Abstract
Development of successful vaccines against glycotopes remains a major challenge. In the current studies, we have successfully developed a novel carrier protein for glycotopes based on the concept of antigen clustering and specific stimulation of T helper cells to mount strong antibody response to glycotopes. The bipartite carrier protein consists of a tandem repeat of a cysteine-rich peptide for docking of clustered glycotopes to effectively activate B cells and an Fc domain for antigen delivery to antigen presenting cells (APCs). To demonstrate its utility, we conjugated the tumor-specific monosaccharide antigen Tn to this novel carrier protein and successfully developed a Tn vaccine against cancer in animal models. The Tn vaccine effectively elicited high-titer IgG1 antibodies against Tn in immunized mice, and effectively suppressed the development of prostate cancer in Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) mice. Our results suggest that this novel bipartite carrier protein could be effectively used for developing anti-glycotope vaccines such as the anticancer Tn vaccine.
Keywords: Glycotope, carrier protein, vaccine, tumor immunotherapy, Tn antigen
1. Introduction
Among the cell surface tumor markers, tumor-associated carbohydrate antigens (TACAs) have spurred intense research into exploiting TACAs for the development of anticancer vaccines [1–6]. Some mucin-related carbohydrate epitopes, such as Tn (GalNAcα1-O-Ser/Thr), STn (NeuAcα2–6GalNAcα1-O-Ser/Thr) and TF (Galβ1–3GalNAcα/β-O-Ser/Thr), are highly overexpressed in carcinomas such as breast, prostate, and ovarian cancers [5, 7, 8], and have been targeted for cancer vaccine development [2, 4, 9, 10].
Glycotopes are haptens which need to be conjugated to carrier proteins for inducing high affinity IgG antibodies. Glycoptope-carrier protein conjugates, through the MHC-II pathway in antigen presenting cells (APCs), can activate type II T helper cells that aid class switching and affinity selection of stimulated B cells leading to secretion of high affinity IgG antibodies[11–13]. Consequently, designing effective carrier proteins for glycotopes is an essential step for developing successful glycotope-based vaccines. Indeed, carrier proteins such as keyhole limpet hemocyanin (KLH), bovine serum albumin (BSA) and Tetanus Toxoid (TT) have been used for developing TACA-conjugated vaccines [3, 14–16]. However, these carrier proteins still present several problems for developing TACA vaccines [10]. For example, the conjugation reaction is difficult to control and the immune response against unconjugated linker leads to epitope suppression [17]. Furthermore, the carrier proteins, which are often huge in size, contain multiple B cell epitopes which can readily induce antibody response against the carrier proteins themselves.
In the current studies, we report a novel synthetic bipartite carrier protein ((Mr = 34 kDa) which contains the IgG Fc domain (referred to as IFD) for aiding uptake/presentation by APCs and a tandem repeated cysteine-rich peptide (referred to as the antigen clustering domain or ACD) for glycotope docking. It has been demonstrated that carrier proteins containing the immunoglobulin (Ig) Fc domain (IFD) facilitate uptake through receptor-mediated endocytosis [13, 18–21] and hence antigen presentation through the MHC II pathway in APCs [22–24]. It has also been demonstrated that carrier proteins containing antigen clustering domain (ACD) are highly effective in inducing strong B cell activation [12, 25–27]. Indeed, we show in the current studies, such a bipartite carrier protein is highly effective in inducing high affinity IgG1 antibody against the glycotope Tn. Our results could suggest the general applicability of using this bipartite carrier protein for developing glycotope-based vaccines.
2. Materials and Methods
2.1. Materials
The animal protocol was approved by Academia Sinica Institutional Animal Care and Utilization Committee. Human prostate cancer specimens for staining with anti-Tn antibody were retrieved from the archives of the Department of Pathology, Tri-Service General Hospital (Taipei, Taiwan). Samples were fully encoded to protect patient privacy and were used through a protocol approved by the Institutional Review Board of Tri-Service General Hospital National Defense Medical Center.
Female New Zealand White rabbits and female BALB/c mice were acquired from the animal center at National Taiwan University.
2.2. Construction of the plasmid encoding the bipartite carrier protein
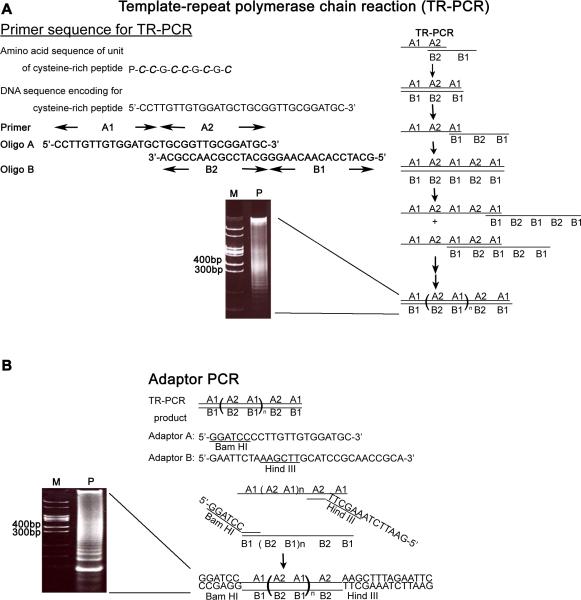
The DNA fragments consisting of tandem repeats of the 30-mer DNA encoding the cysteine-rich peptide (Pro-Cys-Cys-Gly-Cys-Cys-Gly-Cys-Gly-Cys) was generated by template-repeat polymerase chain reaction (TR-PCR) [26]. The seven repeats-containing DNA fragment was excised from the plasmid DNA and subcloned into different expression vectors, which express mFc (GenBank ID: EF392839), rFc (GenBank ID: K00752) or PEIa (GenBank ID: K01397.1). The following plasmids were created: prFc(Cys42)Histag2, pmFc(Cys42)Histag2, and pPEIa(Cys42)Histag2. The plasmid DNA encoding a single repeat was cloned into the GST expression vector to create pGST(Cys6).
2.3. Expression and purification of the bipartite carrier proteins
Plasmids were transformed individually into the E. coli strain BL21(DE3)pLysS, and cultured in LB medium containing ampicillin (50 μg/ml) at 37 °C. After induction, the bacteria were harvested and resuspended in the binding buffer (20 mM sodium phosphate, pH 7.9, and 8 M Urea) containing 1 mM DTT. After sonication and centrifugation, supernatants were applied to the nickel column (Pharmacia). The column was washed with the binding buffer containing 0.2 mM Tris(2-carboxyethyl) phosphine (TCEP) and 100 mM imidazole. Recombinant proteins were then eluted with the binding buffer containing 0.2 mM TCEP and 500 mM imidazole. Plasmid GST(Cys6) was transformed into the E. coli strain TOP10. The transformant was cultured in LB under the same conditions as described above. The bacteria were harvested and resuspended in PBS containing 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1 mg/ml lysozyme. GST(Cys6) was purified by glutathione-agarose affinity column (Pharmacia), and eluted with 50 mM Tris-HCl containing 10 mM glutathione, pH 8.0.
2.4. Preparation of glycotope-carrier protein conjugates
Glycotope, are the carbohydrate epitopes, which are normally the carbohydrate part of a glycoprotein or glycolipid, such as Tn, STn, sialic acid and GM3. The synthesis and purification of Tn, STn, sialic acid and GM3 were described in previously studies [28–30]. Tn was conjugated to rFc(Cys42)Histag2, mFc(Cys42)Histag2 or PEIa(Cys42)Histag2 at a glycotope/carrier protein weight ratio of 5 to 1 (STn was used as example to find an optimal condition for maximum conjugation)(Supplementary Figure 1). Conjugation was performed in elution buffer (20 mM sodium phosphate, pH 7.9, 8 M urea, 500 mM imidazole, and 0.2 mM TCEP). After 48 h, conjugates were refolded against PBS containing 0.2 mM TCEP. GST(Cys6) was dialyzed against PBS containing 0.2 mM TCEP. Different glycotopes and Linker (N-Succinimidyl-6-Maleimidocaproate) were conjugated to GST(Cys6) at 4 °C for 48 h.
2.5. Immunization of mice and rabbits with Tn-carrier protein conjugates
Six- to eight-week-old female BALB/c mice and ten-week-old TRMAP mice (Jackson Laboratory) were immunized subcutaneously with 10 μg of mFc(Cys42-Tn)Histag2 in complete Freund's adjuvant, followed by immunizing with the same dose of conjugates in incomplete Freund's adjuvant three times at biweekly intervals. For immunization of rabbits, sixteen-week-old female New Zealand White rabbits were subcutaneously injected with 100 μg of rFc(Cys42-Tn)Histag2 following the same schedule described above.
2.6. Immunocompetition assay
Approximately 5×105~1×106 HeLa cells (purchased from ATCC) were cultured on coverslips in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (Invitrogen) at 37°C in 5% CO2. Cells were fixed, permeabilized and stained with rabbit anti-Tn serum (1:1000 dilution) which was pre-incubated with different amount of Tn antigen (0, 1, 10, 50, and 100 μg) for 30 min at 37°C. Coverslips were then incubated for 1 h at 37°C. Bound antibodies were visualized following treatment with the goat anti-rabbit Texas Red (1:500 dilution) and incubation at 37°C for 30 min. Nuclei were stained with DAPI (Thermo Scientific) (1:5000 dilution) for 10 min at room temperature.
2.7. Quartz crystal microbalance (QCM) analysis
The 9-MHz gold chips were available from ANT Tech (Taipei, Taiwan). The binding experiments were performed using an ANTQ300 instrument equipped with a flow-injection system. For Kd measurement, PEIa(Cys42-Tn)Histag2 was coated on the chip surface, and serial dilutions of purified anti-Tn IgG antibody ranging from 0.0625 μM to 2 μM were injected onto the coated chip. PBS was used to wash out the unbound molecules at a flow rate of 40 μl/min.
2.8. Immunohistochemical (IHC) staining of cancer tissues with anti-Tn antibody
Tissue sections were dewaxed in xylene and rehydrated in alcohol. Antigen retrieval was carried out by incubating tissue sections in 0.01 mM citrate buffer (pH 6.0) at 95°C for 40 min. Endogenous peroxidase was blocked with 0.3 % hydrogen peroxide for 30 min. Sections were then incubated with 5% normal horse serum in PBS for 30 min at room temperature in order to block nonspecific antibody reaction. After washing with TBS plus 0.1 % Tween 20, slides were incubated for 40 min at 4 °C with anti-Tn antiserum. Sections were then rinsed in TBS plus 0.1 % Tween 20 and incubated for 10 min at room temperature with HRP polymer conjugated secondary antibody (SUPERPICTURE POLYMER KIT, Zymed Laboratories, Inc.). Subsequently, sections were stained with DAB chromogen/substrate, counterstained with Mayer's hematoxylin, dehydrated, and then mounted.
2.9. Isotyping of anti-Tn sera
GST(Cys6-Tn) was coated on 96-well flat-bottomed plates (Falcon) at a concentration of 0.15 μg per well. One-hundred microliter of diluted anti-Tn serum (diluted 5000-fold in PBS containing 0.1 % BSA) was added to each coated well. After incubating at 37°C for 2 h, the wells were washed with PBS three times. Subsequently, different rabbit anti-mouse isotype antibodies (ZYMED Laboratories, Invitrogen) were added and the plates incubated 37°C for 1 hr. After incubation, the plates were washed three times, followed by addition of peroxidase-conjugated goat anti-rabbit immunoglobulin and continued incubation at 37 °C for 1 h. The substrate solution contained 0.54 mg/ml 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) and 0.01% H2O2, and 0.1 M citric acid (pH 4.2). Absorbance was read at 410 nm using the Multiscan RC microplate reader (Labsystems, Finland).
2.10. Statistical analysis
Data were analyzed using the SPSS 13.0 software and a p value of less than 0.05 was considered significant.
3. Results
3.1. Construction of a novel bipartite carrier protein with a synthetic cysteine-rich peptide fused with an IgG Fc fragment
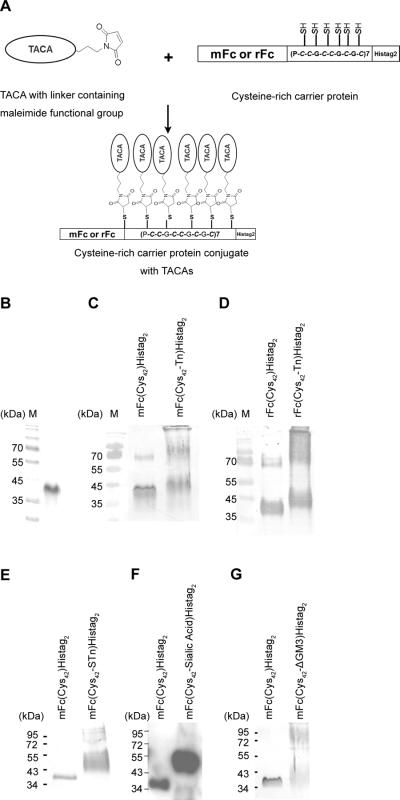
The poor immunogenicity and the inability to activate T-helper cells present major obstacles for the development of effective glycotope-based vaccines. To overcome these hurdles, a small bipartite fusion protein consisting of a synthetic cysteine-rich peptide and an IgG Fc fragment was constructed. The synthetic cysteine-rich peptide is a tandemly repeated synthetic peptide with the basic repeating unit being a 10 amino-acid sequence (Pro-Cys-Cys-Gly-Cys-Cys-Gly-Cys-Gly-Cys) that contains six cysteine residues for glycotope conjugation (Fig. 1). The DNA encoding the cysteine-rich peptide was created by using the template-repeat polymerase chain reaction (TR-PCR) (Fig. 1) which is designed to produce tandem repeated DNA sequences [26]. The PCR products are a mixture of DNA sequences with variable tandem repeats. The 7-repeat DNA fragment was subcloned into the plasmid DNA vector that expresses either rFc (rabbit Fc) or mFc (mouse Fc) at the N-terminus and his-tagged (Histag2) at the C-terminus. The 7-repeat DNA fragment was inserted in-frame between the Fc and Histag2. The fusion protein was purified by the nickel resin, and the purity reached over 95% homogeneity as judged by coomassie-stained SDS-gel [as an example, see purified mFc(Cys42)Histag2 protein in Fig. 2B].
Figure 1. Construction of a bipartite carrier protein for tumor-associated carbohydrate antigens (TACAs).
(A) Generation of DNA fragments encoding tandemly repeated cysteine-rich peptides by template-repeat polymerase chain reaction (TR-PCR). The TR-PCR procedure uses two 30-mer oligos, A1A2 and B2B1, both of which encode the same 10 amino acid cysteine rich peptide (P-C-C-G-C-C-G-C-G-C). A1, A2, B2 and B1 are each 15-mer in size. A1 is complementary to B1, and A2 is complementary to B2. The PCR reactions involved 30 cycles of denaturation at 94 °C for 60s, annealing at 62 °C for 60s, and polymerization at 72 °C for 60s, followed by a final polymerization step at 72 °C for 10 min. The product was analyzed by electrophoresis on 8% polyacrylamide gel (M: marker; P: the product of TR-PCR). (B) Creating the restriction site for subcloning by adaptor-PCR. The products of TR-PCR (1:100 dilution) were subjected to adapter-PCR with the adapter primers A and B. The amplification protocol consisted of 4 cycles of denaturation at 94 °C for 60s, annealing at 54 °C for 60s, and polymerization at 72 °C for 60s, followed by a final polymerization step at 72 °C for 10 min. The products were analyzed by electrophoresis on 8% DNA-PAGE and the DNA fragment containing 7 tandem repeat units (each unit is a 30-mer nucleotide) was cloned into a T vector. M: marker; P: the product of adaptor-PCR.
Figure 2. Single-step conjugation of glycotopes to the bipartite carrier protein.
(A) The single-step conjugation scheme. (B~G) Monitor the conjugation efficiency by immunoblotting. (B) The coomassie-stained of purified mFc(Cys42)Histag2. (C ~G) The conjugation efficiency of Tn, STn, sialic acid or GM3 to mFc(Cys42)Histag2 or rFc(Cys42)Histag2 was monitored by SDS PAGE, followed by immunobloting with anti-His-tag antibody. The increase in high molecular weight species reflects the degree of carbohydrate antigen conjugation.
3.2. An efficient single-step conjugation of tumor-associated carbohydrate antigens (TACAs) to the bipartite carrier protein
Traditionally, TACA-carrier protein conjugation is a two-step reaction which results in low yields. In the first step, KLH or BSA is conjugated to the heterobifunctional (SH, NH2) cross-linker, such as m-maleimidobenzoyl-N-hydroxysuccinimide ester (MBS). In the second step, the KLH-MBS or BSA-MBS reacts with TACA to form TACA-carrier protein conjugates. However, unconjugated linkers, which elicits immune response against the linker, leading to reduced immune response to TACAs [17]. To overcome this problem, we developed a single-step reaction for the conjugation of glycotope to the carrier protein. A glycotope containing the maleimide-functional group was chemically synthesized, and conjugated to the thiol-groups of the cysteine residues in the bipartite carrier protein (Fig. 2A). The efficiency of the single-step conjugation reaction can be monitored, albeit qualitatively, by SDS-PAGE, followed by immunoblotting with the anti-His-tag antibodies. The results of individual conjugation reactions were shown in Fig. 2C–G. The apparent reduced molecular weight of the carrier protein (Mr about 40 kDa) was increased after conjugating with Tn, STn, sialic acid or GM3.
3.3. Glycotope-carrier protein conjugates induce high-affinity anti-glycotope IgG antibodies
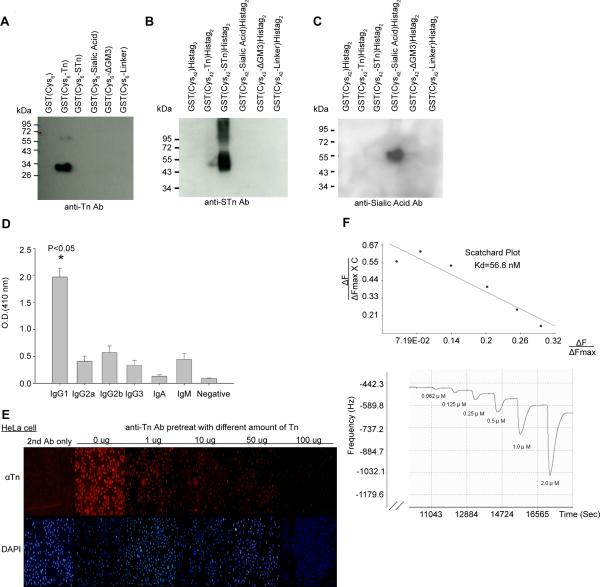
After immunization, the specificity of individual anti-glycotope antibodies was analyzed by Western blotting (Fig. 3A–C). The sera were shown to exhibit a high degree of specificity against Tn, STn or sialic acid respectively. In addition, the mouse sera were analyzed by antibody isotyping. As shown in Fig. 3D, the immunized mouse sera contained primarily IgG rather than IgM antibodies (P < 0.05). The specificity of anti-Tn antisera obtained from mice immunized with mFc(Cys42-Tn)Histag2 was also demonstrated by immunostaining of HeLa cells. As shown in Fig. 3E, the anti-Tn serum was able to stain HeLa cells as monitored by the immunofluorescent assay. Exogenously added Tn was shown to reduce the immunofluorescence in a dose-dependent manner, suggesting that the anti-Tn antiserum is Tn-specific (Fig. 3E). In addition, we have examined the binding specificity of anti-Tn antibody against Tn by flow cytometry. Jurkat cells can be stained by anti-Tn antibody, and the binding can be competed by Tn. (Supplementary Figure 2). We have also determined the binding constant of the purified anti-Tn antibody using quartz crystal microbalance (QCM). As shown in Fig. 3F, Scatchard analysis showed that the Kd value of the anti-Tn antibody for binding Tn is about 56.8 nM.
Figure 3. Glycotope-carrier protein conjugates induce high affinity anti- IgG antibody in mice.
Anti-Tn, -STn and –sialic acid sera from individual mFc(Cys42)Histag2 conjugated products induced highly specific antibodies for their respected glycotope (A to C). GST(Cys6) or GST(Cys42) conjugated with Tn, STn, sialic acid, ΔGM3 (It contains the carbohydrate, but not lipid, part of GM3 ), or linker (N-succinimidyl-6-maleimidocaproate) was analyzed by 12.5% SDS-PAGE, followed by immunoblotting with the sera obtained from the mice immunized with mFc(Cys42)Histag2 - glycotope conjugates.
(D) Isotyping analysis of anti-Tn sera of immunized mice (n=5). Isotyping was performed as described in Materials and Methods. Negative control: secondary antibody is normal rabbit serum. Other secondary antibodies were different rabbit anti-mouse-isotypes antibodies. (E) The binding of anti-Tn antisera to HeLa cells was antagonized by exogenously added Tn. Immunocompetition assay was performed as described in Materials and Methods. (F) Measurement of Kd of anti-Tn IgG antibody using QCM. QCM analysis was performed as described in Materials and Methods. ΔFmax denoted the maximal frequency shift for fully saturated surface (Hz) and ΔF denoted the frequency shift (Hz). C denoted the concentration of anti-Tn IgG antibody.
3.4. The Tn-conjugated vaccine prolongs the survival of TRAMP mice and suppresses prostate tumor metastasis
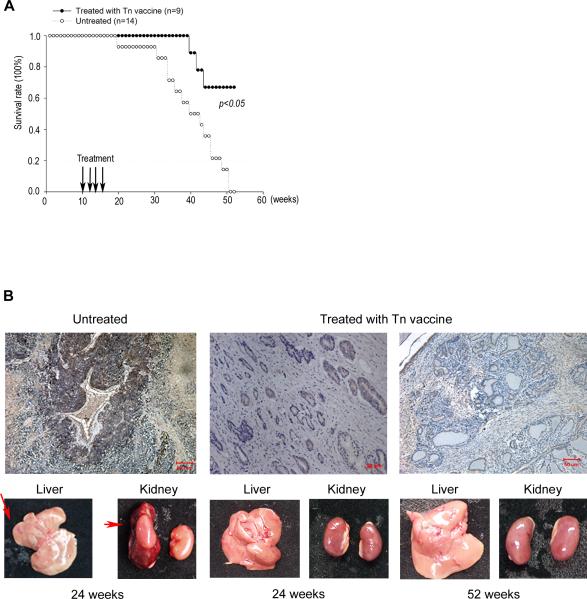
The ability of the Tn-carrier protein conjugates (Tn vaccine) to elicit strong immune response and produce high affinity IgG antibodies prompted us to examine its possible use as an anticancer vaccine in the Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) model [31]. At the 39th week, all immunized TRAMP mice survived, while the survival rate of untreated mice was only 57%. At the 52nd week, 67% of immunized TRAMP mice survived, while all control mice died (Fig. 4A). Furthermore, untreated control TRAMP mice were not only diagnosed with the prostate tumor but also liver and kidney metastases at the 24th week (lower-left panel of Fig. 4B). By contrast, Tn-immunized TRAMP mice exhibited no detectable metastasis in either the liver or the kidney (lower-middle panel of Fig. 4B). Even at the 52nd week, Tn-immunized TRAMP mice exhibited no detectable liver or kidney metastasis (lower-right panel of Fig. 4B). Interestingly, Tn expression in the prostate was decreased in Tn vaccine immunized TRAMP mice as evidenced by the reduced IHC staining of the prostate tissue (upper panels of Fig. 4B). These results demonstrate that the Tn vaccine developed in the current studies is effective to prevent prostate cancer development in the TRAMP model.
Figure 4. Anti-Tn vaccine prolongs the survival of TRAMP mice and suppresses tumor metastasis.
Ten-week old male TRAMP mice immunized subcutaneously with anti-Tn vaccine four times at biweekly intervals. The non-immunized TRAMP mice served as a control group. (A) The survival of Tn vaccine-treated TRAMP mice (n=9) was significantly higher than the untreated mice (n=14). At the 39th week, Tn-immunized TRAMP mice were all viable, but only 57% of control mice survived. At the 52nd week, 67% of the Tn-immunized TRAMP mice survived, while all the control mice died. (B) Tn vaccine suppressed metastasis of prostate tumors in TRAMP mice. Top panels: Comparison of immunohistochemical staining results of the prostate of anti-Tn vaccine-immunized TRAMP mice. Note that Tn expression was significantly reduced in the prostate of Tn vaccine-treated TRAMP mice at both 24th (middle) and 52nd weeks (right) as compared to the control TRAMP mice at the 24th week (left). Bottom panels: Control TRAMP mice showed both liver and kidney metastases (red arrows) at the 24th week. In contrast, no metastasis was detected in the liver or kidney at the 24th week or the 52nd week in the Tn-immunized TRAMP mice. (Magnification × 40, scale bar measured 50 μm)
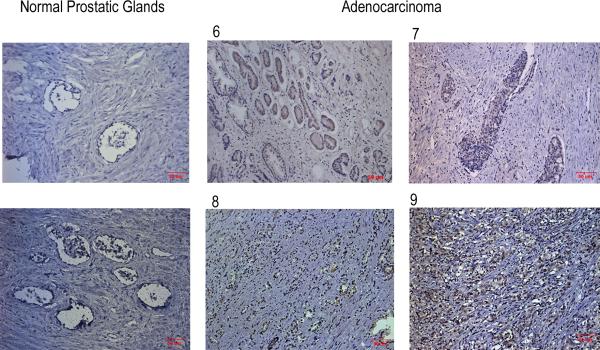
3.5. High-level expression of Tn in human prostate cancer as examined by the anti-Tn antibody
Prostate tumor specimens were obtained from 26 prostate cancer patients and the corresponding clinicopathological features were shown in Table 1a. Initially, Tn expression was examined by immunohistochemical staining of the normal prostate tissue and four prostate cancer tissues with different Gleason scores. As shown in Fig. 5, the Tn signal was positively correlated with the Gleason score of the prostate tumors, suggesting a positive correlation between Tn expression and the degree of malignancy of prostate tumors. We then performed immunohistochemical staining on all 26 prostate tumor tissues. The data were summarized in Table 1b. A strong correlation between Tn expression and the clinicopathological features of prostate tumors was observed. High-level expression of Tn is correlated with poor differentiation (p<0.001), high Gleason score (p<0.001) and high PSA level (p<0.001). This study demonstrates that the anti-Tn antibody can be used effectively to distinguish the pathological stages of prostate cancer tissues.
Table 1a.
Clinicopathological features of 26 prostate cancer patients.
| Total number | 26 |
| Median age | 71 yrs |
| Tumor size | |
| 1 | 7(26.9%) |
| 2 | 10(38.5%) |
| 3 | 7(26.9%) |
| 4 | 2(7.7%) |
| Pathological grading | |
| Well | 2(7.7%) |
| Moderate | 12(46.2%) |
| Poor | 12(46.2%) |
| Gleason score | |
| <7 | 10(35.8%) |
| ≥7 | 16(61.5%) |
| PSA level(ng/ml) | |
| <4 | 3(11.5%) |
| 4~10 | 11(42.3) |
| 10~20 | 6(23.1%) |
| >20 | 6(23.1%) |
The clinicopathological features of 26 prostate cancer patients were classified into several groups according to tumor size, differentiation, Gleason grade, and PSA level.
Figure 5. Tn expression correlates with the histological grading of the human prostate cancers.
Comparison of immunohistochemical staining results of Tn staining between the normal human prostate and cancerous tissue specimens was performed using rabbit anti-Tn antiserum. Four representative prostatic adenocarcinomas graded by the Gleason grading system (scored 6, 7, 8, and 9) showed gradient positivities of Tn staining (shown in both upper and lower middle and right panels), while Tn staining of two normal prostate specimens (shown in the left upper and lower panels) were negative. (Magnification × 100, scale bar measured 50 μm)
Table 1b.
Correlation between clinicopathological features and Tn expression in prostate cancers.
|
|
Tn expression |
|
||
|---|---|---|---|---|
| Clinicopathological features | Number | Low | High | p value |
| Pathological grading | <0.001* | |||
| Well | 2 | 2(100%) | 0(0%) | |
| Moderate | 12 | 9(75%) | 3(25%) | |
| Poor | 12 | 4(33%) | 8(67%) | |
| Gleason score | <0.001* | |||
| <7 | 10 | 10(100%) | 0(0%) | |
| ≥7 | 16 | 3(18.8%) | 13(81.2%) | |
| PSA level ng/ml (mean) | 7.41 | 40.19 | <0.001* | |
The Tn expression was based on the intensity and distribution of Tn estimated from immunohistochemical staining. The representative results are shown in Fig. 5. The data shown in this table were based on 26 prostate tumor tissues. The detail results of Tn levels, Gleason score, and PSA levels of each individual patient are presented in Supplementary Figure 4.
4. Discussion
The bipartite carrier protein developed in the current studies is based on two concepts, clustered antigens for effective B cell activation and Fc-mediated antigen uptake/presentation for T helper activation. Clustered or aggregated antigens are well known to be an effective means to activate B cells through B cell receptor clustering [11, 12, 25, 32, 33]. In fact, our earlier studies have demonstrated that vaccines based on the concept of clustered antigens can induce strong antibody response against weak or self-antigens [26]. Also, prior studies have indicated that clustered TACAs can induce stronger immune response than mono-TACAs [34, 35]. Based on the concept of clustered antigen, our bipartite carrier protein was designed to contain a glycotope docking site that is consisted of seven tandemly repeated 10-mer peptide (P-C-C-G-C-C-G-C-G-C). Conjugation of glycotopes to this docking site can result in highly clustered glycotopes on the carrier protein. This synthetic cysteine-rich peptide can be conveniently redesigned in the future to improve antigen clustering efficiency for B cell activation. For example, the spacing between the cysteine residues and the total number of cysteine residues can ultimately determine the optimal activation of the B cell receptors.
The second design concept of the bipartite carrier protein is based on the Fc-mediated antigen uptake/presentation for T helper activation. Previous studies have demonstrated that antigens fused with the IgG Fc region increase the Fc receptor-mediated antigen uptake by APCs, resulting in type II T helper activation and subsequent class switching and affinity maturation of B cells [36–38]. In addition, fusion of antigen to IgG Fc can additionally extend the serum half-life of the antigen [38, 39]. The use of IgG Fc in the bipartite carrier protein design could represent an improvement over the previous carrier protein design which employs the bacterial toxin receptor-binding domain for facilitating cellular antigen uptake since the former (i.e. IgG Fc-antigen fusion) is more specific for antigen presenting cells (APCs) while the latter is not expected to exhibit any specificity [13, 40, 41]. Consequently, the former is likely to specifically induce antibody response through activation of type II T helper cells and hence the IgG antibody response [13, 36, 42] while the latter may also elicit cell-mediated immunity leading to perhaps tissue damage [40].
The current studies on the design and application of a bipartite carrier protein may have important implications in glycotope-based vaccine development in the future. Firstly, the conjugation reaction to our carrier protein is a single-step reaction which can largely prevent the unconjugated linker on the surface of the carrier protein (Fig.2B~G and Fig. 3A~C). Secondly, the carrier protein is small in size and does not contain extra glycan that can elicit cross-reactive immune response, and the result has shown in Supplementary Figure 3. For example, immunization with KLH alone in rats is known to induce antibodies which recognize the TF antigen and its ß-enantiomer [43]. Our recombinant carrier protein, isolated from Escherichia coli, is free of carbohydrates and can thus avoid this problem. It should also be pointed out that, in addition to its application to glycotopes, the bipartite carrier protein reported here could serve as a platform for inducing antibody response against weak immunogens, and may be useful in general vaccine development.
Our results indicate that TACAs can be efficiently conjugated to the bipartite carrier protein, producing high-affinity and high-specificity anti-glycotope IgG antibodies (Fig. 3). Indeed, we have successfully employed the Tn-bipartite carrier protein conjugates as an anticancer vaccine in animal models. The Tn vaccine reported here is highly effective in preventing the development prostate cancer in the TRAMP mouse model (Fig. 4). It also prolonged the survival of TRAMP mice and essentially eliminated metastasis in the liver and kidney. These results suggest the potential use of the Tn vaccine for cancer prevention and treatment in the future.
Using the anti-Tn antibodies, we have also demonstrated a correlation between Tn expression and the malignancy of the prostate cancer in patients (Fig. 5, and Table 1). These observations not only validate the use of Tn vaccine in prostate cancer prevention and therapy, but also suggest the possible diagnostic use of the anti-Tn antibodies. It should be noted that Tn is highly overexpressed in many tumors [6, 8], suggesting a potential broader therapeutic and diagnostic application of the anti-Tn vaccine.
Supplementary Material
Highlights
A novel carrier protein has been developed for glycotope-based vaccines.
The immunogen can activate both B cells and antigen presenting cells.
This study offers a platform for anti-glycotope vaccine development.
Acknowledgments
This work was supported by grants (to JH) from the Academia Sinica (94C002 K) and the Department of Health (DOH97-TD-I-111-TM027), DOH100-TD-C-111-008 (to Jacqueline Whang-Peng) in Taiwan, and an NIH grant CA39662 (to LFL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ragupathi G. Carbohydrate antigens as targets for active specific immunotherapy. Cancer Immunol Immunother. 1996 Nov;43(3):152–7. doi: 10.1007/s002620050316. [DOI] [PubMed] [Google Scholar]
- [2].Slovin SF, Keding SJ, Ragupathi G. Carbohydrate vaccines as immunotherapy for cancer. Immunol Cell Biol. 2005 Aug;83(4):418–28. doi: 10.1111/j.1440-1711.2005.01350.x. [DOI] [PubMed] [Google Scholar]
- [3].Vliegenthart JF. Carbohydrate based vaccines. FEBS Lett. 2006 May 22;580(12):2945–50. doi: 10.1016/j.febslet.2006.03.053. [DOI] [PubMed] [Google Scholar]
- [4].Dube DH, Bertozzi CR. Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005 Jun;4(6):477–88. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- [5].Zhang S, Zhang HS, Cordon-Cardo C, Reuter VE, Singhal AK, Lloyd KO, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: II. Blood group-related antigens. Int J Cancer. 1997 Sep 26;73(1):50–6. doi: 10.1002/(sici)1097-0215(19970926)73:1<50::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- [6].Zhang S, Cordon-Cardo C, Zhang HS, Reuter VE, Adluri S, Hamilton WB, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int J Cancer. 1997 Sep 26;73(1):42–9. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- [7].Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med. 1997 Aug;75(8):594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- [8].Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984 Jun 15;224(4654):1198–206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- [9].Kobata A, Amano J. Altered glycosylation of proteins produced by malignant cells, and application for the diagnosis and immunotherapy of tumours. Immunol Cell Biol. 2005 Aug;83(4):429–39. doi: 10.1111/j.1440-1711.2005.01351.x. [DOI] [PubMed] [Google Scholar]
- [10].Buskas T, Thompson P, Boons GJ. Immunotherapy for cancer: synthetic carbohydrate-based vaccines. Chem Commun (Camb) 2009 Sep;28(36):5335–49. doi: 10.1039/b908664c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Thyagarajan R, Arunkumar N, Song W. Polyvalent antigens stabilize B cell antigen receptor surface signaling microdomains. J Immunol. 2003 Jun 15;170(12):6099–106. doi: 10.4049/jimmunol.170.12.6099. [DOI] [PubMed] [Google Scholar]
- [12].Puffer EB, Pontrello JK, Hollenbeck JJ, Kink JA, Kiessling LL. Activating B cell signaling with defined multivalent ligands. ACS Chem Biol. 2007 Apr 24;2(4):252–62. doi: 10.1021/cb600489g. [DOI] [PubMed] [Google Scholar]
- [13].Gosselin EJ, Wardwell K, Gosselin DR, Alter N, Fisher JL, Guyre PM. Enhanced antigen presentation using human Fc gamma receptor (monocyte/macrophage)-specific immunogens. J Immunol. 1992 Dec 1;149(11):3477–81. [PubMed] [Google Scholar]
- [14].Kaiser A, Gaidzik N, Westerlind U, Kowalczyk D, Hobel A, Schmitt E, et al. A synthetic vaccine consisting of a tumor-associated sialyl-T(N)-MUC1 tandem-repeat glycopeptide and tetanus toxoid: induction of a strong and highly selective immune response. Angewandte Chemie. 2009;48(41):7551–5. doi: 10.1002/anie.200902564. [DOI] [PubMed] [Google Scholar]
- [15].Kudryashov V, Ragupathi G, Kim IJ, Breimer ME, Danishefsky SJ, Livingston PO, et al. Characterization of a mouse monoclonal IgG3 antibody to the tumor-associated globo H structure produced by immunization with a synthetic glycoconjugate. Glycoconj J. 1998 Mar;15(3):243–9. doi: 10.1023/a:1006992911709. [DOI] [PubMed] [Google Scholar]
- [16].Guo Z, Boons G-J, ebrary Inc . Carbohydrate-based vaccines and immunotherapies. Wiley; Hoboken, N.J.: Oxford, UK: 2009. p. xviii.p. 408. (Wiley series in drug discovery and development). 8 p. of plates. [Google Scholar]
- [17].Buskas T, Li Y, Boons GJ. The immunogenicity of the tumor-associated antigen Lewis(y) may be suppressed by a bifunctional cross-linker required for coupling to a carrier protein. Chemistry. 2004 Jul 19;10(14):3517–24. doi: 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- [18].Snider DP, Kaubisch A, Segal DM. Enhanced antigen immunogenicity induced by bispecific antibodies. J Exp Med. 1990 Jun 1;171(6):1957–63. doi: 10.1084/jem.171.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shen L, Lang ML, Wade WF. The ins and outs of getting in: structures and signals that enhance BCR or Fc receptor-mediated antigen presentation. Immunopharmacology. 2000 Sep;49(3):227–40. doi: 10.1016/s0162-3109(00)00255-1. [DOI] [PubMed] [Google Scholar]
- [20].Yada A, Ebihara S, Matsumura K, Endo S, Maeda T, Nakamura A, et al. Accelerated antigen presentation and elicitation of humoral response in vivo by FcgammaRIIB- and FcgammaRI/III-mediated immune complex uptake. Cell Immunol. 2003 Sep;225(1):21–32. doi: 10.1016/j.cellimm.2003.09.008. [DOI] [PubMed] [Google Scholar]
- [21].Swanson JA, Hoppe AD. The coordination of signaling during Fc receptor-mediated phagocytosis. J Leukoc Biol. 2004 Dec;76(6):1093–103. doi: 10.1189/jlb.0804439. [DOI] [PubMed] [Google Scholar]
- [22].Ota H, Takashima Y, Hayashi Y, Matsumoto Y. A fusion protein of IgG fc and mouse-derived antigen on the surface of pseudorabies virus particles does not accelerate production of harmful auto-reactive antibodies. J Vet Med Sci. 2006 Nov;68(11):1179–83. doi: 10.1292/jvms.68.1179. [DOI] [PubMed] [Google Scholar]
- [23].Stabila PF, Wong SC, Kaplan FA, Tao W. Cell surface expression of a human IgG Fc chimera activates macrophages through Fc receptors. Nat Biotechnol. 1998 Dec;16(13):1357–60. doi: 10.1038/4339. [DOI] [PubMed] [Google Scholar]
- [24].You Z, Huang X, Hester J, Toh HC, Chen SY. Targeting dendritic cells to enhance DNA vaccine potency. Cancer Res. 2001 May 1;61(9):3704–11. [PubMed] [Google Scholar]
- [25].Minguet S, Dopfer EP, Schamel WW. Low-valency, but not monovalent, antigens trigger the B-cell antigen receptor (BCR) Int Immunol. 2010 Mar;22(3):205–12. doi: 10.1093/intimm/dxp129. [DOI] [PubMed] [Google Scholar]
- [26].Hsu CT, Ting CY, Ting CJ, Chen TY, Lin CP, Whang-Peng J, et al. Vaccination against gonadotropin-releasing hormone (GnRH) using toxin receptor-binding domain-conjugated GnRH repeats. Cancer Res. 2000 Jul 15;60(14):3701–5. [PubMed] [Google Scholar]
- [27].Yankai Z, Rong Y, Yi H, Wentao L, Rongyue C, Ming Y, et al. Ten tandem repeats of beta-hCG 109–118 enhance immunogenicity and anti-tumor effects of beta-hCG C-terminal peptide carried by mycobacterial heat-shock protein HSP65. Biochem Biophys Res Commun. 2006 Jul 14;345(4):1365–71. doi: 10.1016/j.bbrc.2006.05.022. [DOI] [PubMed] [Google Scholar]
- [28].Jacques S, Rich JR, Ling CC, Bundle DR. Chemoenzymatic synthesis of GM3 and GM2 gangliosides containing a truncated ceramide functionalized for glycoconjugate synthesis and solid phase applications. Organic & biomolecular chemistry. 2006 Jan 7;4(1):142–54. doi: 10.1039/b513595h. [DOI] [PubMed] [Google Scholar]
- [29].Pan Y, Chefalo P, Nagy N, Harding C, Guo Z. Synthesis and immunological properties of N-modified GM3 antigens as therapeutic cancer vaccines. Journal of medicinal chemistry. 2005 Feb 10;48(3):875–83. doi: 10.1021/jm0494422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kuduk SD, Schwarz JB, Chen X-T, Glunz PW, Sames D, Ragupathi G, et al. Synthetic and Immunological Studies on Clustered Modes of Mucin-Related Tn and TF O-Linked Antigens: The Preparation of a Glycopeptide-Based Vaccine for Clinical Trials against Prostate Cancer†. Journal of the American Chemical Society. 1998 Dec 01;120(48):12474–85. 1998. [Google Scholar]
- [31].Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cairo CW, Gestwicki JE, Kanai M, Kiessling LL. Control of multivalent interactions by binding epitope density. J Am Chem Soc. 2002 Feb 27;124(8):1615–9. doi: 10.1021/ja016727k. [DOI] [PubMed] [Google Scholar]
- [33].Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B cell antigen receptor signaling 101. Mol Immunol. 2004 Jul;41(6–7):599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- [34].Lo-Man R, Vichier-Guerre S, Bay S, Deriaud E, Cantacuzene D, Leclerc C. Anti-tumor immunity provided by a synthetic multiple antigenic glycopeptide displaying a tri-Tn glycotope. Journal of immunology. 2001 Feb 15;166(4):2849–54. doi: 10.4049/jimmunol.166.4.2849. [DOI] [PubMed] [Google Scholar]
- [35].Kagan E, Ragupathi G, Yi SS, Reis CA, Gildersleeve J, Kahne D, et al. Comparison of antigen constructs and carrier molecules for augmenting the immunogenicity of the monosaccharide epithelial cancer antigen Tn. Cancer immunology, immunotherapy : CII. 2005 May;54(5):424–30. doi: 10.1007/s00262-004-0584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zaharatos GJ, Yu J, Pace C, Song Y, Vasan S, Ho DD, et al. HIV-1 and influenza antigens synthetically linked to IgG2a Fc elicit superior humoral responses compared to unmodified antigens in mice. Vaccine. 2011 Dec 9;30(1):42–50. doi: 10.1016/j.vaccine.2011.10.056. [DOI] [PubMed] [Google Scholar]
- [37].Loureiro S, Ren J, Phapugrangkul P, Colaco CA, Bailey CR, Shelton H, et al. Adjuvant-free immunization with hemagglutinin-Fc fusion proteins as an approach to influenza vaccines. J Virol. 2011 Mar;85(6):3010–4. doi: 10.1128/JVI.01241-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang MY, Wang Y, Mankowski MK, Ptak RG, Dimitrov DS. Cross-reactive HIV-1-neutralizing activity of serum IgG from a rabbit immunized with gp41 fused to IgG1 Fc: possible role of the prolonged half-life of the immunogen. Vaccine. 2009 Feb 5;27(6):857–63. doi: 10.1016/j.vaccine.2008.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003 Feb 3;197(3):315–22. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Goletz TJ, Klimpel KR, Leppla SH, Keith JM, Berzofsky JA. Delivery of antigens to the MHC class I pathway using bacterial toxins. Hum Immunol. 1997 May;54(2):129–36. doi: 10.1016/s0198-8859(97)00081-5. [DOI] [PubMed] [Google Scholar]
- [41].Kounnas MZ, Morris RE, Thompson MR, FitzGerald DJ, Strickland DK, Saelinger CB. The alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein binds and internalizes Pseudomonas exotoxin A. J Biol Chem. 1992 Jun 25;267(18):12420–3. [PubMed] [Google Scholar]
- [42].Zizzari IG, Veglia F, Taurino F, Rahimi H, Quaglino E, Belleudi F, et al. HER2-based recombinant immunogen to target DCs through FcgammaRs for cancer immunotherapy. J Mol Med (Berl) 2011 Dec;89(12):1231–40. doi: 10.1007/s00109-011-0794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wirguin I, Suturkova-Milosevic L, Briani C, Latov N. Keyhole limpet hemocyanin contains Gal(beta 1–3)-GalNAc determinants that are cross-reactive with the T antigen. Cancer immunology, immunotherapy : CII. 1995 May;40(5):307–10. doi: 10.1007/BF01519630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.