Abstract
Context:
Reproductive function may improve after bariatric surgery, although the mechanisms and time-related changes are unclear.
Objective:
The objective of the study was to determine whether ovulation frequency/quality as well as associated reproductive parameters improve after Roux en Y gastric bypass surgery.
Design:
This was a prospective cohort study that enrolled female subjects from 2005 to 2008 with study visits at baseline and then 1, 3, 6, 12, and up to 24 months after surgery.
Setting:
The study was conducted at an academic health center.
Patients:
Twenty-nine obese, reproductive-aged women not using confounding medications participated in the study.
Main Outcome Measures:
The primary outcome was integrated levels of urinary progestin (pregnanediol 3-glururonide) from daily urinary collections at 12 months postoperatively. Secondary outcomes were changes in vaginal bleeding, other biometric, hormonal, ultrasound, dual-energy x-ray absorptiometry measures, and Female Sexual Function Index.
Results:
Ninety percent of patients with morbid obesity had ovulatory cycles at baseline, and the ovulatory frequency and luteal phase quality (based on integrated pregnanediol 3-glururonide levels) were not modified by bariatric surgery. The follicular phase was shorter postoperatively [6.5 d shorter at 3 months and 7.9–8.9 d shorter at 6–24 months (P < 0.01)]. Biochemical hyperandrogenism improved, largely due to an immediate postoperative increase in serum SHBG levels(P < 0.01), with no change in clinical hyperandrogenism (sebum production, acne, hirsutism). Bone density was preserved, contrasting with a significant loss of lean muscle mass and fat (P < 0.001), reflecting preferential abdominal fat loss (P < 0.001). Female sexual function improved 28% (P = 0.02) by 12 months.
Conclusions:
Ovulation persists despite morbid obesity and the changes from bypass surgery. Reproductive function after surgery is characterized by a shortened follicular phase and improved female sexual function.
Obesity in women has been linked to a number of adverse reproductive outcomes including anovulation, delayed time to conception, increased rate of miscarriage, and later pregnancy complications (1). Obesity among infertile women seeking pregnancy, and especially abdominal obesity, (2) has been associated with diminished response to ovulation induction and with decreased pregnancy rates (3, 4). Obese women seeking pregnancy are advised first to lose weight before conception (5), and some countries (e.g. New Zealand) deny access to publicly funded infertility treatments to obese women (6).
The mechanisms behind impaired female reproduction in obese women are poorly understood and likely complex. Obesity is associated with impaired ovulatory function and depleted or poor egg quality (1), and endometrial abnormalities that may compromise implantation (7), and female sexual dysfunction may reduce sexual desire and frequency of intercourse (8). A potential model for better understanding the effects of obesity on reproductive function is bariatric surgery because massive weight loss occurs in a relatively short period of time, and each person can serve as their own control when studied sequentially. Women undergoing bariatric surgery may have improved ovulatory function, fertility, and pregnancy outcomes (9–12).
We hypothesized that weight loss per se in obese women undergoing Roux en Y gastric bypass would result in improvements in the frequency and quality of ovulation and sexual function and, furthermore, that such changes would be linked in a dose-response fashion with the amount and distribution of weight lost.
Materials and Methods
Subjects
The protocol was reviewed and approved by the Institutional Review Board at Pennsylvania State College of Medicine. Women were recruited between 2005 and 2008 and were studied for up to 2 yr afterward. All subjects gave written informed consent. Due to the closeout of the grant, the study was terminated on January 1, 2010, and planned visits beyond this date could not be completed.
Inclusion criteria included age 18–40 yr. The 1991 National Institutes of Health guidelines for bariatric surgery were followed (13): body mass index (BMI) above 40 kg/m2 or a BMI between 35 and 39.9 kg/m2 with a weight-related health problem, such as diabetes or high blood pressure, and failed medical weight loss. All subjects were premenopausal with intact ovaries and uterus. Furthermore, subjects were not using hormonal contraceptives or medications known to affect ovulation or sex steroids and remained off of them throughout the study. Exclusion criteria included smoking, a history of alcohol or substance abuse, pregnancy, and an unwillingness to use barrier contraception, if sexually active, after the gastric bypass surgery. Subjects with obesity caused by hypothyroidism, Cushing's syndrome, or by genetic predisposition were excluded. We limited our study to subjects who had undergone Roux en Y gastric bypass.
Visits
There were six visits planned during the study. A preoperative study visit was performed 1 month before gastric bypass surgery and then visits at 1, 3, 6, 12, and 24 months after surgery. At each visit, a history/physical examination was performed, menstrual diaries were collected, facial sebum was measured, fasting blood was obtained, body composition was obtained with dual-energy x-ray absorptiometry (DXA), and daily urine collections were retrieved. Subjects were instructed to collect first void daily urine samples from the preoperative visit until 1 month afterward and then for a month before each subsequent visit. Visits were scheduled to correspond with regular bariatric surgery follow-up and were therefore independent of menstrual phase. We did not obtain ultrasounds or sexual function questionnaires at the 24-month visit.
Physical examination
Height and weight were obtained in the General Clinical Research Center at Penn State University on the same scale and stadiometer throughout the study, and waist circumference was measured. Hirsutism was assessed by trained study personnel using the modified Ferriman-Galwey score (14). Facial lesion counts of open and closed comedones (noninflammatory lesions) were obtained from the forehead, left and right cheeks, nose, and chin by trained study personnel (15). We also obtained sebum measures on the face with a sebumeter from the left, center, and right portions of the forehead and summed the results (15).
Dual-energy x-ray absorptiometry
Body composition data were determined using the fan-beam mode by DXA using a Hologic QDR-4500W bone densitometer (Hologic Inc., Bedford, MA) (16). Subregion analysis of android (abdominal) and gynoid (hip) fat was modeled (17). We could not obtain DXA scans on women weighing greater than 300 lb due to machine limitations.
Ultrasound scan
A transvaginal ultrasound of the pelvis was performed before and 6 and 12 months after surgery (14). Endometrial thickness in the sagittal plane was measured, the largest follicle/cyst on each ovary was measured, and volume of the ovary was calculated using the formula for a prolate ellipsoid (14).
Questionnaire
Before and 12 months after bariatric surgery, subjects filled out the Female Sexual Function Index (FSFI), a brief multidimensional scale for assessing sexual function in women. The scale has received initial psychometric evaluation, including studies demonstrating reliability, convergent validity, and discriminate validity (18).
Assays
Fasting serum from each visit was assayed for estradiol, progesterone, testosterone, and SHBG as previously reported (19). Urinary estrone 3-glucuronide (E13G) and pregnanediol 3-glucuronide (Pd3G) were measured in triplicate using competitive, double-antibody, time-resolved fluoroimmunoassays as previously described, (19, 20), and values were divided by urinary creatinine concentrations to standardize for urine flow rate (19, 20). All assays had a coefficient of variation of 10% or less (19). Urine samples with concentrations of E13G, Pd3G, and creatinine that were each virtually identical for 2 or more sequential collection days were considered to be derived from the same void and omitted from analyses.
End points of menstrual cycle function
Parameters of menstrual cycle function are defined in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org. In brief, menstrual cycle length was defined by the onset of menses as previously described (21). Follicular phase length was defined by the onset of menses through the day of luteal transition, an index of ovulation when the E13G to Pd3G ratio sharply drops with the transition of steroidogenesis (22). Luteal phase length was defined as the cycle length minus follicular phase length. Vaginal bleeding was recorded and analyzed as the number of bleed days (including spotting) within the defined menses (see above), within a bleeding interval that includes menses plus adjacent bleeding days (allowing 2 d nonbleeding before or after menses), and within the cycle. Endocrine endpoints of menstrual cycle function were derived from urinary E13G and Pd3G measurements using previously described algorithms (see Supplemental Table 1) (9, 21, 23).
Statistical analysis
Primary outcome
The primary outcome was the change in area under the curve for urinary Pd3G during the luteal phase (9) for the cycle closest to the monthly visit at 12 months (and for those who continued also at 24 months). We chose this as a measure both of frequency and quality of ovulation [i.e. elevated integrated progestin levels indicate luteal phase adequacy (9)]. A sample size of 20 women was calculated to have at least 86% power to detect an effect size, absolute difference in means divided by the sd, of 0.9 between any two visits using a two-sided test with a familywise type I error of 0.05. The familywise type I error of 0.05 accounts for multiple comparison testing in the final analysis. Secondary outcomes included other parameters of ovulatory function (length of menstrual cycle and follicular phase and luteal phase, rate of ovulation, and urinary endocrine end points) as well as changes in biometric data, DXA data, circulating hormones, and sexual function.
Mixed-effects models were used to test for changes in the primary and secondary end points after surgery. A separate model was fit for each end point. Data were analyzed at six time points: before and 1, 3, 6, 12, and 24 months after surgery. Contrasts were constructed to compare each postsurgery time point to the presurgical time point.
A preliminary analysis was conducted to compare the nonconception cycles of five women who became pregnant during the study with the women who did not become pregnant. Of 46 menstrual cycle function end points (see Supplemental Table 1), only four differences were observed (P < 0.05) for the women who eventually became pregnant: the day of follicular E13G rise onset was delayed, the midluteal Pd3G level was lower, one of five indices of the luteal Pd3G area under the curve was lower, and the rate of midlate luteal Pd3G drop was slower. Therefore, a variable for pregnancy was not included in the final mixed-effects models. All hypothesis tests were two sided, and results were reported as the mean change from before surgery (baseline) with the corresponding 95% confidence interval. All calculations were done with SAS (version 9.2; SAS Institute, Inc., Cary, NC). Statistical significance was considered to be P < 0.05.
Results
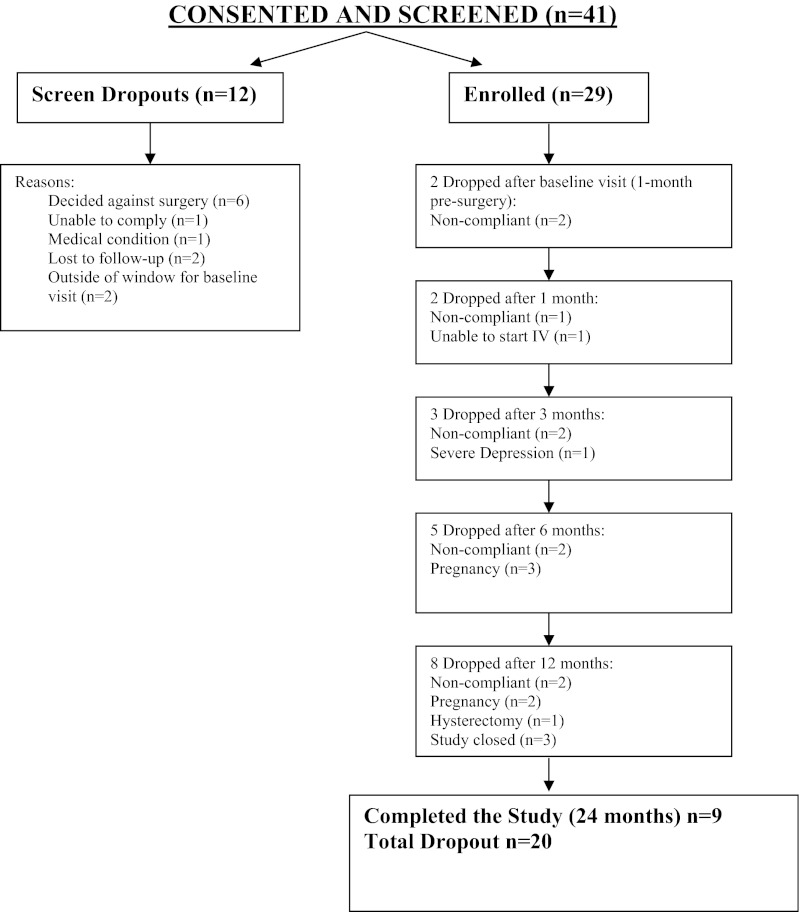
We enrolled 29 subjects in the study (see flow chart in Fig. 1). The mean ± sd age of the enrolled subjects was 34.5 ± 4.3 yr. Forty-eight percent self-reported regular menses and 52% irregular menses. Most women (52%) were also nulligravid at baseline. Five women conceived after surgery, four of whom had prior pregnancies. We noted significant reductions vs. baseline in weight and waist circumference at 12 and 24 months (Table 1).
Fig. 1.
Flow chart of subjects in the study.
Table 1.
Baseline characteristics and change after bariatric surgery in biometric, DXA, and urinary menstrual cycle function parameters
| 1 month before surgery (baseline) Mean (sd) | 1-month postsurgery change from baseline |
3-month postsurgery change from baseline |
6-month postsurgery change from baseline |
12-month postsurgery change from baseline |
24-month postsurgery change from baseline |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | P value | Estimate (95% CI) | P value | Estimate (95% CI) | P value | Estimate (95% CI) | P value | Estimate (95% CI) | P value | ||
| Biometric parameters | |||||||||||
| Weight (kg) | 132 (17) | −15 (−17, −14) | <0.001 | −29 (−31, −27) | <0.001 | −40 (−43, −37) | <0.001 | −50 (−54, −45) | <0.001 | −51 (−59, −44) | <0.001 |
| BMI (kg/m2) | 49 (7) | −6 (−6, −5) | <0.001 | −11 (−11, −10) | <0.001 | −15 (−16, −13) | <0.001 | −18 (−20, −17) | <0.001 | −19 (−22, −16) | <0.001 |
| Waist (cm) | 129 (16) | −8 (−12, −3) | <0.001 | −23 (−28, −17) | <0.001 | −30 (−37, −23) | <0.001 | −37 (−46, −28) | <0.001 | −41 (−53, −29) | <0.001 |
| Ferriman-Gallwey score | 15 (8) | 0.4 (−0.2, 0.9) | 0.20 | 0.6 (−0.1, 1.4) | 0.10 | 0.6 (−0.5, 1.6) | 0.28 | 0.1 (−1.4, 1.5) | 0.92 | 0.0 (−2.5, 2.5) | 0.99 |
| Total acne count (comodones+papules+pustules) | 12 (15) | 3.3 (−0.2, 6.8) | 0.06 | 2.7 (−1.8, 7.1) | 0.24 | 1.1 (−4.4, 6.7) | 0.69 | −1.6 (−8.8, 5.5) | 0.65 | −2.6 (−12.5, 7.3) | 0.60 |
| Total sebumeter reading (left+center+right) | 239 (128) | −38 (−100, 24) | 0.22 | −61 (−135, 13) | 0.10 | −77 (−161, 8) | 0.07 | −63 (−163, 38) | 0.22 | −79 (−199, 41) | 0.19 |
| DXA parameters | |||||||||||
| Total bone mineral density (g/cm2) | 1.19 (0.08) | 0.01 (0.00, 0.02) | 0.22 | 0.01 (−0.01, 0.02) | 0.38 | 0.00 (−0.02, 0.02) | 0.68 | 0.00 (−0.03, 0.02) | 0.87 | −0.03 (−0.07, 0.01) | 0.20 |
| Bone mineral content (g) | 2500 (315) | 32 (4, 59) | 0.02 | 16 (−19, 51) | 0.36 | −24 (−68, 20) | 0.29 | −61 (−123, 1) | 0.05 | −127 (−225, −29) | 0.01 |
| Lean (kg) | 57 (5) | −5 (−7, −4) | <0.001 | −8 (−9, −6) | <0.001 | −9 (−11, −7) | <0.001 | −11 (−14, −8) | <0.001 | −13 (−16, −9) | <0.001 |
| Fat (kg) | 60 (8) | −7 (−9, −6) | <0.001 | −17 (−19, −14) | <0.001 | −26 (−29, −23) | <0.001 | −33 (−37, −29) | <0.001 | −34 (−39, −28) | <0.001 |
| Android fat (kg) | 6 (1) | −1 (−1, 0) | <0.001 | −2 (−2, −1) | <0.001 | −3 (−3, −2) | <0.001 | −4 (−4, −3) | <0.001 | −4 (−4, −3) | <0.001 |
| Gynoid fat (kg) | 8 (2) | −1 (−1, −1) | <0.001 | −2 (−3, −1) | <0.001 | −3 (−4, −3) | <0.001 | −4 (−5, −3) | <0.001 | −4 (−5, −3) | <0.001 |
| Urinary menstrual cycle function parameters | |||||||||||
| Menstrual cycle length (d) | 37.2 (24.6) | −2.5 (−9.4, 4.3) | 0.47 | −2.9 (−8.9, 3.1) | 0.34 | −6.0 (−11.7, −0.3) | 0.04 | −4.4 (−10.3, 1.4) | 0.14 | −4.1 (−10.6, 2.4) | 0.21 |
| Follicular phase length (d) | 22.0 (12.5) | −3.2 (−8.4, 2.1) | 0.23 | −6.5 (−10.5, −2.4) | 0.002 | −8.2 (−12.3, −4.2) | <0.001 | −7.9 (−12.1, −3.7) | <0.001 | −8.9 (−13.9, −3.9) | <0.001 |
| Luteal phase length (d) | 12.2 (2.4) | 3.8 (0.4, 7.2) | 0.03 | 0.0 (−2.6, 2.6) | 0.99 | 0.8 (−1.9, 3.4) | 0.57 | 0.8 (−2.0, 3.7) | 0.56 | 0.0 (−3.4, 3.4) | 1.00 |
| 28-d E13G AUC (ng/mg creatinine) | 565 (338) | −86 (−202, 31) | 0.15 | −22 (−104, 60) | 0.59 | 9 (−72, 90) | 0.82 | 15 (−68, 99) | 0.71 | −38 (−137, 61) | 0.44 |
| Luteal Pd3G AUC 5 (μg/mg creatinine) | 104 (69) | −10 (−48, 28) | 0.59 | 25 (−3, 53) | 0.08 | 0 (−28, 27) | 0.98 | 27 (−3, 57) | 0.08 | 21 (−14, 56) | 0.23 |
| Ovulatory cycles (%) | 90.0 (30.5) | 9.9 (−2.4, 22.3) | 0.11 | 10.0 (−0.4, 20.5) | 0.06 | 10.1 (0.2, 20.0) | 0.05 | 10.0 (−0.5, 20.8) | 0.06 | 0.1 (−13.1, 13.3) | 0.98 |
| Creatinine (mg/ml) | 1.13 (0.35) | 1.16 (0.87, 1.46) | <0.001 | 0.94 (0.64, 1.24) | <0.001 | 0.58 (0.27, 0.89) | <0.001 | 0.40 (0.08, 0.72) | 0.02 | 0.13 (−0.23, 0.49) | 0.47 |
CI, Confidence interval; AUC, area under the curve.
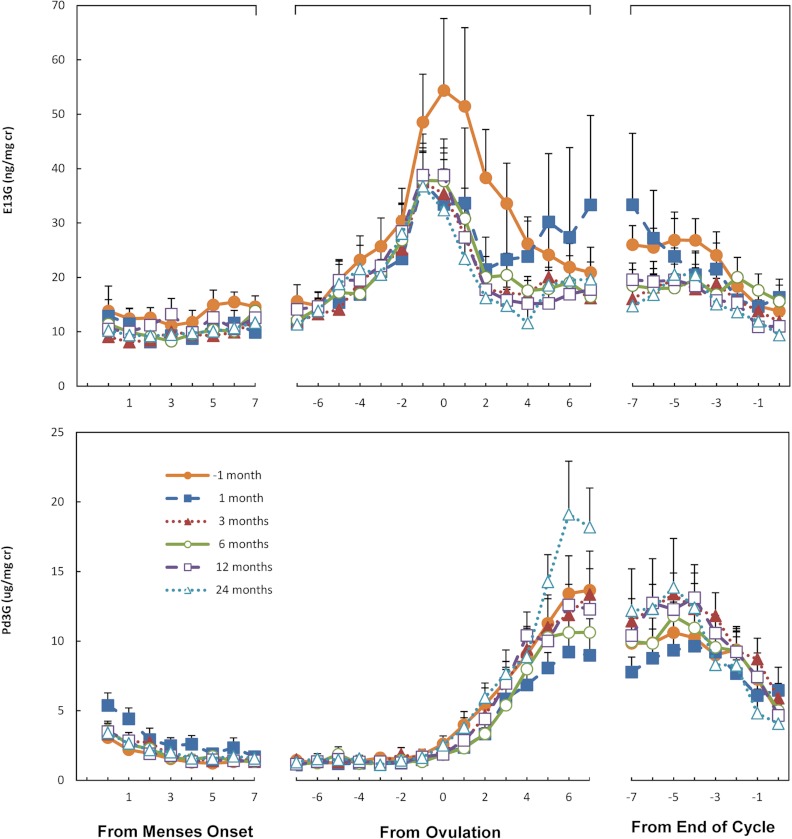
Our primary end point, luteal phase Pd3G, did not change over the course of the study, nor did frequency of ovulation and areas under the curve for whole-cycle E13G (Table 1). Similarly, luteal phase length, vaginal bleeding, and an array of additional endocrine parameters of menstrual cycle function remained consistent over the 24-month study period (Supplemental Table 2). In fact, the menstrual cycle endocrine profiles are remarkably similar at each of the six time intervals across the study (Fig. 2). Urinary creatinine concentrations increased during the initial months after surgery but declined over time (Table 1).
Fig. 2.
Urinary concentrations (means + se) of E13G (top panel) and Pd3G (bottom panel) plotted relative to onset of menses, ovulation, and the end of the cycle for samples collected one month before and 1, 3, 6, 12, and 24 months after women underwent bariatric surgery. Values are corrected for creatinine.
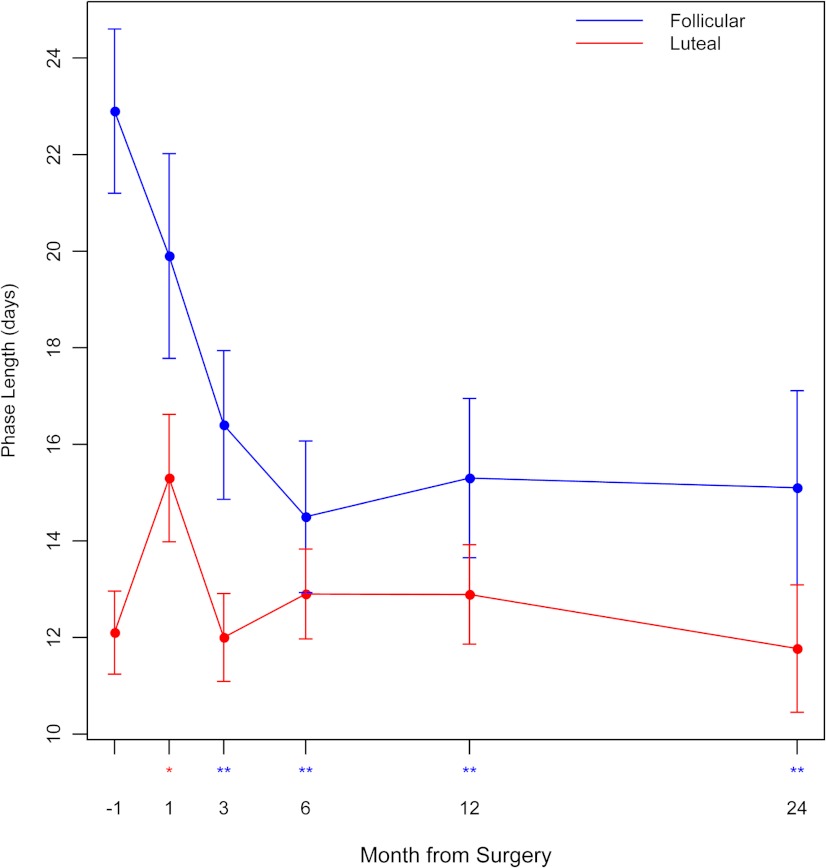
Follicular phase length was 6.5 d shorter within 3 months after surgery and 7.9–8.9 d shorter 6–24 months after surgery (P < 0.001) (Fig. 3 and Table 1). This effect was also manifested in the reduction of three other menstrual cycle function parameters: days from E13G rise onset to ovulation (P = 0.012), day of Pd3G peak (P = 0.002), and suggested by day of E13G peak (P = 0.06) (Supplemental Table 2). We noted a significant decrease in the overall menstrual cycle length at 6 months after surgery (P = 0.04) but not 12 months.
Fig. 3.
Lengths (means ± se) of the follicular phase and luteal phase 1 month before and 1, 3, 6, 12, and 24 months after bariatric surgery. *, P < 0.05 and **, P < 0.01 for change after surgery.
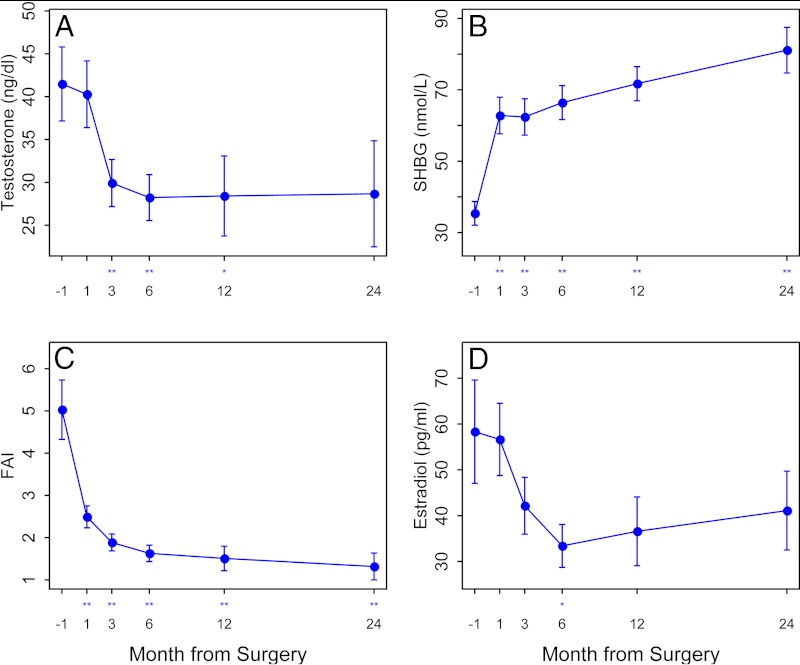
Female sexual function improved significantly at 12 months (21.2 ± 9.6 at baseline vs. 27.1 ± 7.4 at 12 months; P = 0.02). The sexual desire and arousal domains increased the most. We noted no significant change in measures of sebum, hirsutism, or acne scores. However, both testosterone and SHBG serum levels changed significantly at 12 and 24 months. The main increase in SHBG occurred immediately within 1 month of surgery (P < 0.001), whereas testosterone declined primarily in the 3-month postoperative period (P = 0.002) and estradiol only at month 6 (P = 0.03) (Fig. 4). There were no changes in progesterone concentrations over the study (data not shown).
Fig. 4.
Serum testosterone levels (A), serum sex hormone binding globulin (SHBG) levels (B), free androgen index (FAI) (C), serum estradiol levels (D), 1 month before and 1, 3, 6, 12, and 24 months after bariatric surgery. *, P < 0.05 and **, P < 0.01 for change after surgery.
We noted no significant change in total ovarian volume (right plus left ovarian volume) over 12 months (16.1 ± 13.1 cm3 at baseline, 17.2 ± 9.4 at 6 months, and 13.3 ± 6.4 at 12 months, P = 0.70) or in the size of the largest follicle on either ovary (12.0 ± 9.4 mm at baseline, 12.6 ± 7.4 at 6 months, and 8.6 ± 2.9 at 12 months, P = 0.16) or in the endometrial thickness (6.2 ± 3.0 mm at baseline, 6.0 ± 3.2 at 6 months, and 5.7 ± 3.8 at 12 months, P = 0.63).
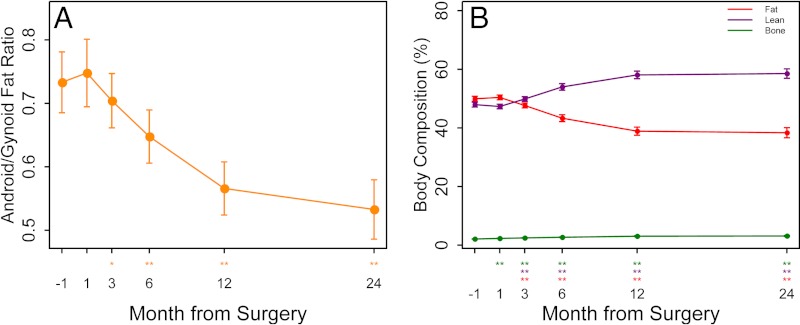
Body composition showed significant decreases in absolute total, fat, and lean mass at 12 and 24 months. In relative terms, the percent lean mass and bone mass increased, whereas the percent fat mass decreased (Fig. 5). Although there was a significant decrease in bone mineral content at 24 months, bone mineral density did not change. We found a preferential loss of gynoid fat early after bariatric surgery, but the ratio of android to gynoid fat continued to decline significantly at 12 and 24 months. We modeled the change in fat by DXA, both total and by subregion (android/gynoid), with change in a number of parameters of reproduction including sebum, acne, and hirsutism scores, serum testosterone, SHBG, and FSFI score and found no significant associations (data not shown).
Fig. 5.
Android/gynoid fat ratio (left), and percent body composition with fat, bone, and lean muscle mass (right) (means ± se) 1 month before and 1, 3, 6, 12, and 24 months after bariatric surgery as determined by DXA. *, P < 0.05 and **, P < 0.01 for change after surgery.
Discussion
Our study followed up a cohort of morbidly obese reproductive-aged women for up to 2 yr after Roux en Y gastric bariatric bypass surgery to examine the changes in reproductive function and body composition. We found that morbid obesity and weight loss had relatively little effect on the ovarian cycle, other than to substantially shorten the follicular phase. Furthermore, we showed no time-related changes, either as a result of surgery and the ensuing decreased caloric intake or as a result of long-term weight loss. We found no skin changes (sebum, acne, or hirsutism scores) with weight loss, and bone mineral density was maintained despite massive weight loss (24). Finally, we noted a significant improvement in female sexual function 12 months after surgery. Our study, despite its limitations, to our knowledge is the largest, most comprehensive (in terms of study of biometric, urinary, serum, ultrasound, and DXA parameters), and longest study (up to 24 months postoperatively) of female reproductive function before and after Roux en Y gastric bariatric surgery.
The high ovulation rates (≥90%) at baseline and throughout the postoperative period exceeded our expectations, especially because so many subjects had reported irregular menses at baseline. We attribute this to two main factors. One is our selection criteria of obese healthy women off confounding medications likely to be used by women with menstrual disorders at baseline (such as hormonal contraceptives). The second is the unreliability of a menstrual history as a marker of ovulation in the light of our finding of prolonged follicular phases with baseline obesity. Thus, a prolonged intermenstrual interval may not reflect anovulation, analogous to the unreliability of the self-report of regular menstrual cycles in the context of female hyperandrogenism, in which studies have shown that 20–50% of these self-reported regular cycles may be anovulatory (25–27).
Obesity is associated with increased length of menstrual cycles, primarily due to lengthening of the follicular phase, i.e. beyond 14 d (28, 29). The luteal phase is characterized by corpus luteum progesterone production, with a rise in serum and urinary progestins, and its duration is typically less variable than the follicular phase (30). However, the luteal phase may shorten with increasing BMI (31). The most detailed previous study of the effects of weight loss after bariatric surgery on the ovarian cycle noted an increase in luteal urinary Pd3G levels and a shortening of the time to peak levels, although both of these were less than in a control group of normal-weight women (9). We noted no change in the luteal phase function with weight loss, either by our primary outcome of peak urinary luteal progestin levels or other parameters of luteal function.
Despite a shortening of the follicular phase by 8–9 d, weight loss caused by bariatric surgery was not associated with significant changes in menstrual cycle (except at 6 months postoperatively) or luteal phase lengths or amount of vaginal bleeding. The failure to detect this at 12 months may be due to a limited sample size due to dropout from pregnancy and other reasons. The impact of a shortened follicular phase on fertility has been associated with varying outcomes in the literature, although most commonly it is associated with reduced fecundity, often in the context of ovarian aging. Khalil et al. (32, 33) reported lower fertility with longer and shorter follicular phases in intrauterine insemination. Shorter follicular phases in infertile women have been associated with lower pregnancy rates (34). Broom et al. (35) reported no link between fertility and follicular phase length. Given these inclusive reports, we may surmise that the shortening of the follicular phase with weight loss represented a normalization of this parameter rather than an abnormality.
The mechanism for a shorter follicular phase affecting fertility is beyond the scope of this study, although it could affect both the endometrium or oocyte quality. The possibility of increased fecundity in our cohort after bypass is suggested by the observation that five women achieved pregnancy spontaneously in our cohort. Yet ovulation rates started and remained high and mostly unaffected by weight loss and most who conceived (80%) had done so in the past. In fact, endocrine outcomes of menstrual cycle function were similar between women who became pregnant and those who did not. The most significant change in reproductive function was the marked improvement in sexual function as detected by the FSFI, similar to that noted by other groups (8). This was independent of changes in hormonal parameters and body composition. We did not track intercourse or the individual desire to conceive during this study, but improved sexual desire may have led to increased frequency of intercourse. Furthermore, it is possible that other previously unexplored factors, such as loss of perineal fat allowing for deeper vaginal penetration during intercourse and a shorter distance of the ejaculate to the oocyte, may also be critical factors for pregnancy.
Our subjects had evidence of abdominal obesity, which has been associated with a relative hyperandrogenism due to increased testosterone levels and suppressed SHBG levels (36). We noted a modest improvement in biochemical hyperandrogenemia but no significant changes in clinical hyperandrogenism despite massive weight loss and preferential loss of central obesity. Furthermore, the rapid increase in circulating SHBG is analogous to immediate improvements in glucose and insulin levels in type 2 diabetes after gastric bypass, (37) and are likely related to these changes (38, 39).
Our study has several potential weaknesses. These include our limitation of studying only one type of bariatric surgery procedure (i.e. Roux en Y), subject dropout, lack of a control group, a focus on surrogate outcomes of reproduction rather than pregnancy and live birth, and our interesting findings of urinary creatinine. We narrowed our study to Roux en Y bypass surgery because the effects on weight loss, satiety, and endocrine function may vary between bariatric procedures, and we wanted to eliminate this confounder (40). Furthermore, this is the most commonly performed bariatric procedure in the United States. Dropout was due both to noncompliance and unexpected pregnancy. Our retention rates were, however, superior to other detailed studies in the field, and our duration of 2 yr follow-up exceeds comparable studies (9). We did not have a control group because our study was designed such that each subject would serve as her own control. A randomized trial would offer a better design, but there are few adequate randomized studies of bariatric surgery (41).
One of the strengths of our study are the well-validated urinary steroid assays, especially our Pd3G assay, which underwent intensive validation including correlation with other urinary assays, serum levels, and ultrasound monitoring of cycle changes (20). More frequent appropriately timed serum hormonal measures and ultrasound determinations of follicular development would have added further support to our urinary findings of frequent ovulation, but we deemed the additional study visits to be an undue burden to subjects in the immediate postoperative recovery period and to those traveling a long distance for their care. This assay in diverse female populations has reliably measured appropriate profiles of Pd3G in many hundreds of menstrual cycles that range from ovulatory to anovulatory (19, 21–23, 42).
Urinary hormone levels were corrected for urine flow rate using creatinine concentrations, which doubled within 1 month after surgery but returned to presurgical levels by 24 months, and may have confounded our results. We suspect this postoperative increase in creatinine levels may be due to a combination of reduced hydration secondary to gastric restriction and/or increased catabolism of fat and lean muscle mass during the period of exponential weight loss (43). If creatinine production increased due to heightened catabolism, this could potentially mask endocrine patterns after adjustment. Importantly, however, endocrine indices remained unaffected by surgery at 24 months after surgery when urinary creatinine levels had returned to presurgical levels. Assay specificity characteristics make it unlikely that measurement of creatinine was biased by a urine matrix effect that may have potentially occurred due to altered metabolism after surgery (44).
In conclusion, we found the effects of weight loss on reproductive function to be more modest than we hypothesized. Ovulation rates and the quality of ovulation appear to be relatively unaffected by morbid obesity or body composition, severe caloric restriction after gastric bypass surgery, or long-term weight loss. Thus, in terms of ovulation, there appears to be no fertile window in relation to the surgery, but rather the door appears open at all times. Furthermore, hyperandrogenism changes minimally. Sexual function, in comparison, improves significantly. Future studies should aim to more carefully study sexual function or other mechanisms of improved fertility in women who undergo bariatric surgery. In the interim, our study suggests that pregnancy is possible in all women considering or undergoing gastric bypass surgery.
Supplementary Material
Acknowledgments
In addition to the authors, we acknowledge the study coordinator, Sandra Eyer, who conducted the study with care. We also thank Chris Hamilton in the Core Endocrine Laboratory for his expertise in running the serum assays and Christina Stetter for assisting in statistical analyses and graphics. In particular, we are grateful to the women who volunteered for this study. The findings and conclusions in this report are those of the authors and do not necessarily represent views of the National Institute for Occupational Safety and Health.
This project is funded, in part, under a grant from the Pennsylvania Department of Health using Tobacco CURE Funds, by a Clinical and Translational Science Award from the National Center for Research Resources and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1RR033184 and a National Institutes of Health Construction Grant C06 RR016499 (to Pennsylvania State University). The funding agencies specifically disclaim responsibility for any analyses, interpretations, or conclusions.
Disclosure Summary: R.S.L., W.C.D., C.L.G., E.F.K., A.M.R., and R.S.H. have nothing to declare. S.J.E. reported consultancy for Intuitive Surgical (da vinci) and Hologic (Myosure) and has received royalties as author for UptoDate, Inc. A.R.K. has reported an investment in Merck stock. J.W.M. and J.S.K. received monies paid to the Centers for Disease Control Foundation to support sample analysis. R.N.C. has reported honorarium for a lecture on how gastric bypass surgery improves diabetes to a research group.
For editorial see page 4352
- BMI
- Body mass index
- DXA
- dual-energy x-ray absorptiometry
- E13G
- estrone 3-glucuronide
- FSFI
- Female Sexual Function Index
- Pd3G
- pregnanediol 3-glucuronide.
References
- 1. Brewer CJ, Balen AH. 2010. The adverse effects of obesity on conception and implantation. Reproduction 140:347–364 [DOI] [PubMed] [Google Scholar]
- 2. Kuchenbecker WK, Groen H, Zijlstra TM, Bolster JH, Slart RH, van der Jagt EJ, Kobold AC, Wolffenbuttel BH, Land JA, Hoek A. 2010. The subcutaneous abdominal fat and not the intraabdominal fat compartment is associated with anovulation in women with obesity and infertility. J Clin Endocrinol Metab 95:2107–2112 [DOI] [PubMed] [Google Scholar]
- 3. Rausch ME, Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, Steinkampf MP, McGovern PG, Cataldo NA, Gosman GG, Nestler JE, Giudice LC, Leppert PC, Myers ER, Coutifaris C. 2009. Predictors of pregnancy in women with polycystic ovary syndrome. J Clin Endocrinol Metab 94:3458–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R. 2011. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod 26:245–252 [DOI] [PubMed] [Google Scholar]
- 5. Anderson K, Norman RJ, Middleton P. 2010. Preconception lifestyle advice for people with subfertility. Cochrane Database of Syst Rev 14:CD008189. [DOI] [PubMed] [Google Scholar]
- 6. Johnson NP, Stewart AW, Falkiner J, Farquhar CM, Milsom S, Singh VP, Okonkwo QL, Buckingham KL. 2010. PCOSMIC: a multi-centre randomized trial in women with PolyCystic Ovary Syndrome evaluating Metformin for Infertility with Clomiphene. Hum Reprod 25:1675–1683 [DOI] [PubMed] [Google Scholar]
- 7. Bellver J, Martínez-Conejero JA, Labarta E, Alamá P, Melo MA, Remohi J, Pellicer A, Horcajadas JA. 2011. Endometrial gene expression in the window of implantation is altered in obese women especially in association with polycystic ovary syndrome. Fertil Steril 95:2335–2341, 2341.e1–e8 [DOI] [PubMed] [Google Scholar]
- 8. Bond DS, Wing RR, Vithiananthan S, Sax HC, Roye GD, Ryder BA, Pohl D, Giovanni J. 2011. Significant resolution of female sexual dysfunction after bariatric surgery. Surg Obes Relat Dis 7:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rochester D, Jain A, Polotsky AJ, Polotsky H, Gibbs K, Isaac B, Zeitlian G, Hickmon C, Feng S, Santoro N. 2009. Partial recovery of luteal function after bariatric surgery in obese women. Fertil Steril 92:1410–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maggard MA, Yermilov I, Li Z, Maglione M, Newberry S, Suttorp M, Hilton L, Santry HP, Morton JM, Livingston EH, Shekelle PG. 2008. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA 300:2286–2296 [DOI] [PubMed] [Google Scholar]
- 11. Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, Sancho J, San Millán JL. 2005. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 90:6364–6369 [DOI] [PubMed] [Google Scholar]
- 12. Eid GM, Cottam DR, Velcu LM, Mattar SG, Korytkowski MT, Gosman G, Hindi P, Schauer PR. 2005. Effective treatment of polycystic ovarian syndrome with Roux-en-Y gastric bypass. Surg Obes Relat Dis 1:77–80 [DOI] [PubMed] [Google Scholar]
- 13. NIH conference 1991. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 115:956–961 [PubMed] [Google Scholar]
- 14. Legro RS, Chiu P, Kunselman AR, Bentley CM, Dodson WC, Dunaif A. 2005. Polycystic ovaries are common in women with hyperandrogenic chronic anovulation but do not predict metabolic or reproductive phenotype. J Clin Endocrinol Metab 90:2571–2579 [DOI] [PubMed] [Google Scholar]
- 15. Thiboutot D, Zaenglein A, Weiss J, Webster G, Calvarese B, Chen D. 2008. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol 59:792–800 [DOI] [PubMed] [Google Scholar]
- 16. Ladson G, Dodson WC, Sweet SD, Archibong AE, Kunselman AR, Demers LM, Williams NI, Coney P, Legro RS. 2011. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril 95:1059–1066.e1–e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. 1996. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes 45:633–638 [DOI] [PubMed] [Google Scholar]
- 18. Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, D'Agostino R, Jr, 2000. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 26:191–208 [DOI] [PubMed] [Google Scholar]
- 19. Legro RS, Pauli JG, Kunselman AR, Meadows JW, Kesner JS, Zaino RJ, Demers LM, Gnatuk CL, Dodson WC. 2008. Effects of continuous versus cyclical oral contraception: a randomized controlled trial. J Clin Endocrinol Metab 93:420–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kesner JS, Knecht EA, Krieg EF, Jr, Barnard G, Mikola HJ, Kohen F, Gani MM, Coley J. 1994. Validations of time-resolved fluoroimmunoassays for urinary estrone 3-glucuronide and pregnanediol 3-glucuronide. Steroids 59:205–211 [DOI] [PubMed] [Google Scholar]
- 21. Reutman SR, LeMasters GK, Kesner JS, Shukla R, Krieg EF, Jr, Knecht EA, Lockey JE. 2002. Urinary reproductive hormone level differences between African American and Caucasian women of reproductive age. Fertil Steril 78:383–391 [DOI] [PubMed] [Google Scholar]
- 22. Baird DD, Weinberg CR, Zhou H, Kamel F, McConnaughey DR, Kesner JS, Wilcox AJ. 1999. Preimplantation urinary hormone profiles and the probability of conception in healthy women. Fertil Steril 71:40–49 [DOI] [PubMed] [Google Scholar]
- 23. Venners SA, Liu X, Perry MJ, Korrick SA, Li Z, Yang F, Yang J, Lasley BL, Xu X, Wang X. 2006. Urinary estrogen and progesterone metabolite concentrations in menstrual cycles of fertile women with non-conception, early pregnancy loss or clinical pregnancy. Hum Reprod 21:2272–2280 [DOI] [PubMed] [Google Scholar]
- 24. Scibora LM, Ikramuddin S, Buchwald H, Petit MA. 2012. Examining the link between bariatric surgery, bone loss, and osteoporosis: a review of bone density studies. Obes Surg 22:654–667 [DOI] [PubMed] [Google Scholar]
- 25. Carmina E. 1998. Prevalence of idiopathic hirsutism. Eur J Endocrinol 139:421–423 [DOI] [PubMed] [Google Scholar]
- 26. Carmina E, Lobo RA. 1999. Do hyperandrogenic women with normal menses have polycystic ovary syndrome? Fertil Steril 71:319–322 [DOI] [PubMed] [Google Scholar]
- 27. Azziz R, Waggoner WT, Ochoa T, Knochenhauer ES, Boots LR. 1998. Idiopathic hirsutism: an uncommon cause of hirsutism in Alabama. Fertil Steril 70:274–278 [DOI] [PubMed] [Google Scholar]
- 28. Rowland AS, Baird DD, Long S, Wegienka G, Harlow SD, Alavanja M, Sandler DP. 2002. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology 13:668–674 [DOI] [PubMed] [Google Scholar]
- 29. Kato I, Toniolo P, Koenig KL, Shore RE, Zeleniuch-Jacquotte A, Akhmedkhanov A, Riboli E. 1999. Epidemiologic correlates with menstrual cycle length in middle aged women. Eur J Epidemiol 15:809–814 [DOI] [PubMed] [Google Scholar]
- 30. Lenton EA, Landgren BM, Sexton L. 1984. Normal variation in the length of the luteal phase of the menstrual cycle: identification of the short luteal phase. Br J Obstet Gynaecol 91:685–689 [DOI] [PubMed] [Google Scholar]
- 31. Santoro N, Lasley B, McConnell D, Allsworth J, Crawford S, Gold EB, Finkelstein JS, Greendale GA, Kelsey J, Korenman S, Luborsky JL, Matthews K, Midgley R, Powell L, Sabatine J, Schocken M, Sowers MF, Weiss G. 2004. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: the Study of Women's Health across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab 89:2622–2631 [DOI] [PubMed] [Google Scholar]
- 32. Khalil MR, Rasmussen PE, Erb K, Laursen SB, Rex S, Westergaard LG. 2001. Homologous intrauterine insemination. An evaluation of prognostic factors based on a review of 2473 cycles. Acta Obstet Gynecol Scand 80:74–81 [DOI] [PubMed] [Google Scholar]
- 33. Khalil MR, Rasmussen PE, Erb K, Laursen SB, Rex S, Westergaard LG. 2001. Intrauterine insemination with donor semen. An evaluation of prognostic factors based on a review of 1131 cycles. Acta Obstet Gynecol Scand 80:342–348 [DOI] [PubMed] [Google Scholar]
- 34. Check JH, Adelson H, Lurie D, Jamison T. 1992. Effect of the short follicular phase on subsequent conception. Gynecol Obstet Invest 34:180–183 [DOI] [PubMed] [Google Scholar]
- 35. Broom TJ, Matthews CD, Cooke ID, Ralph MM, Seamark RF, Cox LW. 1981. Endocrine profiles and fertility status of human menstrual cycles of varying follicular phase length. Fertil Steril 36:194–200 [DOI] [PubMed] [Google Scholar]
- 36. Pasquali R, Casimirri F, Balestra V, Flamia R, Melchionda N, Fabbri R, Barbara L. 1991. The relative contribution of androgens and insulin in determining abdominal body fat distribution in premenopausal women. J Endocrinol Invest 14:839–846 [DOI] [PubMed] [Google Scholar]
- 37. Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. 2009. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122:248–256.e5 [DOI] [PubMed] [Google Scholar]
- 38. Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, Clore JN, Blackard WG. 1991. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab 72:83–89 [DOI] [PubMed] [Google Scholar]
- 39. Ding EL, Song Y, Malik VS, Liu S. 2006. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 295:1288–1299 [DOI] [PubMed] [Google Scholar]
- 40. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. 2004. Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 41. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. 2012. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 366:1577–1585 [DOI] [PubMed] [Google Scholar]
- 42. Cragin LA, Kesner JS, Bachand AM, Barr DB, Meadows JW, Krieg EF, Reif JS. 2011. Menstrual cycle characteristics and reproductive hormone levels in women exposed to atrazine in drinking water. Environ Res 111:1293–1301 [DOI] [PubMed] [Google Scholar]
- 43. Tamboli RA, Hossain HA, Marks PA, Eckhauser AW, Rathmacher JA, Phillips SE, Buchowski MS, Chen KY, Abumrad NN. 2010. Body composition and energy metabolism following Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 18:1718–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Serpa Neto A, Bianco Rossi FM, Dal Moro Amarante R, Alves Buriti N, Cunha Barbosa Saheb G, Rossi M. 2009. Effect of weight loss after Roux-en-Y gastric bypass, on renal function and blood pressure in morbidly obese patients. J Nephrol 22:637–646 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.