Abstract
Context:
Impaired vitamin D metabolism may contribute to the development and progression of diabetic kidney disease.
Objective:
The aim of the study was to test associations of circulating vitamin D metabolites with risks of incident microalbuminuria, impaired glomerular filtration rate (GFR), and hypertension in type 1 diabetes.
Design:
We performed a cohort study of 1193 participants in the Diabetes Control and Complications Trial (DCCT), a randomized clinical trial of intensive diabetes therapy, and its observational follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. We measured plasma concentrations of 25-hydroxyvitamin D [25(OH)D], 1,25-dihydroxyvitamin D, and 24,25-dihydroxyvitamin D by mass spectrometry at the end of the DCCT and tested associations with incident microalbuminuria, impaired GFR, and hypertension over up to 16 yr of EDIC follow-up.
Results:
At the time metabolites were measured, mean age was 32.4 yr; mean duration of diabetes, 7.5 yr; mean iothalamate GFR, 132.9 ml/min/1.73 m2; and geometric mean albumin excretion rate, 11.8 mg/24 h. Over follow-up, 166 cases of microalbuminuria, 54 cases of impaired GFR, and 541 cases of hypertension were observed. Compared with 25(OH)D of at least 30 ng/ml, 25(OH)D below 20 ng/ml was associated with a 65% higher risk of microalbuminuria (95% confidence interval, 7 to 154%) in adjusted analyses. Low concentrations of 24,25-dihydroxyvitamin D, but not 1,25-dihydroxyvitamin D, were also associated with increased risk of microalbuminuria. No circulating vitamin D metabolite was associated with risk of impaired GFR or hypertension.
Conclusions:
Low plasma concentrations of 25(OH)D and 24,25-dihydroxyvitamin D are associated with increased risk of microalbuminuria in type 1 diabetes. In contrast, we did not find evidence linking impaired vitamin D metabolism to early GFR loss or the development of hypertension.
In type 1 diabetes, microalbuminuria or impaired glomerular filtration rate (GFR) occurs in up to 40% of patients (1–4). These manifestations of diabetic kidney disease (DKD) are associated with hypertension and increased cardiovascular disease risk and can progress to end-stage renal disease (4–6). Intensive glucose control can prevent kidney disease, and inhibitors of the renin-angiotensin system (RAS) can slow its progression (7–10). However, renal complications remain an important cause of morbidity and mortality.
Impaired vitamin D metabolism may be a novel therapeutic target to prevent the development and progression of DKD. In animal models, including models of diabetic glomerulopathy, treatment with 1,25-dihydroxyvitamin D [1,25(OH)2D; the active vitamin D hormone] or its analogs prevents albuminuria, glomerulosclerosis, and progressive loss of kidney function (11–17). Mechanisms include suppression of the RAS, and effects are synergistic with RAS blockade (11–18). Among people with type 2 diabetes and urine albumin excretion of 100-3000 mg/g creatinine, 48 wk of treatment with paricalcitol, a 1,25(OH)2D analog, lowered albuminuria by 15% (19).
However, long-term effects of vitamin D interventions in humans remain untested, and it is not clear whether there is renal benefit to supplement forms of vitamin, i.e. vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) (20). Epidemiological studies demonstrate that low circulating concentrations of total 25-hydroxyvitamin D [25(OH)D], which reflect total intake of vitamins D3 and D2 from cutaneous synthesis and dietary consumption (21), are associated with increased risks of albuminuria, GFR loss, and hypertension (22–24). Such studies assessing vitamin D metabolism and DKD have been limited by cross-sectional design and measurement of single vitamin D metabolites.
We measured circulating concentrations of 25(OH)D, 1,25(OH)2D, and 24,25-dihydroxyvitamin D [24,25 (OH)2D, the most abundant product of 25(OH)D catabolism] in a well-characterized type 1 diabetes population using novel mass spectrometry methods. We hypothesized that lower plasma concentrations of 25(OH)D, 1,25(OH)2D, and 24,25(OH)2D—representing interrelated deficiencies in vitamin D intake, activation, and catabolism, respectively—would each be associated with increased risks of microalbuminuria, impaired GFR, and hypertension over long-term follow-up.
Subjects and Methods
Study population
The Diabetes Control and Complications Trial (DCCT) enrolled 1441 persons with type 1 diabetes from 1983–1989 to determine the effects of intensive diabetes therapy on the long-term complications of diabetes (25). Participants were randomly assigned to intensive diabetes therapy aimed at lowering glucose concentrations as close as safely possible to the normal range or to conventional therapy aimed at preventing symptoms of hyperglycemia and hypoglycemia. In 1994, after completion of the DCCT, 1375 participants (96% of the surviving cohort) agreed to participate in the Epidemiology of Diabetes Interventions and Complications Study (EDIC). During EDIC, diabetes therapy and glycemic control as measured by hemoglobin A1c became similar in the two original DCCT treatment groups, and yearly follow-up has continued through the present time (9). The current study includes all participants with available frozen plasma collected at or near the end of the DCCT (EDIC baseline), excluding seven participants who were pregnant at the time of plasma collection (n = 1193; 83% of randomized DCCT participants).
Circulating vitamin D metabolites
Concentrations of 25(OH)D, 1,25(OH)2D, and 24,25(OH)2D were measured in plasma samples that were obtained at or soon before the end of the DCCT. Plasma samples were stored continuously at −80 C. Vitamin D metabolites are known to be stable during long-term storage (26). HPLC-tandem mass spectrometry was used to quantify total 25(OH)D [the sum of 25(OH)D2 and 25(OH)D3)], total 1,25(OH)2D [the sum of 1,25(OH)2D2 and 1,25(OH)2D3], and 24,25(OH)2D [specifically 24,25(OH)2D3] (27–29). Interassay imprecision was 4.40% at 10.4 ng/ml for 25(OH)D3, 4.35% at 9.7 ng/ml for 25(OH)D2, 9.6% at 33.2 pg/ml for 1,25(OH)2D2, 12.2% at 36.4 pg/ml for 1,25(OH)2D3, and 8.58% at 1.5 ng/ml for 24,25(OH)2D3. The calibration for the measurement of 25(OH)D was verified using SRM 972 from National Institute of Standards and Technology (30) with accuracy of 91–95% for 25(OH)D3 and 100–116% for 25(OH)D2.
Plasma concentrations of intact PTH and fibroblast growth factor-23 (FGF-23) were measured in a subset of participants to describe their interrelationships with circulating vitamin D metabolites. We selected a subset of participants with available concurrent iothalamate GFR measurements (n = 300) to facilitate accurate study of biomarkers across GFR. Plasma PTH was measured with the Beckman-Coulter DxI automated immunoassay. Plasma FGF-23 was measured using the Kainos intact FGF-23 ELISA.
Study outcomes
Study outcomes included times from vitamin D specimen collection to development of persistent microalbuminuria, impaired GFR, and incident hypertension. Microalbuminuria was defined as albumin excretion rate (AER) of at least 30 mg/24 h on two consecutive study visits (4). AER was measured yearly during the DCCT and every other year during EDIC by 4-h timed urine collection using fluoroimmunoassay (coefficient of variation, 9.4%) (7). Impaired GFR was defined as estimated GFR (eGFR) below 60 ml/min/1.73 m2 on two consecutive study visits (9). Serum creatinine was measured yearly during DCCT and EDIC (9), and the Chronic Kidney Disease-Epidemiology equation was used to estimate GFR (31). Because the number of cases of impaired GFR events was small, we also examined change in eGFR, evaluated as a continuous outcome to maximize power. Hypertension was defined as systolic blood pressure of at least 140 mm Hg, diastolic blood pressure of at least 90 mm Hg, or use of antihypertensive medications to treat high blood pressure (5). Blood pressure was measured quarterly during the DCCT and yearly during EDIC. During the DCCT, use of RAS antagonists was prohibited, and use of antihypertensive medications was prohibited before a diagnosis of hypertension. Antihypertensive medication use was ascertained yearly during EDIC (5).
Covariates
Demographic characteristics and smoking were ascertained by questionnaire. Solar irradiation of DCCT site was quantified as the mean annual UV index in 1992, as published online by the National Weather Service. Hemoglobin A1c was measured using HPLC (32). GFR was measured as the urinary clearance of I125 iothalamate (7). To increase precision, baseline AER for this study was calculated as the geometric mean of the AER measured concurrently with vitamin D metabolites and the AER preceding vitamin D measurement, usually by 1 yr. Data on vitamin D supplement use were not available.
Statistical methods
Correlations of circulating vitamin D metabolites with clinical characteristics and other biomarkers were assessed using Kendall's τ statistic. Associations of circulating vitamin D metabolites with time to study outcomes were assessed using Cox proportional hazards model. Time at risk began at the time vitamin D metabolites were measured. Participants with an outcome present at the time of vitamin D metabolite measurement (prevalent cases) were excluded from analyses of that outcome. For those who remained free of an outcome event throughout follow-up, the censoring time was the time of the last study visit for which the outcome assessment was conducted; this could vary for each outcome. We included outcome data from all study visits that occurred after measurement of vitamin D metabolites, including DCCT study visits when vitamin D metabolites were measured before DCCT closeout, through EDIC year 16 (September 2009 to April 2010).
Because threshold concentrations of 25(OH)D may exist below which adequate conversion to 1,25(OH)2D cannot be maintained, plasma 25(OH)D concentrations were examined in categories associated with risk of clinically relevant disease outcomes in prior studies: 30 or greater, 20–29, and less than 20 ng/ml (21, 33). Because no such categories exist for 24,25 (OH)2D and 1,25(OH)2D, these metabolites were examined in quartiles. Adjusted hazard ratios and corresponding 95% confidence intervals were generated for each category of vitamin D metabolite, and P values were generated evaluating each vitamin D metabolite as a continuous variable. AER was examined in discrete 2-yr intervals to reflect the timing of its collection during EDIC. Generalized estimating equations were used to examine eGFR (intercept) and its change over time (slope) by categories of vitamin D metabolites.
Models were adjusted for variables (measured at the time of plasma collection) that could confound associations of circulating vitamin D metabolites with study outcomes: age (continuous), sex, race (white vs. non-white), duration of diabetes (categories), DCCT treatment assignment (allowing separate baseline hazards in Cox models), season (May-October vs. November-April), solar irradiation of DCCT site (continuous), body mass index (continuous), AER (continuous), and eGFR (continuous). Results were similar when AER and eGFR measured at the time of plasma collection were not included as covariates. P values less than 0.05 were considered statistically significant. All analyses were performed using R version 2.13.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participant characteristics
Of the 1193 participants included in this study, 47% were women and 96% were white. At the time of biomarker measurement (at or near the end of the DCCT), mean age was 32.4 yr, mean duration of diabetes was 7.5 yr, mean iothalamate GFR was 132.9 ml/min/1.73 m2, and geometric mean AER was 11.8 mg/24 h (Table 1). At EDIC yr 1 and 16, use of RAS inhibitors (angiotensin converting enzyme inhibitors or angiotensin II receptor blockers) was reported by 6.2 and 54.9% of participants, respectively, whereas use of calcium channel blockers was reported by 2.5 and 8.4% of participants, respectively. Throughout EDIC, the prevalence of RAS inhibitor use was similar or slightly higher for participants with lower baseline serum vitamin D metabolite concentrations (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Table 1.
Clinical characteristics at the time of biomarker measurements (at or near the end of the DCCT) for all DCCT/EDIC participants with measurement of vitamin D metabolites (n = 1193) and for the subset of DCCT/EDIC participants with additional measurement of PTH and FGF-23 (n = 300)
| All measured participants | Subset of participants with PTH and FGF-23 | |
|---|---|---|
| n | 1193 | 300 |
| Age (yr) | 32.4 (2.6) | 32.3 (2.6) |
| Duration of diabetes (yr) | 7.5 (0.6) | 5.8 (0.6) |
| Female sex, n (%) | 564 (47%) | 113 (44%) |
| Non-white race, n (%) | 42 (4%) | 15 (5%) |
| DCCT intensive diabetes therapy, n (%) | 596 (50%) | 157 (48%) |
| Current smoking, n (%) | 236 (18%) | 58 (17%) |
| Hypertension, n (%) | 96 (8%) | 14 (5%) |
| Body mass index (kg/m2) | 25.7 (1.9) | 25.9 (1.9) |
| Systolic blood pressure (mm Hg) | 114.9 (3.4) | 114.8 (3.4) |
| Diastolic blood pressure (mm Hg) | 74.0 (2.9) | 74.2 (2.8) |
| Hemoglobin A1c (%) | 8.1 (1.3) | 8.3 (1.3) |
| AER (mg/24 h) | 11.8 (1.6) | 10.3 (1.4) |
| Serum creatinine (mg/dl) | 0.7 (1.2) | 0.7 (1.2) |
| eGFR (ml/min/1.73 m2) | 107.7 (16.2) | 109.6 (15.3) |
| Iothalamate GFR (ml/min) | 133.0 (4.9) | |
| Total 25(OH)D (ng/ml) | 25.4 (3.0) | 25.6 (3.1) |
| 24,25(OH)2D3 (ng/ml) | 4.1 (1.5) | 4.1 (1.5) |
| Total 1,25(OH)2D (pg/ml) | 41.3 (3.4) | 44.9 (3.5) |
| FGF-23 (pg/ml) | 31.4 (3.0) | |
| PTH (pg/ml) | 30.8 (3.7) |
Cell contents are expressed as number (percentage) or mean (sd), except for AER, which is summarized as geometric mean (geometric sd).
Baseline correlates of circulating vitamin D metabolites
1,25(OH)2D correlated weakly with 25(OH)D (Kendall's τ = 0.16), whereas 24,25(OH)2D correlated strongly with both total 25(OH)D and 25(OH)D3 (τ = 0.70 and τ = 0.73, respectively). 25(OH)D, 24,25(OH)2D, and 1,25(OH)2D concentrations each correlated negatively with age and body mass index and were lower with non-white race (Table 2). Women had lower mean 25(OH)D and 24,25(OH)2D concentrations but higher mean 1,25 (OH)2D concentration. Participants assigned to DCCT intensive diabetes therapy had lower mean concentrations of 25(OH)D and 24,25(OH)2D; these associations were attenuated and not statistically significant after adjustment for demographic variables, time from DCCT randomization, and body mass index at plasma collection and DCCT baseline (Supplemental Table 2). 25(OH)D displayed expected seasonal variation (Supplemental Fig. 1).
Table 2.
Correlations of circulating biomarkers of mineral metabolism with clinical characteristics among DCCT/EDIC participants with type 1 diabetes
| 25(OH)D (ng/ml) | 24,25(OH)2D (ng/ml) | 1,25(OH)2D (pg/ml) | PTH (pg/ml) | FGF-23 (pg/ml) | |
|---|---|---|---|---|---|
| n | 1193 | 1193 | 1149 | 300 | 300 |
| Dichotomous clinical characteristics (β coefficients listed) | |||||
| Female sex | −1.46b | −0.6b | 2.5b | −1.52 | 5.18b |
| Non-white race | −9.25b | −2.08b | 3.25b | −2.03 | 5.7 |
| DCCT intensive diabetes therapy | −1.78b | −0.24b | −0.9 | 1.79b | 3.74b |
| Continuous clinical characteristics (correlation coefficients listed) | |||||
| Age | −0.04b | −0.05b | −0.06b | 0.06b | −0.07b |
| Duration of diabetes | −0.07b | −0.07b | −0.06b | 0.01 | 0.03 |
| Body mass index | −0.07b | −0.07b | −0.04b | 0.08b | 0.12b |
| Systolic blood pressure | −0.04 | −0.03 | −0.02 | −0.03 | −0.01 |
| Diastolic blood pressure | −0.05b | −0.03 | 0.01 | 0.01 | 0.02 |
| Hemoglobin A1c | 0 | −0.02 | −0.03 | 0.02 | −0.02 |
| AER | −0.06b | −0.03 | 0 | 0.08b | −0.04b |
| Iothalamate GFR | 0.04 | 0.08b | −0.03 | −0.06 | 0.01 |
| 25(OH)D | 0.7b | 0.16b | −0.21b | 0.12b | |
| 24,25(OH)2D | 0.7b | 0.1b | −0.22b | 0.13b | |
| 1,25(OH)2D | 0.16b | 0.1b | 0 | −0.17b | |
| PTHa | −0.21b | −0.22b | 0 | −0.03 | |
| FGF-23a | 0.12b | 0.13b | −0.17b | −0.03 |
For dichotomous clinical characteristics, cell contents are differences in mean values of the circulating biomarkers of mineral metabolism. For continuous clinical characteristics, cell contents are Kendall's τ statistic (unitless).
n = 300 for all columns.
P < 0.05.
24,25(OH)2D concentrations tended to be lower below an iothalamate GFR of approximately 120 ml/min/1.73 m2, but other biomarkers did not vary significantly with GFR (Table 2 and Supplemental Fig. 2). Concentrations of 25(OH)D, 24,25(OH)2D, and 1,25(OH)2D did not vary with AER when AER was below 300 mg/24 h but tended to be lower with AER above 300 mg/24 h (Supplemental Fig. 3). Concentrations of 25(OH)D and 24,25(OH)2D but not 1,25(OH)2D correlated inversely with plasma PTH concentration (Supplemental Fig. 4). FGF-23 correlated negatively with 25(OH)D and 24,25(OH)2D and positively with 1,25(OH)2D (Table 2).
Incident microalbuminuria
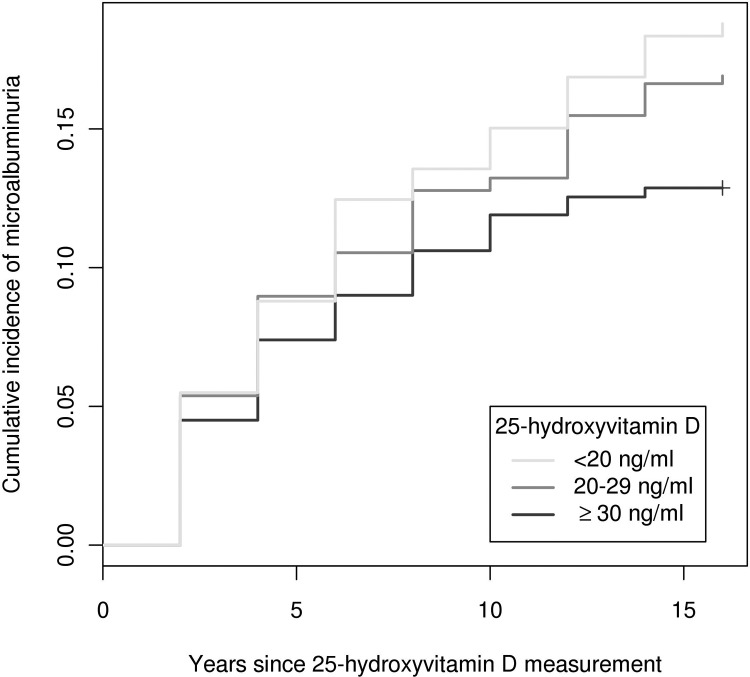
A total of 166 cases of incident microalbuminuria were observed over 17-yr median follow-up. Participants with 25(OH)D below 20 ng/ml had a 65% higher risk of microalbuminuria compared with those with 25(OH)D of at least 30 ng/ml (95% confidence interval, 7 to 154%; Table 3 and Fig. 1). In parallel, each 10 ng/ml decrement in 25(OH)D was associated with a 23% higher risk of microalbuminuria (95% confidence interval, 2 to 50%; P = 0.03). In comparison to 24,25(OH)2D of at least 5.3 ng/ml, participants with lower 24,25(OH)2D concentrations also had higher risks of microalbuminuria, but not in a monotonic fashion (Table 3). Each 1 ng/ml decrement in 24,25(OH)2D was associated with a 7% higher risk of microalbuminuria, which was not statistically significant (95% confidence interval, 1% decreased risk to 16% increased risk; P = 0.09). Further adjustment for 25(OH)D concentration somewhat attenuated the association of 24,25(OH)2D with risk of microalbuminuria. Plasma 1,25(OH)2D concentration was not associated with risk of microalbuminuria (Table 3).
Table 3.
Associations of circulating vitamin D metabolites measured at the end of the DCCT with incident microalbuminuria, incident impaired GFR, and incident hypertension during the EDIC Study
| Vitamin D metabolite | Microalbuminuria |
Impaired GFR |
Hypertension |
|||
|---|---|---|---|---|---|---|
| No. of cases (unadjusted %) | Adjusted hazard ratio (95% CI) | No. cases (unadjusted %) | Adjusted hazard ratio (95% CI) | No. cases (unadjusted %) | Adjusted hazard ratio (95% CI) | |
| 25(OH)D | ||||||
| ≥30 ng/ml | 40 (12.9) | 1 (ref) | 16 (4.5) | 1 (ref) | 153 (46.8) | 1 (ref) |
| 20–29 ng/ml | 75 (16.8) | 1.43 (0.97 to 2.1) | 22 (4.3) | 1.01 (0.53 to 1.94) | 243 (51.6) | 1.22 (0.99 to 1.49) |
| <20 ng/ml | 51 (18.7) | 1.65 (1.07 to 2.54) | 16 (4.9) | 1.10 (0.53 to 2.26) | 145 (48.5) | 1.14 (0.9 to 1.44) |
| P value | 0.03 | 0.76 | 0.27 | |||
| 24,25(OH)2D | ||||||
| ≥5.3 ng/ml | 28 (11.2) | 1 (ref) | 8 (2.8) | 1 (ref) | 129 (48.5) | 1 (ref) |
| 3.9–5.3 ng/ml | 46 (18.4) | 1.88 (1.17 to 3.02) | 19 (6.7) | 2.52 (1.1 to 5.79) | 130 (49.2) | 1.03 (0.8 to 1.31) |
| 2.6–3.9 ng/ml | 42 (15.8) | 1.59 (0.98 to 2.58) | 11 (3.6) | 1.34 (0.53 to 3.35) | 143 (50.0) | 1.08 (0.85 to 1.38) |
| <2.6 ng/ml | 47 (18.5) | 1.98 (1.22 to 3.23) | 16 (5.3) | 1.88 (0.78 to 4.54) | 132 (49.1) | 1.03 (0.8 to 1.33) |
| P value | 0.09 | 0.38 | 0.49 | |||
| 1,25(OH)2D | ||||||
| ≥47 pg/ml | 35 (14.4) | 1 (ref) | 9 (3.2) | 1 (ref) | 125 (47.7) | 1 (ref) |
| 39–47 pg/ml | 47 (17.7) | 1.30 (0.84 to 2.03) | 15 (5.2) | 1.61 (0.7 to 3.71) | 132 (48.9) | 0.99 (0.77 to 1.26) |
| 34–39 pg/ml | 37 (17.1) | 1.23 (0.77 to 1.96) | 11 (4.2) | 1.34 (0.55 to 3.25) | 122 (50.8) | 1.07 (0.83 to 1.38) |
| <34 pg/ml | 39 (14.7) | 1.17 (0.73 to 1.86) | 15 (4.8) | 1.58 (0.68 to 3.66) | 136 (48.1) | 0.98 (0.77 to 1.26) |
| P value | 0.39 | 0.22 | 0.96 | |||
All models are adjusted for age, duration of diabetes, season, body mass index, AER, and eGFR at the time of biomarker measurement as well as sex, race, DCCT treatment assignment, and solar irradiation of DCCT clinic site. P values were generated evaluating each vitamin D metabolite as a continuous variable. CI, Confidence interval.
Fig. 1.
Cumulative incidence of microalbuminuria by baseline 25(OH)D concentration.
Impaired GFR
Fifty-four cases of incident impaired GFR were observed over 17-yr median follow-up. Circulating vitamin D metabolites were not associated with risk of impaired GFR (Table 3). During follow-up, overall mean eGFR was slightly higher with lower 25(OH)D concentration, but no circulating vitamin D metabolite was associated with change in eGFR over time (slope, Table 4).
Table 4.
Associations of circulating vitamin D metabolites measured at the end of the DCCT with the mean eGFR and change in eGFR over time during the EDIC study
| Vitamin D metabolite | Overall mean eGFR (ml/min/1.73 m2) |
Change in mean eGFR (ml/min/1.73 m2/yr)a |
||
|---|---|---|---|---|
| Unadjusted mean (95% CI) | Adjusted mean difference (95% CI) | Unadjusted mean change (95% CI) | Adjusted mean difference in change (95% CI) | |
| 25(OH)D | ||||
| ≥30 ng/ml | 116.0 (115.2 to 116.9) | 0 (ref) | −1.31 (−1.31 to −1.3) | 0 (ref) |
| 20–29 ng/ml | 117.0 (116.4 to 117.5) | 1.2 (−0.3 to 2.7) | −1.24 (−1.25 to −1.24) | 0.06 (−0.07 to 0.2) |
| <20 ng/ml | 117.4 (116.0 to 118.8) | 1.9 (0.1 to 3.7) | −1.35 (−1.36 to −1.34) | −0.04 (−0.21 to 0.13) |
| P value | <0.001 | 0.90 | ||
| 24,25(OH)2D | ||||
| Q1 (highest) | 117.5 (116.5 to 118.5) | 0 (ref) | −1.30 (−1.31 to −1.3) | 0 (ref) |
| Q2 | 115.9 (114.9 to 116.8) | −0.4 (−2.1 to 1.3) | −1.25 (−1.25 to −1.24) | 0.03 (−0.14 to 0.2) |
| Q3 | 117.4 (116.5 to 118.3) | 0.6 (−1.1 to 2.4) | −1.32 (−1.33 to −1.31) | −0.02 (−0.18 to 0.14) |
| Q4 | 115.7 (114.2 to 117.1) | −0.1 (−2.0 to 1.9) | −1.29 (−1.3 to −1.28) | 0.01 (−0.17 to 0.18) |
| P value | 0.29 | 0.88 | ||
| 1,25(OH)2D | ||||
| Q1 (highest) | 118.5 (117.3 to 119.6) | 0 (ref) | −1.26 (−1.26 to −1.25) | 0 (ref) |
| Q2 | 117.2 (116.1 to 118.2) | −0.6 (−2.2 to 1.1) | −1.27 (−1.28 to −1.26) | −0.01 (−0.18 to 0.15) |
| Q3 | 116.0 (114.7 to 117.4) | −1.2 (−3.1 to 0.7) | −1.23 (−1.24 to −1.22) | 0.00 (−0.16 to 0.16) |
| Q4 | 115.4 (114.2 to 116.5) | −0.9 (−2.6 to 0.9) | −1.35 (−1.36 to −1.34) | −0.12 (−0.29 to 0.05) |
| P value | 0.32 | 0.44 | ||
All models are adjusted for age, duration of diabetes, season, body mass index, and AER at the time of biomarker measurement as well as sex, race, DCCT treatment assignment, and solar irradiation of DCCT clinic site. P values were generated evaluating each vitamin D metabolite as a continuous variable.
Negative numbers represent loss of creatinine clearance over time.
Incident hypertension
There was a weak inverse correlation of 25(OH)D concentration with diastolic blood pressure at the time of biomarker measurement, but 25(OH)D was not correlated with systolic blood pressure, and 24,25(OH)2D and 1,25(OH)2D were not correlated with systolic or diastolic blood pressure (Table 2). Although 518 cases of incident hypertension were observed over 20-yr median follow-up, circulating vitamin D metabolites were not associated with risk of hypertension (Table 3).
Discussion
Low plasma concentrations of 25(OH)D and 24,25 (OH)2D were associated with increased risks of developing new-onset microalbuminuria in a large and well-characterized type 1 diabetes population. Plasma 1,25(OH)2D concentration was not associated with risk of microalbuminuria, and no circulating vitamin D metabolite was associated with risk of impaired GFR, change in eGFR, or risk of hypertension. These results suggest that insufficient intake of vitamin D from cutaneous synthesis and oral consumption may increase the risk of microalbuminuria in type 1 diabetes but do not support a role for impaired vitamin D metabolism in early GFR loss or the development of hypertension in this population.
In a related study of 227 Steno Diabetes Center patients with type 1 diabetes, very low plasma 25(OH)D3 concentrations (<6.2 ng/ml) were not significantly associated with risk of microalbuminuria (34). However, the lack of a significant association in the Steno study may be related to the relatively fewer observed cases of microalbuminuria (n = 81). The wide confidence interval reported in the Steno study includes substantial differences in microalbuminuria risk, including that observed in our study.
It is important to note that the association of 25(OH)D concentration with microalbuminuria may be confounded by unmeasured variables, such as differences in lifestyle or defects in tubular reabsorption of filtered 25(OH)D. RAS inhibitors may have lowered urine albumin excretion in some participants because as use increased over time. However, less frequent RAS inhibitor use cannot explain the observed associations of low 25(OH)D and 24,25(OH)2D concentrations with greater microalbuminuria risk because it was slightly more frequent among participants with lower baseline vitamin D metabolite concentrations. Plasma 25(OH)D correlated negatively with PTH but positively with FGF-23, implying that the association of low 25(OH)D with increased risk of microalbuminuria could be mediated or confounded by high PTH but not by high FGF-23. Most importantly, many factors influence the development of microalbuminuria in type 1 diabetes, and any causal impact of 25(OH)D, if present, may be small. Before widespread 25(OH)D screening or treatment, the long-term effects of vitamin D supplementation on diabetes complications should be evaluated in well-controlled clinical trials.
Low plasma 24,25(OH)2D concentration was also associated with increased microalbuminuria risk, although this association was not as consistent as the association of low plasma 25(OH)D concentration with microalbuminuria risk. As the primary product of 25(OH)D metabolism by CYP24A1, 24,25(OH)2D is a biomarker of 25(OH)D turnover. In a population with impaired GFR, 24,25 (OH)2D concentration was directly correlated with eGFR, suggesting that low 24,25(OH)2D concentration, alone or relative to 25(OH)D, reflects impairment of renal vitamin D metabolism (29). In the current study population, GFR was normal or high, and the correlation of 25(OH)D with 24,25(OH)2D was also high. In this setting, 24,25(OH)2D concentration appears to provide little information beyond 25(OH)D concentration.
Interestingly, plasma 1,25(OH)2D concentration was not clearly associated with microalbuminuria risk. 1,25(OH)2D is the potent hormonal form of vitamin D through which 25(OH)D would be expected to exert any biological effect (21). It is possible that local conversion of 25(OH)D to 1,25(OH)2D results in beneficial autocrine or paracrine effects that are not captured by circulating 1,25(OH)2D concentration. Alternatively, circulating 1,25(OH)2D may be important for disease prevention, but inaccurate ascertainment of 1,25(OH)2D status may bias results to the null. Our assay measures plasma 1,25 (OH)2D more accurately and precisely than prior assays (28), but substantial misclassification may still result from the combination of short circulating half-life (8–12 h) and single time of measurement.
Circulating vitamin D metabolites were not associated with risk of impaired GFR or change in eGFR in the present study. This contrasts with results from a community-based population of older adults, in which lower 25(OH)D concentration was associated with increased risk of GFR loss, particularly among participants with type 2 diabetes (23). Given our small number of impaired GFR events and the fact that overall mean decline in eGFR was near that expected for normal aging, our current results may represent a type 2 error. Alternatively, low 25(OH)D may promote or be confounded by endothelial dysfunction and related processes that contribute more to microalbuminuria than to progressive parenchymal kidney disease.
In contrast to prior studies (22, 24), circulating vitamin D metabolites were not significantly related to blood pressure in cross-section or to risk of hypertension during follow-up. It is possible that blood pressure in type 1 diabetes is so strongly influenced by other factors, such as glycemia and albuminuria (5), that marginal risk conferred by insufficient vitamin D is not relevant. However, our clear null result should question the notion that low 25(OH)D is a risk factor for hypertension. It is possible that prior studies were confounded by adiposity or other elements of the metabolic syndrome because distributions of these important covariates varied more widely than in our population and are difficult to account for precisely.
Although our focus was on how circulating vitamin D metabolites affect risk of kidney disease, it is clear that kidney disease also determines vitamin D metabolism. To examine this, we described relationships of GFR (measured by iothalamate clearance) and AER with circulating vitamin D metabolites and related regulatory hormones in cross-sectional analyses. GFR was entirely within the normal or high range. Within this range, there was no correlation of GFR with 25(OH)D, 1,25(OH)2D, PTH, or FGF-23. There was a suggestion that 24,25(OH)2D concentrations were lower below a GFR threshold of approximately 120 ml/min/1.73 m2, mirroring the positive correlation of eGFR with 24,25(OH)2D in the setting of impaired GFR (29) and suggesting that impaired 25(OH)D catabolism may be an early sign of disturbed renal mineral metabolism in type 1 diabetes. Concentrations of 25(OH)D, 1,25(OH)2D, and 24,25(OH)2D were lower among the small number of participants with AER above 300 mg/24 h but did not correlate with AER when AER was less than 300 mg/24 h. It is possible that vitamin D metabolites are lost along with vitamin D binding protein with high levels of albuminuria (35).
Strengths of this study include the assessment of a type 1 diabetes population at high risk of hypertension and kidney disease, large sample size, use of novel and specific mass spectrometry assays for three complementary circulating vitamin D metabolites, long duration of follow-up, use of detailed phenotype data to assess clinically important outcomes with confidence, and a combination of detailed baseline covariate data and a relatively homogenous study population that minimizes the risk of confounding. Limitations include the availability of vitamin D metabolite measurements at only one point in time. 25(OH)D concentrations have been observed to be relatively stable within individuals over time (36), and our study was conducted during a period in which vitamin D supplementation was not common, but unmeasured changes in vitamin D metabolite concentrations over time could still lead to misclassification and bias results toward the null. Most importantly, our study is observational in nature, which allows for residual confounding and prevents assumptions of causality. As a result, we do not recommend routine measurements of circulating vitamin D metabolites or routine treatment with vitamin D on the basis of these results.
This study supports further investigation into the use of vitamin D supplementation to prevent microalbuminuria in type 1 diabetes. We did not find evidence linking impaired vitamin D metabolism to early GFR loss or the development of hypertension.
Supplementary Material
Acknowledgments
This work was supported by Grants RC4DK090766, R01DK088762, R01HL096875, P30DK035816, and P01DK02456 from the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, and Blood Institute. The DCCT/EDIC project is supported by contracts with the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases, National Eye Institute, National Institute of Neurological Disorders and Stroke, the General Clinical Research Centers Program and the Clinical and Translational Science Awards Program, National Center for Research Resources, and by Genentech through a Cooperative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases. A complete list of participants in the DCCT/EDIC research group can be found in the appendix of Ref. 9.
Clinical Trial Registration no.: NCT00360893 and NCT00360815.
Disclosure Summary: I.H.d.B. receives research funding from Abbott Laboratories. J.M.L. receives consulting fees from Reata Pharmaceuticals, Eli Lilly, and Novartis Pharmaceuticals. M.E.M. receives consulting fees from Abbott Laboratories, Novo Nordisk, and CVS Caremark and grant funding from Sanofi-Aventis and Eli Lilly. M.W.S. receives consulting fees from Pharma Diagnostic. The other authors have nothing to declare.
Footnotes
- AER
- Albumin excretion rate
- DKD
- diabetic kidney disease
- eGFR
- estimated GFR
- FGF-23
- fibroblast growth factor-23
- GFR
- glomerular filtration rate
- 25(OH)D
- 25-hydroxyvitamin D
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- 24,25(OH)2D
- 24,25-dihydroxyvitamin D
- RAS
- renin-angiotensin system.
References
- 1. Parving HH, Oxenbøll B, Svendsen PA, Christiansen JS, Andersen AR. 1982. Early detection of patients at risk of developing diabetic nephropathy. A longitudinal study of urinary albumin excretion. Acta Endocrinol (Copenh) 100:550–555 [DOI] [PubMed] [Google Scholar]
- 2. Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. 1982. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1:1430–1432 [DOI] [PubMed] [Google Scholar]
- 3. Mogensen CE. 1984. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med 310:356–360 [DOI] [PubMed] [Google Scholar]
- 4. de Boer IH, Rue TC, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Sun W, Zinman B, Brunzell JD, White NH, Danis RP, Davis MD, Hainsworth D, Hubbard LD, Nathan DM. 2011. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med 171:412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Boer IH, Kestenbaum B, Rue TC, Steffes MW, Cleary PA, Molitch ME, Lachin JM, Weiss NS, Brunzell JD. 2008. Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch Intern Med 168:1867–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borch-Johnsen K, Kreiner S. 1987. Proteinuria: value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed) 294:1651–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. 1995. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications (DCCT) Research Group. Kidney Int 47:1703–1720 [DOI] [PubMed] [Google Scholar]
- 8. 2003. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Zinman B. 2011. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 365:2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. 1993. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 329:1456–1462 [DOI] [PubMed] [Google Scholar]
- 11. Schwarz U, Amann K, Orth SR, Simonaviciene A, Wessels S, Ritz E. 1998. Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int 53:1696–1705 [DOI] [PubMed] [Google Scholar]
- 12. Makibayashi K, Tatematsu M, Hirata M, Fukushima N, Kusano K, Ohashi S, Abe H, Kuze K, Fukatsu A, Kita T, Doi T. 2001. A vitamin D analog ameliorates glomerular injury on rat glomerulonephritis. Am J Pathol 158:1733–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirata M, Makibayashi K, Katsumata K, Kusano K, Watanabe T, Fukushima N, Doi T. 2002. 22-Oxacalcitriol prevents progressive glomerulosclerosis without adversely affecting calcium and phosphorus metabolism in subtotally nephrectomized rats. Nephrol Dial Transplant 17:2132–2137 [DOI] [PubMed] [Google Scholar]
- 14. Kuhlmann A, Haas CS, Gross ML, Reulbach U, Holzinger M, Schwarz U, Ritz E, Amann K. 2004. 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol 286:F526–F533 [DOI] [PubMed] [Google Scholar]
- 15. Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC. 2008. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci USA 105:15896–15901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deb DK, Sun T, Wong KE, Zhang Z, Ning G, Zhang Y, Kong J, Shi H, Chang A, Li YC. 2010. Combined vitamin D analog and AT1 receptor antagonist synergistically block the development of kidney disease in a model of type 2 diabetes. Kidney Int 77:1000–1009 [DOI] [PubMed] [Google Scholar]
- 17. Li YC. 2010. Renoprotective effects of vitamin D analogs. Kidney Int 78:134–139 [DOI] [PubMed] [Google Scholar]
- 18. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 2002. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D. 2010. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376:1543–1551 [DOI] [PubMed] [Google Scholar]
- 20. de Boer IH, Kestenbaum B. 2008. Vitamin D in chronic kidney disease: is the jury in? Kidney Int 74:985–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. 2011. Dietary reference intakes for calcium and vitamin D. In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Washington, DC: The National Academies Press; [PubMed] [Google Scholar]
- 22. de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 2007. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 50:69–77 [DOI] [PubMed] [Google Scholar]
- 23. de Boer IH, Katz R, Chonchol M, Ix JH, Sarnak MJ, Shlipak MG, Siscovick DS, Kestenbaum B. 2011. Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clin J Am Soc Nephrol 6:2141–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. 2007. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 49:1063–1069 [DOI] [PubMed] [Google Scholar]
- 25. 1993. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329:977–986 [DOI] [PubMed] [Google Scholar]
- 26. Agborsangaya C, Toriola AT, Grankvist K, Surcel HM, Holl K, Parkkila S, Tuohimaa P, Lukanova A, Lehtinen M. 2010. The effects of storage time and sampling season on the stability of serum 25-hydroxyvitamin D and androstenedione. Nutr Cancer 62:51–57 [DOI] [PubMed] [Google Scholar]
- 27. Hoofnagle AN, Laha TJ, Donaldson TF. 2010. A rubber transfer gasket to improve the throughput of liquid-liquid extraction in 96-well plates: application to vitamin D testing. J Chromatogr B Analyt Technol Biomed Life Sci 878:1639–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strathmann FG, Laha TJ, Hoofnagle AN. 2011. Quantification of 1α,25-dihydroxy vitamin D by immunoextraction and liquid chromatography-tandem mass spectrometry. Clin Chem 57:1279–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bosworth CR, Levin G, Robinson-Cohen C, Hoofnagle AN, Ruzinski J, Young B, Schwartz SM, Himmelfarb J, Kestenbaum B, de Boer IH. 2012. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int 82:693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phinney KW. 2008. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr 88:511S–512S [DOI] [PubMed] [Google Scholar]
- 31. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steffes M, Cleary P, Goldstein D, Little R, Wiedmeyer HM, Rohlfing C, England J, Bucksa J, Nowicki M. 2005. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem 51:753–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Boer IH, Levin G, Robinson-Cohen C, Biggs ML, Hoofnagle AN, Siscovick DS, Kestenbaum B. 2012. Serum 25-hydroxyvitamin D concentration and risk for major clinical disease events in a community-based population of older adults: a cohort study. Ann Intern Med 156:627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Joergensen C, Hovind P, Schmedes A, Parving HH, Rossing P. 2011. Vitamin D levels, microvascular complications, and mortality in type 1 diabetes. Diabetes Care 34:1081–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thrailkill KM, Jo CH, Cockrell GE, Moreau CS, Fowlkes JL. 2011. Enhanced excretion of vitamin D binding protein in type 1 diabetes: a role in vitamin D deficiency? J Clin Endocrinol Metab 96:142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. 2010. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol 171:903–908 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.