Abstract
Context:
Despite tremendous interest in hypoparathyroidism, large cohort studies describing typical treatment patterns, laboratory parameters, and rates of complications are lacking.
Objective:
Our objective was to characterize the course of disease in a large cohort of hypoparathyroid patients.
Design and Setting:
We conducted a chart review of patients with permanent hypoparathyroidism identified via a clinical patient data registry. Patients were seen at a Boston tertiary-care hospital system between 1988 and 2009.
Patients:
We identified 120 patients. Diagnosis was confirmed by documented hypocalcemia with a simultaneous low or inappropriately normal PTH level for at least 1 yr. Mean age at the end of the observation period was 52 ± 19 (range 2–87) yr, and the cohort was 73% female.
Main Outcome Measure:
We evaluated serum and urine laboratory results and renal and brain imaging.
Results:
We calculated time-weighted average serum calcium measurements for all patients. The time-weighted average for calcium was between 7.5 and 9.5 mg/dl for the majority (88%) of patients. Using linear interpolation, we estimated the proportion of time within the target calcium range for each patient with a median of 86% (interquartile range 67–98%). Of those with a 24-h urine collection for calcium (n = 53), 38% had at least one measurement over 300 mg/d. Of those with renal imaging (n = 54), 31% had renal calcifications, and 52% of those with head imaging (n = 31) had basal ganglia calcifications. Rates of chronic kidney disease stage 3 or higher were 2- to 17-fold greater than age-appropriate norms.
Conclusions:
Hypoparathyroidism and its treatment carry a large burden of disease. Renal abnormalities are particularly common.
Hypoparathyroidism is a rare disorder characterized by low or inappropriately normal levels of PTH in the setting of hypocalcemia (1, 2). The most common cause of hypoparathyroidism is inadvertent damage or removal of the parathyroid glands during thyroidectomy (3). Rarer causes include parathyroidectomy, autoimmunity, DiGeorge syndrome, mitochondrial disease, and mutations in the calcium-sensing receptor (CaSR), the transcription factor glial cells missing B, and PTH itself (1, 4–7).
Hypoparathyroidism is one of few endocrine disorders not currently treated by replacement of the missing hormone. Standard treatment consists of vitamin D analogs and calcium supplementation. This regimen, however, can lead to hypercalciuria, because PTH insufficiency impairs renal calcium reabsorption. The goal of treatment, thus, is to maintain serum calcium at the low end of the normal range. Although undertreatment can cause symptomatic hypocalcemia, overtreatment and associated hypercalciuria can cause nephrolithiasis, nephrocalcinosis, and renal insufficiency (8, 9).
A few cross-sectional studies have examined serum and urine biochemical values in cohorts of patients with hypoparathyroidism either on therapy (10–12) or at presentation (13). However, few longitudinal studies are available to assess rates of complications. Here, we present results for a longitudinal cohort of 120 patients with hypoparathyroidism of diverse etiologies treated within a single tertiary-care hospital system. We performed chart reviews and describe patients' biochemical parameters and rates of complications including symptomatic hypocalcemia, hypercalciuria, and renal disease.
Patients and Methods
Patients
We identified patients with permanent hypoparathyroidism seen at least once at Massachusetts General Hospital or Brigham and Women's Hospital from 1988 through 2009. Patients were included if they had documented hypocalcemia with a simultaneous low or inappropriately normal PTH level and disease persisting for more than 1 yr. Patients were identified using the Research Patient Data Registry, comprised of electronic medical data for more than 4.5 million patients treated within the Partners Healthcare System (14). We initially queried the database for all patients with an International Classification of Diseases (ICD)-9 diagnosis of hypoparathyroidism (252.1), identifying 1178 patients. Detailed chart reviews of a subset of these patients revealed that only approximately 25% had permanent hypoparathyroidism by the criteria above. We thus restricted this query to patients with at least one recorded PTH level no higher than 25 pg/ml, identifying 409 patients; chart reviews found 167 patients (41%) with permanent hypoparathyroidism. Patients with equivocal diagnoses were reviewed by two authors (D.M.M. and M.M.) and a consensus diagnosis made. Because terminal illnesses can have profound effects on calcium and phosphorus values, patients who died before the end of the study period were excluded (n = 29). Cause of death was recorded in 20 patients and was unrelated to hypoparathyroidism. Patients were also excluded if their records contained minimal information relevant to their hypoparathyroidism (n = 10) or if they had metastatic malignancy or renal failure (unrelated to hypoparathyroidism) due to an anticipated independent effect on mineral ion metabolism (n = 8). Our final cohort thus included 120 patients. Two patients were seen only once within our system but had extensive medical documentation including previous laboratory testing and were thus included. The remaining 118 patients were seen at least twice within our system. This study was approved by the Partners Human Research Committee.
Data collection
Clinic notes and hospital discharge summaries were reviewed to determine the onset and etiology of disease, medications, and adverse events. Data were censored before the onset of disease, defined as at birth for patients with congenital hypoparathyroidism, at the date of surgery for patients with postsurgical hypoparathyroidism, and at the onset of symptoms for patients with other acquired forms of hypoparathyroidism. Laboratory data obtained at outside facilities but noted in the medical record were included. Radiological reports including renal/abdominal ultrasounds, abdominal computed tomography (CT) scans, and head CT scans were reviewed. Findings were confirmed by independent review by one member of the study team (M.R.C.).
Statistical analyses
Data are presented as mean ± sd unless not normally distributed, in which case median and interquartile range (IQR) are reported. Because serum calcium and phosphorus levels were obtained at variable intervals, time-weighted averages (avgtw) were computed assuming a linear trend between measurements, calculating the area under the curve using the trapezoidal rule, and dividing by the time between the first and last measurements (15). Calcium-phosphate product calculations were limited to patients over 12 yr of age due to physiologically higher levels of phosphorus in children. To calculate the number of days during which calcium was out of the target range, we assumed a linear trend between calcium measurements and computed when the interpolated line crossed the indicated thresholds. Intervals of more than 12 months between calcium measurements (5% of 4671 total observed intervals) were censored because the assumption of linear change over large intervals becomes increasingly inaccurate. The proportions of observed time with low, target-range, and high serum calcium did not differ significantly whether or not intervals longer than 12 months were included. Correlations of serum and urine calcium values included varying numbers of observations per patient and were adjusted for repeated measures using a random-effects generalized least-squares regression. Estimated glomerular filtration rates (eGFRs) were calculated using the Modification of Diet in Renal Disease (MDRD) equation (16), and univariate regressions were performed with the following predictors: age, duration of disease, avgtw serum calcium, avgtw serum phosphorus, average calcium-phosphate product, and estimated proportion of time with serum calcium over 9.5 mg/dl. Predictors with a univariate P < 0.1 were entered into a multivariate linear regression model for eGFR using backward stepwise selection. Rates of chronic kidney disease stage 3 or higher were compared with age-matched normative values from the National Health and Nutrition Examination Survey with one-sample t tests. Statistical analyses were performed using Stata version 11 (StataCorp., College Station, TX).
Results
Cohort description
A total of 120 patients with permanent hypoparathyroidism were identified. The mean length of follow-up was 7.4 ± 5.1 yr, calculated as the interval between earliest and latest visit based on billing codes. Each patient had at least one PTH level measured with a median value of 8 pg/ml and an IQR of 5–14 pg/ml. There was no significant association of PTH values with serum calcium levels (data not shown). Table 1 describes the demographic makeup and the etiologies and duration of disease in the cohort. Most of the cohort had postsurgical hypoparathyroidism. Other etiologies included parathyroid autoimmunity (four patients with confirmed AIRE mutations and five patients clinically diagnosed with autoimmunity), genotyped activating mutations of the CaSR, and complex genetic syndromes. Twenty-three patients had no identified etiology for their disease (idiopathic), none of whom had recorded genetic testing.
Table 1.
Demographics of patient cohort
| n = 120 for cohort | |
|---|---|
| Age at end of study period (yr) | 52 ± 19 (2–87) |
| Sex | |
| Male | 32 (27%) |
| Female | 88 (73%) |
| Race | |
| White | 97 (81%) |
| Black | 6 (5%) |
| Hispanic | 6 (5%) |
| Asian | 3 (3%) |
| Other/not recordeda | 8 (7%) |
| Age at onset of hypoparathyroidism (yr) | 35 ± 21 (0–76) |
| Duration of hypoparathyroidism (yr) | 17 ± 16 (1–59) |
| Etiology | |
| Total acquired | 107 (89%) |
| Postsurgical | 79 (66%) |
| Autoimmune | 9 (7%) |
| Idiopathic (acquired) | 19 (16%) |
| Total congenital | 13 (11%) |
| Activating CaSR mutation | 5 (4%) |
| DiGeorge syndrome | 3 (3%) |
| Kearns-Sayre syndrome | 1 (1%) |
| Idiopathic (congenital) | 4 (3%) |
Data are presented as mean ± sd (range) for age at end of study period, age at onset, and duration of hypoparathyroidism. Data are presented as n (percent) for subgroup totals.
Includes American Indian (n = 1), other (n = 1), and not recorded (n = 6).
Medical therapy
Table 2 describes the prescribed medications at each patient's last recorded clinic or hospital visit. The majority of patients were taking calcium supplements with a median frequency of three times daily. Of the seven patients not taking calcium, three were maintaining serum calcium levels with calcitriol and dietary intake alone, three declined recommended supplements, and one had mistakenly discontinued calcium under the direction of a nonendocrine healthcare provider. The majority were also prescribed calcitriol. A minority was prescribed high-dose vitamin D2 or D3. Two patients were taking teriparatide: one after a renal transplant for nephrocalcinosis in an attempt to reduce hypercalciuria and the other for treatment of glucocorticoid-associated osteoporosis. Twenty percent of patients were taking a thiazide diuretic at their last recorded visit; we were unable to distinguish whether this was prescribed for hypertension, hypercalciuria, or both. No patients were taking phosphate binders other than calcium carbonate. Hypoparathyroidism was managed by an endocrinologist for 108 patients (90%) and by a primary care provider or other specialist for 12 patients (10%).
Table 2.
Medication usage
| n (%) | Dosea | |
|---|---|---|
| Medication | ||
| Calcium supplementation (mg/d) | 113 (94) | 2048 ± 1507 (mode, 1500; range, 300–9450) |
| High-dose vitamin D (IU/wk) | 7 (6) | 135,714 ± 149,204 (mode, 50,000; range, 50,000–400,000) |
| Calcitriol (μg/d)b | 106 (88) | 0.50 ± 0.44 (mode, 0.25; range, 0.125–4.00) |
| Teriparatide (μg/d) | 2 (2) | Patient 74, 40 μg 5 d/wk, 60 μg 2 d/wk; patient 101, 20 μg daily |
| Thiazide | 24 (20) | |
| Hydrochlorothiazide (mg/d) | 23 (19) | 31 ± 15 (mode, 25; range, 12.5–50) |
| Chlorthalidone (mg/d) | 1 (1) | 25 |
| Medication management | ||
| Prescriber | ||
| Endocrinologist | 108 (90) | |
| Primary care physician | 12 (10) |
Excluding pediatric patients (<18 yr, n = 5).
Of patients prescribed calcitriol, 62% were taking it once daily, 27% twice daily, 5% three times daily, 2% four times daily, and 4% with alternating regimens.
Serum calcium and phosphorus levels
The median interval over which calcium values were observed for each patient was 7.0 yr (IQR 3.6–13.5 yr). The median number of serum calcium observations per patient was 23 (IQR 12–49) with a median frequency of 3.7 times per year (IQR 2.3–7.8). The median number of phosphorus observations per patient was 13 (IQR 5–23) with a median frequency of 2.6 observations per year (IQR 1.4–6.9).
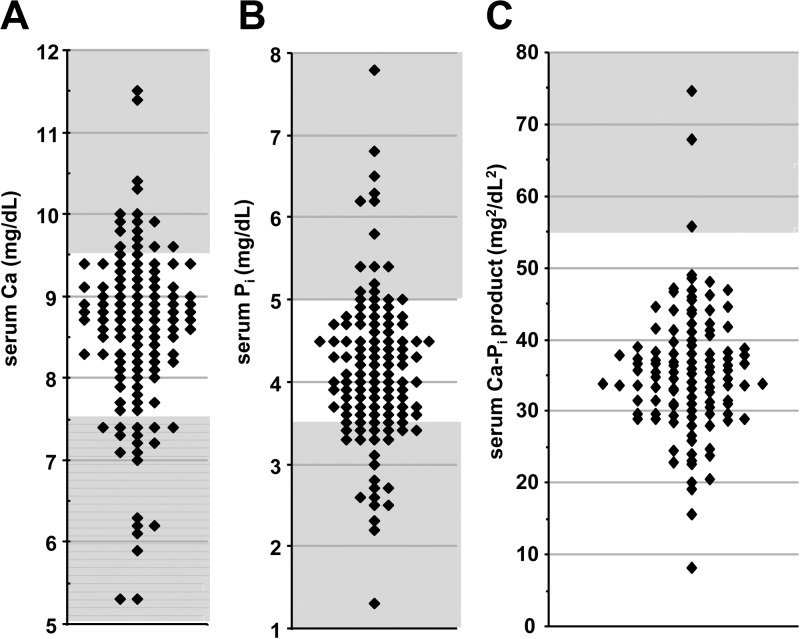
For patients with hypoparathyroidism, experts recommend a goal serum calcium in the low-normal range and a calcium-phosphate product of less than 55 mg2/dl2 (1, 2). Figure 1, A and B, shows the distribution of the most recently measured calcium and phosphorus levels, respectively. Mean calcium was 8.6 ± 1.1 (range 5.3–11.5) mg/dl and mean phosphorus was 4.2 ± 0.9 (range 1.3–7.8) mg/dl. For the purpose of subsequent analyses, we defined a target serum calcium range as 7.5–9.5 mg/dl, which encompasses the lower half of the reference range as well as mild hypocalcemia. The most recently measured calcium level was within the target range for 71% of patients, below the target for 16%, and above the target for 13%. Two percent of patients were frankly hypercalcemic (>10.5 mg/dl). Simultaneous calcium and phosphorus levels were available in 114 patients over 12 yr of age. As shown in Fig. 1C, the average most recently measured calcium-phosphate product was 35.4 ± 9.0 mg2/dl2 with three patients (3%) having values over 55 mg2/dl2. Over the duration of the study, 25 patients (22%) had at least one recorded calcium-phosphate product over 55 mg2/dl2.
Fig. 1.
Distribution plot of the most recently recorded serum calcium (A), serum phosphorus (B), and serum calcium-phosphate product (C) for each patient in the cohort. Unshaded areas represent target ranges for each measurement.
To further evaluate calcium and phosphorus values, we used linear interpolation to determine avgtw and to calculate the proportion of time spent in the target range for each patient (see Patients and Methods). Avgtw calcium was 8.4 ± 0.7 (range 5.6–10.0) mg/dl, and avgtw phosphorus was 4.2 ± 0.8 (range 2.9–7.8) mg/dl. Avgtw calcium differed by disease etiology (P for ANOVA < 0.001). Specifically, postsurgical patients had higher avgtw calcium (8.6 ± 0.5 mg/dl) than autoimmune patients (7.9 ± 1.1 mg/dl) and patients with CaSR mutations (7.6 ± 0.3 mg/dl) (P = 0.04 and P = 0.02 for pair-wise comparisons, respectively, after Bonferroni correction). There was no difference in avgtw phosphorus by etiology of disease.
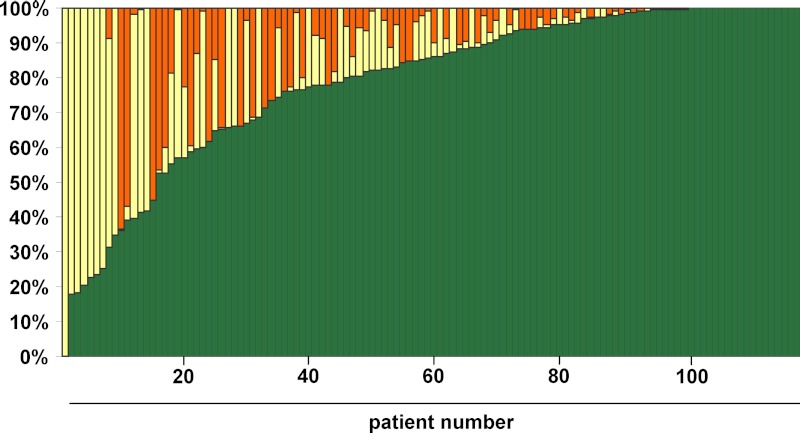
Figure 2 shows the estimated proportion of time spent with serum calcium within, below, and above the target range (7.5–9.5 mg/dl) for each patient. The estimated proportion of time in the target range had a median of 86% (IQR 67–98%). The mean proportion of time in the target range varied by disease etiology (P = 0.02 for ANOVA) and was highest in postsurgical patients (84%), intermediate in autoimmune patients (65%), and lowest in patients with CaSR mutations (55%). These pair-wise differences were not significant after correction for multiple testing. There were 19 recorded 25-hydroxyvitamin D levels greater than 100 ng/ml, 18 of which were in the context of taking high-dose vitamin D. Three episodes of mild hypercalcemia (range 10.2–10.6 mg/dl) were associated with these elevated 25-hydroxyvitamin D levels.
Fig. 2.
Plot of proportion of observed time with serum calcium between 7.5 and 9.5 mg/dl (green), <7.5 mg/dl (yellow), or >9.5 mg/dl (orange). Each column represents one patient in the cohort.
Urine calcium levels
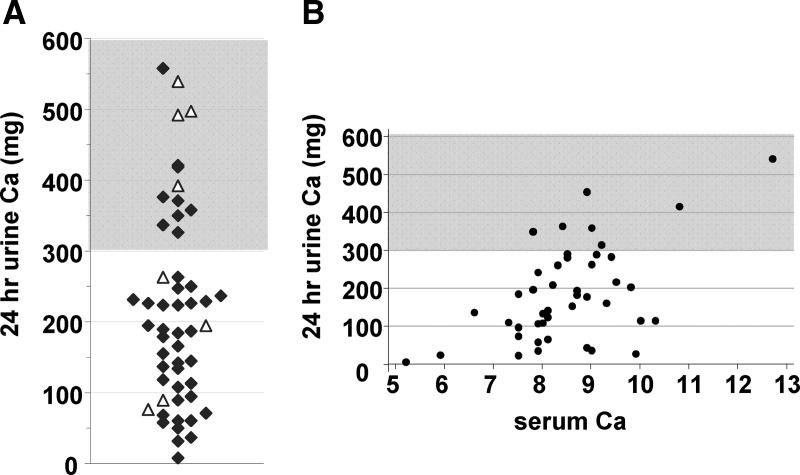
Fifty-three patients (44% of cohort) had at least one 24-h urine calcium collection. Figure 3A shows the distribution of the most recent 24-h urine calcium values. After excluding one outlier with elevated urinary calcium (1980 mg) in the setting of sepsis and multiple iv calcium boluses, the mean 24-h urine calcium level was 216 ± 140 (range 8–557) mg. Of the most recent measurements, 14 (26% of measured) were hypercalciuric with urinary calcium over 300 mg (1, 2). Overall, 20 patients (38% of those measured) had at least one 24-h urine calcium over 300 mg. Patients prescribed a thiazide diuretic had higher urinary calcium levels than those not prescribed a thiazide (mean 318 vs. 197 mg, P = 0.02).
Fig. 3.
Urine calcium parameters. A, Distribution plot of the most recently recorded 24-h urine calcium value (n = 53); ▵, patients prescribed a thiazide medication. B, Scatterplot of all 24-h urine calcium levels with serum calcium levels recorded the same day (n = 44, representing 22 patients). Unshaded areas represent target range (<300 mg).
We identified serum calcium levels drawn within 24 h of a 24-h urine calcium collection (n = 44) (Fig. 3B). We excluded patients prescribed a thiazide as they would be expected to have relatively lower urine calcium levels for a given serum calcium level (17). No patient with a CaSR mutation had a 24-h urine collection. As expected, higher serum calcium levels were associated with higher urine calcium values (increase of 50 mg/d urine calcium for each increase in serum calcium of 1 mg/dl, P < 0.001 after correction for repeated measures). However, there was wide variation, and five of the seven observed urine calcium levels over 300 mg were associated with low-normal serum calcium levels.
Renal complications
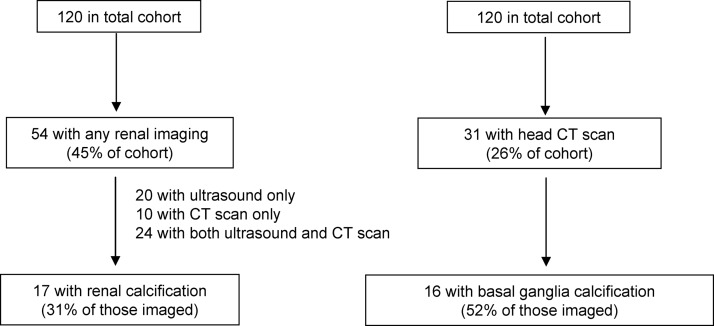
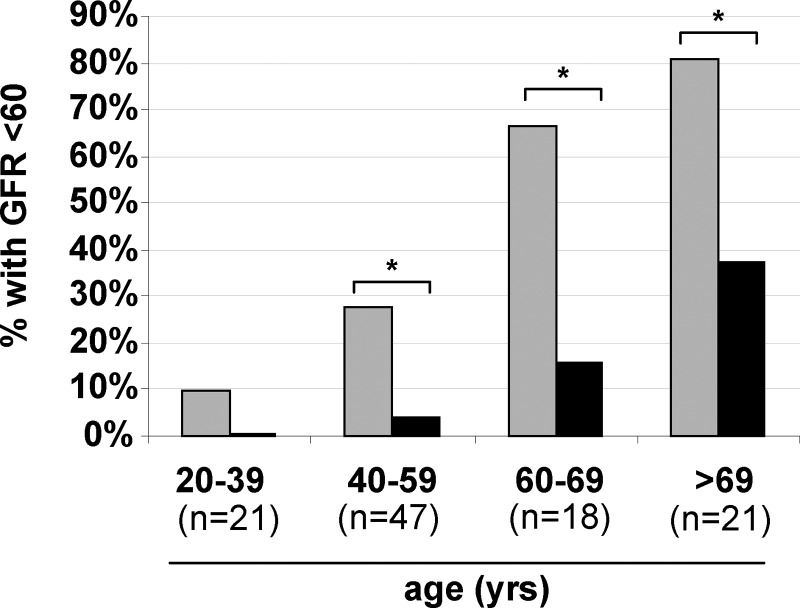
Two patients in our cohort required renal transplant due to nephrocalcinosis: one at age 23 with congenital idiopathic hypoparathyroidism and one at age 71 with acquired idiopathic hypoparathyroidism of 11 yr duration. To investigate the effect of hypoparathyroidism on renal physiology and function, we examined radiological data for evidence of renal calcification. As noted in Fig. 4, 54 patients had renal imaging at least once, of whom 17 (31% of the subgroup with imaging) had either renal stones or nephrocalcinosis. Of these 17, five were symptomatic. We calculated eGFRs after excluding patients less than 18 yr old (for whom this calculation is not valid) and those with previous renal transplant, leaving a final group of 107 (16). Overall, 44 (41%) had an eGFR of 60 ml/min · 1.73 m2 or below, consistent with chronic kidney disease stage 3 or higher. We compared rates of eGFR of 60 ml/min · 1.73 m2 or below with age-matched norms from National Health and Nutrition Examination Survey 1999–2006 (18) and found significantly higher rates of renal impairment in our cohort as compared with healthy controls (Fig. 5). In univariate analyses, age, duration of disease, avgtw calcium, and estimated proportion of time with serum calcium higher than 9.5 mg/dl (relative hypercalcemia) were each negatively correlated with eGFR. Avgtw phosphorus and average calcium-phosphate product were not correlated with eGFR (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). After entering these predictors into a multivariate regression model, age, duration of disease, and proportion of time with relative hypercalcemia remained significantly associated with eGFR (Supplemental Table 1). In the subsets of patients with available data, no association was seen between eGFR and either urine calcium values or presence of renal calcification.
Fig. 4.
Flowchart representing number of patients with renal and head imaging and the number with renal and basal ganglia calcifications.
Fig. 5.
Proportion of patients with eGFR below 60 ml/min · 1.73 m2 by age group. Gray columns, hypoparathyroid cohort; black columns, population norms. *, P < 0.001 for comparison by one-sample t test.
Other complications
Forty-two patients (33%) required at least one emergency department visit or hospital admission for complications of hypoparathyroidism, including eight (7%) during the last year of observation and 16 (13%) during the last 2 yr. Reasons for visits included symptomatic hypocalcemia (62% of visits), symptomatic hypercalcemia (12%), and other causes (26%), including renal stones, complications of hemodialysis, and complications of iv calcium extravasation. After excluding the first 30 d after diagnosis, 25 patients (21%) ever required emergency treatment of hypocalcemia. Ten patients (8%) had a report of a hypocalcemic seizure, including eight (7%) after diagnosis and initiation of therapy. One patient had dilated cardiomyopathy attributed to chronic hypocalcemia. As shown in Fig. 4, 16 of 31 patients with brain imaging were found to have basal ganglia calcification (BGC). After exclusion of one pediatric patient due to physiologically higher serum phosphorus levels in children, avgtw serum phosphorus was quantitatively higher in patients with BGC compared with those without BGC (4.4 ± 0.7 vs. 4.0 ± 0.9), but this difference was not statistically significant.
Skeletal health
A subset of 42 patients had bone mineral density evaluations by dual-energy x-ray absorptiometry. As has been previously reported, the z-scores of these patients were significantly higher than population norms by one-sample t test (11), with means ranging from 0.9 ± 1.4 for the total hip (n = 38, P < 0.001) to 2.4 ± 1.4 for the lateral spine (n = 11, P < 0.001). Twenty-one patients sustained a total of 44 reported fractures: 12 vertebral, eight rib, five ankle, five digit, five arm, four metatarsal, two nose, and one each wrist, hip, and clavicle. Two traumatic incidents (a fall down stairs and a motor vehicle rollover) accounted for 10 of these fractures.
Discussion
Our cohort of 120 patients with hypoparathyroidism is one of the largest to date and includes a comprehensive description of treatment regimens, laboratory parameters, and complications. The longitudinal nature of our study is distinct from previously published cohorts and thus gives new insight into the frequency of hypocalcemic and hypercalcemic episodes. Studies have been hindered by the relative rarity of this disorder. In a preliminary report of the Rochester Epidemiology Project, the prevalence of hypoparathyroidism was found to be 37 in 100,000, which would translate to approximately 100,000 patients in the United States (19). Overall, our analysis demonstrates a high rate of complications of hypoparathyroidism, including symptomatic episodes of both hypocalcemia and hypercalcemia and significant rates of renal calcification and impairment. These complications were observed despite the fact that serum calcium was maintained between 7.5 and 9.5 mg/dl on average 86% of the time. Note that a calcium level of 9.5 mg/dl is high for most patients with hypoparathyroidism.
Similar to previous reports, the predominant cause of hypoparathyroidism in our cohort was postsurgical (11, 19). Our patients with idiopathic disease may have had an identifiable disorder had genetic testing been undertaken. Medication regimens were also similar to those described previously (10, 11) with most patients requiring multiple medications at frequent intervals.
Treatment of hypoparathyroid patients with calcium and vitamin D analogs increases the risk of hypercalciuria; hypercalciuria is a risk factor for nephrocalcinosis, nephrolithiasis, and impaired renal function. The rates of these complications, however, have been difficult to estimate given the lack of large natural history studies. In one cross-sectional study of 25 patients with postsurgical hypoparathyroidism, 23% had 24-h urine calcium excretion higher than 320 mg and 8% had asymptomatic nephrolithiasis noted on renal ultrasound (10). Renal function was normal in all patients. In another cross-sectional study of 33 patients with hypoparathyroidism of diverse etiologies, 15% reported a history of nephrolithiasis (11). Higher rates of renal complications have been observed in patients participating in clinical studies at the National Institutes of Health (20–22). In one National Institutes of Health cohort (n = 27), 41% had nephrocalcinosis and 33% had an eGFR below 60 ml/min · 1.73 m2 (22). Our data are longitudinal and, thus, distinct from these previous studies. Notably, only a minority of our patients had a recorded 24-h urine calcium measurement, which may reflect the absence of formal treatment guidelines.
BGC is a well-known complication of hypoparathyroidism (23). The prevalence of BGC in our cohort was high at 52% of the small subset with imaging to review. Previously reported rates of BGC in hypoparathyroidism have varied widely. In one cohort of 33 patients, 12% had BGC (11). In another cohort of 25 patients with CaSR mutations, 36% had BGC (12). In neither of these studies were patients systematically screened for BGC. In contrast, in a cohort of 145 patients with nonsurgical hypoparathyroidism, all of whom had CT scans, 74% had BGC (13). The prevalence of BGC in the general population is not well established, but estimates are significantly lower at 2–12.5% (24, 25). Intriguingly, a mutation in a type III sodium-phosphate transporter leading to impaired cellular uptake of inorganic phosphate has recently been shown to be a cause of familial idiopathic BGC (26). This finding suggests that extracellular accumulation of phosphate in the setting of chronic hyperphosphatemia may contribute to BGC in hypoparathyroidism.
Several groups are investigating the use of PTH replacement therapy for hypoparathyroidism; this approach may reduce treatment-associated hypercalciuria and renal complications. Initial data have been promising using either full-length PTH or the N-terminal 1–34 fragment (20–22, 27–31). Our data may provide a benchmark with which to compare complication rates with future therapies.
Our study has limitations. Our search criteria likely did not capture all patients with hypoparathyroidism in the database. In particular, patients with PTH values measured before 1988 or at an outside facility would have been missed. Additionally, patients assigned alternate billing codes such as hypocalcemia (ICD-9 275.41) or disorder of calcium metabolism (ICD-9 275.40) would not be included. Patients in our cohort were seen at a tertiary academic medical system and thus may have been more severely affected with higher rates of complications than the overall population (referral bias). Because this is a retrospective chart review, testing was obtained at the discretion of medical providers. Thus, patients with more severe disease may have been monitored more intensively, leading to a higher apparent rate of complications due to observation bias. There were few pediatric patients, restricting the generalizability of our findings to adults. Finally, comparison of laboratory values over a 21-yr time span might be affected by changes in assay technology and performance. That being said, we would not anticipate calcium or phosphorus values to be significantly affected by interval laboratory changes. Indeed, there were no meaningful differences in serum calcium or phosphorus values observed over time (data not shown).
Overall, the striking proportion of patients with renal disease in the present study suggests that monitoring and optimizing therapy to preserve renal function is of critical importance for patients with hypoparathyroidism. The association of relative hypercalcemia with impaired renal function reinforces the existing recommendations to maintain serum calcium in the low-normal range. Although our data do not formally establish an association between urinary calcium excretion and renal impairment, this is a plausible link, and hypercalciuria is also the most likely cause of the high prevalence of renal calcification. We believe that careful monitoring and adjustment of treatment to reduce urinary calcium excretion is a key part of the medical management of these patients. Because risk of nephrolithiasis increases linearly with increasing urinary calcium (32), avoiding urinary excretion of more than 300 mg/d may be insufficient. Monitoring for renal calcification through renal ultrasound and more formal measurements of creatinine clearance may be warranted. In summary, our study finds a high rate of complications in patients with chronic hypoparathyroidism and underscores the need for large, prospective longitudinal studies of this population as well as for therapies that are more effective in preventing disease complications.
Supplementary Material
Acknowledgments
We thank Harald Jüppner, M.D., Robert Neer, M.D., and Henry Kronenberg, M.D., for helpful comments on data analysis and preparation of the manuscript.
This research was supported by National Institutes of Health Grants K08DK081669-01 to M.M. and T32DK007028-37 to D.M.M.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- avgtw
- Time-weighted averages
- BGC
- basal ganglia calcification
- CaSR
- calcium-sensing receptor
- CT
- computed tomography
- eGFR
- estimated glomerular filtration rate
- IQR
- interquartile range.
References
- 1. Shoback D. 2008. Clinical practice. Hypoparathyroidism. N Engl J Med 359:391–403 [DOI] [PubMed] [Google Scholar]
- 2. Bilezikian JP, Khan A, Potts JT, Jr, Brandi ML, Clarke BL, Shoback D, Jüppner H, D'Amour P, Fox J, Rejnmark L, Mosekilde L, Rubin MR, Dempster D, Gafni R, Collins MT, Sliney J, Sanders J. 2011. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res 26:2317–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marx SJ. 2000. Hyperparathyroid and hypoparathyroid disorders. N Engl J Med 343:1863–1875 [DOI] [PubMed] [Google Scholar]
- 4. Parkinson DB, Thakker RV. 1992. A donor splice site mutation in the parathyroid hormone gene is associated with autosomal recessive hypoparathyroidism. Nat Genet 1:149–152 [DOI] [PubMed] [Google Scholar]
- 5. Pearce SH, Williamson C, Kifor O, Bai M, Coulthard MG, Davies M, Lewis-Barned N, McCredie D, Powell H, Kendall-Taylor P, Brown EM, Thakker RV. 1996. A familial syndrome of hypocalcemia with hypercalciuria due to mutations in the calcium-sensing receptor. N Engl J Med 335:1115–1122 [DOI] [PubMed] [Google Scholar]
- 6. Thakker RV. 2001. Genetic developments in hypoparathyroidism. Lancet 357:974–976 [DOI] [PubMed] [Google Scholar]
- 7. Ding C, Buckingham B, Levine MA. 2001. Familial isolated hypoparathyroidism caused by a mutation in the gene for the transcription factor GCMB. J Clin Invest 108:1215–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Santos F, Chan JC. 1986. Idiopathic hypoparathyroidism: a case study on the interactions between exogenous parathyroid hormone infusion and 1,25-dihydroxyvitamin D. Pediatrics 78:1139–1141 [PubMed] [Google Scholar]
- 9. Weber G, Cazzuffi MA, Frisone F, de Angelis M, Pasolini D, Tomaselli V, Chiumello G. 1988. Nephrocalcinosis in children and adolescents: sonographic evaluation during long-term treatment with 1,25-dihydroxycholecalciferol. Child Nephrol Urol 9:273–276 [PubMed] [Google Scholar]
- 10. Arlt W, Fremerey C, Callies F, Reincke M, Schneider P, Timmermann W, Allolio B. 2002. Well-being, mood and calcium homeostasis in patients with hypoparathyroidism receiving standard treatment with calcium and vitamin D. Eur J Endocrinol 146:215–222 [DOI] [PubMed] [Google Scholar]
- 11. Rubin MR, Dempster DW, Zhou H, Shane E, Nickolas T, Sliney J, Jr, Silverberg SJ, Bilezikian JP. 2008. Dynamic and structural properties of the skeleton in hypoparathyroidism. J Bone Miner Res 23:2018–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raue F, Pichl J, Dorr HG, Schnabel D, Heidemann P, Hammersen G, Jaursch-Hancke C, Santen R, Schofl C, Wabitsch M, Haag C, Schulze E, Frank-Raue K. 2011. Activating mutations in the calcium-sensing receptor: genetic and clinical spectrum in 25 patients with autosomal dominant hypocalcaemia: a German survey. Clin Endocrinol (Oxf) 75:760–765 [DOI] [PubMed] [Google Scholar]
- 13. Goswami R, Sharma R, Sreenivas V, Gupta N, Ganapathy A, Das S. 2012. Prevalence and progression of basal ganglia calcification and its pathogenic mechanism in patients with idiopathic hypoparathyroidism. Clin Endocrinol (Oxf) 77:200–206 [DOI] [PubMed] [Google Scholar]
- 14. Nalichowski R, Keogh D, Chueh HC, Murphy SN. 2006. Calculating the benefits of a Research Patient Data Repository. AMIA Annu Symp Proc 2006:1044. [PMC free article] [PubMed] [Google Scholar]
- 15. Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. 2003. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–931 [DOI] [PubMed] [Google Scholar]
- 16. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470 [DOI] [PubMed] [Google Scholar]
- 17. Porter RH, Cox BG, Heaney D, Hostetter TH, Stinebaugh BJ, Suki WN. 1978. Treatment of hypoparathyroid patients with chlorthalidone. N Engl J Med 298:577–581 [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clarke B, Leibson C, Emerson J, Ransom J, Lagast H, Co-morbid medical conditions associated with prevalent hypoparathyroidism: a population-based study. American Society of Bone and Mineral Research Annual Meeting, San Diego, CA, 2011, p S182 (Abstract SA0170) [Google Scholar]
- 20. Winer KK, Yanovski JA, Cutler GB., Jr 1996. Synthetic human parathyroid hormone 1–34 vs calcitriol and calcium in the treatment of hypoparathyroidism. JAMA 276:631–636 [PubMed] [Google Scholar]
- 21. Winer KK, Yanovski JA, Sarani B, Cutler GB., Jr 1998. A randomized, cross-over trial of once-daily versus twice-daily parathyroid hormone 1–34 in treatment of hypoparathyroidism. J Clin Endocrinol Metab 83:3480–3486 [DOI] [PubMed] [Google Scholar]
- 22. Winer KK, Ko CW, Reynolds JC, Dowdy K, Keil M, Peterson D, Gerber LH, McGarvey C, Cutler GB., Jr 2003. Long-term treatment of hypoparathyroidism: a randomized controlled study comparing parathyroid hormone-(1–34) versus calcitriol and calcium. J Clin Endocrinol Metab 88:4214–4220 [DOI] [PubMed] [Google Scholar]
- 23. Eaton LM, Camp JD, Love JG. 1939. Symmetric cerebral calcification, particularly of the basal ganglia, demonstrable roentgenographically. Arch Neurol Psychiatry 41:921–942 [Google Scholar]
- 24. Fénelon G, Gray F, Paillard F, Thibierge M, Mahieux F, Guillani A. 1993. A prospective study of patients with CT detected pallidal calcifications. J Neurol Neurosurg Psychiatry 56:622–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gomille T, Meyer RA, Falkai P, Gaebel W, Königshausen T, Christ F. 2001. [Prevalence and clinical significance of computerized tomography verified idiopathic calcinosis of the basal ganglia]. Radiologe 41:205–210 (German) [DOI] [PubMed] [Google Scholar]
- 26. Wang C, Li Y, Shi L, Ren J, Patti M, Wang T, de Oliveira JR, Sobrido MJ, Quintáns B, Baquero M, Cui X, Zhang XY, Wang L, Xu H, Wang J, Yao J, Dai X, Liu J, Zhang L, Ma H, Gao Y, Ma X, Feng S, Liu M, Wang QK, Forster IC, Zhang X, Liu JY. 2012. Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat Genet 44:254–256 [DOI] [PubMed] [Google Scholar]
- 27. Winer KK, Sinaii N, Peterson D, Sainz B, Jr, Cutler GB., Jr 2008. Effects of once versus twice-daily parathyroid hormone 1–34 therapy in children with hypoparathyroidism. J Clin Endocrinol Metab 93:3389–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Winer KK, Sinaii N, Reynolds J, Peterson D, Dowdy K, Cutler GB., Jr 2010. Long-term treatment of 12 children with chronic hypoparathyroidism: a randomized trial comparing synthetic human parathyroid hormone 1–34 versus calcitriol and calcium. J Clin Endocrinol Metab 95:2680–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rubin MR, Sliney J, Jr, McMahon DJ, Silverberg SJ, Bilezikian JP. 2010. Therapy of hypoparathyroidism with intact parathyroid hormone. Osteoporos Int 21:1927–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sikjaer T, Rejnmark L, Rolighed L, Heickendorff L, Mosekilde L. 2011. The effect of adding PTH(1–84) to conventional treatment of hypoparathyroidism: a randomized, placebo-controlled study. J Bone Miner Res 26:2358–2370 [DOI] [PubMed] [Google Scholar]
- 31. Winer KK, Zhang B, Shrader JA, Peterson D, Smith M, Albert PS, Cutler GB., Jr 2012. Synthetic human parathyroid hormone 1–34 replacement therapy: a randomized crossover trial comparing pump versus injections in the treatment of chronic hypoparathyroidism. J Clin Endocrinol Metab 97:391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Curhan GC, Willett WC, Speizer FE, Stampfer MJ. 2001. Twenty-four-h urine chemistries and the risk of kidney stones among women and men. Kidney Int 59:2290–2298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.