Abstract
Context:
Monocarboxylate transporter 8 (MCT8) is a thyroid hormone-specific cell membrane transporter. MCT8 deficiency causes severe psychomotor retardation and abnormal thyroid tests. The great majority of affected children cannot walk or talk, and all have elevated serum T3 levels, causing peripheral tissue hypermetabolism and inability to maintain weight. Treatment with thyroid hormone is ineffective. In Mct8-deficient mice, the thyroid hormone analog, diiodothyropropionic acid (DITPA), does not require MCT8 to enter tissues and could be an effective alternative to thyroid hormone treatment in humans.
Objective:
The objective of the study was to evaluate the effect and efficacy of DITPA in children with MCT8 deficiency.
Methods:
This was a multicenter report of four affected children given DITPA on compassionate grounds for 26–40 months. Treatment was initiated at ages 8.5–25 months, beginning with a small dose of 1.8 mg, increasing to a maximal 30 mg/d (2.1–2.4 mg/kg · d), given in three divided doses.
Results:
DITPA normalized the elevated serum T3 and TSH when the dose reached 1 mg/kg · d and T4 and rT3 increased to the lower normal range. The following significant changes were also observed: decline in SHBG (in all subjects), heart rate (in three of four), and ferritin (in one of four). Cholesterol increased in two subjects. There was no weight loss and weight gain occurred in two. None of the treated children required a gastric feeding tube or developed seizures. No adverse effects were observed.
Conclusion:
DITPA (1–2 mg/kg · d) almost completely normalizes thyroid tests and reduces the hypermetabolism and the tendency for weight loss. The effects of earlier commencement and long-term therapy remain to be determined.
In 2004 Dumitrescu et al. (1) and Friesema et al. (2) reported that mutations in the monocarboxylate transporter 8 (MCT8, SLC16A2) gene located on the X chromosome produces, in young males, severe psychomotor deficits and unusual thyroid function test abnormalities. Their investigation was prompted by the publication, a year earlier, about a homolog of the human MCT8 in the rat with thyroid hormone (TH)-specific cell membrane transporter properties (3). Shortly thereafter (4) it became apparent that MCT8 gene defects were responsible for the clinical manifestations described 60 yr earlier by Allan, Herndon, and Dudley (5). In this syndrome boys present in infancy or early childhood with feeding difficulties, severe cognitive deficiency, hypotonia, and poor head control. They develop progressive spastic quadriplegia, diminished muscle mass with weakness, joint contractures, and dystonia. Not known until the discovery of the MCT8 gene defect were the characteristic serum thyroid test abnormalities, namely high serum T3, low rT3, low T4, and normal or slightly elevated TSH. This constellation of thyroid test abnormalities is a manifestation of multiple changes in TH physiology and metabolism due to the defective TH transport across cell membranes (6, 7).
The hormonal deficiency is variable among tissues and cell types because the requirement of MCT8 for adequate TH transfer depends on whether other TH transporters are present. In the absence of adequately functioning MCT8, tissues such as the brain manifest the effects of TH deprivation. In contrast, a tissue such as liver, with abundance of other TH transporters, shows the consequences of hormonal excess due to the high levels of circulating T3 (8). Patients with MCT8 deficiency therefore have coexisting TH deficiency and excess (9, 10). This precludes treatment with supraphysiological doses of TH. Therefore, an analog of TH, transported independently of MCT8, was sought for the treatment of subjects with this transporter deficiency.
We were able to demonstrate that the same dose of the TH analog diiodothyropropionic acid (DITPA) was able to rescue Mct8 deficient and wild-type animals from the effect of TH deprivation in central and peripheral tissues (11). Being a compound that has formerly been used in humans for other purposes (12, 13), DITPA was administered on a compassionate basis to four children with MCT8 deficiency for a period from 2.1–3.3 yr. This preliminary experience is the subject of this report.
Patients and Methods
Case reports (for details see Supplemental Data, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org)
Family 8 (subjects A1 and A2)
The affected monozygotic twin boys, hereafter referred to as subjects A1 and A2, were born in Israel, to Ashkenasi, nonconsanguineous parents living in Australia. Born at 36–37 wk gestation, weights of twins 1 and 2, respectively, were 2490 and 2733 g (10th to 25th centile). Delay in the ability to make eye contact until 3 months and to smile until 4 months brought the twins to medical attention.
At 18 months subjects A1 and A2 had, respectively, serum T3 of 54 and 60% above the upper limit of normal, T4 26 and 20% below the lower limit of normal (LLN), and rT3 44 and 37% below the LLN with normal TSH of 4.0 and 3.7 mU/liter. The MCT8 gene harbored the mutation c.962 C>T; P321L. The mother is heterozygous for the mutation.
Although length progressed between the 10th and the 25th centile, by the age of 6 months, their weight dropped below the first centile.
Treatment with DITPA was started at 25 months of age in both twins.
Family 10 (subject B)
The affected boy, hereafter referred to as subject B, was born at term to nonconsanguineous white European (Swiss) parents. His birth weight and length were 2840 g and 47.5 cm. Neonatal screen TSH was less than 15 mU/liter (the cutoff value for the program). Hypotonia was noted at 1 month of age and thyroid tests at 4 months showed a free T4 (FT4) 15% below the LLN for age and total T3 63% above the upper limit of normal. The MCT8 gene had the mutation c.733 C>T; R245X. The mother was heterozygous, and a maternal uncle, now 22 yr old, does not walk or talk and has seizures and presumably harbors the same MCT8 defect.
Treatment with DITPA was started at 8.5 months of age.
Family 11 (subject C)
The affected boy, hereafter referred to as subject C, was born in Canada to nonconsanguineous Iranian parents. His term birth weight and length were 3875 g and 50 cm. Neonatal screen TSH was less than 17 mU/liter. Poor head control was noted by 4 months, and by 5 months hypotonia was obvious. A de novo MCT8 gene mutation, c.1238 C>T, produces a stop codon (Q380X). The child was growing normally between the 50th and 75th centile for length and between the 10th and 25th centile for weight. At 21 months his basal metabolic rate was +79%.
Treatment with DITPA was started at 25 months of age.
Source of DITPA and permission for its use
DITPA was produced by Sigma (St. Louis, MO) and purchased in each case by the parents from different suppliers. Permissions were granted for the compassionate use of DITPA. These are detailed in the Supplemental Data.
Treatment protocol
The protocol was designed to determine the short-term effectiveness of DITPA and, if well tolerated, over the longer term. It was continued with minor deviations in dose (dependent on the capsule formulation), timing of testing (depending on the proximity of the testing facility), and laboratory tests (depending on the available local laboratory technology). The initial dose was 1.8 or 2 mg once daily for 1 wk, twice daily for 1 month, and then three times daily. This dose, given three times daily, represented 0.53–0.71 mg DITPA per kilogram body weight per day, or 14–19% of the dose previously given to adults without MCT8 deficiency, 3.75 mg/kg · d (13, 14). Thereafter the dose was increased by 1.8 or 2 mg for one of the three daily doses, then the second, and finally all three doses, reaching over the period of 4–10 months, the schedule of 3.6 or 4 mg three times daily (0.97–1.21 mg/kg · d). The same manner of stepwise dose increment was used to reach, over the period of 15–29 months, the maximum dose 9 or 10 mg three times daily (2.0–2.4 mg/kg · d).
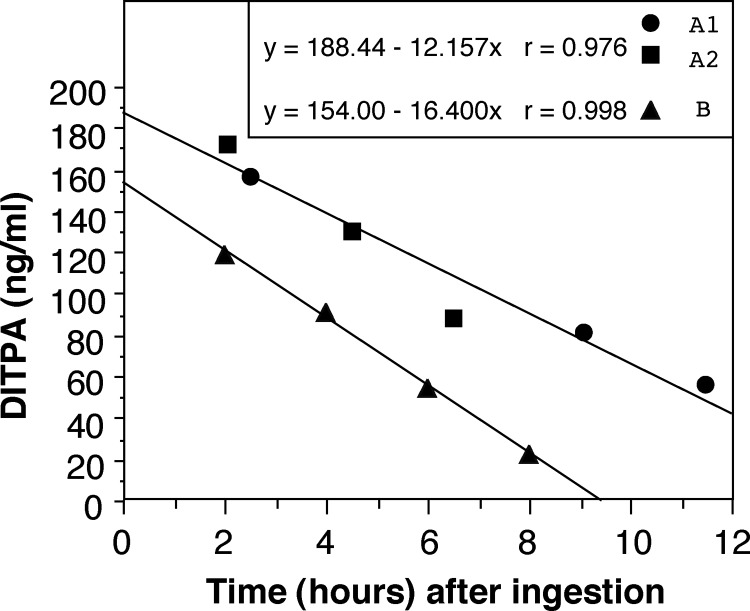
The decision to administer DITPA three times a day, or as close as every 8 h as possible (usually 7–7-10 h), was based on the measurement of its half-life, which ranged from 5 to 8 h (Fig. 1).
Fig. 1.
In vivo clearance of DITPA determined in children receiving two different doses of DITPA. Children were treated with 3.6 mg/d (0.4 mg/kg · d for subjects A1 and A2) or 9 mg/d (1.1 mg DITPA/kg · d for subject B). After withholding DITPA for 12 h, a single dose of 1.8 mg (subjects A1 and A2) or 3.6 mg (subject B) was given orally, and blood samples were obtained for the measurement of DITPA at the indicated times. The half-life of DITPA was 5 h for subject B and 8 h for subjects A1 and A2. The lower serum concentration of DITPA in subject B, despite administration of double the dose given to subjects A1 and A2, may be due to differences in absorption or distribution volume.
Blood tests
Tests were obtained at the hospital clinical laboratories of the respective study sites, using automated methods. Markers of TH action were: cholesterol, SHBG, ferritin, alkaline phosphatase, creatine kinase (CK), and osteocalcin (OSTC). Thyroid tests performed locally included total T4, T3 and rT3, FT4 and free T3 (FT3), TSH, and thyroglobulin (TG). Other tests were complete blood count and differential, serum sodium, potassium, chloride, carbon dioxide, blood urea nitrogen, creatinine, bilirubin alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase, lactate, and ammonium.
Thyroid function tests
In addition to the determinations carried out on-site, an aliquot from all samples was forwarded to Chicago for the following measurements: total T4, total T3, and TSH by chemiluminescence immunometric (Elecsys Automated System, Indianapolis, IN); total rT3 by RIA (Adaltis, Rome, Italy); and serum TG by an in-house RIA. Serum FT4 and FT3 indexes were calculated as the product of the total serum concentrations of each iodothyronine and the normalized resin T4 uptake ratio.
Measurement of DITPA in serum
DITPA was measured by liquid chromatography (Shimadzu, Columbia, MD) followed by tandem mass spectrometry (LC-MS/MS; API 4000; Applied Biosystems, Foster City, CA) as follows. After addition of the internal standard 3,3′,5-triiodothyropropionic acid (TITPA) to the serum sample, DITPA and TITPA were extracted using conditioned methanol and water Oasis HLB extraction cartridge, 30 mg, 1 ml (Waters Co., Milford, MA). DITPA and TITPA eluted from the cartridge with acetonitril and methanol were evaporated to dryness under a stream of nitrogen at 40 C, and the residues were reconstituted in acetonitril: 2 mm ammonium acetate (1:1). Ten microliters were injected into a 3.3 cm × 3 mm, 3 μm SUPELCOSIL LC-18 DB analytical column (SUPELCO, Bellefonte, PA). DITPA and TITPA were chromatographed using a gradient elution with 2 mm ammonium acetate (pH 3.7) and acetonitril and were detected by monitoring ions at a mass to charge ratio 509 to 127 (DITPA) and 635 to 127 (TITPA), respectively. The intraassay coefficient of variation is 5.1%.
Standard curves were also constructed to assess the linearity over the range of 20–600 ng/ml DITPA by the addition of DITPA to human serum. DITPA was obtained from Sigma-Aldrich (Milwaukee, WI) and TITPA from Toronto Research Chemicals (North York, Ontario, Canada).
Correction of DITPA interference in the T3 assay
Because DITPA interferes with the measurement of T3 by immunoassay but not T4 and rT3, correction factors were established as follows. Human sera were enriched with incremental amounts of DITPA, and T3 was measured in these sera by each of three different T3 assays as well as DITPA by LC-MS/MS. Curves correlating the amounts of T3 measured in excess of that present endogenously were plotted against DITPA concentration and formulas were established. The validity of the T3 correction for the presence of DITPA was verified by measurement of both T3 and DITPA by LC-MS/MS.
Data presentation
Grouped data are expressed as mean ± se and statistics used ANOVA. Regression analysis used the method of least mean square. Weight sd scores (SDS) were calculated using United States Centers for Disease Control and Prevention data from 2000.
Results
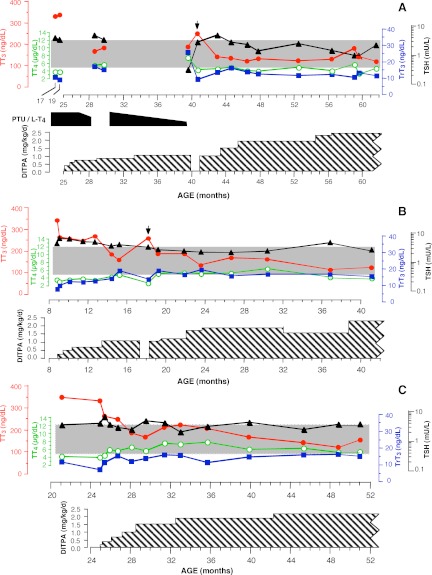
Four children received treatment with DITPA over the period of 26–40 months (subjects A1 and A2 for 40 months, subject B for 37 months, and subject C for 26 months). Treatment was initiated at the age of 8.5 months (subject B) and 25 months (subjects A1, A2, and C). Maximal doses reached were from 2.0 to 2.4 mg/kg · d. Before the institution of DITPA treatment, three children received a combination of l-T4 and propylthiouracil (PTU). Previously used in a child with MCT8 deficiency (15), our rationale was to elevate the serum T4 concentration, making it more readily available to the brain, and reducing or preventing further increase in T3, with the PTU. In subject C, this treatment was given for 1 month (from 21 to 22 months of age) but was discontinued because of development of hypogranulocytosis. The other two children, subjects A1 and A2, received the combined PTU/l-T4 treatment for 18 months (from 19 to 28 months of age and again from 30.5 to 39.9 months of age). During the second period overlapping with the administration of DIPTA, PTU/l -T4 was gradually tapered (see Fig. 2A).
Fig. 2.
The evolution of thyroid tests during the course of treatment with DITPA. The shaded area indicates the normal range for all four determinations. DITPA dose, expressed in milligrams per kilogram body weight per day, was calculated at the time the dose was changed. It is indicated by the cross-hatched area. Arrows indicate tests obtained 1 month after interruption of DITPA treatment. Note the simultaneous treatment of the twins (A) with PTU/l-T4. It is for this reason that TSH was suppressed and T4 was relatively higher on the sample obtained at age 39.5 months. In panel A, the values from measurements in sera of the twins (A1 and A2) were averaged at each data point, while panels B and C show data for subjects B and C, respectively. T3 levels are corrected for interference from DITPA, as described in Patients and Methods.
No adverse effects of DITPA were observed. In particular, there was no weight loss or gastrointestinal problems, as previously observed in adults (13, 14). All tests not directly associated with TH action remained unchanged. More specifically, there were no significant changes in blood count (hemoglobin, hematocrit, red blood cells, white blood cells, and differential or platelets). The same applied to serum electrolytes (Na, K, Cl, and HCO3−), renal tests (creatinine and blood urea nitrogen), liver tests (bilirubin, alanine transaminase, and aspartate transaminase), ammonium, and lactate.
Thyroid function tests
The evolution of serum iodothyronine concentrations as well as TSH are graphed in Fig. 2, with T3 levels corrected for interference by DITPA, as described above. In all four subjects, treatment with DITPA normalized or near normalized the thyroid function tests by reducing the serum T3 and TSH concentrations and increasing those of T4 and rT3. When the DITPA dose reached 1 mg/kg · d, T3 values were normal in all four subjects and TSH was normal in three of the four. Although rT3 and T4 concentrations rose, they remained low normal or slightly below normal. The FT4 and FT3 concentrations followed the same pattern as the total hormones (data not shown). TG levels declined in parallel with those of TSH, showing little decline in subject C, in whom changes in TSH were minimal (data not shown). Most dramatic was the decline of the T3 to T4 ratio (Table 1), reaching the normal range of 8–33 ng/μg, with DITPA doses above 1 mg/kg · d. On 1.9 mg/kg · d DITPA, the urinary iodide concentration was 26,130 μg/liter in twin A1 and 39,690 μg/liter in twin A2.
Table 1.
Parameters of thyroid hormone action on and off DITPA
| Subject | DITPA Rx | T3/T4 | SHBG | Ferritin | Cholesterol | ALP | OSTC | HR | Age range (months) |
|---|---|---|---|---|---|---|---|---|---|
| A1+A2 | Off | 87 ± 1 | 311 ± 17 | 24 ± 2 | 3.5 ± 0.1 | 202 ± 3 | 22 ± 5 | 101 ± 1 | 17–19.6 |
| On | 28 ± 1 | 275 ± 14 | 19 ± 2 | 4.2 ± 0.1 | 173 ± 8 | 23 ± 1 | 84 ± 2 | 44.6–46.5 | |
| P | <0.0001 | <0.05 | NS | <0.01 | NS | NS | <0.0.01 | ||
| B | Off | 88 ± 3 | 75 ± 1 | 111 ± 30 | 3.4 ± 0.1 | 190 ± 9 | 126 ± 2 | 8.8–9.4 | |
| On | 30 ± 3 | 18 ± 3 | 11 ± 5 | 3.6 ± 0.2 | 176 ± 4 | 94 ± 1 | 19.2–21.9 | ||
| P | <0.0002 | <0.004 | <0.02 | NS | NS | <0.001 | |||
| C | Off | 80 ± 2 | 254 ± 3 | 36 ± 4 | 3.1 ± 0.1 | 153 ± 3 | 15.6 ± 3.8 | 87 ± 1 | 18–25 |
| On | 29 ± 1 | 186 ± 12 | 24 ± 4 | 3.2 ± 0.2 | 223 ± 56 | 13.5 ± 3.8 | 96 ± 3 | 28–31 | |
| P | <0.0001 | <0.03 | NS | NS | NS | NS | NS |
With the exception of T4 and T3, all measurements were carried out in different laboratories for different subjects but the same for a given subject. Values obtained at the same time from twins A1 and A2 of family 8 were averaged. Means are calculated from three to four data points at baseline (immediately prior to DITPA treatment) and on DITPA (when the dose reached 1 mg/kg · d). ALP, alkaline phosphatase; NS, not significant.
Treatment with DITPA was interrupted for 1 month in three of the subjects (A1, A2, and B). This resulted in the return of the thyroid tests toward the pretreatment baseline (Fig. 2, arrows).
Parameters of TH action
Measurements of parameters of TH action are shown in Table 1. Because the values of most tests listed change with age, measurements at baseline closest to initiation of DITPA treatment and after the dose reached 1 mg/kg body weight were used for comparison and for statistical analysis. Significant changes were observed in the following measurements: a decline in SHBG in all subjects, sleeping heart rate in three of the four, and ferritin in 1 of the four. Serum cholesterol increased in two of the subjects. Collectively these results suggest reduced peripheral tissue TH action or improvement of the thyrotoxic effect of T3 excess. In contrast to adults without MCT8 defects, DITPA did not produce an increase in serum OSTC (14).
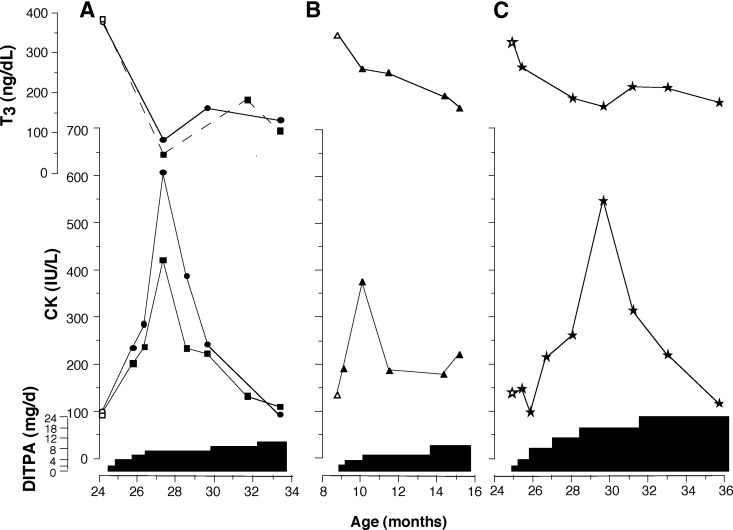
A marked increase in CK was observed in all four children early in the initiation of DITPA administration. It reached a peak 1.5–5 months into the treatment and then returned to baseline (Fig. 3). Measurement of CK isoenzymes by electrophoresis in subjects A1 and A2 showed that greater than 97% originated from skeletal muscle. The cardiac isoenzyme was 3–5% (normal < 6) and the brain isoenzyme was less than 1% (normal < 1%). Troponin I was less than 0.1 μg/liter (normal 0–0.1).
Fig. 3.
Transient increase in serum CK during the course of DITPA treatment and its correlation with the decline in serum T3. Results from the twins of family 8 are plotted separately in panel A. T3 levels are corrected for interference from DITPA, as described in Patients and Methods.
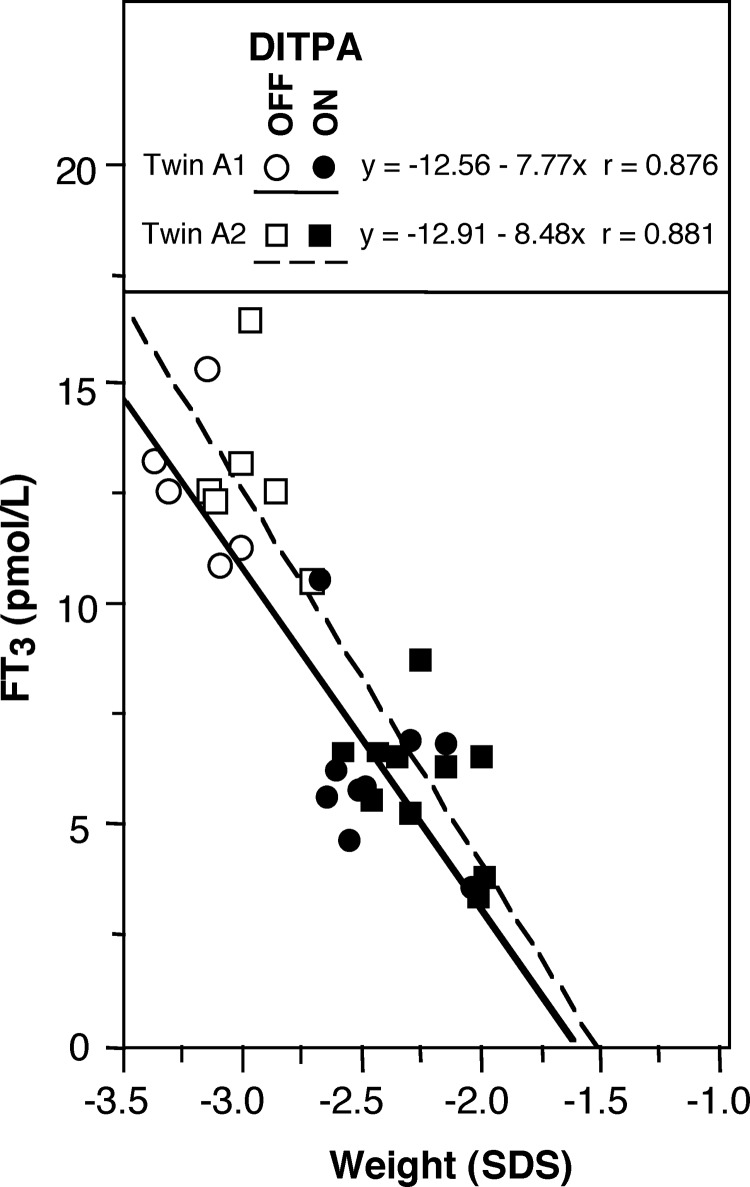
Of interest is the weight gain in the twins of family 8 (subjects A1 and A2), who had severe failure to thrive; baseline SDS −3.1 and −3.3, respectively, compared with −2.7 for subject B and −1.3 for subject C. Improved weight SDS in twins A1 and A2 showed a close correlation with the decline of T3 on DITPA treatment (Fig. 4). A less important trend of weight change was observed in subject C ranging from −1.0 to −1.5 SDS before and from −0.8 to −1.2 SDS after DITPA treatment. Subject B, on the other hand, sustained a gradual weight loss during DITPA treatment; from −2.7 SDS before treatment to −3.5 SDS by the end of 25 months of treatment.
Fig. 4.
Correlation of serum free T3 concentration and body weight in the twins of family 8. Weights, expressed as SDS, obtained from age 17 to 25 months, before treatment, and from 25 to 43 months while on DITPA are plotted (excluding periods when PTU and l-T4 were administered). Serum free T3 levels are corrected for interference from DITPA, as described in Patients and Methods.
Bone age assessed in the twins of family 8 was initially slightly advanced, 48 months at chronological age (CA) 38 months, with ratio of bone age to CA 1.26. Later it was similar to CA (60 months at CA 57 months, ratio 1.05). Bone age of subject B was slightly delayed (6 months at CA 10 months, ratio 0.6). Subject C had bone age 36 months at CA 31 months, ratio 1.16. Bone age was available (published and our cases) in nine children, not treated with DITPA, examined at ages 9–36 months. It was delayed in one, advanced in two, and normal in six.
Psychomotor development and brain
The Bayley III scale was used to assess the psychomotor development before and throughout the treatment with DITPA. Results are shown in Table 2. Progression was observed only in the twins of family 8 (subjects A1 and A2). However, their advance remained at about the same level when expressed as percent of the CA. Subject C maintained the same percent of cognitive function but fell in all other domains. Subject B showed no change and actual regression relative to advancing age.
Table 2.
Bayley developmental scores (months) before and after treatment with DITPA
| Subject | Time on DITPA | Age (months) | Cognitive | Receptive language | Expressive language | Fine motor | Gross motor |
|---|---|---|---|---|---|---|---|
| A1, A2 | 0 | 26 | 7, 10 | 13, 10 | 6, 6 | 8, 6 | 3.3, 3.3 |
| 33% | 44% | 23% | 27% | 13% | |||
| 6 | 32 | 10, 11 | 13, 11 | 8, 7 | 9, 9 | 5, 4.2 | |
| 33% | 38% | 23% | 28% | 14% | |||
| 16 | 42 | 15, 17 | 19, 20 | 8, 8 | 11, 11 | 4.75, 5 | |
| 38% | 46% | 19% | 26% | 12% | |||
| 24 | 48 | 17, 17 | 19, 20 | 8, 8 | 11, 9 | 6, 6 | |
| 35% | 41% | 17% | 21% | 13% | |||
| 30 | 54 | 17, NA | 19, 22 | 8, 10 | 11, 11 | 6, 6 | |
| 31% | 40% | 17% | 20% | 11% | |||
| B | 0 | 8 | 1 | 6 | 1 | 1 | 1 |
| 13% | 75% | 13% | 13% | 13% | |||
| 2 | 11 | 1 | 6 | 1 | 1 | 1 | |
| 9% | 55% | 9% | 9% | 9% | |||
| 13.5 | 22.5 | 1 | 6 | 1 | 1 | 1 | |
| 4% | 27% | 4% | 4% | 4% | |||
| C | 0 | 21 | 2 | 2 | 6 | 3 | 2 |
| 10% | 10% | 29% | 14% | 10% | |||
| 0 | 25 | 3.5 | 2 | 6 | 3 | 2 | |
| 14% | 8% | 24% | 12% | 8% | |||
| 7 | 32 | 3.5 | 2 | 6 | 3 | 2 | |
| 11% | 6% | 19% | 9% | 6% |
For each subject, serial assessments were performed by the same observer. Bayley scores indicate the developmental age attained (in months) for each domain. Percent figures show the Bayley score for each domain expressed as a percentage of CA at the time of the assessment (percentage figures for identical twins A1 and A2 of family 8 were averaged). NA, Not available.
Twins A1 and A2 showed abnormally delayed myelination on magnetic resonance imaging at age 9 months (see Supplemental Data). Repeat magnetic resonance imaging at age 47 months, after 22 months of DITPA treatment, showed late myelination but was now within the normal range for age. The head circumference is tracking near the 50th percentile for both twins.
Discussion
DITPA is a TH agonist that binds to the TH receptor-α and -β with almost the same affinity. However, compared with T3, the affinity to TH receptor-β is 350-fold lower (16). Similarly, compared with T4 and T3, DITPA has lower metabolic activity in vivo as shown by the reduced stimulation of hepatic α-glycerolphosphate dehydrogenase activity when given to rats in equivalent doses as T4 (17). DITPA also has nongenomic action, as demonstrated by its angiogenic effect (18).
In humans, DITPA underwent phase II clinical trials for treatment of chronic heart failure (13, 14). Twenty-one adult patients, without MCT8 deficiency but with heart failure, completed a 24-wk course of DITPA, given in incremental doses of 90 mg/d (45 mg twice daily) each for 2 wk, reaching a maximal dose of 360 mg (on the average 3.75 mg/kg · d) on the eighth week. By the fourth week, serum TSH was suppressed below normal as were, although to a lesser degree, serum T3 and T4, without an effect on the heart rate throughout the entire period of treatment. Serum total and low-density lipoprotein cholesterol, TG, and body weight declined, whereas SHBG and OSTC increased, indicating the manifestations of TH excess. These results contrast with those we observed in MCT8-deficient children, in whom the effect was the opposite, indicating a net decrease in the baseline level of TH action. Indeed, heart rate and SHBG declined significantly in all four children, whereas cholesterol increased in two and ferritin declined in one. These results should be interpreted with caution because all measurements are influenced by age and some by the state of nutrition, which could not be controlled under the conditions of the study. Body weight (SDS) increased or remained unchanged. Although the precise reason for the transient increase in CK is unclear, it could be due to the rapid decline in T3. This has been reported in children with chronic hyperthyroidism undergoing treatment with antithyroid drugs (19). The progressive weight loss of MCT8 deficiency, often requiring gastric tube feeding, was not observed in any of the children during the entire course of treatment. As a matter of fact, two gained weight in parallel with the reduction of serum T3.
The difference in the effect of DITPA in adults compared with MCT8-deficient children could be a result of the underlying genetic defect but is more likely due to a difference in the dose as well as the frequency of administration. The maximum dose of 2.4 mg/kg · d is much lower, considering that optimal drug doses in children in terms of body weight are higher than in adults. DITPA was administered to the children three times, rather than twice daily, maintaining more constant blood level and lesser peaks, given the relatively short half-life of this TH analog. Although we cannot exclude a reduced thyromimetic effect of DITPA in the presence of MCT8 deficiency, this was not observed in the Mct8-deficient mouse, which responded to DITPA in the same manner as the normal mouse. The hypothalamo-pituitary axis was not suppressed by DITPA in the MCT8-deficient children because the TSH declined only slightly and the T4 did not decline, even on the highest dose.
The observed effects of DITPA represent direct actions of this TH analog because interruption of treatment produced a trend toward a return of thyroid tests toward the baseline. This also suggests a reversible action of DITPA and that continuous administration may be required to maintain its effect.
The mode of DITPA action is undoubtedly similar if not identical to that of T3. Its availability to the central and peripheral tissues reduces the generation of T3 by deiodination through a decrease in both D1 and D2, as shown in the Mct8-deficient mouse (11). The result is normalization of the serum T3 and decrease of its metabolic effect, resulting in the reduction of the thyrotoxic state in peripheral tissue of the MCT8-deficient children. The failure of serum T4 to fully normalize despite the reduction in T3 generation is likely due to decreased MCT8-dependent secretion of T4 from the thyroid gland, increased metabolism in the kidneys, and loss of TH in the urine (20, 21). The large amount of iodine derived from DITPA degradation may also affect thyroidal secretion. Based on data from mice, DITPA corrects cerebral markers of TH deprivation (9, 21). Yet no important improvements in psychomotor function were observed by the Bayley III score. Some progress was observed in the children of family 8, whereas this was not observed in the other two children, despite the equal effect of DITPA on peripheral tissues. Several hypotheses may be advanced. First, the twins of family 8 have a missense mutation, whereas the other two have nonsense mutations producing truncated MCT8 molecules. Second, the twins have been undergoing intensive physical, mental, and occupational therapy. Third, brain damage due to TH deprivation may have occurred in embryonic life, and therefore, the treatment with DITPA was started too late in all four children to have an effect. Fourth, DITPA may not reach specific brain cells, as can occur for T3 in mouse brain (22, 23). Lastly, MCT8 may have other functions than transport of TH. On the other hand, none of the four children developed seizures when one third of MCT8-deficient subjects do. Timing of initiation of therapy may be important in preventing some of the abnormalities of TH deprivation, which become subsequently irreversible.
In summary, on the basis of this limited experience, it can be concluded that DITPA given in doses of 1–2 mg/kg · d to children with MCT8 deficiency produces no important adverse effects. It almost fully normalizes the serum thyroid tests, reduces the hypermetabolism, the tendency for weight loss, and the need for gastric tube feeding. The effect of long-term and early institution of treatment remains to be determined.
Supplementary Material
Acknowledgments
We thank Dr. Tally Lerman-Sagie (neurologist, Wolfson Medical Center, Holon, Israel) for the referral of family 8; Dr. Theo Visser (Erasmus University Medical Center, Rotterdam, The Netherlands) for the sequencing of the MCT8 gene of family 10; and Dr. Annette Feigenbaum (geneticist, The Hospital of Sick Children, Toronto, Ontario, Canada) for the referral of family 11. The developmental assessments were performed by Dr. Lee Sutton in Australia and Dr. Eva Mamak in Canada. We also acknowledge the assistance of Dr. Evgeny Berdyshev in developing the method for the measurement of DITPA by mass spectroscopy.
This work was supported in part by grants R37DK15070 and P60DK20595 from the National Institute of Diabetes and Digestive and Kidney Diseases and also Grant 5M01RR04999. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. Special thanks are due for funds donated by the Smile Foundation, with support from the Sherman family and the Esformes Research Foundation.
Current address for C.D.C.: Dipartimento di Endocrinologia e Metabolismo, Ospedale di Cisanello, Via Paradisa, 2, Pisa, 56124 Italy.
Results from this work were presented at the 8th Joint Meeting of the Lawson Wilkins Pediatric Endocrine Society and the European Society for Pediatric Endocrinology.
Disclosure Summary: All authors have nothing to declare.
For editorial see page 4362
- CA
- Chronological age
- CK
- creatine kinase
- DITPA
- diiodothyropropionic acid
- FT3
- free T3
- FT4
- free T4
- LC-MS/MS
- tandem mass spectrometry
- LLN
- lower limit of normal
- MCT8
- monocarboxylate transporter 8
- OSTC
- osteocalcin
- PTU
- propylthiouracil
- SDS
- sd score
- TG
- thyroglobulin
- TH
- thyroid hormone
- TITPA
- 3,3′,5-triiodothyropropionic acid.
References
- 1. Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. 2004. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ. 2004. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364:1435–1437 [DOI] [PubMed] [Google Scholar]
- 3. Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. 2003. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 278:40128–40135 [DOI] [PubMed] [Google Scholar]
- 4. Schwartz CE, May MM, Carpenter NJ, Rogers RC, Martin J, Bialer MG, Ward J, Sanabria J, Marsa S, Lewis JA, Echeverri R, Lubs HA, Voeller K, Simensen RJ, Stevenson RE. 2005. Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet 77:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allan W, Herndon CN, Dudley FC. 1944. Some examples of the inheritance of mental deficiency: apparently sex-linked idiocy and microcephaly. Am J Ment Defic 48:325–334 [Google Scholar]
- 6. Schwartz CE, Stevenson RE. 2007. The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome. Best Pract Res Clin Endocrinol Metab 21:307–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Visser WE, Friesema EC, Visser TJ. 2011. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol 25:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heuer H, Visser TJ. 2009. Minireview: pathophysiological importance of thyroid hormone transporters. Endocrinology 150:1078–1083 [DOI] [PubMed] [Google Scholar]
- 9. Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S. 2006. Tissue specific thyroid hormone deprivation and excess in Mct8 deficient mice. Endocrinology 147:4036–4043 [DOI] [PubMed] [Google Scholar]
- 10. Trajkovic M, Visser TJ, Mittag J, Horn S, Lukas J, Darras VM, Raivich G, Bauer K, Heuer H. 2007. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest 117:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Di Cosmo C, Liao XH, Dumitrescu AM, Weiss RE, Refetoff S. 2009. A thyroid hormone analogue with reduced dependence on the monocarboxylate transporter 8 (MCT8) for tissue transport. Endocrinology 150:4450–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morkin E, Pennock G, Spooner PH, Bahl JJ, Underhill Fox K, Goldman S. 2002. Pilot studies on the use of 3,5-diiodothyropropionic acid, a thyroid hormone analog, in the treatment of congestive heart failure. Cardiology 97:218–225 [DOI] [PubMed] [Google Scholar]
- 13. Goldman S, McCarren M, Morkin E, Ladenson PW, Edson R, Warren S, Ohm J, Thai H, Churby L, Barnhill J, O'Brien T, Anand I, Warner A, Hattler B, Dunlap M, Erikson J, Shih MC, Lavori P. 2009. DITPA (3,5-diiodothyropropionic acid), a thyroid hormone analog to treat heart failure. Phase II Trial Veterans Affairs Cooperative Study. Circulation 119:3093–3100 [DOI] [PubMed] [Google Scholar]
- 14. Ladenson PW, McCarren M, Morkin E, Edson RG, Shih MC, Warren SR, Barnhill JG, Churby L, Thai H, O'Brien T, Anand I, Warner A, Hattler B, Dunlap M, Erikson J, Goldman S. 2010. Effects of the thyromimetic agent diiodothyropropionic acid on body weight, body mass index, and serum lipoproteins: a pilot prospective, randomized, controlled study. J Clin Endocrinol Metab 95:1349–1354 [DOI] [PubMed] [Google Scholar]
- 15. Wémeau JL, Pigeyre M, Proust-Lemoine E, d'Herbomez M, Gottrand F, Jansen J, Visser TJ, Ladsous M. 2008. Beneficial effects of propylthiouracil plus L-thyroxine treatment in a patient with a mutation in MCT8. J Clin Endocrinol Metab 93:2084–2088 [DOI] [PubMed] [Google Scholar]
- 16. Chapo J, Peng Y, Pitts KR. 2007. A phosphorimager-based filter binding thyroid hormone receptor competition assay for chemical screening. J Pharmacol Toxicol Methods 56:28–33 [DOI] [PubMed] [Google Scholar]
- 17. Pennock GD, Raya TE, Bahl JJ, Goldman S, Morkin E. 1992. Cardiac effects of 3,5-diiodothyropropionic acid, a thyroid hormone analog with inotropic selectivity. J Pharmacol Exp Ther 263:163–169 [PubMed] [Google Scholar]
- 18. Mousa SA, O'Connor L, Davis FB, Davis PJ. 2006. Proangiogenesis action of the thyroid hormone analog 3,5-diiodothyropropionic acid (DITPA) is initiated at the cell surface and is integrin mediated. Endocrinology 147:1602–1607 [DOI] [PubMed] [Google Scholar]
- 19. Mizuno H, Sugiyama Y, Nishi Y, Ueda N, Ohro Y, Togari H. 2006. Elevation of serum creatine kinase in response to medical treatment of Graves' disease in children. Acta Paediatr 95:243–245 [DOI] [PubMed] [Google Scholar]
- 20. Di Cosmo C, Liao XH, Dumitrescu AM, Philp NJ, Weiss RE, Refetoff S. 2010. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J Clin Invest 120:3377–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trajkovic-Arsic M, Visser TJ, Darras VM, Friesema EC, Schlott B, Mittag J, Bauer K, Heuer H. 2010. Consequences of monocarboxylate transporter 8 deficiency for renal transport and metabolism of thyroid hormones in mice. Endocrinology 151:802–809 [DOI] [PubMed] [Google Scholar]
- 22. Hernandez A, Quignodon L, Martinez ME, Flamant F, St. Germain DL. 2010. Type 3 deiodinase deficiency causes spatial and temporal alterations in brain T3 signaling that are dissociated from serum thyroid hormone levels. Endocrinology 151:5550–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hernandez A, Morte B, Belinchón MM, Ceballos A, Bernal J. 2012. Critical role of types 2 and 3 deiodinases in the negative regulation of gene expression by T(3) in the mouse cerebral cortex. Endocrinology 153:2919–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.