted a 5-yr, multicenter prospective study of 2792 CS and CS-like patients, all of whom had comprehensive PTEN analysis. Analysis of PTEN and downstream proteins by immunoblotting was performed on total protein lysates from patient-derived lymphoblast lines. We compared blood PTEN protein levels between PTENmut+ patients and those with variants of unknown significance or wild-type PTEN (PTENwt/vus).
Main Outcome Measures:
We assessed the utility of PTEN protein levels in predicting germline PTEN mutations.
Results:
Of 2792 CS/CS-like patients, 721 patients had thyroid cancer; 582 of them (81%) had blood PTEN protein analyzed. PTEN germline pathogenic mutations were present in 27 of 582 patients (4.6%). Ninety-six percent (26 of 27) of PTENmut+ patients had blood PTEN protein levels in the lowest quartile as compared with 25% (139 of 555) of PTENwt/vus patients (P < 0.001). Low blood PTEN levels predicted for PTENmut+ cases with a 99.76% negative predictive value (95% confidence interval = 98.67–99.96) and a positive test likelihood ratio of 3.84 (95% confidence interval = 3.27–4.52).
Conclusions:
Our study shows that low blood PTEN protein expression could serve as a screening molecular correlate to predict for germline PTEN mutation in CS and CS-like presentations of thyroid cancer.
Thyroid cancer is the most rapidly rising incident cancer in women and the second most rapidly rising incident cancer in men in the United States. In 2011, according to the American Cancer Society, approximately 48,000 new cases of thyroid cancer were diagnosed and caused mortality in more than 1700 patients, a number that is rising yearly despite aggressive therapy with surgery, radioactive iodine, and TSH suppression with l-T4. Importantly, 2012 SEER (surveillance epidemiology and end results) data note prevalence of thyroid cancer in more than 496,000 individuals, of which approximately 300,000 are females, with almost 90,000 (20%) having recurrent or residual disease (1). Cancer registry data have also demonstrated a very high familiality and, thus, a strong genetic component. Epithelial thyroid carcinomas are a major component of Cowden and related syndromes characterized by germline mutations in the PTEN tumor suppressor gene, and collectively known as PTEN hamartoma tumor syndromes (PHTS) (2, 3). The syndromes encompassed by PHTS include Cowden syndrome (CS), Bannayan-Riley-Ruvalcaba syndrome, and Proteus-like syndrome (4). These disorders share phenotypic similarities but are clinically distinct, highlighting the protean spectrum of PHTS. Clinical criteria for CS are based on guidelines developed by the International Cowden Consortium (5, 6) and subsequently adopted by the National Comprehensive Cancer Network and include pathognomonic, major, and minor criteria (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org) (7). CS is an autosomal dominant disorder characterized by the development of multiple hamartomas, and importantly carcinomas, of the thyroid, breast, endometrium, and kidney (3, 8). Based on early epidemiological studies, nonmedullary thyroid cancer was identified as a major feature and benign thyroid disorders such as thyroid adenoma and nodular hyperplasia were included as minor features toward clinical diagnosis (9). In a recent large prospective study, we confirmed and quantified that germline mutations in PTEN increased the risk of epithelial thyroid cancer by more than 70-fold when compared with that of the general population (2). In contrast, the prevalence of germline PTEN mutations in unselected differentiated thyroid cancer is low (<1%) (10, 11). It is important to efficiently identify PTEN-related thyroid cancers among all presentations of epithelial thyroid cancer because of the increased risks of extrathyroidal cancers in PHTS, namely 85% lifetime risk of breast cancer, 28% of uterine cancer, and 33% of renal cancer (3).
In addition to complete loss of function, alterations of tumor suppressor genes in cancer can lead to partial loss-of-function, gain-of-function, or dominant-negative phenotypes. Inheriting a mutation in PTEN predisposes to cancer, but the inactivation of phosphate and tensin homolog deleted on chromosome 10 (PTEN) has been shown to occur via multiple diverse mechanisms across the different tumor types (12–15). The germline mutational spectrum in PHTS is broad with mutations scattered across the PTEN coding region (16, 17). Correlations between specific germline PTEN mutations, shown or predicted to result in loss of function or dysfunction, and disease severity in PHTS have been suggested but will require further study (16, 18).
There is growing interest in understanding how various monoallelic inactivating tumor suppressor alterations lead to tumorigenesis. Experimental data from animal models suggest that lower Pten protein dosage influences carcinogenesis, with a Pten dose reduction of as little as 20% leading to murine mammary cancer development (19). Others have shown that homozygous deletion of Pten in mouse thyroid cells led to benign follicular adenomas in female mice (20), whereas hemizygous deletion of Pten was seen to accelerate thyroid adenocarcinoma formation when additional genetic alterations were present, such as loss of p27 (21) and in the setting of mutant thyroid hormone receptor-β (20). Based on these mouse studies, it does appear that decreased gene dosage of PTEN is indeed important for thyroid cancer progression in the presence of other genetic alterations. We demonstrated that, on the whole, reduced PTEN protein dose in CS lymphoblast-derived cell lines tended to occur in the presence of an underlying germline PTEN mutation and tended to correlate with increasing clinical phenotypic load of CS (17). Therefore, we sought to determine whether lowered levels of PTEN protein is predictive of an underlying germline PTEN mutation among CS and CS-like individuals who presented with thyroid cancer and, if so, whether loss of PTEN protein expression in affected malignant thyroid tissue can aid in the identification of patients in whom germline PTEN testing is warranted.
Materials and Methods
A total of 2792 research participants were prospectively accrued into and provided informed consent for Cleveland Clinic Institutional Review Board protocol 8458. Probands who met at least the relaxed International Cowden Consortium operational criteria for CS were eligible. Relaxed criteria are defined as full criteria minus one criterion, and such individuals are referred to as CS-like. These patients were recruited from both community and academic medical centers throughout North America, Europe, and Asia. For each patient, the medical record was examined by cancer genetics professionals, and when possible, primary documentation of medical records/pathology reports were obtained for confirmation of the thyroid cancer and precise histology, with the patients' consent.
PTEN mutation and deletion analysis
All research participants underwent PTEN (NM_000314.4) mutation analysis as described as follows. Genomic DNA was extracted from peripheral blood leukocytes using standard methods (22). Scanning of genomic DNA samples for PTEN mutations was performed as previously reported with a combination of denaturing gradient gel electrophoresis, high-resolution melting curve analysis (Idaho Technology, Salt Lake City, UT) and direct Sanger sequencing (ABI 3730xl; Life Technologies, Carlsbad, CA) (23). Deletion analysis using the multiplex ligation-dependent probe amplification (MLPA) assay (24) was performed with the P158 MLPA kit (MRC-Holland, Amsterdam, The Netherlands) according to the manufacturer's protocol. All patients underwent PCR-based Sanger sequencing of the PTEN promoter region (Supplemental Table 2 for primer sequences) as previously described (25). Promoter mutations were defined as previously reported (25, 26) except for −1084T→C. This variant has been reported in population controls of European descent (two in 150) (27), and additional work is required to characterize it. We consider it as a variant of unknown clinical significance (VUS) at this time.
Analysis of PTEN and other downstream proteins by immunoblotting
Human immortalized lymphoblast-derived cell lines were obtained from each patient and cultured in RPMI 1640 supplemented with 20% fetal bovine serum. All cell lines were cultured at 37 C and 5% CO2. Whole-cell lysates were prepared with mammalian protein extraction reagent (ThermoFisher Scientific, Waltham, MA) supplemented with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Lysates were separated by SDS-PAGE and transferred onto nitrocellulose. Antibodies used included anti-PTEN mouse monoclonal (Cascade Biosciences, Portland, OR) at 1:5000, anti-phospho-AKT rabbit polyclonal (Cell Signaling, Danvers, MA) at 1:1000, anti-phospho-MAPK1/2 rabbit polyclonal (Cell Signaling) at 1:20,000, anti-glyceraldehyde-3-phosphate dehydrogenase rabbit monoclonal (Cell Signaling) at 1:20,000, and anti-actin mouse monoclonal (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:20,000. The blots were scanned digitally with the Odyssey imaging system (Li-Cor Biotechnology, Lincoln, NE). Detected fluorescence intensities for protein bands were background adjusted and normalized between gels with the median expression of individual proteins on each blot. Blood PTEN protein levels were classified as PTENQ1, PTENQ2, PTENQ3, and PTENQ4 with PTENQ1 corresponding to the lowest quartile of PTEN expression seen and PTENQ4 being the highest.
Immunohistochemical staining of thyroid tissue for PTEN
Four-micrometer-thick formalin-fixed paraffin-embedded tissue sections were cut, and immunohistochemistry was performed after pressure cooker antigen retrieval [0.01 m citrate buffer (pH 6.4) for 20 min]. To block endogenous peroxidase activity, the sections were incubated with 0.3% hydrogen peroxide in methanol for 30 min after cooling to room temperature. After blocking for 30 min in 0.75% normal horse serum, the sections were incubated with a mouse anti-PTEN monoclonal antibody (1:50 dilution; Cascade Biosciences, Portland, OR). Primary antibody binding was localized by using an avidin-biotin-peroxidase kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's instruction. PTEN expression was independently evaluated by two of the authors (J.N. and X.H.) under a light microscope and randomly spot evaluated by a third investigator (C.E.). Both authors were blinded to the clinical history and underlying germline mutation. Intensity of PTEN staining was classified separately for the nucleus and the cytoplasm and graded strong (3+), moderate (2+), weak (1+), or absent (−).
Statistical analysis
The χ2 test was used to measure the association between germline PTEN mutation status and categorical clinicopathological variables. Univariate and multivariate logistic regression models were used to test for the association between PTEN expression status, clinicopathological features, and germline PTEN mutation status. Odds ratios and 95% confidence intervals (CIs) were calculated. Likelihood ratio tests were used to evaluate the significance of each covariate. Performance of PTENQ1 in predicting PTEN mutation status was evaluated using receiver-operating characteristic (ROC) methodology to give measurements of sensitivity, specificity, accuracy, and positive and negative predictive values. Calibration to obtain bias-corrected estimates of predicted vs. observed values was performed with 500 bootstraps. Statistical tests were two sided with P ≤ 0.05 considered significant. All analyses were performed using SPSS version 19.0 software (SPSS Inc., Chicago, IL) and on R version 2.11.1 (28).
Results
Patient characteristics by germline PTEN status and blood PTEN protein category
Of the 2792 CS/CS-like patients, 721 had epithelial thyroid cancer confirmed by medical and/or pathology records. Germline PTEN status and blood PTEN protein levels were determined in all patients. Germline PTEN pathogenic mutations were found in 35 of 721 (5%) CS/CS-like patients (PTENmut+) with thyroid cancer. Human immortalized lymphoblast-derived cell lines were obtained from 582 patients (81%), and PTEN protein analyses were performed. Of 27 PTENmut+ patients who had PTEN protein analyses performed, 26 (96%) had blood PTEN expression levels in the lowest quartile (PTENQ1, Table 1), compared with 139 of 555 (25%) patients who did not harbor a germline PTEN mutation (P < 0.001). Age of thyroid cancer onset and presence of benign thyroid adenomas or thyroiditis was not associated with significantly different blood PTEN protein expression levels (Table 1), whereas male thyroid cancer and follicular histology subtype were more likely to be associated with a low blood PTENQ1 expression level (P = 0.05) (Table 1).
Table 1.
Relationship between clinicopathological characteristics of Cowden-related thyroid cancer patients, PTEN mutation status, and blood PTEN expression levels
| Characteristics | No. of patients (n = 582) | Blood PTEN expression levels |
P | |||
|---|---|---|---|---|---|---|
| PTENQ1 | PTENQ2 | PTENQ3 | PTENQ4 | |||
| Age of thyroid cancer onset | 0.29 | |||||
| ≤18 yr | 15 | 6 | 4 | 2 | 3 | |
| >18 yr | 567 | 155 | 140 | 132 | 140 | |
| Sex | 0.05 | |||||
| Male | 35 | 16 | 10 | 5 | 5 | |
| Female | 547 | 149 | 134 | 124 | 140 | |
| Histology | 0.05 | |||||
| Follicular | 56 | 19 | 7 | 10 | 20 | |
| Nonfollicular | 526 | 146 | 137 | 119 | 124 | |
| Benign thyroid disease | ||||||
| Thyroiditis | 84 | 30 | 22 | 14 | 18 | 0.29 |
| Follicular adenoma/goiter | 201 | 60 | 52 | 38 | 52 | 0.59 |
| Germline PTEN mutation status | <0.001 | |||||
| Pathogenic mutation | 27 | 26 | 0 | 0 | 1 | |
| VUS/SNP/WT | 555 | 139 | 144 | 129 | 143 | |
SNP, Single-nucleotide polymorphism; WT, wild type.
Multivariate logistic regression analysis of the 582 patients with blood PTEN protein expression was performed using the clinical features that were found to significantly predict for germline PTEN mutation by univariate analysis: age of thyroid cancer onset below 18 yr, male, presence of macrocephaly, follicular thyroid cancer histology and presence of coexisting thyroiditis, and/or thyroid adenoma and blood PTENQ1 expression. We found that five of the seven factors were independent predictors of the presence of a germline PTEN mutation (Table 2). Patients with blood PTENQ1 levels had a 73-fold increased risk of having an underlying germline PTEN mutation (P < 0.001). Presence of coexisting thyroid adenoma and a follicular thyroid cancer subtype were not found to be independent predictors in this model.
Table 2.
Multivariate analysis of predictive factors for pathogenic germline PTEN mutations in CS patients presenting with thyroid cancer
| Factor | OR | 95% CI | P |
|---|---|---|---|
| Male vs. female | 4.96 | 1.35–18.18 | 0.02 |
| Age of onset ≤18 vs. >18 yr | 15.94 | 1.63–155.35 | 0.02 |
| Macrocephaly vs. no macrocephaly | 5.24 | 1.84–14.98 | 0.02 |
| History of thyroiditis vs. no thyroiditis | 3.31 | 1.04–10.57 | 0.04 |
| PTENQ1 vs. PTENQ2,Q3,orQ4 | 73.51 | 9.27–583.17 | <0.001 |
Relationship between type of pathogenic germline PTEN mutation and blood PTEN protein levels
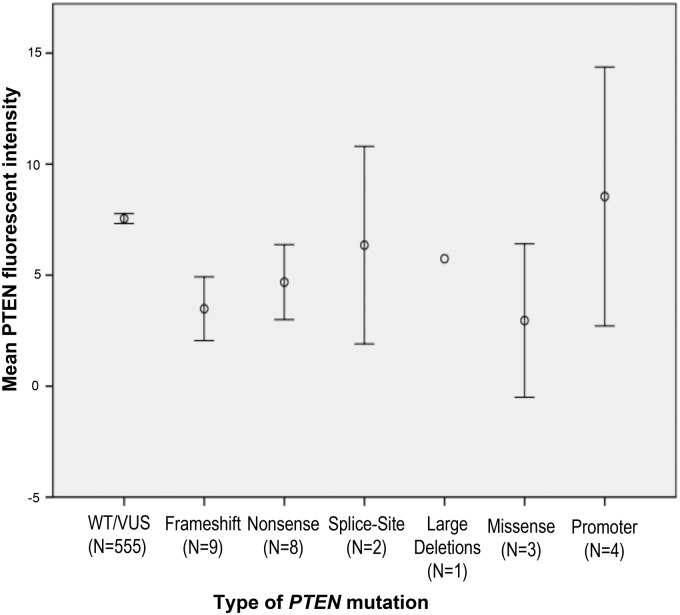
PTEN frameshift and nonsense mutations accounted for 63% (17 of 27) of mutations. Of the 27 PTENmut+patients who had blood PTEN protein analyses performed, all patients with frameshift mutations (nine of nine), splice-site (two of two), missense (three of three), or nonsense mutations (eight of eight) had blood PTEN protein levels in the lowest quartile. Only one patient with a promoter mutation had protein levels that were higher than blood PTENQ1 (Fig. 1).
Fig. 1.
Mean PTEN fluorescent intensity according to PTEN mutation status and type of mutation. Error bars are representative of 95% CI. WT, Wild type.
Performance of blood PTENQ1 in predicting for germline PTEN mutations
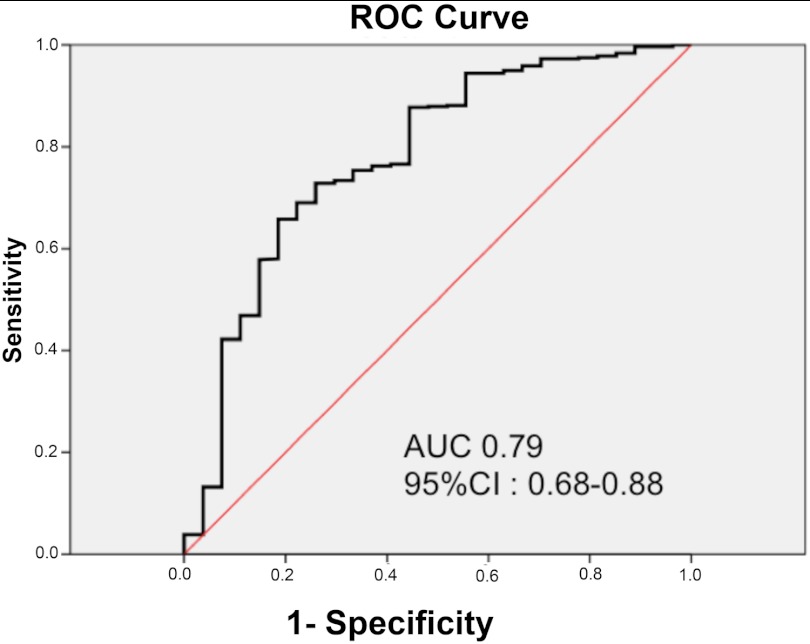
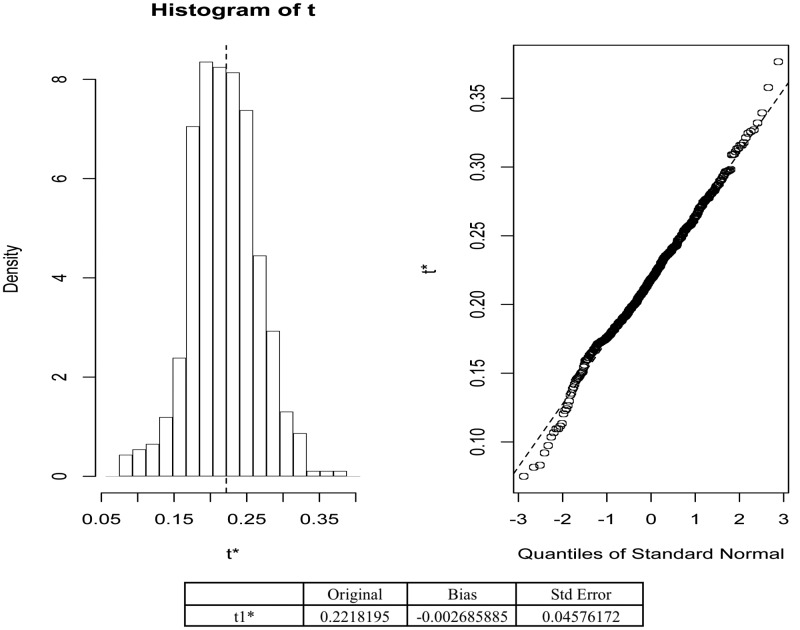
A ROC curve was constructed to assess the ability of blood PTENQ1 to predict germline PTEN mutations. The area under the curve was 0.79 (95% CI = 0.68–0.88; Fig. 2). Low blood PTEN levels predicted for PTENmut+ cases with a sensitivity of 96.30% (95% CI = 80.97–99.38%), specificity of 74.95% (95% CI = 71.13–78.51%). Blood PTENQ1 gave a positive predictive value of 15.76% (95% CI = 10.56–22.23%) and a 99.76% negative predictive value (95% CI = 98.67–99.96%) and a positive test likelihood ratio of 3.84 (95% CI = 3.27–4.52). We used bootstrap validation to confirm that blood PTENQ1 within the dataset predicted for PTENmut+ cases (n = 500), demonstrating excellent calibration (Fig. 3).
Fig. 2.
ROC curve of low blood PTEN levels (PTENQ1) in predicting germline PTEN mutation in CS patients who present with thyroid cancer. AUC, Area under the curve.
Fig. 3.
Calibration plot for PTENQ1 predicted probabilities of PTEN mutation with actual outcomes.
Correlation between blood PTENQ1 and PTEN expression in affected thyroid tissue
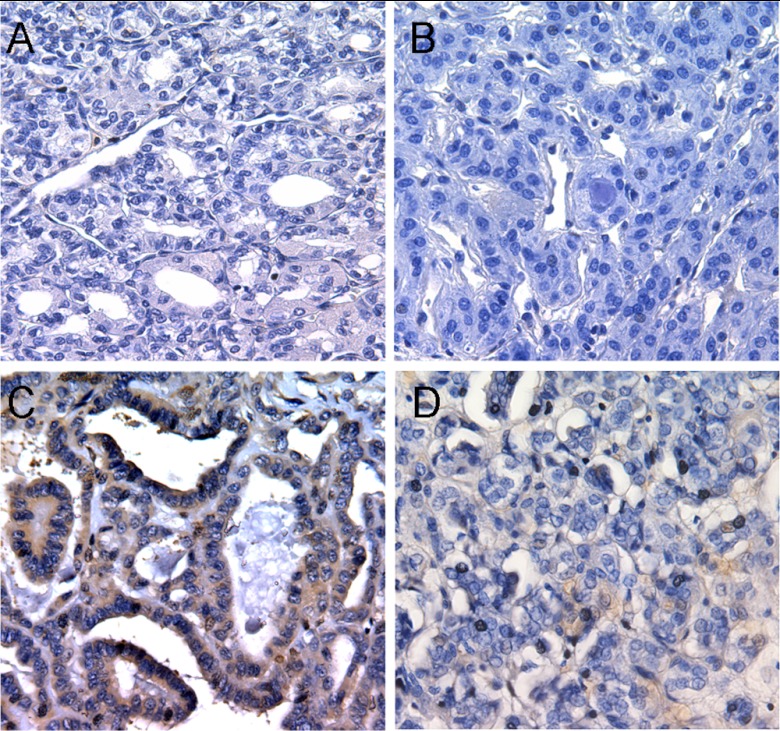
Affected thyroid cancer tissues (n = 12) underwent PTEN immunohistochemical staining; seven from PTENmut+ cases showed global loss of PTEN protein by immunohistochemistry. Absent or weak PTEN expression on immunohistochemistry were seen in all cases and correlated strongly with patient-derived blood PTENQ1 protein levels (Fig. 4, A and B). Five cases of PTENwt/vus-related thyroid cancer showed strong cytoplasmic or nuclear PTEN staining (Fig. 4, C and D).
Fig. 4.
PTEN immunohistochemistry of germline PTENmut+-related thyroid cancer showing both nuclear and cytoplasmic loss of PTEN distinct from that seen in PTENwt/vus-related thyroid cancer (×40 magnification). A, Papillary thyroid cancer with absent (−) PTEN staining; underlying c.210–1G→A germline PTEN mutation with blood PTENQ1 expression; B, follicular variant of papillary thyroid cancer with weak (+) nuclear PTEN staining; underlying c.80-?_164+?del germline PTEN mutation with blood PTENQ1 expression; C, papillary thyroid cancer with strong (3+) cytoplasmic PTEN staining; PTEN wild-type with blood-PTENQ3 expression; D, papillary thyroid cancer with moderate (2+) nuclear PTEN staining; PTEN wild-type with blood-PTENQ1 expression.
Discussion
Germline mutations in PTEN support a haploinsufficient tumor suppressor model given that heterozygosity in CS patients lead to clinical phenotype and increased cancer susceptibility even in the absence of detectable biallelic mutation of the PTEN gene in tumors derived from CS patients (29, 30). Small changes in the expression levels of tumor suppressor genes have been proposed to influence susceptibility to cancer (19, 31). Our findings suggest that the functional PTEN dose is important. Our results show that in the setting of epithelial thyroid cancer presentation, changes resulting in low blood PTEN expression level was seen in 96% of PTENmut+ patients (26 of 27) and could be used to accurately exclude the absence of a germline PTEN mutation. In particular, it predicted for 100% of patients with frameshift, splice-site, large-deletion, missense, and nonsense mutations (Fig. 1).
All three patients with missense PTEN mutations had lower blood PTEN expression compared with patients who were PTENwt/vus. This makes sense given that all three of these mutations are located either close to the active site pocket of the phosphatase domain (C136R and S170I) or at the C-terminal region of PTEN (K254T) and are predicted to be pathogenic, consistent with in vitro functional studies (32, 33) showing mutations in these regions resulting in destabilization and decreased expression level. Therefore, it is not surprising to see low blood PTEN expression levels in patients harboring these germline alterations.
For clinicians, importantly, for the first time, we now have clinical predictors for underlying germline PTEN mutation in CS/CS-like patients presenting with thyroid cancer. Of all the factors examined, only four clinical factors were significant in independently predicting a germline PTEN mutation after multivariate logistic regression: presence of macrocephaly, male thyroid cancer, history of thyroiditis, and onset of thyroid cancer under the age of 18 yr, the presence of any of which should alert the clinician to the possibility of a germline PTEN mutation in CS/CS-like patients presenting with thyroid cancer.
Notably, blood PTENQ1 level was a stronger predictor than any clinical features. Impressively, a low blood PTEN expression level missed only one case with a germline PTEN mutation with a negative predictive value in excess of 99%. This suggests that perhaps blood PTEN protein expression levels could serve as a reliable molecular correlate for selecting patients in whom genetic testing may not be warranted. The likelihood of someone with a low blood PTENQ1 expression having an underlying germline PTEN mutation is 3.84-fold greater than in those who had higher blood PTEN expression levels. However, one limitation of this study is the absence of a normal range for blood PTEN expression levels. In our study, we were able to quantify the blood PTEN expression of the entire cohort of 721 CS and CS-like patients presenting with thyroid cancer and classified them into quartiles of blood PTEN expression for comparison between patients who had an underlying germline mutation and those who did not. Although this has performed well in this study, our data are preliminary and validation in unselected populations in the future should lead to direct clinical application.
Among patients who did not harbor any germline PTEN mutation, 25% of them had blood PTENQ1 levels. Of the 139 patients, two had a PTEN VUS, 32 with common PTEN single-nucleotide polymorphisms and the remaining 105 were PTEN wild-type. This affected both the positive predictive value and specificity of using blood PTENQ1 in predicting for patients with germline PTEN mutations. In the absence of germline mutations, other mechanisms that may lead to progressive loss of PTEN expression include epigenetic events and posttranslational modifications on PTEN, misguided PTEN subcellular localization, and PTEN-specific microRNA up-regulation (34–39). Blood PTENQ1 expression could be used to better characterize patients meeting criteria for CS/CS-like who do not harbor pathogenic germline PTEN mutations to help drive more research into other mechanisms by which PTEN is negatively regulated in these patients.
We saw a close correlation between low blood PTEN levels and weak/absent PTEN expression in affected thyroid tissue of PTENmut+ patients. We and others have shown that PTEN is strongly expressed in normal thyroid epithelium, in particular in the nucleus and to a lesser extent in the cytoplasm (13, 40, 41). Barletta et al. (42) recently showed that eight of nine patients who had low PTEN expression in a thyroidectomy series had clinical features suggestive of CS. Consistent with these findings, we saw globally (nuclear and cytoplasmic) reduced PTEN expression in affected thyroid adenomas and thyroid cancers. Although we were limited by the availability of affected PTENmut+ thyroid cancer tissue in this study, collectively, our and others' data suggest that thyroid tissue PTEN expression such as blood PTEN expression could serve as a clinically useful molecular correlate for germline PTEN mutation and warrants to be studied prospectively to see whether it can complement or surpasses current clinical predictors.
The classic two-hit model of tumor suppressor inactivation was originally established by mathematical modeling of cancer incidence and had implied that tumorigenesis requires complete loss of function of tumor-suppressor genes. Increasingly, we are beginning to see that the nuanced functional importance of PTEN dosage affects PTEN in its role as a tumor suppressor. We provide here both patient-derived support for preclinical data that PTEN protein dose may indeed influence tumorigenesis and a potential role whereby PTEN protein expression in thyroid tissue/blood can serve as a molecular correlate for selecting patients in whom genetic assessment and testing may be warranted.
Supplementary Material
Acknowledgments
We thank all our research participants and their clinicians who contributed to this study over the last 6 yr. We thank the Genomic Medicine Biorepository of the Cleveland Clinic Genomic Medicine Institute and our database and clinical research teams for their meticulous upkeep and auditing of the clinical databases.
This work was supported in part by Grants P01CA124570 and R01CA118980 from the National Cancer Institute, Bethesda, MD (both to C.E.). J.N. is the National Medical Research Council (Singapore) Fellow and an Ambrose Monell Foundation Cancer Genomic Medicine Clinical Fellow at the Cleveland Clinic Genomic Medicine Institute. C.E. is the Sondra J. and Stephen R. Hardis Chair of Cancer Genomic Medicine at the Cleveland Clinic and is an American Cancer Society Clinical Research Professor, generously funded in part by the F. M. Kirby Foundation.
Disclosure Summary: C.E. receives royalties from Quest Diagnostics in regard to intellectual property related to RNA profiles to distinguish malignant from benign thyroid neoplasias. No other author had any financial or personal relationships that could inappropriately influence or bias this work.
Footnotes
- CI
- Confidence interval
- CS
- Cowden syndrome
- PHTS
- PTEN hamartoma tumor syndromes
- PTEN
- phosphate and tensin homolog deleted on chromosome 10
- ROC
- receiver-operating characteristic
- VUS
- variant of unknown clinical significance.
References
- 1. Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, Altekruse S, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner M, Lewis D, Chen H, Feuer E, Cronin KA. 2012. SEER data submission SEER Cancer Statistics Review, 1975–2009 (vintage 2009 populations). Bethesda, MD: National Cancer Institute [Google Scholar]
- 2. Ngeow J, Mester J, Rybicki LA, Ni Y, Milas M, Eng C. 2011. Incidence and clinical characteristics of thyroid cancer in prospective series of individuals with Cowden and Cowden-like syndrome characterized by germline PTEN, SDH, or KLLN alterations. J Clin Endocrinol Metab 96:E2063–E2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. 2012. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 18:400–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zbuk KM, Eng C. 2007. Cancer phenomics: RET and PTEN as illustrative models. Nat Rev Cancer 7:35–45 [DOI] [PubMed] [Google Scholar]
- 5. Eng C. 2000. Will the real Cowden syndrome please stand up: revised diagnostic criteria. J Med Genet 37:828–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pilarski R, Eng C. 2004. Will the real Cowden syndrome please stand up (again)? Expanding mutational and clinical spectra of the PTEN hamartoma tumour syndrome. J Med Genet 41:323–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daly MB, Axilbund JE, Buys S, Crawford B, Farrell CD, Friedman S, Garber JE, Goorha S, Gruber SB, Hampel H, Kaklamani V, Kohlmann W, Kurian A, Litton J, Marcom PK, Nussbaum R, Offit K, Pal T, Pasche B, Pilarski R, Reiser G, Shannon KM, Smith JR, Swisher E, Weitzel JN. 2010. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw 8:562–594 [DOI] [PubMed] [Google Scholar]
- 8. Mester JL, Zhou M, Prescott N, Eng C. 2012. Papillary renal cell carcinoma is associated with PTEN hamartoma tumor syndrome. Urology 79:1187.e1–1187.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Starink TM, van der Veen JP, Arwert F, de Waal LP, de Lange GG, Gille JJ, Eriksson AW. 1986. The Cowden syndrome: a clinical and genetic study in 21 patients. Clin Genet 29:222–233 [DOI] [PubMed] [Google Scholar]
- 10. Dahia PL, Marsh DJ, Zheng Z, Zedenius J, Komminoth P, Frisk T, Wallin G, Parsons R, Longy M, Larsson C, Eng C. 1997. Somatic deletions and mutations in the Cowden disease gene, PTEN, in sporadic thyroid tumors. Cancer Res 57:4710–4713 [PubMed] [Google Scholar]
- 11. Nagy R, Ganapathi S, Comeras I, Peterson C, Orloff M, Porter K, Eng C, Ringel MD, Kloos RT. 2011. Frequency of germline PTEN mutations in differentiated thyroid cancer. Thyroid 21:505–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marsh DJ, Zheng Z, Zedenius J, Kremer H, Padberg GW, Larsson C, Longy M, Eng C. 1997. Differential loss of heterozygosity in the region of the Cowden locus within 10q22–23 in follicular thyroid adenomas and carcinomas. Cancer Res 57:500–503 [PubMed] [Google Scholar]
- 13. Gimm O, Perren A, Weng LP, Marsh DJ, Yeh JJ, Ziebold U, Gil E, Hinze R, Delbridge L, Lees JA, Mutter GL, Robinson BG, Komminoth P, Dralle H, Eng C. 2000. Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am J Pathol 156:1693–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dahia PL, Aguiar RC, Alberta J, Kum JB, Caron S, Sill H, Marsh DJ, Ritz J, Freedman A, Stiles C, Eng C. 1999. PTEN is inversely correlated with the cell survival factor Akt/PKB and is inactivated via multiple mechanisms in haematological malignancies. Hum Mol Genet 8:185–193 [DOI] [PubMed] [Google Scholar]
- 15. Perren A, Weng LP, Boag AH, Ziebold U, Thakore K, Dahia PL, Komminoth P, Lees JA, Mulligan LM, Mutter GL, Eng C. 1999. Immunohistochemical evidence of loss of PTEN expression in primary ductal adenocarcinomas of the breast. Am J Pathol 155:1253–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marsh DJ, Coulon V, Lunetta KL, Rocca-Serra P, Dahia PL, Zheng Z, Liaw D, Caron S, Duboué B, Lin AY, Richardson AL, Bonnetblanc JM, Bressieux JM, Cabarrot-Moreau A, Chompret A, Demange L, Eeles RA, Yahanda AM, Fearon ER, Fricker JP, Gorlin RJ, Hodgson SV, Huson S, Lacombe D, Eng C, et al. 1998. Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum Mol Genet 7:507–515 [DOI] [PubMed] [Google Scholar]
- 17. Tan MH, Mester J, Peterson C, Yang Y, Chen JL, Rybicki LA, Milas K, Pederson H, Remzi B, Orloff MS, Eng C. 2011. A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am J Hum Genet 88:42–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marsh DJ, Kum JB, Lunetta KL, Bennett MJ, Gorlin RJ, Ahmed SF, Bodurtha J, Crowe C, Curtis MA, Dasouki M, Dunn T, Feit H, Geraghty MT, Graham JM, Jr, Hodgson SV, Hunter A, Korf BR, Manchester D, Miesfeldt S, Murday VA, Nathanson KL, Parisi M, Pober B, Romano C, Eng C, et al. 1999. PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet 8:1461–1472 [DOI] [PubMed] [Google Scholar]
- 19. Alimonti A, Carracedo A, Clohessy JG, Trotman LC, Nardella C, Egia A, Salmena L, Sampieri K, Haveman WJ, Brogi E, Richardson AL, Zhang J, Pandolfi PP. 2010. Subtle variations in Pten dose determine cancer susceptibility. Nat Genet 42:454–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeager N, Klein-Szanto A, Kimura S, Di Cristofano A. 2007. Pten loss in the mouse thyroid causes goiter and follicular adenomas: insights into thyroid function and Cowden disease pathogenesis. Cancer Res 67:959–966 [DOI] [PubMed] [Google Scholar]
- 21. Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP. 2001. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat Genet 27:222–224 [DOI] [PubMed] [Google Scholar]
- 22. Eng C, Thiele H, Zhou XP, Gorlin RJ, Hennekam RC, Winter RM. 2001. PTEN mutations and proteus syndrome. Lancet 358:2079–2080 [DOI] [PubMed] [Google Scholar]
- 23. van der Stoep N, van Paridon CD, Janssens T, Krenkova P, Stambergova A, Macek M, Matthijs G, Bakker E. 2009. Diagnostic guidelines for high-resolution melting curve (HRM) analysis: an interlaboratory validation of BRCA1 mutation scanning using the 96-well LightScanner. Hum Mutat 30:899–909 [DOI] [PubMed] [Google Scholar]
- 24. Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. 2002. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res 30:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Teresi RE, Zbuk KM, Pezzolesi MG, Waite KA, Eng C. 2007. Cowden syndrome-affected patients with PTEN promoter mutations demonstrate abnormal protein translation. Am J Hum Genet 81:756–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou XP, Waite KA, Pilarski R, Hampel H, Fernandez MJ, Bos C, Dasouki M, Feldman GL, Greenberg LA, Ivanovich J, Matloff E, Patterson A, Pierpont ME, Russo D, Nassif NT, Eng C. 2003. Germline PTEN promoter mutations and deletions in Cowden/Bannayan-Riley-Ruvalcaba syndrome result in aberrant PTEN protein and dysregulation of the phosphoinositol-3-kinase/Akt pathway. Am J Hum Genet 73:404–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buxbaum JD, Cai G, Chaste P, Nygren G, Goldsmith J, Reichert J, Anckarsäter H, Rastam M, Smith CJ, Silverman JM, Hollander E, Leboyer M, Gillberg C, Verloes A, Betancur C. 2007. Mutation screening of the PTEN gene in patients with autism spectrum disorders and macrocephaly. Am J Med Genet B Neuropsychiatr Genet 144B:484–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hornik K. 2012. The R FAQ. http://cran.r-project.org/doc/FAQ/R-FAQ.html
- 29. Dahia PM, Gimm O, Chi H, Marsh DJ, Reynolds PR, Eng C. 2000. Absence of germline mutations in MINPP1, a phosphatase encoding gene centromeric of PTEN, in patients with Cowden and Bannayan-Riley-Ruvalcaba syndrome without germline PTEN mutations. J Med Genet 37:715–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marsh DJ, Dahia PL, Coulon V, Zheng Z, Dorion-Bonnet F, Call KM, Little R, Lin AY, Eeles RA, Goldstein AM, Hodgson SV, Richardson AL, Robinson BG, Weber HC, Longy M, Eng C. 1998. Allelic imbalance, including deletion of PTEN/MMACI, at the Cowden disease locus on 10q22-23, in hamartomas from patients with Cowden syndrome and germline PTEN mutation. Genes Chromosomes Cancer 21:61–69 [DOI] [PubMed] [Google Scholar]
- 31. Yan H, Dobbie Z, Gruber SB, Markowitz S, Romans K, Giardiello FM, Kinzler KW, Vogelstein B. 2002. Small changes in expression affect predisposition to tumorigenesis. Nat Genet 30:25–26 [DOI] [PubMed] [Google Scholar]
- 32. Han SY, Kato H, Kato S, Suzuki T, Shibata H, Ishii S, Shiiba K, Matsuno S, Kanamaru R, Ishioka C. 2000. Functional evaluation of PTEN missense mutations using in vitro phosphoinositide phosphatase assay. Cancer Res 60:3147–3151 [PubMed] [Google Scholar]
- 33. Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H. 1999. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Sci USA 96:10182–10187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salmena L, Carracedo A, Pandolfi PP. 2008. Tenets of PTEN tumor suppression. Cell 133:403–414 [DOI] [PubMed] [Google Scholar]
- 35. Zhou XP, Kuismanen S, Nystrom-Lahti M, Peltomaki P, Eng C. 2002. Distinct PTEN mutational spectra in hereditary non-polyposis colon cancer syndrome-related endometrial carcinomas compared to sporadic microsatellite unstable tumors. Hum Mol Genet 11:445–450 [DOI] [PubMed] [Google Scholar]
- 36. Planchon SM, Waite KA, Eng C. 2008. The nuclear affairs of PTEN. J Cell Sci 121:249–253 [DOI] [PubMed] [Google Scholar]
- 37. Jacob AI, Romigh T, Waite KA, Eng C. 2009. Nuclear PTEN levels and G2 progression in melanoma cells. Melanoma Res 19:203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. 2007. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133:647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ma X, Kumar M, Choudhury SN, Becker Buscaglia LE, Barker JR, Kanakamedala K, Liu MF, Li Y. 2011. Loss of the miR-21 allele elevates the expression of its target genes and reduces tumorigenesis. Proc Natl Acad Sci USA 108:10144–10149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Di Loreto C, Tell G, Pestrin M, Pandolfi M, Damante G, Puglisi F. 2005. PTEN and Egr-1 expression in thyroid proliferative lesions. Cancer Lett 224:105–109 [DOI] [PubMed] [Google Scholar]
- 41. Gimm O, Attié-Bitach T, Lees JA, Vekemans M, Eng C. 2000. Expression of the PTEN tumour suppressor protein during human development. Hum Mol Genet 9:1633–1639 [DOI] [PubMed] [Google Scholar]
- 42. Barletta JA, Bellizzi AM, Hornick JL. 2011. Immunohistochemical staining of thyroidectomy specimens for PTEN can aid in the identification of patients with Cowden syndrome. Am J Surg Pathol 35:1505–1511 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.