Abstract
Context:
Patients with congenital adrenal hyperplasia (CAH) often suffer from long-term complications secondary to chronic glucocorticoid therapy and suboptimal treatment regimens.
Objective:
The aim of the study was to describe clinical characteristics of a large cohort of pediatric and adult CAH patients.
Design and Setting:
We conducted a cross-sectional study of 244 CAH patients [183 classic, 61 nonclassic (NC)] included in a Natural History Study at the National Institutes of Health.
Main Outcome Measure(s):
Outcome variables of interest were height sd score, obesity, hypertensive blood pressure (BP), insulin resistance, metabolic syndrome, bone mineral density, hirsutism (females), and testicular adrenal rest (TART).
Results:
The majority had elevated or suppressed androgens, with varied treatment regimens. Mean adult height sd score was −1.0 ± 1.1 for classic vs. −0.4 ± 0.9 for NC patients (P = 0.015). Obesity was present in approximately one third of patients, across phenotypes. Elevated BP was more common in classic than NC patients (P ≤ 0.01); pediatric hypertensive BP was associated with suppressed plasma renin activity (P = 0.001). Insulin resistance was common in classic children (27%) and adults (38% classic, 20% NC); 18% of adults had metabolic syndrome. The majority (61%) had low vitamin D; 37% of adults had low bone mineral density. Hirsutism was common (32% classic; 59% NC women). TART was found in classic males (33% boys; 44% men).
Conclusions:
Poor hormonal control and adverse outcomes are common in CAH, necessitating new treatments. Routine monitoring of classic children should include measuring BP and plasma renin activity. Osteoporosis prophylaxis and TART screening should begin during childhood. A longitudinal study is under way.
Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive disorders characterized by a defect in cortisol biosynthesis (1). 21-Hydroxylase deficiency accounts for approximately 95% of the cases. There is a wide spectrum of phenotypes determined by the residual 21-hydroxylase activity, with a continuum between the classic or severe form and the mild nonclassic (NC) form. The incidence of classic CAH ranges from 1 in 10,000 to 20,000 live births worldwide and is subclassified into salt-wasting (SW) and simple virilizing (SV), reflecting the degree of aldosterone deficiency (2). The NC form is much more common, with an estimated prevalence of 1 in 1000 (3).
Clinical symptoms in CAH patients are due to a combination of the disease-related glucocorticoid and mineralocorticoid deficiencies, the accumulation of steroid precursors that are shunted into the androgen synthesis pathway resulting in androgen excess, and treatment-related side effects. By consensus, hydrocortisone is the treatment of choice for children (2), but several glucocorticoid preparations are used for adults (4). Optimal therapeutic regimens are difficult to achieve, and often short- and long-term complications related to glucocorticoid and/or androgen excess arise, reflecting over- or undertreatment.
The management of CAH patients changes throughout the life cycle. During childhood, the focus is on achieving normal growth and development, whereas the management of the adult is aimed at preventing long-term complications such as obesity, osteoporosis, and metabolic syndrome. We report here the clinical features of a large cross-sectional cohort of children and adults with CAH due to 21-hydroxylase deficiency.
Patients and Methods
Patients
In 2006, we began a Natural History Study of patients with androgen excess at the National Institutes of Health (NIH) Clinical Center (Bethesda, MD) (www.ClinicalTrials.gov Identifier no. NCT00250159). Here, we report clinical data from the baseline evaluation of 244 patients with 21-hydroxylase deficiency. Patients were recruited via listings on www.Clinicaltrials.gov, a national support group for patients and families with CAH (Congenital Adrenal Hyperplasia Research Education & Support Foundation) and via self-referral. Approximately 5% were physician referrals. Patient compensation was not provided, although the full evaluation was performed free of charge. All patients were examined by one of two pediatric endocrinologists (M.S.K. or D.P.M.). The diagnosis was confirmed with hormonal testing and genotyping (5). The study was approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. All adult patients and parents of participating children gave written informed consent, and all minors at least 8 yr old gave assent.
Phenotypic classification was determined based on clinical and hormonal criteria and review of medical records. Good genotype-phenotype correlation was observed and has been previously reported (5). Anthropometric measurements were obtained in the morning. Blood pressure (BP) was measured and repeated if elevated. Ferriman-Gallwey score was determined for postpubertal females, and a total score of 8 or more indicated hirsutism.
Assays
Morning fasting blood was collected before taking medications and was analyzed at the NIH Clinical Center Laboratory (Bethesda, MD), unless otherwise specified. Testosterone, ACTH, androstenedione (Mayo Medical Labs, Rochester, MN), and insulin were measured by chemiluminescence immunoassay; 17-hydroxyprogesterone (17-OHP) by liquid chromatography-tandem mass spectrometry (Mayo Medical Labs); plasma renin activity (PRA) by RIA (Mayo Medical Labs); resting catecholamines and metanephrines by HPLC; and vitamin D by liquid chromatography mass spectrometry. Free testosterone was calculated based on previously derived constants.
Radiological studies
A dual-energy x-ray absorptiometry scan was performed in 67 children (age, ≥8 yr) and 42 adults. Posterior-anterior spine (L1–L4), femoral, and whole-body bone mineral density (BMD) were assessed using a Hologic Densitometer QDR 4500 (Hologic, Bedford, MA). Left hand x-ray was obtained in patients over 2 yr old, and bone age was determined by the Greulich-Pyle method. Testicular sonography was performed in males starting at 4 yr of age.
Clinical data and definitions
Height was expressed as a sd score (SDS) based on National Health and Nutrition Examination Survey data (6), and adult height SDS was based on data at 20 yr. Predicted adult height was calculated according to the Bayley-Pinneau method. Corrected height SDS was defined as: final height SDS − midparental height SDS (7). Short stature was defined as height SDS no greater than −2.0. Pediatric patients with fused epiphyses were included in the adult cohort for stature analyses. Of the 149 pediatric patients who had open epiphyses, 25 were excluded from the stature analyses due to previous administration of GH or an aromatase inhibitor. Of the 95 patients who had reached adult height, eight were excluded due to previous administration of GH or an aromatase inhibitor.
Body mass index (BMI) percentile was calculated using U.S. anthropometric reference data (6). Obesity was defined as BMI in the 95th percentile or greater for children and BMI of at least 30 kg/m2 for adults.
For children, hypertensive BP was defined as systolic BP (SBP) or diastolic BP (DBP) in the 95th percentile or greater for age, sex, and height, and prehypertensive BP as SBP or DBP in the 90th to 95th percentiles (8). For adults, hypertensive BP was defined as SBP of at least 140 mm Hg or DBP of at least 90 mm Hg, and prehypertensive BP as SBP between 120 and 140 mm Hg or DBP between 80 to 90 mm Hg (9).
Glucocorticoid dose equivalencies were calculated based on their growth-suppressing effects in comparison to hydrocortisone. Prednisone dose was multiplied by 5 (10), and dexamethasone dose was multiplied by 80 (11).
17-OHP was considered suppressed if below 100 ng/dl. Androstenedione, testosterone, and PRA were categorized as suppressed, normal, and elevated based on the respective age- and sex-specific normal range.
Insulin sensitivity was assessed according to the homeostasis model assessment of insulin resistance (HOMA-IR) method, calculated as: insulin (μU/ml) × glucose (mmol/liter)/22.5 (12). Elevated HOMA-IR index was defined as above 2.5 in adults and above 3.16 in adolescents (13).
Vitamin D deficiency was defined as 25-hydroxyvitamin D no greater than 20 ng/ml, and insufficiency as 21–29 ng/ml (14).
For children, a modified Weiss (15) definition was used to identify patients at least 4 yr old with metabolic syndrome and included three or more of the following: fasting glucose of at least 100 mg/dl, high-density lipoprotein (HDL) below the fifth percentile (16), triglycerides above the 95th percentile (16), hypertensive BP, or BMI of at least 95%. For adults, metabolic syndrome was defined based on a modified International Diabetes Federation definition (17): fasting insulin in the 75th percentile or higher or fasting glucose of at least 100 mg/dl, plus two or more of the following: HDL below 40 mg/dl (males) or below 50 mg/dl (females) or on lipid-lowering drug; triglycerides of at least 150 mg/dl; SBP of at least 130 mm Hg or DBP of at least 80 mm Hg or on antihypertensive medication.
In children, low BMD was defined as a Z score of −2 sd or less at the lumbar spine and/or whole body adjusted for age, gender, and body size, as appropriate (18). For adults, osteopenia was defined as a T score of less than −1.0 and at least −2.5 sd, and osteoporosis was defined as a T score of −2.5 sd or less at the femoral neck according to World Health Organization criteria (19).
Statistical analysis
Data were described by frequency distributions or descriptive statistics and are reported as percentage or as mean ± sd. Differences between phenotypic groups were evaluated. Certain analyses combined the SW and SV groups if no differences were observed between them, and were compared with NC. Other outcome variables of interest were: height SDS, corrected height SDS, short stature, obesity, hypertensive BP, insulin resistance, metabolic syndrome, low BMD, irregular menses (females), hirsutism (females), and the presence of testicular adrenal rest (TART) (males). All continuous data were assessed for approximate normality; 17-OHP and androstenedione were log-transformed. Continuous data between and among groups were compared by either t tests or ANOVA or, if appropriate, by nonparametric Wilcoxon rank-sum or Kruskal-Wallis tests. Categorical data were compared by χ2 or Fisher's exact tests, as appropriate, and singly ordered categorical data were analyzed by the Kruskal-Wallis test. Correlation analyses between two sets of continuous data used Pearson's correlation coefficient or Spearman's rho, as appropriate. If ANOVA could not be used for pooling data, corrections for multiple comparisons were carried out by the Stepdown Bonferroni method, and corrected P values are reported. Analyses were performed separately for pediatric and adult cohorts. P values ≤0.05 were considered statistically significant. Data were analyzed using SAS system software version 9.2 (SAS Institute, Inc., Cary, NC).
Results
Patients
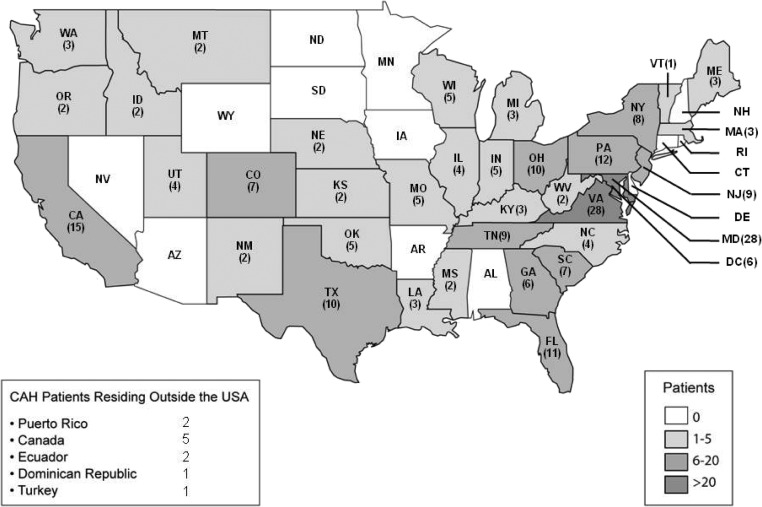
Patients were from 36 states and Puerto Rico, and nine patients were from other countries (Canada, Ecuador, Turkey, and Dominican Republic) (Fig. 1). The cohort included 170 children (ages 0.6 to 17 yr) and 74 adults, defined as age of at least 18 yr (18 to 68 yr) (Table 1).
Fig. 1.
Geographic distribution of the CAH patient population (n = 244).
Table 1.
Clinical characteristics of pediatric and adult patients with CAH due to 21-hydroxylase deficiency according to phenotype
| SW | SV | NC | |
|---|---|---|---|
| Pediatric patients | |||
| n | 97 | 42 | 31 |
| Female, n (%) | 44 (45.4) | 13 (31.0) | 21 (67.7) |
| Age (yr) | 8.0 ± 5.5 | 9.9 ± 4.6 | 10.5 ± 3.0 |
| Height SDS | −0.63 ± 1.1 | 0.76 ± 1.6 | 0.46 ± 1.4 |
| BMI percentile | 0.71 ± 0.3 | 0.84 ± 0.2 | 0.79 ± 0.2 |
| BA − CA (yr) | 0.81 ± 1.6 | 3.47 ± 2.7 | 2.36 ± 1.5 |
| Midparental height SDS | 0.22 ± 0.8 | 0.23 ± 0.98 | 0.29 ± 1.0 |
| Age at diagnosis (yr) | |||
| Females | 0 ± 0.3 | 0.9 ± 1.6 | 7.4 ± 3.6 |
| Males | 0 ± 0.2 | 3.9 ± 2.2 | 6.3 ± 2.7 |
| Hydrocortisone (mg/m2 · d)a | 14.0 ± 4.8 | 15.5 ± 5.5 | 10.5 ± 3.0 |
| Adult patients | |||
| n | 26 | 18 | 30 |
| Females, n (%) | 14 (53.8) | 11 (61.1) | 29 (96.7) |
| Age (yr) | 29.5 ± 12.5 | 34.3 ± 13.7 | 33.9 ± 13.0 |
| Height SDS | −1.0 ± 1.1 | −1.3 ± 1.1 | −0.46 ± 0.94 |
| BMI (kg/m2) | 30.6 ± 8.2 | 26.6 ± 4.7 | 26.1 ± 5.6 |
| Midparental height SDS | 0.18 ± 0.7 | −0.34 ± 0.8 | −0.11 ± 0.9 |
| Age at diagnosis (yr) | |||
| Females | 0 ± 0.03 | 1.4 ± 3.1 | 20.4 ± 11.9 |
| Males | 0 ± 0.2 | 3.2 ± 2.0 | 27.0b |
| Hydrocortisone (mg/m2 · d) | 16.4 ± 4.6 (n = 7) | 17.7 ± 6.9 (n = 7) | 16.6 ± 10.3 (n = 8) |
| Dexamethasone (μg/d) | 398 ± 164 (n = 10) | 362 ± 129 (n = 6) | 363 ± 150 (n = 10) |
| Prednisone (mg/d) | 6.5 ± 2.2 (n = 9) | 8.2 ± 7.3 (n = 5) | 6.3 ± 2.6 (n = 5) |
Values are expressed as means ± sd unless otherwise specified.
Patients receiving a long-acting glucocorticoid or no glucocorticoid therapy are omitted.
Due to n = 1, a sd could not be computed.
Treatment
As expected, the majority of children were receiving hydrocortisone (Fig. 2A), whereas adults were treated with a variety of medications. The mean glucocorticoid equivalence dose in classic patients was 15.0 ± 5.9 mg/m2 · d in children and 17.9 ± 7.6 mg/m2 · d in adults. For adult classic patients, treatment was similarly distributed between hydrocortisone, prednisone, and dexamethasone (Fig. 2A). Glucocorticoid was being given in a reverse circadian manner in 28% of patients, and 2% were receiving a combination of short-acting and long-acting glucocorticoids. Twenty-four percent of adult NC females were not receiving glucocorticoid therapy, 28% were on oral contraceptive pill, and 14% were on spironolactone.
Fig. 2.
Prevalence of treatment and hormonal characteristics according to phenotype in pediatric and adult patients with CAH due to 21-hydroxylase deficiency. A, Glucocorticoid therapy (HC, hydrocortisone; Pred, prednisone/prednisolone; Dex, dexamethasone). B, 17-OHP in nanograms per deciliter. To convert to SI units, multiply 17-OHP by 0.0302. C, Androstenedione. D, Testosterone. Suppressed, normal, and elevated levels are based on age- and sex-specific normal range.
Adrenal hormones
Overtreatment (suppressed adrenal hormones) and undertreatment (hyperandrogenism) were common in both pediatric and adult patients. Approximately 30% of classic patients (pediatric and adult) and half of the NC women were in acceptable hormonal control based on early morning 17-OHP between 100 and 1200 ng/dl (Fig. 2B). About half of the patients had either elevated or suppressed androstenedione (Fig. 2C). Testosterone was elevated for age and sex in approximately 15 to 20% of pediatric and adult patients (Fig. 2D). In addition, PRA was either elevated or suppressed in 44% of classic patients, especially in SW patients. In children, bone age advancement was associated with higher androstenedione (r2 = 0.456; P < 0.001). Bone age advancement was also associated with elevated testosterone levels (suppressed or normal vs. elevated, P = 0.02).
Significant differences were observed between classic and NC CAH patients for both epinephrine (pediatric, 15.0 ± 14.2 vs. 26.7 ± 17.2 pg/ml, P = 0.001; adult, 10.0 ± 6.5 vs. 15.3 ± 9.8 pg/ml, P = 0.021; normal range, 4–83 pg/ml) and free metanephrine (pediatric, 18.6 ± 12.3 vs. 36.2 ± 11.1 pg/ml, P < 0.001; adult, 16.2 ± 10.7 vs. 24.7 ± 11.1 pg/ml, P = 0.002; normal range, 12–61 pg/ml). As expected (20), SW patients had the lowest and NC patients had the highest values [patients with values below normal range (SW vs. SV vs. NC), pediatric—epinephrine, 12.7 vs. 6.1 vs. 3.9%; metanephrine, 47.6 vs. 17.6 vs. 4.2%; adults—epinephrine, 21.7 vs. 7.1 vs. 0%; metanephrine, 56.5 vs. 14.3 vs. 6.7%].
Stature
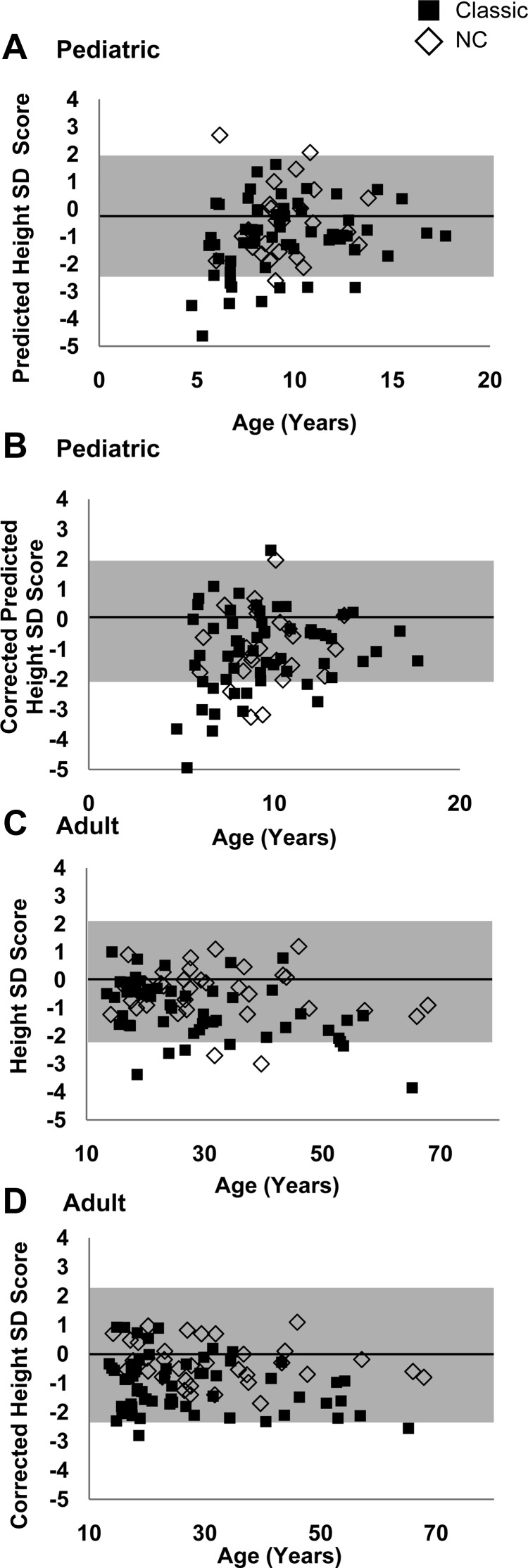
In children, 20% of classic and 7% of NC patients had a predicted adult height SDS of −2.0 or less (Fig. 3A). Children with predicted adult short stature were more likely to have lower midparental height Z scores (P = 0.001), have a larger discrepancy between bone age and chronological age (P < 0.001), and be receiving a higher glucocorticoid dose (P = 0.013). Lower corrected predicted height was not associated with sex, age, phenotype, bone age advancement, glucocorticoid type/dose, or age at diagnosis.
Fig. 3.
A, Predicted height SDS by age according to phenotype in pediatric patients with CAH. B, Corrected predicted height SDS by age according to phenotype in pediatric patients with CAH. C, Adult height SDS by age according to phenotype in patients with CAH. D, Corrected adult height SDS by age according to phenotype in patients with CAH. Normal range is marked in gray.
Mean adult height SDS was −1.0 ± 1.1 for classic vs. −0.4 ± 0.9 for NC patients (P = 0.015) (Fig. 3B); mean corrected height SDS was −1.1 ± 0.9 for classic vs. −0.3 ± 0.7 for NC patients (P < 0.001) (Fig. 3C). No differences were observed between the SW and SV groups. Lower adult height SDS was correlated with older age (r2 = −0.30; P = 0.006), especially in classic patients (r2 = −0.43; P = 0.001). Lower corrected adult height (Fig. 3C) was associated with male sex (P = 0.003) and the classic phenotype (P < 0.001), but not with glucocorticoid type/dose, age, or age at diagnosis.
Cardiovascular disease risk factors
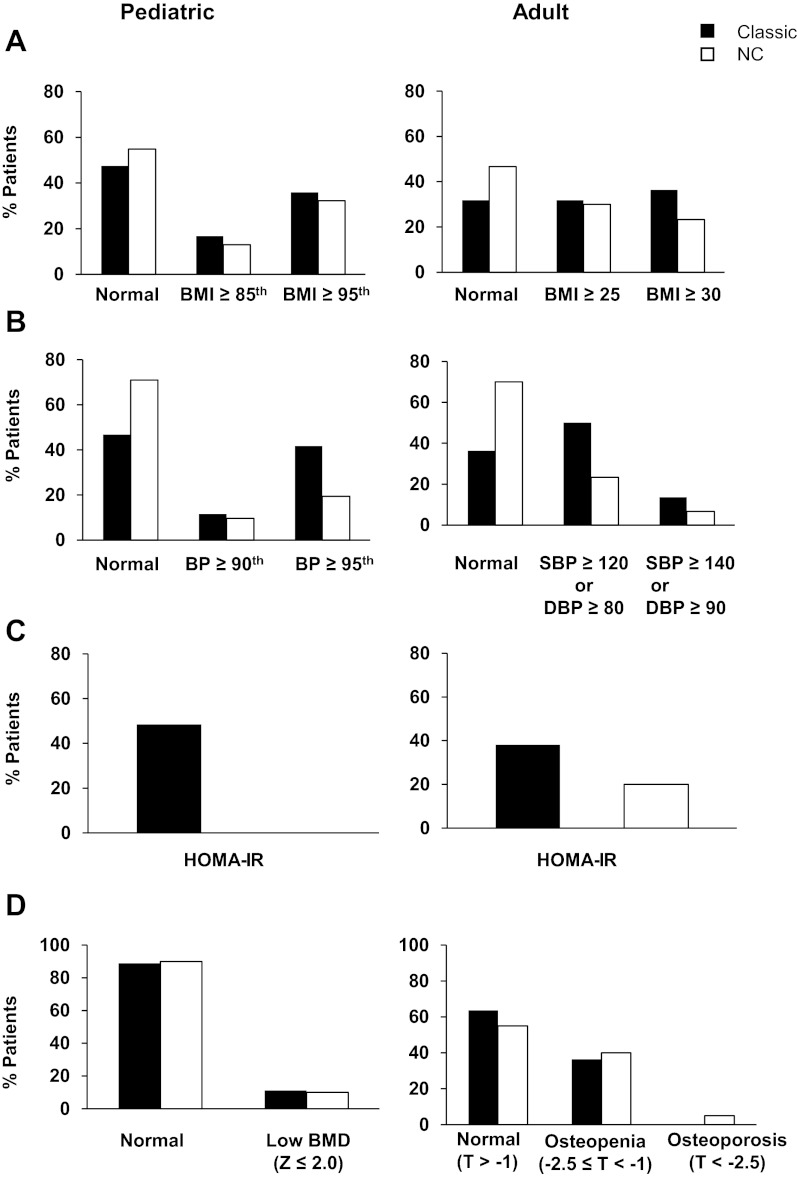
Obesity
Approximately 35% of children were obese, with no differences observed between the classic and NC groups (Fig. 4A). Obese compared with nonobese children were more likely to be on a long-acting glucocorticoid (P <0.05), but did not differ in glucocorticoid dose, family history of obesity, sex, or age. However, obese children had higher insulin (P < 0.001), HOMA-IR (P = 0.009), leptin (P < 0.001), 17-OHP (P = 0.04), and androstenedione (P = 0.002) than normal or overweight patients.
Fig. 4.
Prevalence of clinical characteristics according to phenotype in pediatric and adult patients with CAH due to 21-hydroxylase deficiency. A, BMI. B, BP, SBP, and DBP. C, Insulin resistance based on HOMA-IR (13); pediatric includes adolescents (≥13 yr). D, BMD; pediatric includes those at least 8 yr old.
Approximately one third of adults were obese, and one third had a normal BMI (Fig. 4A). In adults, obesity was associated with higher glucocorticoid dose (P = 0.004), but not type. Obese compared with nonobese adults had higher leptin (P < 0.001); however, no difference was found in CAH type, androgens, age, sex, or family history of obesity.
Blood pressure
Classic children were more likely to have elevated BP than NC children (P = 0.017) (Fig. 4B). In addition, in classic children, hypertensive BP was associated with younger age (P < 0.001) and suppressed PRA (P = 0.001), but not with fludrocortisone dose, obesity, or BMI.
In adults, elevated BP was more likely in classic than NC patients (P = 0.009). There was no difference between the SW and SV groups. In adults, factors associated with elevated BP were elevated 17-OHP (P = 0.009), and male sex (P = 0.017). No associations were found with obesity, fludrocortisone dose, BMI, PRA, or family history of hypertension.
Metabolic syndrome
Metabolic syndrome was observed in 18% of adults and associated with older age (P = 0.015); no association was found with androgens, glucocorticoid type, or dose. Metabolic syndrome was found in only one pediatric patient.
Insulin sensitivity, assessed by HOMA-IR, was more likely to be impaired in classic than in NC children (P = 0.004) (Fig. 4C). For children, 2% of patients had elevated cholesterol, whereas approximately 10% of both classic and NC patients had decreased HDL.
Insulin sensitivity did not differ between CAH types among adult patients (Fig. 4C). In adults, 6% had elevated cholesterol, whereas approximately 15% had decreased HDL.
Bone mineral density
The majority of children had normal BMD (Fig. 4D). Patients with a low BMD (Z score < −2) did not differ from patients with a normal BMD in terms of age, sex, CAH phenotype, or hydrocortisone dose; however, 17-OHP was lower (P = 0.007) in patients with low BMD.
Low BMD was a common finding in adult patients with both classic and NC CAH (Fig. 4D). Older age was associated with lower femoral neck T-score in classic adults (P = 0.007), but not in NC adults. No other associations were observed.
Vitamin D
In children, vitamin D deficiency was observed in 19% of classic patients and in none of the NC patients; insufficiency was found in 42% of classic and 67% of NC patients. In adults, vitamin D deficiency was found in 28% of classic and 11% of NC patients; insufficiency was found in 40% of classic and 39% of NC patients.
Female reproductive issues
In adult females, the majority of NC and SV women reported sexual activity (96 and 91%, respectively), whereas only 57% of SW women reported a history of ever having had a sexual partner. Of those women who were heterosexual and sexually active (eight SW, 10 SV, 25 NC), the number of women with one or more pregnancies was lowest for the SW group [SW, one (12.5%); SV, five (50%); NC: 12 (48%)], with 14 intentional pregnancies (SW, one; SV, two; NC, 11).
One third of women had irregular menses, with no differences between classic and NC groups. Women with irregular menses were more likely to have elevated 17-OHP (P = 0.008), androstenedione (P = 0.014), and testosterone (P = 0.028). History of irregular menses was not associated with hirsutism or elevated fasting insulin.
Hirsutism
Hirsutism was present in 46% of females and, although not statistically significant, was more common among NC women (32% classic vs. 59% NC; P = 0.06). Prior history of hirsutism was reported in 56% classic and 72% NC women. Many patients reported having received prior treatment for hirsutism.
Testicular adrenal rest
The prevalence of TART was 33% in boys and 44% in men with classic CAH. The youngest boy with TART was 4.1 yr old. None of the NC males had evidence of TART. Boys with TART were more likely to have higher androstenedione (P = 0.007). In classic men, TART was not associated with age at diagnosis, phenotype, adrenal hormones, or glucocorticoid type or dose.
Discussion
We performed comprehensive phenotyping of a large cohort of patients with CAH due to 21-hydroxylase deficiency enrolled in a Natural History Study at the NIH Clinical Center in Bethesda, Maryland. We report here the baseline evaluation of this cohort. Long-term adverse health outcomes were frequently observed in both pediatric and adult patients.
In this cross-sectional study of CAH patients, adult height was lower than the population norm. On average, classic patients lost approximately 7.5 cm of genetic height potential, whereas NC patients lost approximately 2 cm. Obesity and hypertension were common in children with both classic and NC CAH, whereas metabolic syndrome was commonly observed in adults. Low BMD was observed among adults, whereas the majority of children had normal BMD. Hyperandrogenism and decreased sexual activity were common findings in SW women, and TART was common among classic males, even prepubertal boys. Hirsutism was common in women of all subtypes.
In our pediatric cohort, patients were predominantly treated with hydrocortisone, as expected given the pediatric consensus statement endorsing the use of short-acting rather than longer-acting glucocorticoid for children with CAH (21). However, there is currently no standard regimen for treating CAH adults, which was reflected in the observed varied glucocorticoid regimens. Good hormonal control is difficult to achieve, especially in classic patients. Thus, glucocorticoid over- and undertreatment was observed. Our findings are in agreement with a cross-sectional study of adults with CAH in the United Kingdom in which varied glucocorticoid regimens and suboptimal hormonal control were reported (4).
Patients with CAH often fail to reach their midparental height as a result of hyperandrogenism, hypercortisolism, or a combination of these two undesirable states. Adult height of patients from our cohort was similar to reports from other centers. Mean adult height SDS achieved by our classic CAH patients was −1.0, with a mean corrected height SDS of −1.1; a meta-analysis of 35 studies of patients with classic CAH revealed a mean final height SDS of −1.38 and mean corrected height SDS of −1.03 (7). Approximately 20% of our patients had an adult or predicted adult height more than 2 SDS below the population mean, and height outcome was worse for classic males. Although we did not find a statistical association between age at diagnosis and height outcome, the fact that our cohort was mostly diagnosed before the initiation of neonatal screening, with late diagnosis of SV males, could have contributed to this finding. Final adult height has been negatively correlated with childhood glucocorticoid dosing (22). We were unable to evaluate lifetime exposure to glucocorticoid dosing due to the cross-sectional nature of our study.
Patients with CAH commonly have risk factors for cardiovascular disease including obesity, hypertension, and insulin resistance (23). These risk factors were especially prevalent in our pediatric patients. Approximately one third of our pediatric and adult patients of all CAH phenotypes were obese. This prevalence is increased compared with the U.S. population estimate of 17% childhood obesity but similar to the adult U.S. obesity rate of 36% (6). Some studies of CAH report a positive correlation between increasing BMI with age (24, 25), but this was not observed in our study. Our data support the contribution of glucocorticoid therapy to the development of obesity. Higher doses were associated with obesity in adults, and approximately 20% of adults had hormonal measurements consistent with overtreatment. Conversely, higher adrenal androgens were commonly observed in our pediatric obese patients, indicating the coexistence of both hyperandrogenism and hypercortisolism in a subgroup of difficult-to-treat children. Interestingly, family history of obesity was not associated with having obesity, confirming the importance of disease- or treatment-related factors.
Classic CAH children have been shown to have a higher prevalence of elevated SBP and absent physiological nocturnal SBP nadir on 24-h ambulatory monitoring (26, 27). Two thirds of children with SW CAH in our cohort had hypertensive BP, compared with the 3% estimate of children ages 8–17 yr in the United States with increased BP (28). In our study, there was no association between hypertensive BP and elevated BMI or fludrocortisone dose, but hypertension was associated with suppressed PRA in children, highlighting the importance of routine laboratory monitoring. There are minimal data on hypertension in adult patients with CAH; however, women with CAH have been found to have increased BP compared with controls (4, 29). In our adult cohort, hypertensive BP was most common in classic men, and nearly two thirds of classic CAH adults had an elevated BP, comparable to the estimated 29% hypertensive and 37% prehypertensive adults in the general U.S. population (28).
Patients with CAH have been reported to have increased prevalence of insulin resistance, a major component of the metabolic syndrome (29–32). Insulin sensitivity was decreased in children with classic CAH and adults of all phenotypes. Interestingly, approximately one third of NC adults with decreased insulin sensitivity were not receiving glucocorticoid therapy, similar to prior findings of insulin resistance in untreated NC women (31).
Chronic glucocorticoid therapy is a well-known risk factor for osteoporosis and a possible contributor to decreased BMD in CAH (33–35). An alarmingly high prevalence of vitamin D deficiency and insufficiency was found in our CAH cohort; 21% of classic and 7% of NC patients were deficient; 41% of classic and 48% of NC patients were insufficient. However, a similarly high prevalence of vitamin D deficiency has been described in the overall U.S. population, with 32% of the overall population having vitamin D deficiency (36). Thus, our finding of low vitamin D may reflect population trends rather than a disease-specific association. However, CAH patients are at risk for decreased BMD and our findings underscore the need for preventative measures. Osteoporosis prophylaxis including physical activity and calcium and vitamin D supplementation should be implemented at a young age.
Adult women with classic CAH, especially those with SW CAH, have been reported to be less sexually active and less likely to have children compared with the general population (37, 38). Similarly, we found that the majority of SV females were sexually active and experienced a number of successful pregnancies, whereas the SW women were less likely to have sexual relations, and only one had become pregnant.
In males, TART (39, 40) can eventually affect fertility and testicular functioning, with impaired spermatogenesis and Leydig cell failure (41). Similar to other studies (41, 42), we report a significant prevalence of TART by ultrasound in both our adult and pediatric cohorts with classic CAH.
Our study had limitations because patients came from multiple centers leading to heterogeneity in our study population. We report here patients' baseline evaluation; measurements are from one evaluation. Extensive medical records were not available for most patients; thus, we were unable to estimate long-term glucocorticoid exposure. An additional limitation of this study is the possible self-referral bias of a more motivated cohort than the general population of CAH patients.
Our study is unique in that comprehensive phenotyping was performed in a large cohort that spans a wide age range, from infancy to the elderly. Based on our findings and clinical experience with this large cohort of CAH patients, we recommend several clinical approaches. Routine monitoring of children with classic CAH should include measuring BP and PRA; fludrocortisone dose should be adjusted to maintain PRA in the mid-normal range. Glucocorticoid dose should be minimized to reduce risk of obesity and preserve BMD. Osteoporosis prophylaxis including physical activity and calcium and vitamin D supplementation should also be implemented at a young age, and screening dual-energy x-ray absorptiometry scans should begin in early adulthood. Screening testicular ultrasound should be performed in classic males beginning in childhood; NC males are not at risk for adrenal rest. NC CAH represents a mild form of the disease; adult height is mostly preserved; sexual function is normal; but insulin resistance is commonly found, even in those not receiving glucocorticoid therapy, and hirsutism is common among affected adult females. Our recommendations are in agreement with the 2010 Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline (2), but they also highlight the importance of BP monitoring, osteoporosis prophylaxis, obesity prevention, and TART screening—all of which should begin during childhood.
Difficulty achieving optimal metabolic control with the available therapeutic regimens is common. A long-term longitudinal study that aims to elucidate hormone-mediated and disease-specific phenotypic characterizations is currently under way. Such data are of major importance in improving the management of patients with CAH and in acquiring further knowledge for use in the design of novel therapeutic interventions that aim to improve patient outcome.
Acknowledgments
The authors are grateful to the patients and their parents for participating in this study. We thank numerous fellows and staff in the 1H Pediatric and OP9 Clinics and the adult and pediatric day hospitals at the National Institutes of Health for their assistance in the clinical evaluation of these patients.
This work was supported by the Intramural Research Programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institutes of Health Clinical Center; and by The Congenital Adrenal Hyperplasia Research, Education and Support Foundation.
Clinical Trial Registration Identifier no. NCT00250159; www.ClinicalTrials.gov.
Disclosure Summary: Authors have nothing to disclose.
Footnotes
- BMD
- Bone mineral density
- BMI
- body mass index
- BP
- blood pressure
- CAH
- congenital adrenal hyperplasia
- DBP
- diastolic BP
- HDL
- high-density lipoprotein
- HOMA-IR
- homeostasis model assessment of insulin resistance
- NC
- nonclassic
- 17-OHP
- 17-hydroxyprogesterone
- PRA
- plasma renin activity
- SBP
- systolic BP
- SDS
- sd score
- SV
- simple virilizing
- SW
- salt-wasting
- TART
- testicular adrenal rest.
References
- 1. Merke DP, Bornstein SR. 2005. Congenital adrenal hyperplasia. Lancet 365:2125–2136 [DOI] [PubMed] [Google Scholar]
- 2. Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC. 2010. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 95:4133–4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Speiser PW, Dupont B, Rubinstein P, Piazza A, Kastelan A, New MI. 1985. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet 37:650–667 [PMC free article] [PubMed] [Google Scholar]
- 4. Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, Han TS, Carroll PV, Conway GS, Rees DA, Stimson RH, Walker BR, Connell JM, Ross RJ. 2010. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab 95:5110–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, McDonnell NB, Merke DP. 2011. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 96:E161–E172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. 2009–2010. National Health and Nutrition Examination Survey. Centers for Disease Control and Prevention: National Center for Health Statistics. http://www.cdc.gov/nchs/data/factsheets/fact_sheet_obesity.pdf
- 7. Muthusamy K, Elamin MB, Smushkin G, Murad MH, Lampropulos JF, Elamin KB, Abu Elnour NO, Gallegos-Orozco JF, Fatourechi MM, Agrwal N, Lane MA, Albuquerque FN, Erwin PJ, Montori VM. 2010. Clinical review: adult height in patients with congenital adrenal hyperplasia: a systematic review and metaanalysis. J Clin Endocrinol Metab 95:4161–4172 [DOI] [PubMed] [Google Scholar]
- 8. Falkner B. 2005. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Bethesda, MD: National Heart Lung and Blood Institute; 1–48 [Google Scholar]
- 9. Chobanian A. 2004. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Heart Lung and Blood Institute; 1–86 [PubMed] [Google Scholar]
- 10. Hindmarsh PC. 2009. Management of the child with congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab 23:193–208 [DOI] [PubMed] [Google Scholar]
- 11. Rivkees SA, Crawford JD. 2000. Dexamethasone treatment of virilizing congenital adrenal hyperplasia: The ability to achieve normal growth. Pediatrics 106:767–773 [DOI] [PubMed] [Google Scholar]
- 12. Matsuda M, DeFronzo RA. 1999. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 13. Rodden AM, Diaz VA, Mainous AG, 3rd, Koopman RJ, Geesey ME. 2007. Insulin resistance in adolescents. J Pediatr 151:275–279 [DOI] [PubMed] [Google Scholar]
- 14. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. 2011. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 15. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. 2004. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350:2362–2374 [DOI] [PubMed] [Google Scholar]
- 16. Hickman TB, Briefel RR, Carroll MD, Rifkind BM, Cleeman JI, Maurer KR, Johnson CL. 1998. Distributions and trends of serum lipid levels among United States children and adolescents ages 4–19 years: data from the Third National Health and Nutrition Examination Survey. Prev Med 27:879–890 [DOI] [PubMed] [Google Scholar]
- 17. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. 2005. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 18. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy 2001. Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–79511176917 [Google Scholar]
- 19. 1994. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 843:1–129 [PubMed] [Google Scholar]
- 20. Verma S, Green-Golan L, VanRyzin C, Drinkard B, Mehta SP, Weise M, Eisenhofer G, Merke DP. 2010. Adrenomedullary function in patients with nonclassic congenital adrenal hyperplasia. Horm Metab Res 42:607–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. 2002. Consensus statement on 21-hydroxylase deficiency from the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. J Clin Endocrinol Metab 87:4048–4053 [DOI] [PubMed] [Google Scholar]
- 22. Grigorescu-Sido A, Bettendorf M, Schulze E, Duncea I, Heinrich U. 2003. Growth analysis in patients with 21-hydroxylase deficiency influence of glucocorticoid dosage, age at diagnosis, phenotype and genotype on growth and height outcome. Horm Res 60:84–90 [DOI] [PubMed] [Google Scholar]
- 23. Kim MS, Merke DP. 2009. Cardiovascular disease risk in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Semin Reprod Med 27:316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cornean RE, Hindmarsh PC, Brook CG. 1998. Obesity in 21-hydroxylase deficient patients. Arch Dis Child 78:261–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Völkl TM, Simm D, Beier C, Dörr HG. 2006. Obesity among children and adolescents with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics 117:e98–e105 [DOI] [PubMed] [Google Scholar]
- 26. Roche EF, Charmandari E, Dattani MT, Hindmarsh PC. 2003. Blood pressure in children and adolescents with congenital adrenal hyperplasia (21-hydroxylase deficiency): a preliminary report. Clin Endocrinol (Oxf) 58:589–596 [DOI] [PubMed] [Google Scholar]
- 27. Völkl TM, Simm D, Dötsch J, Rascher W, Dörr HG. 2006. Altered 24-hour blood pressure profiles in children and adolescents with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 91:4888–4895 [DOI] [PubMed] [Google Scholar]
- 28. Ostchega Y, Carroll M, Prineas RJ, McDowell MA, Louis T, Tilert T. 2009. Trends of elevated blood pressure among children and adolescents: data from the National Health and Nutrition Examination Survey1988–2006. Am J Hypertens 22:59–67 [DOI] [PubMed] [Google Scholar]
- 29. Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjöld A, Hagenfeldt K, Thorén M. 2007. Metabolic profile and body composition in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 92:110–116 [DOI] [PubMed] [Google Scholar]
- 30. Williams RM, Deeb A, Ong KK, Bich W, Murgatroyd PR, Hughes IA, Acerini CL. 2010. Insulin sensitivity and body composition in children with classical and nonclassical congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 72:155–160 [DOI] [PubMed] [Google Scholar]
- 31. Saygili F, Oge A, Yilmaz C. 2005. Hyperinsulinemia and insulin insensitivity in women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency: the relationship between serum leptin levels and chronic hyperinsulinemia. Horm Res 63:270–274 [DOI] [PubMed] [Google Scholar]
- 32. Charmandari E, Weise M, Bornstein SR, Eisenhofer G, Keil MF, Chrousos GP, Merke DP. 2002. Children with classic congenital adrenal hyperplasia have elevated serum leptin concentrations and insulin resistance: potential clinical implications. J Clin Endocrinol Metab 87:2114–2120 [DOI] [PubMed] [Google Scholar]
- 33. Bachelot A, Chakhtoura Z, Samara-Boustani D, Dulon J, Touraine P, Polak M. 2010. Bone health should be an important concern in the care of patients affected by 21 hydroxylase deficiency. Int J Pediatr Endocrinol pii:326275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elnecave RH, Kopacek C, Rigatto M, Keller Brenner J, Sisson de Castro JA. 2008. Bone mineral density in girls with classical congenital adrenal hyperplasia due to CYP21 deficiency. J Pediatr Endocrinol Metab 21:1155–1162 [DOI] [PubMed] [Google Scholar]
- 35. King JA, Wisniewski AB, Bankowski BJ, Carson KA, Zacur HA, Migeon CJ. 2006. Long-term corticosteroid replacement and bone mineral density in adult women with classical congenital adrenal hyperplasia. J Clin Endocrinol Metab 91:865–869 [DOI] [PubMed] [Google Scholar]
- 36. Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. 2011. Vitamin D status: United States, 2001–2006. NCHS Data Brief 59:1–8 [PubMed] [Google Scholar]
- 37. Gastaud F, Bouvattier C, Duranteau L, Brauner R, Thibaud E, Kutten F, Bougnères P. 2007. Impaired sexual and reproductive outcomes in women with classical forms of congenital adrenal hyperplasia. J Clin Endocrinol Metab 92:1391–1396 [DOI] [PubMed] [Google Scholar]
- 38. Jääskeläinen J, Hippeläinen M, Kiekara O, Voutilainen R. 2000. Child rate, pregnancy outcome and ovarian function in females with classical 21-hydroxylase deficiency. Acta Obstet Gynecol Scand 79:687–692 [PubMed] [Google Scholar]
- 39. Claahsen-van der Grinten HL, Hermus AR, Otten BJ. 2009. Testicular adrenal rest tumours in congenital adrenal hyperplasia. Int J Pediatr Endocrinol 2009:624823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Claahsen-van der Grinten HL, Otten BJ, Stikkelbroeck MM, Sweep FC, Hermus AR. 2009. Testicular adrenal rest tumours in congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab 23:209–220 [DOI] [PubMed] [Google Scholar]
- 41. Stikkelbroeck NM, Otten BJ, Pasic A, Jager GJ, Sweep CG, Noordam K, Hermus AR. 2001. High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J Clin Endocrinol Metab 86:5721–5728 [DOI] [PubMed] [Google Scholar]
- 42. Claahsen-van der Grinten HL, Sweep FC, Blickman JG, Hermus AR, Otten BJ. 2007. Prevalence of testicular adrenal rest tumours in male children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol 157:339–344 [DOI] [PubMed] [Google Scholar]