Abstract
Context:
The stages of the menopause transition are characterized by changes in ovarian hormones and increased cardiovascular disease (CVD) risk factors and vasomotor symptoms that may adversely affect vascular health.
Objective:
We tested the hypothesis that endothelial function, a predictor of CVD, would be reduced across the stages of the menopause transition, independent of CVD risk factors and vasomotor symptoms.
Design, Setting, and Participants:
This was a cross-sectional study of 132 healthy women from the general community aged 22–70 yr, categorized as premenopausal (n = 33, 32 ± 6 yr; mean ± sd), early perimenopausal (n = 20, 49 ± 3 yr) or late perimenopausal (n = 22, 50 ± 4 yr), or early (n = 30, 55 ± 3 yr) or late postmenopausal (n = 27, 61 ± 4 yr).
Main Outcome:
Endothelial-dependent vasodilation was measured by brachial artery flow-mediated dilation (FMD) using ultrasound.
Results:
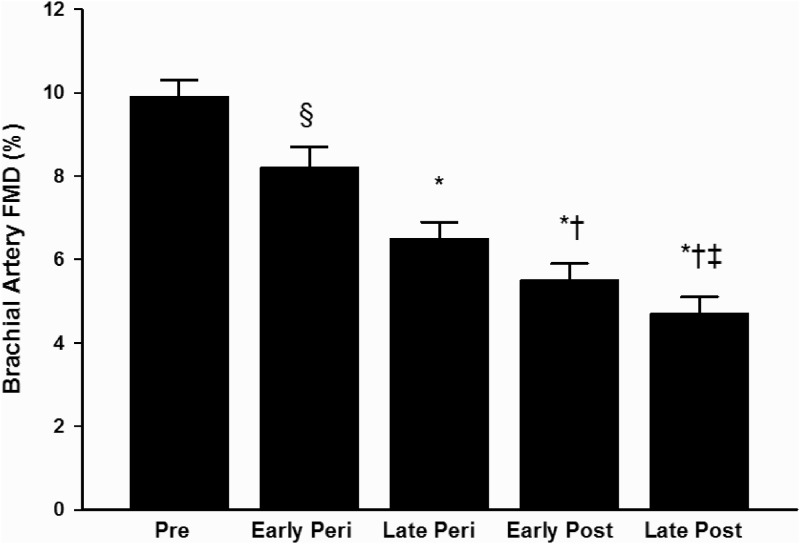
Brachial artery FMD was significantly different among the groups (P < 0.001). It was highest in premenopausal women (9.9 ± 2.1%) with progressive decrements in perimenopausal (early: 8.2 ± 2.5%; late: 6.5 ± 1.9%) and postmenopausal women (early: 5.5 ± 1.9%; late: 4.7 ± 1.7%). Adjustment for risk factors, vasomotor symptoms, and sex hormones did not alter the association (P < 0.001). In subgroup analyses of women aged 50–59 yr, brachial artery FMD was lower in late peri- and early and late postmenopausal compared with early perimenopausal women (P < 0.001) but was not different between late perimenopausal and either early or late postmenopausal women.
Conclusions:
Our findings suggest that a decline in endothelial function begins during the early stages of menopause (perimenopause) and worsens with the loss of ovarian function and prolonged estrogen deficiency. These data add to the accumulating evidence that the perimenopausal window is a critical time period for adverse changes in CVD risk.
Even though cardiovascular disease (CVD) death rates have been declining, CVD is still a major public health concern in women (1). The prevalence of CVD increases with age in both women and men but is less prevalent in women than men until midlife. Thereafter the gap narrows until the sixth decade, when the prevalence of CVD no longer differs between the sexes (1). The female advantage in younger women has been attributed to vascular protection by estrogens, which is lost with menopause and resulting estrogen deficiency. Although recent clinical trial data do not support cardioprotective effects of conjugated equine estrogens when initiated 10–20 yr after the menopause (2, 3), it is plausible based on studies in nonhuman primates that initiating hormone therapy (HT) at the time of menopause is cardioprotective (4).
Vascular aging is a major risk factor for the development of CVD, in that it combines with other known risk factors to create an age-disease interaction (5). Endothelial dysfunction, characterized by reduced endothelial-dependent vasodilation, is a biomarker of aging and a significant predictor of cardiovascular events in women (6). Vascular aging in women is unique in that arteries may also be exposed to adverse changes in traditional CVD risk factors (i.e. central adiposity, hypertension, dyslipidemia) and vasomotor symptoms mediated by changes in the hormonal environment across the stages of the menopause transition (7–10). Indeed, the age-related decline in endothelial function appears to be delayed and/or occur at a slower rate in premenopausal women than in men, but catches up during the postmenopausal period, particularly in estrogen-deficient women (11, 12). However, it is unclear how changes in ovarian hormones during the potentially critical perimenopausal years contribute to the age-associated decline in endothelial function. If the menopause transition accelerates vascular aging, this would have important implications for aggressively promoting intervention strategies for CVD prevention in women during the perimenopausal years.
Accordingly, the present study tested the hypothesis that endothelial function is impaired across the stages of the menopause transition in healthy women. Additionally, we determined whether the decline in endothelial function with the menopause transition was related to traditional CVD risk factors and the occurrence of hot flashes.
Materials and Methods
Study population
One hundred thirty-two healthy women were studied: 33 premenopausal (22–43 yr), 42 perimenopausal (43–56 yr), and 57 postmenopausal (49–70 yr). Menopausal status was determined by self-reported menstrual cycle characteristics. Premenopausal women had regular menstrual cycles with no change in cycle length (21–35 d), confirmed by menstrual cycle calendars and ovulation prediction kits. Perimenopausal women were categorized as either early (n = 20; more than two cycles with cycle length changes of ≥7 d) or late (n = 22; ≥2 but <12 months of amenorrhea) transition, as defined by the Stages of Reproductive Aging Workshop (STRAW) (13). Postmenopausal women had gone 1 yr or longer without menses and were further categorized as early (n = 30; ≤5 yr) or late (n = 27; >5 yr) postmenopause stages according to STRAW criteria (13). Women had not used hormonal contraceptives, HT, or other treatments for menopausal symptoms for at least 6 months and vitamin supplements or antiinflammatory medications for at least 4 wk. Women were included if they were normotensive (resting fasted blood pressure <140/90 mm Hg), nondiabetic, had a fasted glucose less than 110 mg/dl, were sedentary or recreationally active (<3 d/wk vigorous exercise), were nonsmokers, were not taking cardiovascular or lipid-lowering medications, and were healthy as determined by a medical history and physical examination and standard blood chemistries (i.e. normal kidney, liver and thyroid function). Women with a history of or active estrogen-dependent neoplasms, cancer, acute liver or gallbladder disease, vaginal bleeding, venous thromboembolism, hysterectomy/oophorectomy, hypertriglyceridemia, and CVD were excluded. All women gave their written informed consent to participate in the study. All procedures were reviewed and approved by the institutional review board.
Measurements
For the vascular assessments, participants were studied after an overnight fast with proper hydration (water drinking only) and abstinence from caffeine. Normal dietary patterns, including sodium intake, were maintained for 2 d immediately before the measurements. Because of irregular cycles in perimenopausal women, premenopausal and perimenopausal (when possible) women were tested 7–10 d after the onset of menses (i.e. midfollicular phase) so that vascular comparisons between premenopausal and perimenopausal would be representative across the menstrual cycle. However, late perimenopausal women were tested regardless of menstrual cycle phase after 2 months of amenorrhea. The study took place at the University of Colorado Denver Colorado Clinical and Translational Sciences Institute Clinical and Translational Research Center.
Vascular endothelial-dependent vasodilation
Before vascular assessment, supine blood pressure was measured with a semiautomated device (Dinamap; Johnson & Johnson, New Brunswick, NJ) until at least three stable measures were achieved. Brachial artery flow-mediated dilation (FMD) was measured as described in detail previously (14) and was performed in accordance to current guidelines for assessing FMD in humans (15). Endothelium-independent dilation was determined by measuring brachial artery dilation in response to sublingual nitroglycerine [glyceryl trinitrate (GTN), 0.4 mg] as previously described (16). Because some women declined the procedure and GTN was not given to women with a systolic blood pressure less than 90 mm Hg, this measure was obtained in only 61 women.
Seated blood pressure, body composition, aerobic power, and physical activity
Seated brachial arterial blood pressure was measured in triplicate with a semiautomated device in the morning after an overnight fast, as previously described (17). The total (percent of total mass) and regional (percent of mass in trunk region) body fat were determined using dual-energy x-ray absorptiometry (Hologic Discovery, version 12.6; Hologic Inc., Bedford, MA). Minimal waist and hip circumferences were measured according to previously guidelines, and waist to hip ratio (WHR) was calculated (18). In a subset of women (premenopausal, n = 24; early perimenopausal, n = 20; late perimenopausal, n = 22; early postmenopausal, n = 20; and late postmenopausal, n = 20), the peak oxygen consumption, a measure of the peak aerobic exercise capacity, was determined using an incremental treadmill exercise protocol as described previously (19). Leisure time physical activity was determined by the Modifiable Activity Questionnaire (20).
Metabolic risk factors and sex hormones
Fasting plasma concentrations of glucose, insulin, total cholesterol (Roche Diagnostic Systems, Indianapolis, IN) and high-density lipoprotein (HDL) cholesterol (Diagnostic Chemicals, Ltd., Oxford, CT) were determined using enzymatic/colorimetric methods, and low-density lipoprotein (LDL) cholesterol was determined using the Friedewald equation (21). Serum FSH, estradiol, progesterone, and SHBG were measured using chemiluminescence, estrone using RIA, and total testosterone using a one-step competitive assay. Serum norepinephrine with EGTA/glutathione preservative added was measured using HPLC and plasma endothelin-1 was measured using an enzyme-linked immunoassay. All metabolic risk factors and sex hormones were measured on the vascular testing day. All assays were performed by the University of Colorado Denver Colorado Clinical and Translational Sciences Institute Clinical and Translational Research Center core laboratory. The intra- and interassay coefficients of variation for all assays have been published previously (22, 23).
Vasomotor symptoms
As part of the medical history, women were asked whether they had experienced hot flashes in the past year (yes or no). Additionally, in a subsample of women (n = 100), the frequency and severity scores of menopausal symptoms in the preceding 3 months were determined from the nine-item vasosomatic subscale of the Menopausal Symptom List (24).
Statistical analysis
All data elements were examined using descriptive statistics. Parameters with skewed distributions were log transformed and are presented as median and interquartile range. One-way ANOVA was used to assess group differences in characteristics. ANOVA and post hoc tests using a Bonferroni correction were used to compare brachial artery FMD, GTN-mediated vasodilation, and brachial artery parameters. Pearson and Spearman rho correlation analyses were used to test for linear bivariate relations of interest (i.e. potential modulators of vascular function) with brachial artery FMD- and GTN-mediated vasodilation. Analysis of covariance was used to compare brachial artery FMD- and GTN-mediated vasodilation, with covariates associated with FMD at P < 0.10 and prior and duration of HT and hormonal contraceptive use considered as potential confounders. Because menopausal stages and age were highly correlated (r = 0.88), brachial artery FMD was compared in peri- and postmenopausal women aged 50–59 yr to distinguish the independent effect of menopausal stage. P < 0.05 was considered significant. All data are reported as mean ± sd unless otherwise stated. Data analysis was performed with IBM SPSS Statistics, version 19.0 (White Plains, NY).
Results
Participant characteristics
A total of 84.8% of the participants were Caucasian. The average reported age of menopause and time since menopause was 52.8 ± 0.7 and 3.0 ± 1.4 yr, respectively, for early postmenopausal and 49.2 ± 0.8 and 11.7 ± 4.3 yr, respectively, for late postmenopausal women. Thirty percent (n = 9) of early postmenopausal were prior HT users with an average duration of 2.7 ± 2.2 yr, whereas 74% (n = 20) of late postmenopausal women had used HT in the past for an average duration of 4.0 ± 2.9 yr. Most of the premenopausal (78.8%) and early (80%) and late (95.5%) perimenopausal women had used hormonal contraceptives in the past for an average duration of 5.2 ± 5.0, 6.5 ± 5.3, and 8.9 ± 7.8 yr, respectively.
Total body and trunk fat, seated systolic blood pressure, endothelin-1 (ET-1), norepinephrine, FSH, glucose, and total and LDL cholesterol concentrations were higher, and maximal aerobic power, estradiol, estrone, progesterone, and testosterone concentrations were lower across the stages of the menopause transition (all P < 0.001, Table 1). Additionally, the likelihood of hot flashes were greater across the stages of the menopause transition, with 6% of premenopausal, 50% of early perimenopausal, 74% of late perimenopausal, 70% of early postmenopausal, and 52% of late postmenopausal women reporting hot flashes in the previous year (P < 0.001). Similarly, vasomotor frequency and severity scores were greater across the stages of the menopause transition (Table 1; both P < 0.001).
Table 1.
Participant characteristics
| Variable | Pre | Early Peri | Late Peri | Early Post | Late Post | P |
|---|---|---|---|---|---|---|
| n | 33 | 20 | 22 | 30 | 27 | |
| Age (yr) | 32 ± 4 | 49 ± 3 | 50 ± 4 | 55 ± 3 | 61 ± 4 | <0.001 |
| Body mass (kg) | 66.4 ± 14.2 | 69.0 ± 10.8 | 67.2 ± 13.1 | 71.5 ± 13.1 | 69.8 ± 12.3 | 0.55 |
| BMI (kg/m2) | 24.2 ± 5.8 | 25.6 ± 3.6 | 24.2 ± 4.3 | 27.0 ± 5.0 | 26.9 ± 4.3 | 0.062 |
| Total body fat (%) | 31 ± 8 | 34 ± 6 | 36 ± 7 | 38 ± 6 | 40 ± 5 | <0.001 |
| Trunk fat (%) | 28 ± 9 | 33 ± 7 | 35 ± 8 | 38 ± 8 | 39 ± 6 | <0.001 |
| Waist circumference (cm) | 78 ± 9 | 83 ± 10 | 82 ± 13 | 87 ± 14 | 83 ± 15 | 0.12 |
| WHR | 0.79 ± 0.06 | 0.80 ± 0.06 | 0.80 ± 0.06 | 0.82 ± 0.08 | 0.81 ± 0.05 | 0.47 |
| Seated systolic BP (mm Hg) | 108 ± 9 | 114 ± 11 | 115 ± 13 | 119 ± 14 | 121 ± 13 | 0.001 |
| Seated diastolic BP (mm Hg) | 69 ± 7 | 74 ± 8 | 72 ± 8 | 74 ± 10 | 73 ± 10 | 0.14 |
| Resting HR (bpm) | 66 ± 10 | 63 ± 8 | 64 ± 10 | 63 ± 6 | 63 ± 7 | 0.54 |
| Total cholesterol (mg/dl) | 154 ± 29 | 163 ± 27 | 167 ± 32 | 192 ± 31 | 193 ± 30 | <0.001 |
| LDL cholesterol (mg/dl) | 89 ± 24 | 94 ± 27 | 99 ± 31 | 114 ± 27 | 119 ± 28 | <0.001 |
| HDL cholesterol (mg/dl) | 49 ± 12 | 51 ± 14 | 50 ± 9 | 55 ± 15 | 53 ± 15 | 0.43 |
| Triglycerides (mg/dl)a | 68 (55–87) | 84 (63–103) | 86 (78–107) | 83 (68–135) | 95 (67–131) | 0.06 |
| Fasting insulin (μIU/ml) | 7.6 ± 5.1 | 6.2 ± 4.0 | 6.6 ± 4.4 | 9.8 ± 7.6 | 8.7 ± 7.8 | 0.21 |
| Fasting glucose (mg/dl) | 84 ± 8 | 85 ± 8 | 81 ± 8 | 89 ± 10 | 87 ± 10 | 0.03 |
| FSH (μIU/ml) | 5.5 ± 3.1 | 23.4 ± 32.6 | 67.3 ± 36.6 | 77.0 ± 29.8 | 86.4 ± 31.2 | <0.001 |
| Estradiol (pg/ml)a | 64 (38–92) | 54 (35–135) | 38 (10–113) | 11 (10–19) | 12 (10–17) | <0.001 |
| Progesterone (ng/ml)a | 0.6 (0.3–0.9) | 0.6 (0.3–1.1) | 0.4 (0.3–0.5) | 0.3 (0.2–0.5) | 0.2 (0.1–0.4) | <0.001 |
| Estrone (ng−1/dl)a | 46 (35–68) | 52 (34–73) | 41 (27–72) | 25 (10–35) | 25 (20–33) | <0.001 |
| SHBG (nmol/liter) | 59 ± 26 | 80 ± 54 | 70 ± 37 | 66 ± 39 | 59 ± 31 | 0.32 |
| Testosterone (ng/dl)a,b | 32 (22–46) | 22 (17–35) | 20 (17–25) | 19 (17–28) | 17 (17–32) | 0.002 |
| ET-1 (pg/ml) | 5.4 ± 0.3 | 5.9 ± 0.3 | 6.1 ± 0.3 | 6.5 ± 0.3 | 6.5 ± 0.3 | 0.03 |
| Norepinephrine (pg/ml)c | 198 ± 159 | 238 ± 82 | 248 ± 112 | 284 ± 154 | 311 ± 137 | 0.08 |
| LTPA (MET h/wk)d | 17.5 ± 11.9 | 19.1 ± 12.5 | 14.7 ± 11.5 | 17.1 ± 21.9 | 11.8 ± 13.1 | 0.52 |
| VO2peak (ml/kg · min)e | 33.4 ± 7.3 | 27.6 ± 4.5 | 27.4 ± 5.3 | 24.7 ± 3.1 | 22.6 ± 4.0 | <0.001 |
| Vasosomatic frequencyf | 4.2 ± 3.8 | 8.3 ± 5.1 | 15.6 ± 11.2 | 10.4 ± 5.1 | 14.6 ± 9.2 | <0.001 |
| Vasosomatic severityf | 4.9 ± 3.2 | 8.3 ± 5.6 | 14.8 ± 10.1 | 10.3 ± 6.0 | 13.2 ± 7.8 | <0.001 |
Data are mean ± sd, unless otherwise indicated. Conversion factors to SI units: total, HDL, and LDL cholesterol (0.0259); triglycerides (0.0113); insulin (6.945); glucose (0.0555); FSH (1); estradiol (3.671); progesterone (3.18); estrone (37); testosterone (0.0347); and norepinephrine (0.00591). Pre, Premenopausal; Peri, perimenopausal; Post, postmenopausal; BP, blood pressure; HR, heart rate; bpm, beats per minute; LTPA, leisure time physical activity; MET, metabolic equivalent; VO2peak, peak aerobic power.
Data are median (interquartile range).
Sample sizes of 105.
Sample sizes of 102.
Sample sizes of 119.
Sample sizes of 106.
Sample sizes of 100.
Brachial artery FMD and GTN-mediated vasodilation
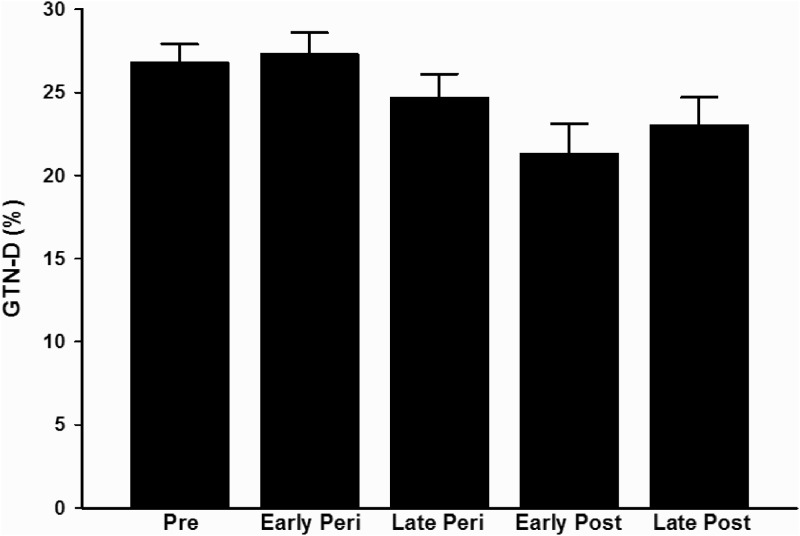
Brachial artery FMD was reduced across the stages of the menopause transition (P < 0.001, Fig. 1). When compared with premenopausal women, brachial artery FMD (%Δ) was lower in early (P = 0.03) and late perimenopausal (P < 0.001) and early and late postmenopausal (both P < 0.001). Similar results were obtained when the data were expressed as absolute (millimeters) change in FMD, with the exception of the comparison between premenopausal and early perimenopausal women (P = 0.10, see on-line supplement). Additional differences among the groups are designated in Fig. 1. GTN-mediated vasodilation (both %Δ and mmΔ) was also associated with menopause stages (P = 0.04; Fig. 2); however, these associations were driven by a tendency toward lower GTN-mediated vasodilation in the early postmenopausal compared with the early perimenopausal women (%Δ, P = 0.09 and mmΔ, P = 0.06). Although baseline brachial artery diameter (for FMD measure) was associated with menopause stage, this association was driven by a tendency toward smaller brachial diameters in premenopausal compared with late postmenopausal women (P = 0.06) and smaller diameters in late perimenopausal compared with late postmenopausal women (P = 0.09). There were no differences in baseline GTN brachial diameter or peak shear rate among the groups (Supplemental Table 1).
Fig. 1.
Brachial artery FMD in premenopausal (Pre), early (Early Peri) and late perimenopausal (Late Peri), and early (Early Post) and late postmenopausal (Late Post) women. *, P < 0.001 and §, P = 0.03 vs. premenopausal women; †, P < 0.001 vs. early perimenopausal; ‡, P < 0.001 vs. late perimenopausal.
Fig. 2.
GTN-mediated vasodilation in premenopausal (Pre), early (Early Peri) and late perimenopausal (Late Peri), and early (Early Post) and late postmenopausal (Late Post) women.
Correlates of brachial artery FMD
Higher seated systolic blood pressure, total body and trunk fat, body mass index (BMI), total and LDL cholesterol, ET-1, norepinephrine and FSH concentrations, and lower estradiol, estrone, and progesterone concentrations and peak aerobic capacity were associated with a lower brachial artery FMD (Table 2). Higher vasosomatic symptom frequency, but not severity scores, tended to be associated with a lower brachial artery FMD. The association of menopausal stage with brachial artery FMD remained highly significant (P < 0.001) after adjustment for these correlates, brachial artery baseline diameter, and hot flash reporting the previous year. Age was a significant correlate of brachial artery FMD, but analyses were not age adjusted because age and menopausal stage were highly correlated (r = 0.88). However, menopausal stage was more strongly associated with brachial artery FMD (%Δ, r = −0.70, and mmΔ, r = −0.68, both P < 0.001) than age (%Δ, r = −0.64, and mmΔ, r = −0.61, both P < 0.001). Adjusting for the prior and duration of HT and hormonal contraceptives did not alter the association with brachial artery FMD (all P > 0.20).
Table 2.
Correlations of CVD risk factors, sex hormone concentrations, vasoconstrictors, and hot flashes with brachial artery FMD
| Variables | Brachial artery FMD |
|
|---|---|---|
| r | P | |
| Seated systolic BP | −0.38 | <0.001 |
| BMI | −0.19 | 0.03 |
| Total body fat | −0.40 | <0.001 |
| Trunk body fat | −0.37 | <0.001 |
| Waist circumference | −0.11 | 0.23 |
| Total cholesterol | −0.33 | <0.001 |
| LDL cholesterol | −0.29 | 0.001 |
| Glucose | −0.12 | 0.17 |
| FSH | −0.57 | <0.001 |
| Estradiol | 0.46 | <0.001 |
| Progesterone | 0.29 | 0.001 |
| Estrone | 0.35 | <0.001 |
| SHBG | 0.01 | 0.91 |
| Testosterone | 0.09 | 0.36 |
| ET-1 | −0.20 | 0.02 |
| Norepinephrine | −0.30 | 0.002 |
| VO2peak | 0.35 | <0.001 |
| Vasosomatic frequency | −0.17 | 0.08 |
| Vasosomatic severity | −0.12 | 0.24 |
BP, Blood pressure; VO2peak, peak aerobic power.
Subsample analysis of women aged 50–59 yr
Similar to the larger cohort, total body fat and FSH were higher (P = 0.006) and estradiol and estrone concentrations were lower across the stages of the menopause transition (Table 3, P < 0.001). There were no other differences in clinical characteristics across the groups. Brachial artery FMD was reduced in late perimenopausal and early and late postmenopausal compared with early perimenopausal women (P < 0.001) but was not different between late perimenopausal and either early or late postmenopausal women (Fig. 3). Adjusting for age, total body fat, brachial diameter, and sex hormones did not influence the results (P < 0.001).
Table 3.
Participant characteristics of perimenopausal and postmenopausal women aged 50–59 yr
| Variable | Early Peri | Late Peri | Early Post | Late Post | P |
|---|---|---|---|---|---|
| n | 9 | 12 | 27 | 9 | |
| Age (yr) | 52 ± 2 | 52 ± 2 | 55 ± 2 | 57 ± 2 | <0.001 |
| Body mass (kg) | 63.6 ± 6.3 | 68.2 ± 15.7 | 71.3 ± 13.2 | 72.5 ± 14.1 | 0.42 |
| BMI (kg/m2) | 23.7 ± 2.6 | 24.8 ± 5.0 | 26.7 ± 4.7 | 28.4 ± 5.4 | 0.12 |
| Total body fat (%) | 30 ± 5 | 36 ± 7 | 38 ± 6 | 40 ± 5 | 0.006 |
| Waist circumference (cm) | 78 ± 6 | 82 ± 15 | 86 ± 14 | 86 ± 13 | 0.46 |
| WHR | 0.80 ± 0.07 | 0.80 ± 0.08 | 0.82 ± 0.09 | 0.80 ± 0.05 | 0.81 |
| Systolic BP (mm Hg) | 111 ± 12 | 120 ± 14 | 118 ± 14 | 118 ± 16 | 0.53 |
| Diastolic BP (mm Hg) | 72 ± 3 | 76 ± 3 | 73 ± 2 | 74 ± 3 | 0.82 |
| Total cholesterol (mg/dl) | 166 ± 24 | 173 ± 41 | 192 ± 32 | 194 ± 30 | 0.11 |
| LDL cholesterol (mg/dl) | 101 ± 27 | 106 ± 38 | 113 ± 28 | 119 ± 26 | 0.54 |
| HDL cholesterol (mg/dl) | 48 ± 6 | 50 ± 7 | 55 ± 16 | 54 ± 13 | 0.52 |
| Triglycerides (mg/dl)a | 81 (68–99) | 94 (56–112) | 85 (69–149) | 101 (78–129) | 0.55 |
| Fasting insulin (μIU/ml) | 4.9 ± 4.1 | 5.1 ± 2.8 | 10.1 ± 7.8 | 9.9 ± 10.8 | 0.11 |
| Fasting glucose (mg/dl) | 85 ± 4 | 80 ± 10 | 88 ± 10 | 87 ± 9 | 0.09 |
| FSH (μIU/ml) | 15.3 ± 24.6 | 80.5 ± 34.5 | 77.2 ± 29.8 | 89.7 ± 24.4 | <0.001 |
| Estradiol (pg/ml)a | 98 (43–137) | 28 (10–92) | 13 (10–19) | 15 (10–19) | <0.001 |
| Progesterone (ng/ml)a | 0.6 (0.3–0.6) | 0.4 (0.3–0.6) | 0.3 (0.1–0.5) | 0.3 (0.1–0.4) | 0.06 |
| Estrone (ng/dl) | 54 ± 10 | 43 ± 9 | 28 ± 6 | 31 ± 10 | 0.002 |
| SHBG (nmol/liter) | 86 ± 52 | 72 ± 41 | 65 ± 41 | 67 ± 32 | 0.60 |
| Testosterone (ng/dl)b | 22 (17–37) | 20 (17–24) | 18 (17–30) | 25 (17–53) | 0.27 |
| ET-1 (pg/ml) | 5.8 ± 1.4 | 6.4 ± 1.9 | 6.4 ± 1.6 | 6.0 ± 1.2 | 0.72 |
| Norepinephrine (pg/ml)b | 271 ± 60 | 264 ± 123 | 291 ± 155 | 273 ± 134 | 0.95 |
| LTPA (MET h/wk)a,b | 17.4 (5.0–30.5) | 12.9 (5.8–22.7) | 9.5 (5.2–20.0) | 6.3 (3.1–9.2) | 0.12 |
| VO2peak (ml/kg · min)b | 28.1 ± 5.0 | 27.8 ± 6.8 | 24.7 ± 3.3 | 22.8 ± 5.1 | 0.09 |
Data are mean ± sd, unless otherwise indicated. Peri, Perimenopausal; Post, postmenopausal; BP, Blood pressure; LTPA, leisure time physical activity; MET, metabolic equivalent; VO2peak, peak aerobic power.
Data are median (interquartile range).
Sample size of 45.
Fig. 3.
Brachial artery FMD in early (Early Peri) and late perimenopausal (Late Peri) and early (Early Post) and late postmenopausal (Late Post) and postmenopausal women aged 50–59 yr. *, P < 0.001 vs. early perimenopausal.
Discussion
The menopause transition is characterized by menstrual cycle irregularities, ovarian hormone changes, and increased risk for the development of CVD. To our knowledge, the present study was the first to examine vascular endothelial function across the stages of the menopause transition. The primary findings indicate that the stages of the menopause transition are associated with impaired endothelial function, independent of traditional CVD risk factors and hot flashes. The impairment in endothelial function is more evident during the late than early perimenopausal transition when controlling for age and becomes worse with prolonged estrogen deficiency during the postmenopausal years.
The loss of normal endothelial function is believed to be a critical step in the initiation and progression of atherosclerosis (5). Although endothelial dysfunction is a biomarker of vascular aging, in women, the age-related decline in endothelial function is evident only after menopause, suggesting that estrogen may play a key role in maintaining a healthy, functional endothelium (11, 12). However, it was unclear how changes in ovarian hormones during the perimenopausal years influenced endothelial function. In the present study, endothelial function was impaired across the stages of the menopause transition, providing the first evidence that endothelial protection may be lost with declines in ovarian function. We found that the impairment in endothelial function was more pronounced during the late perimenopausal transition as indicated by a greater impairment in brachial artery FMD (from premenopausal levels) in late perimenopausal compared with early perimenopausal women of similar age (∼35 vs. ∼17% impairment, respectively). Moreover, a lower brachial artery FMD was strongly related to higher FSH and lower estradiol concentrations that characterize the menopausal transition. Our findings suggest that ovarian hormone levels in the early perimenopausal period may be sufficient to provide some level of endothelial protection and that declines in ovarian function and estrogen levels in the late perimenopausal transition initiate the rapid deterioration in endothelial function that worsens with prolonged estrogen deficiency. These findings are consistent with previous observations demonstrating that menstrual cycle irregularity and hypoestrogenemia related to the loss of ovarian function are associated with endothelial dysfunction and increased CVD risk (25, 26).
Because menopausal stage was highly correlated with age in the present study, it is plausible that the decline in endothelial function was mediated by aging. However, the correlation with brachial artery FMD was stronger with menopausal stage than age. Additionally, when we examined endothelial function in a subgroup of women aged 50–59 yr, brachial artery FMD was lower in late perimenopausal and postmenopausal women compared with early perimenopausal women. Although these findings suggest that declining ovarian function rather than age mediates impaired endothelial function in healthy middle-aged women, within the current cross-sectional study, we cannot completely discern the effects of declining ovarian function from that of age.
In the present study, we examined whether CVD risk factors were related to the endothelial dysfunction across the stages of the menopause transition. Both prospective and longitudinal studies have shown that the menopausal transition is associated with changes in CVD risk factors known to adversely affect endothelial function, including adiposity, blood lipids, and lipoproteins (9, 10, 27). In the prospective Healthy Women Study, LDL cholesterol, triglycerides, and BMI showed greater increases during the perimenopausal transition than after menopause, whereas increases in systolic blood pressure and fasted glucose were greater after menopause (27). Similarly, the Study of Women's Health Across the Nation (SWAN) showed increases in total and LDL cholesterol that were greater 1 yr before the final menstrual period compared with increases observed during the early perimenopausal or postmenopausal period, suggesting that the late perimenopausal transition may be the most critical time period for adverse changes in lipids (9). In the present study, adiposity (total and trunk fat), systolic blood pressure and total and LDL cholesterol were higher across the stages of the menopause transition and were inversely correlated with brachial artery FMD. However, adjusting for these risk factors did not alter the association between menopause stage and endothelial dysfunction. Moreover, in the subgroup analyses of women aged 50–59 yr in whom brachial artery FMD was lower in late perimenopausal and postmenopausal compared with early perimenopausal women, there were no differences in CVD risk factors across the groups.
We also examined whether the endothelial dysfunction across the stages of the menopause transition was related to the presence of hot flashes. Bechlioulis et al. (8) reported that early (<3 yr) postmenopausal women reporting moderate to severe hot flashes had reduced brachial artery FMD compared with age-matched early postmenopausal women reporting no or mild hot flashes, with the latter group not different from premenopausal controls. Similarly, in the Study of Women's Health Across the Nation Heart Study, women with hot flashes had reduced brachial artery FMD compared with women without hot flashes, independent of CVD risk factors, menopausal status, and estradiol concentrations (7). In the present study, although menopause stage, particularly late perimenopausal, was associated with a greater reporting of hot flashes in the previous year and with higher vasosomatic frequency and severity scores, we found no association between hot flashes and brachial artery FMD. Adjustment for the vasosomatic frequency scores and hot flashes did not alter the association between menopausal stage and endothelial dysfunction. These discordant findings may be related to differences in the health status of the population studied and measurement of brachial artery FMD (i.e. cuff occlusion duration) and/or hot flashes from the previous studies (7, 8). Because self-report measures of hot flashes may underestimate the frequency and/or severity, it is possible that an association may have been observed if we used objective measures of hot flashes (i.e. sternal skin conductance).
We can only speculate as to the mechanisms underlying endothelial dysfunction across the stages of the menopause transition. Reduced endothelial-dependent vasodilation is characterized by reduced nitric oxide (NO) production and by increased ET-1 activity and norepinephrine release (28, 29). Because estradiol preserves endothelial function in part by enhancing NO release (30) and by modulating the vascular effects of ET-1 and norepinephrine (31, 32), it is plausible that the endothelial dysfunction observed across the stages of the menopause transition is a consequence of reduced NO bioavailability and increased ET-1 and norepinephrine bioactivity. In the present study, ET-1 and norepinephrine concentrations were elevated across the stages of the menopause transition and were inversely correlated with brachial artery FMD. However, adjustment for ET-1 and norepinephrine did not alter the association between menopause stage and brachial artery FMD. The norepinephrine findings are in contrast to those reported by Kaplon et al. (33), who found that elevated plasma norepinephrine was an independent predictor of endothelial function with aging in women. The reason for this finding could be related to different populations of women. The present study included women across all the menopausal stages, whereas Kaplon et al. included only premenopausal and postmenopausal women. Nonetheless, because plasma concentrations do not necessarily reflect the differences at the local vascular level or vascular responsiveness to these factors, we cannot rule out that these or other vasoactive factors were modulated across the stages of the menopause transition. Other potential mechanisms include oxidative stress, tetrahydrobiopterin deficiency (a critical cofactor for NO production), and impaired estrogen receptor-α and endothelial nitric oxide synthase intracellular signaling (14, 34).
There were some limitations of the present study. First, the cross-sectional design precludes any conclusions about causality. Perimenopausal and postmenopausal status was determined by self-reported menstrual cycle characteristics; thus, group classifications may have been inaccurate. However, sex hormone concentrations at each menopausal stage were consistent with data from observational and longitudinal investigations of the menopause transition (35–37). Second, our findings can only be generalized to apparently healthy, mostly Caucasian, nonsmoking, normotensive, and sedentary/recreationally active women. Third, this study was done before the release of the STRAW+10 menopausal staging system (38). We also applied the previous STRAW criteria to women with a BMI greater than 30 kg/m2, which is not recommended (13). However, excluding women with BMI greater than 30 kg/m2 (n = 22) did not alter our findings (data not shown). Finally, the Pathobiological Determinants of Atherosclerosis in Youth Study found the presence of subclinical atherosclerosis in the abdominal aorta and coronary arteries in women 35 yr of age (39), and although our population was screened for overt disease, it is plausible that some women had subclinical, progressing atherosclerosis.
Clinical significance and conclusions
Endothelial dysfunction is a critical factor in the etiology of CVD and a significant predictor of cardiovascular events in postmenopausal women (6). As women age, they endure an additional insult in that their arteries are exposed to adverse changes in CVD risk factors (i.e. blood pressure, lipids, adiposity) during a time of profound changes in the hormonal environment. Because the menopause transition may be a triggering event that leads to increased vascular vulnerability as women age, a better understanding of the mechanisms mediating endothelial dysfunction in women as they lose the protective effects of estrogen on the vasculature is needed. Although recent clinical trials do not support cardioprotective benefits of HT in older postmenopausal women (2, 3), it is plausible that the timing of HT during a critical window may offer cardioprotection. The present study's findings add to the accumulating evidence that the most critical time period for adverse changes in CVD risk may be the late perimenopausal transition (9, 10, 40). Future investigations should examine whether maintaining estrogen concentrations and/or implementing lifestyle or therapeutic prevention strategies in the early stages of the menopause transition can preserve or attenuate the decline in endothelial function and decrease CV events in women.
Supplementary Material
Acknowledgments
We thank Chelsea Bergman, Lauren Tobin, and Tracy Swibas for their technical assistance.
This work was supported by the National Institutes of Health Awards AG027678 and AG20683, General Clinical Research Center Grant RR-00051, Colorado Clinical Translational Sciences Institute Grant RR-025780, Colorado Nutrition Obesity Research Center Grant P30 DK048520, and University of Colorado Denver Center for Women's Health Research and University of Colorado Women's Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- %Δ
- Absolute change (percentage)
- BMI
- body mass index
- CVD
- cardiovascular disease
- ET-1
- endothelin-1
- FMD
- flow-mediated dilation
- GTN
- glyceryl trinitrate
- HDL
- high-density lipoprotein
- HT
- hormone therapy
- LDL
- low-density lipoprotein
- mmΔ
- absolute change (millimeters)
- NO
- nitric oxide
- STRAW
- Stages of Reproductive Aging Workshop
- WHR
- waist to hip ratio.
References
- 1. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. 2012. Executive Summary: heart disease and stroke statistics—2012 update. Circulation 125:188–197 [DOI] [PubMed] [Google Scholar]
- 2. Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. 2003. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 349:523–534 [DOI] [PubMed] [Google Scholar]
- 3. Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. 2004. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 291:1701–1712 [DOI] [PubMed] [Google Scholar]
- 4. Clarkson TB. 2007. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause 14:373–384 [DOI] [PubMed] [Google Scholar]
- 5. Lakatta EG, Levy D. 2003. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107:139–146 [DOI] [PubMed] [Google Scholar]
- 6. Rossi R, Nuzzo A, Origliani G, Modena MG. 2008. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol 51:997–1002 [DOI] [PubMed] [Google Scholar]
- 7. Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. 2008. Hot flashes and subclinical cardiovascular disease. Circulation 118:1234–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bechlioulis A, Kalantaridou SN, Naka KK, Chatzikyriakidou A, Calis KA, Makrigiannakis A, Papanikolaou O, Kaponis A, Katsouras C, Georgiou I, Chrousos GP, Michalis LK. 2010. Endothelial function, but not carotid intima-media thickness, is affected early in menopause and is associated with severity of hot flushes. J Clin Endocrinol Metab 95:1199–1206 [DOI] [PubMed] [Google Scholar]
- 9. Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, Sutton-Tyrrell K. 2009. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol 54:2366–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wildman RP, Colvin AB, Powell LH, Matthews KA, Everson-Rose SA, Hollenberg S, Johnston JM, Sutton-Tyrrell K. 2008. Associations of endogenous sex hormones with the vasculature in menopausal women: the Study of Women's Health Across the Nation (SWAN). Menopause 15:414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. 1994. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24:471–476 [DOI] [PubMed] [Google Scholar]
- 12. Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. 1996. Menopause is associated with endothelial dysfunction in women. Hypertension 28:576–582 [DOI] [PubMed] [Google Scholar]
- 13. Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. 2001. Stages of Reproductive Aging Workshop (STRAW). J Womens Health Gend Based Med 10:843–848 [DOI] [PubMed] [Google Scholar]
- 14. Moreau KL, Meditz A, Deane KD, Kohrt WM. 2012. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol 302:H1211–H1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. 2002. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39:257–265 [DOI] [PubMed] [Google Scholar]
- 16. Eskurza I, Monahan KD, Robinson JA, Seals DR. 2004. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. 2000. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102:1270–1275 [DOI] [PubMed] [Google Scholar]
- 18. Lohman TG, Roche AF, Martorell R. 1988. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics [Google Scholar]
- 19. Tanaka H, Desouza CA, Jones PP, Stevenson ET, Davy KP, Seals DR. 1997. Greater rate of decline in maximal aerobic capacity with age in physically active vs. sedentary healthy women. J Appl Physiol 83:1947–1953 [DOI] [PubMed] [Google Scholar]
- 20. Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC, Zmuda JM. 1997. A collection of physical activity questionnaires for health-related research. Med Sci Sports Exerc 29:S1–205 [PubMed] [Google Scholar]
- 21. Friedewald WT, Levey RI, Frederickson DS. 1972. Estimation of the concentration of LDL-C in plasma without the use of the preparative ultracentrifuge. Clin Chem 18:499–502 [PubMed] [Google Scholar]
- 22. Gavin KM, Jankowski CM, Kohrt WM, Stauffer BL, Seals DR, Moreau KL. Hysterectomy is associated with large artery stiffening in estrogen-deficient postmenopausal women. Menopause 19:1000–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moreau KL, Gavin KM, Plum AE, Seals DR. 2006. Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause 13:951–958 [DOI] [PubMed] [Google Scholar]
- 24. Perz JM. 1997. Development of the menopause symptom list: a factor analytic study of menopause associated symptoms. Women Health 25:53–69 [DOI] [PubMed] [Google Scholar]
- 25. Kalantaridou SN, Naka KK, Papanikolaou E, Kazakos N, Kravariti M, Calis KA, Paraskevaidis EA, Sideris DA, Tsatsoulis A, Chrousos GP, Michalis LK. 2004. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab 89:3907–3913 [DOI] [PubMed] [Google Scholar]
- 26. Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC, Speizer FE, Manson JE. 2002. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab 87:2013–2017 [DOI] [PubMed] [Google Scholar]
- 27. Matthews KA, Kuller LH, Sutton-Tyrrell K, Chang YF. 2001. Changes in cardiovascular risk factors during the perimenopause and postmenopause and carotid artery atherosclerosis in healthy women. Stroke 32:1104–1111 [DOI] [PubMed] [Google Scholar]
- 28. Bourque SL, Davidge ST, Adams MA. 2011. The interaction between endothelin-1 and nitric oxide in the vasculature: new perspectives. Am J Physiol Regul Integr Comp Physiol 300:R1288–R1295 [DOI] [PubMed] [Google Scholar]
- 29. Zanzinger J, Czachurski J, Seller H. 1994. Inhibition of sympathetic vasoconstriction is a major principle of vasodilation by nitric oxide in vivo. Circ Res 75:1073–1077 [DOI] [PubMed] [Google Scholar]
- 30. Mendelsohn ME. 2000. Mechanisms of estrogen action in the cardiovascular system. J Steroid Biochem Mol Biol 74:337–343 [DOI] [PubMed] [Google Scholar]
- 31. Sudhir K, Ko E, Zellner C, Wong HE, Hutchison SJ, Chou TM, Chatterjee K. 1997. Physiological concentrations of estradiol attenuate endothelin 1-induced coronary vasoconstriction in vivo. Circulation 96:3626–3632 [DOI] [PubMed] [Google Scholar]
- 32. Sudhir K, Elser MD, Jennings GL, Komesaroff PA. 1997. Estrogen supplementation decreases norepinephrine-induced vasoconstriction and total body norepinephrine spillover in perimenopausal women. Hypertension 30:1538–1543 [DOI] [PubMed] [Google Scholar]
- 33. Kaplon RE, Walker AE, Seals DR. 2011. Plasma norepinephrine is an independent predictor of vascular endothelial function with aging in healthy women. J Appl Physiol 111:1416–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gavin KM, Seals DR, Silver AE, Moreau KL. 2009. Vascular endothelial estrogen receptor α is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab 94:3513–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. 2007. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab 92:3060–3067 [DOI] [PubMed] [Google Scholar]
- 36. Randolph JF, Jr, Zheng H, Sowers MR, Crandall C, Crawford S, Gold EB, Vuga M. 2011. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab 96:746–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sowers MR, Zheng H, McConnell D, Nan B, Harlow SD, Randolph JF., Jr 2008. Estradiol rates of change in relation to the final menstrual period in a population-based cohort of women. J Clin Endocrinol Metab 93:3847–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ, for the SCG 2012. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause 19:387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Strong JP, Malcom GT, McMahan CA, Tracy RE, Newman WP, 3rd, Herderick EE, Cornhill JF. 1999. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA 281:727–735 [DOI] [PubMed] [Google Scholar]
- 40. Santoro N, Sutton-Tyrrell K. 2011. The SWAN song: Study of Women's Health Across the Nation's recurring themes. Obstet Gynecol Clin North Am 38:417–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.