Abstract
Purpose
Iodine-125 decay induces localized DNA damage by three major mechanisms: 1) direct damage by the emitted Auger electrons, 2) indirect damage by diffusible free radicals, and 3) charge neutralization of the residual, highly positively charged, tellurium daughter atom by stripping electrons from neighboring residues. The charge neutralization mechanism of 125I-induced DNA damage is poorly understood. Charge transport along DNA molecule can occur by either a hopping mechanism initiated by charge injection into DNA and propagated by charge migration through DNA bases along the DNA length, or by a tunneling mechanism in which charge transfers directly from a donor to an acceptor residue. In the first case additional damage in DNA nucleotides can be inflicted by the traveling charge; therefore, it is important to learn if charge hopping plays a role in 125I-decay-induced DNA damage. In our previous work, we determined that at 193K the charge hopping mechanism was not an appreciable component of the mechanism of 125I-induced DNA damage. However, the question whether this is also the case at higher temperatures remained open.
Methods
In the current study we used a well-known chemical barrier for charge hopping, 8-oxo-7, 8,-dihydroguanine (8-oxo-G), to assess the role of this mechanism in 125I-decay-induced DNA damage at the following temperatures: 198K, 253K, 277K and 298K.
Results
We found that varying the temperature had little effect on the distribution of 125I-induced DNA breaks, as well as on the breaks found at the 8-oxo-G probe both with and without piperidine treatment.
Conclusions
We thus conclude that charge transport by the hopping mechanism is not a major factor in 125I-decay-induced DNA damage at biologically relevant temperatures.
Keywords: DNA, Charge transport, Auger electron emitters
INTRODUCTION
Charges travelling along DNA molecules (either radical cations or anions) (Berlin et al., 2001, Bixon and Jortner, 2001, Jortner et al., 1998, Roginskaya et al., 2004, Boon and Barton, 2002, Giese and Biland, 2002, Giese, 2002, Giese and Wessely, 2001) can cause damage to DNA nucleotides (Burrows and Muller, 1998, Armitage, 1998, Cai and Sevilla, 2003). The two main mechanisms of charge transport along DNA molecules are tunneling (or superexchange) and hopping (Berlin et al., 2001, Jortner et al., 1998, Giese, 2000, Boon and Barton, 2002, Conwell, 2004). Tunneling is the direct transport of charge from a donor to an acceptor within DNA, while hopping is the transport of a charge through discrete steps along the DNA nucleotides (Berlin et al., 2001, Jortner et al., 1998, Boon and Barton, 2002, Giese and Biland, 2002, Roginskaya et al., 2004). Of these two mechanisms the charge hopping is more temperature dependent because it requires thermal energy to overcome the shallow barriers that trap charge at low temperatures (Berlin et al., 2001, Bixon and Jortner, 2001, Conwell, 2004, Cai et al., 2000). Upon acquiring enough energy to overcome these energy barriers, charge is able to escape the shallow traps and migrate along DNA until it either recombines, or reacts with a base (trapped), or is quenched by the environment (water, oxygen, etc) (Schuster, 2000).
There are a number of charge injectors that have been used to generate holes or excess electrons in DNA. For research purposes, the charge generated within DNA comes from sources such as photosensitizers, chemical oxidants or ionizing radiation (Boon and Barton, 2002, Giese and Biland, 2002, Cai and Sevilla, 2004, Debije et al., 2000, Razskazovskiy et al., 2000). Although the sources often used to generate the charge within DNA are different they result in relatively similar effects on DNA. In this study, the charge generated in DNA was produced as a result of the decay of a radionuclide, 125I. The average 125I decay event results in the emission of 21 electrons (Charlton and Booz, 1981), over a short time-window, which overlaps with the time-period during which the corresponding positive charge on the daughter Te is dissipated. Significant damage in adjacent molecular structures is the immediate effect. Decay of 125I incorporated into one strand of a DNA duplex produces strand breaks located within 10 bp from the decay site in both strands with an efficiency close to one double-strand break (DSB) per decay (Martin and Haseltine, 1981).
For the purposes of this study, we determined whether 125I decay-induced charge injection into DNA proceeded by the hopping mechanism using a hole transport probe, 8-oxo-7, 8-dihydroguanine (8-oxo-G). As a product of the oxidation of guanine, 8-oxo-G is more commonly known for its role as an oxidative stress marker, (Cai and Sevilla, 2003). However, for our purposes, its lower oxidation potential than unmodified bases is the key feature of interest that makes 8-oxo-G more readily oxidized by other sources. This attribute makes 8-oxo-G a suitable probe for the hole transport in DNA. The preferential trapping at 8-oxo-G is revealed by piperidine treatment due to unmodified 8-oxo-G reacting slowly with piperidine to form DNA strand breaks, while 8-oxo-G bases that have already undergone oxidation by a radical cation generate additional oxidative products that are much more piperidine labile (Kanvah and Schuster, 2005). This leads to a visible increase in damage at this position when compared to background damage. Earlier studies using electron spin resonance showed that 8-oxo-G bases were still able to trap holes at low temperatures around 77K (Cai and Sevilla, 2003). In this same study, low temperature was shown to cause hole transfer to occur primarily by tunneling mechanism alone (below 190K). Other studies showed that hole transfer occurred by both tunneling and hopping mechanisms at elevated temperatures about 300K (Schuster and Gasper, 1997, Kanvah and Schuster, 2005).
In our previous study, we found that the charge hopping was not a factor in the mechanism of 125I decay induced DNA damage at 193K (−80°C) (Ndlebe et al., 2010). We determined this by using chemical barriers such as 8-oxo-G and 5-bromo-2′-deoxyuridine (BrdU) to probe for the charge hopping at this temperature. In the current study, 8-oxo-G probe was the main focus because it was the well characterized within this temperature range (198K to 298K). Although other studies showed that charge transport through excess electron transfer or hole transport mechanisms still occurred at around 200K (Cai and Sevilla, 2004), the question of whether temperature was a factor in our earlier observation of a lack of apparent charge transport via the hopping mechanism through DNA as a result of 125I decay at 193K still remained. Further, most previous studies on hole transport in DNA using 8-oxo-G as a hole trap were performed at elevated temperatures around 298K (Schuster and Gasper, 1997, Kanvah and Schuster, 2005). Therefore, this study attempts to address the question if the charge hopping contributes to DNA damage after decay of 125I at temperatures between 198K and 298K using 8-oxo-G as a probe for hole transport.
MATERIALS AND METHODS
DNA Sample Preparation
DNA samples were synthesized on an ABI 394 Synthesizer (Applied Biosystems, Foster City, CA, USA). their sequences are shown in Table 1. Modified oligonucleotides containing 5'-Dimethoxytrityl-N2-isobutyryl-8-oxo-deoxyGuanosine-3'-[(2-cyanoethyl)-(N,N-diisopropyl)] (8-oxo-G) phosphoramidite were synthesized as specified by the manufacturer (Glen Research, Sterling, VA, USA). All samples were further purified with 12% denaturing polyacrylamide gel electrophoresis (PAGE) (National Diagnostics, Atlanta, GA, USA), and gel filtration through MicroSpin G-25 or MicroSpin G-50 columns (GE Healthcare, Piscataway, NJ, USA) depending on their size.
Table 1.
Sequences of DNA hairpins
| Name | 125I | Barrier | Hairpin Sequence a |
|---|---|---|---|
| C | − | − | |
| Ci | + | − | |
| X | − | 8-oxo-G | |
| Xi | + | 8-oxo-G |  |
“*” - 32P radiolabel; Z – 125I-dC; X – 8-oxo-G
DNA Radiolabeling
All short oligomers (22-mers) were first 5’ phosphorylated with T4 polynucleotide kinase (Fermentas, Glen Burnie, MD, USA) in reaction buffer supplemented with 1 mM ATP prior to annealing or ligation. The short oligomers were then 3’-end radiolabeled with 32P and Terminal deoxynucleotidyl transferase (Fermentas). Samples were also purified with MicroSpin G-25 columns to remove unincorporated radionucleotides. Samples of hairpin template (HT, Figure 1) were labeled with 125I by incorporating 125I-dCTP (Perkin Elmer, Waltham, MA, USA) to the 3’ end by the Klenow Fragment of DNA polymerase I (Fermentas). In addition to 125I-dCTP, the 3’ end of HT was capped by incorporation of either a dGTP or TTP depending on the HT sequence (Table 1). The control samples were obtained by ligation between unlabeled hairpin templates (without 125I) and 3’ 32P-labeled short oligomers. These control HT samples also underwent Klenow Fragment template extension except unlabeled dCTP was used instead of 125I-dCTP.
Figure. 1. Full Hairpin Sequence.
Full hairpin is comprised of the short oligomer (italic) annealed to the hairpin template (HT) (plain). Underlined is the ligation site between HT and short oligomer; Z - 125I-dCTP; X – 8-oxo-G.
DNA Samples Annealing and Accumulation of DNA Damage
Equimolar mixtures of HT and 22-mer short oligonucleotides (Figure 1) were annealed by cooling from 90°C to room temperature for 1hr in 10mM Tris•HCl, pH 7.4 and 1 mM ethylenediaminetetraacetic acid (TE) buffer with 50 mM NaCl. HT and 22-mers were then ligated using T4 DNA ligase (Fermentas) and resulted hairpins were purified from denaturing polyacrylamide gel as was described (Ndlebe et al., 2010). Gel-purified samples were incubated for 2 weeks at 198K, 253K, 277K and 298K with or without 10% dimethlysulfoxide (DMSO). Then they were thawed at room temperature for ca. 10 minutes, and analyzed for DNA breaks using 12% denaturing PAGE. DNA bands were quantified using a BAS-2500 Bioimager (FUJI Medical Systems USA, Stamford, CT, USA). Where indicated, samples were treated with piperidine by heating at 90°C in the presence of 10% piperidine for 30 minutes, then they were lyophilized. The purpose of the piperidine treatment was to expose base labile lesions.
DNA Strand Break Analysis
DNA strand breaks were quantified using a BAS-2500 Bioimager and ImageGauge software as previously described (Panyutin and Neumann, 1997, Ndlebe et al., 2010). Briefly, an intensity profile was generated for each lane in the gel image and the frequency of breaks were defined as a fraction of the peak height of the band intensity for each base over the peak height for the band intensity that corresponded with the total uncut DNA for the short oligomer for that lane, respectively. The probability of a strand break in the hairpin was calculated based on the assumption that the probability of 125I-induced breaks is 1. The recursive formula used for the probability of breaks in i-th position was:
| (1) |
where Fi is the frequency of breaks at the nucleotide in i-th position from the 3’-end. The formula (1) describes the probability of breaks at one base in relation to the probability of breaks for its 3’ neighboring bases. The estimated experimental error for the breaks frequency measurements was in the range of 10%, based on three independent analyses.
RESULTS
All the samples used within this study comprised of hairpin template (HT, 48mer) that was annealed and ligated to a short oligomer (22mer) (see Figure 1). The HT contained either 125I-dC (samples marked with i) or cold dC (samples without i) at the position marked Z (Figure 1). The 22-mers either contained 8-oxo-G (samples marked with X), or not (samples marked with C). Thus, for example, experimental sample Xi contained both 8-oxo-G in the 22-mer and 125I-dC in the HT. We placed 8-oxo-G four base pairs away from the 125I-dC because at this distance we could assess both 8-oxo-G oxidation and its effect on DNA breaks frequency. All the samples were incubated at the following temperatures: 277K, 298K, 253K and 198K. DNA hairpin sequences used for this study were essentially the same as the DNA hairpins we used in our previous study (see Table 1 in (Ndlebe et al., 2010)).
Effect of Temperature on Iodine-125 induced 8-oxo-G oxidative damage
After two weeks of incubation at the respective temperatures, the samples were analyzed by polyacrylamide gel electrophoresis (Figures 2 and 3). The characteristic pattern of 125I-induced DNA breaks was observed for all the samples contained 125I-dC at all four temperatures (Figure 2 and 3, lanes 2, 3, 6, 7, 10, 11, 14, 15, [bracketed region]). Treatment with piperidine of the samples contained 8-oxo-G (lanes 7, 8, 15, 16) give rise to the corresponding band (marked with arrow). Upon piperidine treatment, unmodified 8-oxo-G reacts slowly with piperidine resulting in DNA strand breaks at this position. However, if 8-oxo-G is modified by oxidation with a radical cation, it generates additional oxidative products, which react more readily with piperidine and should result in more strand cleavage at this position, as compared to unmodified 8-oxo-G. As a result, an observation of any increase in the piperidine labile products at the 8-oxo-G base after both 125I decay and piperidine treatment would suggest that oxidation occurred at this position and would provide evidence for charge transport via the hopping mechanism (hole transport) upon 125I decay in DNA
FIG. 2. Analysis of DNA breaks in samples incubated at 298K and 253K.
Autoradiograph of 12% PAGE. Sample abbreviations are listed in Table 1. Temperature of sample incubation is shown on the top. “+“ or “−” under the sample abbreviation shows if the were treated or not treated with piperidine. The arrow shows the position of a fragment corresponding to cut at 8-oxo-G. Brackets show region of 125I-induced DNA breaks.
FIG. 3. Analysis of DNA breaks in samples incubated at 277K and 198K.
Autoradiograph of 12% PAGE. Sample abbreviations are listed in Table 1. Temperature of sample incubation is shown on the top. “+“ or “−” under the sample abbreviation shows if the were treated or not treated with piperidine. The arrow shows the position of a fragment corresponding to cut at 8-oxo-G. Brackets show region of 125I-induced DNA breaks.
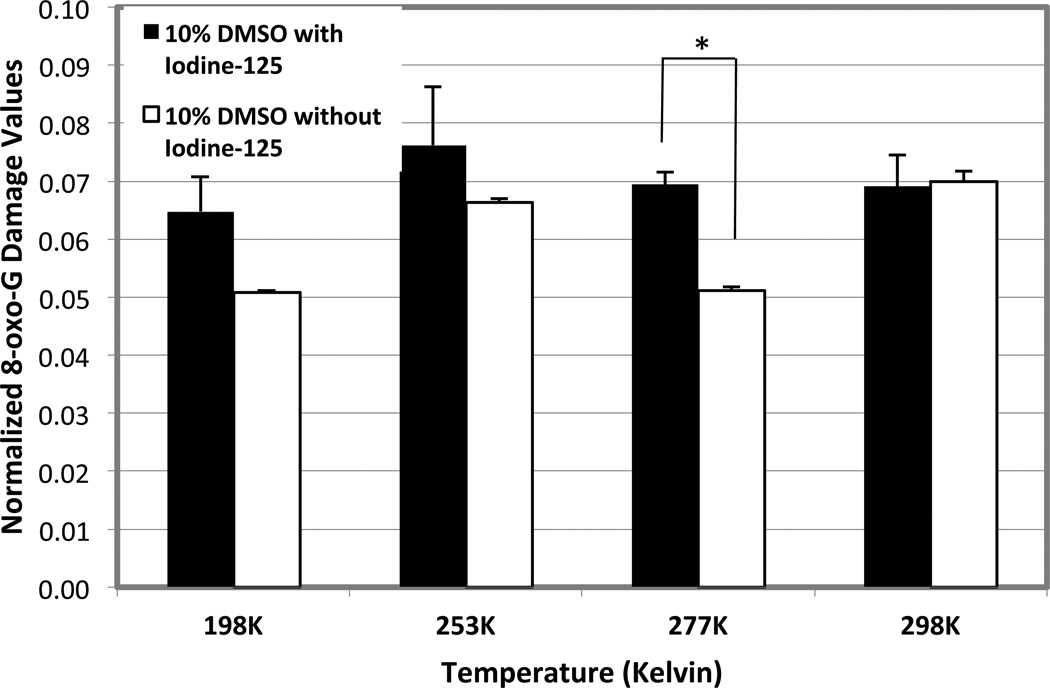
We carefully measured the intensity of the DNA damage bands shown in Figures 2 and 3. The values obtained at each 8-oxo-G were normalized by dividing the peak heights obtained at the 8-oxo-G base by the peak height of the uncut DNA peak. Figure 4 shows the normalized 8-oxo-G damage values for samples incubated at each temperature in the presence of 125I or absence. There was an evident increase in the overall amount of normalized 8-oxo-G damage as the temperature increased from 193K to 277 for samples with and without 125I. This suggests that as temperature increased, there was more 8-oxo-G cleavage due to temperature dependent factors. However, there was very little difference between the values observed for the samples with and without 125I stored at 198K, 253K or 298K. The only statistically significant difference was observed at 277K (n=3, p value = 0.03), where the normalized 8-oxo-G damage in the 125I-contained sample exceeded that of the sample without 125I.
FIG 4. Effect of Temperature on the amount of 8-oxo-G oxidative damage.
Temperature was plotted against the normalized 8-oxo-G damage for each 8-oxo-G containing sample, either with (Xi, black) or without 125I (X). The student’s t-test analysis was performed for the samples (n=3). Only the samples incubated at 277K have statistically significant difference (p=0.03). Error bars indicate the standard error of the mean (SEM) for n = 2 independent experiments.
In order to determine whether the observed effect at the higher temperatures was due to an increase in scavengeable radicals, the experiments were repeated in the presence of the hydroxyl radical scavenger DMSO (Figure. 5). Overall, in the presence of DMSO as the temperature was elevated the normalized 8-oxo-G values did not exhibit any measurable increase as observed for samples without DMSO (compare Figures 4 and 5). This suggests that scavengeable radicals were the major factor in the observed increase in normalized 8-oxo-G oxidative damage values over temperature. There was no significant difference between the normalized oxidative damage at 8-oxo-G for samples with and without 125I in the presence of DMSO at 198K, 253K or 298K. However, again a statistically significant difference was observed for samples incubated at 277K (n=3, p-value =0.02). There was more observable oxidation at the 8-oxo-G base in the 125I-containing sample at this temperature. It is also worth mentioning that we observed more 8-oxo-G oxidation in the presence than in the absence of DMSO in the frozen samples (198K and 253K), however we do not have explanation to this effect.
FIG 5. Effect of Temperature on the amount of 8-oxo-G oxidative in 10% DMSO.
Temperature was plotted against the normalized 8-oxo-G damage for each 8-oxo-G containing sample, either with (Xi, black) or without 125I (X). The student’s t-test analysis was performed for the samples (n=3). Only the samples incubated at 277K have statistically significant difference (p=0.02). Error bars indicate the standard error of the mean (SEM) for n = 3 independent experiments
Effect of Temperature on the Probability of Breaks for iodine-125 induced DNA damage
If travelling charges contribute significantly to the overall yield of DNA breaks produced by decay of 125I then the presence of the trap, 8-oxo-G, should affect the distribution of such breaks along DNA. Previously we showed that there was no difference in the probability of breaks distribution between samples with and without 8-oxoG at 198K. Here we extended these studies and compared probabilities of breaks in 8-oxo-G containing samples at different temperatures.
The probabilities of breaks at individual DNA nucleotides were determined from the data presented in Figures 2 and 3 as outlined in the Materials and Methods. Overall, we found that the samples not treated with piperidine did not show any appreciable difference in the probability of breaks distributions at the different temperatures (Figure 6A). Comparison of the samples with and without 8-oxo-G showed that the presence of the trap had no apparent effect on the overall probability of breaks at different temperatures (data not shown).
FIG 6. Probability of Breaks for untreated (A) and piperidine treated samples (B).
The probability of breaks (after background correction) for iodine-125 containing samples at 298K (grey patch), 277K (black), 253K (white) and 193K (grey) at individual nucleotides.
We then assessed the effect of 8-oxo-G on the distribution of breaks due to 125I decay in DNA at various temperatures after piperidine treatment, which should reveal not only frank DNA breaks but also alkali-labile DNA damages. Piperidine treatment results in considerable amount of breaks even in the sample without 125I-dC especially at the 8-oxo-G position (Ci, lines 8 and 16 in Figures 2 and 3). Therefore, we subtracted this background damage from the intensity of the bands in the experimental samples (Xi, lanes 7 and 15 in Figures 2 and 3) prior to calculations of the probability of breaks due to 125I decay.
Figure 6B shows the probability of breaks for the piperidine treated samples at each of the four temperatures after the background correction. As expected, there was an increase in the overall probability of breaks for the piperidine treated samples (Figure 6B) compared to the untreated samples (Figure 6A). This was due to the additional breaks at the piperidine labile sites. A slight increase in the piperidine-labile breaks at the 8-oxo-G most likely due to an artifact of the background correction as a result of subtraction of two large numbers. Although, there were minor variations between the probability of breaks for the Xi samples at different temperatures, overall, temperature had little overall effect on the 125I induced DNA damage observed.
DISCUSSION
It is generally considered that the major mechanism of charge transfer in DNA at the low temperatures in tunneling (Cai and Sevilla, 2004). However, at higher temperatures trapped ions can overcome barriers and start hopping (Cai and Sevilla, 2004). In our previous study we addressed the question of contribution of charge hopping to DNA damage after decay of 125I incorporated into DNA molecule at 193K. We showed that the presence in DNA of various impediments to the charge transfer by hopping, such as abasic site, mismatches, BrdU and 8-oxo-G, did not have a measurable effect on the distribution of damaged nucleotides along DNA molecules near to the 125I decay site. Therefore, we concluded that the charge transfer by hopping was not a contributing factor to DNA damage at 193K. However, this temperature is considered on the borderline between predominantly tunneling and combination of tunneling and hopping mechanisms of charge transfer. Hence, the question remained open if the charge hopping is the contributing factor at higher temperatures.
To address this question we carried out experiments with DNA molecules containing both 125I and 8-oxo-G at 198K, 253K, 277K and 298K. We did not detect any significant changes in the distribution of probability of frank and piperidine-labile DNA breaks with increase of the temperature of sample incubation. We observed an increase in the oxidative damage at 8-oxo-G with the increase of the temperature. We ascribed this increase to diffusible free radicals (including those produced by 32P) because the experiments repeated in the presence of free radical scavenger DMSO showed no such increase. However, we did not see a statistically significant difference in oxidative damage at 8-oxo-G between 125I-labeled and non-labeled samples at 198K 253K and 298K. Therefore, we concluded that at these temperatures the contribution to nucleotide damages by charges travelling along DNA is below our detection limit. The exception is the 25% increase in 8-oxo-G damage we observed in the 125I-labeled sample vs. non-labeled at 277K (Figure 5). However, if we take into account 125I-produced damage by Auger electrons to G in the similar position in the sample without 8-oxo-G (Ci) then the value of increase will drop from 25% to less than 5%. Therefore, even at 277K if there is a contribution to DNA damage by charges hopping along DNA then it is relatively small. Nevertheless, the anomalous increase in 8-oxo-G oxidation at 277K warrants further investigation.
Nevertheless, the charge generated by decay of 125I has to be neutralized somehow. We believe that neutralization the highly positively charged tellurium daughter atom involves drawing of electrons from neighboring atoms by tunneling mechanism even at the elevated biologically relevant temperatures. This is supported by the earlier experimental works that concluded that the charge neutralization component of the Auger effect plays a role at short distances less than 1.0 nm from the decay site, and therefore, only nucleotides located immediately next to the 125I-containing residue are affected by this component (Lobachevsky and Martin, 2000a, Lobachevsky and Martin, 2000b, Li et al., 2004). DNA damages observed at longer distances from the 125I decay site observed in other studies most likely have other explanations (Linz and Stocklin, 1985, Datta et al., 2005).
In conclusion, we demonstrated that the charge hopping mechanism has no measurable contribution to DNA damage in close proximity (4 nucleotides) to 125I decay site in wide temperature range. However, we cannot exclude that at longer distances, more than 10 nucleotides away from 125I, where contribution of other components of Auger effect is strongly diminished, charge transfer along DNA may play a role in DNA damage. Future experiments using more sensitive techniques should address this issue. We hope that our experimental data will help in developing theoretical models describing charge neutralization after decay of Auger electron emitting radionuclides.
ACKNOWLEGEMENTS
This work was supported by the Intramural Research Program of the National Institutes of Health, Clinical Center.
Footnotes
DECLARATION OF INTEREST
Authors declare no conflicts of interest.
REFERENCES
- Armitage B. Photocleavage of Nucleic Acids. Chem Rev. 1998;98:1171–1200. doi: 10.1021/cr960428+. [DOI] [PubMed] [Google Scholar]
- Berlin YA, Burin AL, Ratner MA. Charge hopping in DNA. Journal of the American Chemical Society. 2001;123:260–268. doi: 10.1021/ja001496n. [DOI] [PubMed] [Google Scholar]
- Bixon M, Jortner J. Charge transport in DNA via thermally induced hopping. Journal of the American Chemical Society. 2001;123:12556–12567. doi: 10.1021/ja010018p. [DOI] [PubMed] [Google Scholar]
- Boon EM, Barton JK. Charge transport in DNA. Current Opinion in Structural Biology. 2002;12:320–329. doi: 10.1016/s0959-440x(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Burrows CJ, Muller JG. Oxidative Nucleobase Modifications Leading to Strand Scission. Chemical Reviews. 1998;98:1109–1152. doi: 10.1021/cr960421s. [DOI] [PubMed] [Google Scholar]
- Cai Z, Gu Z, Sevilla MD. Electron spin resonance study of the temperature dependence of electron transfer in DNA: Competitive processes of tunneling, protonation at carbon, and hopping. Journal of Physical Chemistry B. 2000;104:6. [Google Scholar]
- Cai Z, Sevilla MD. Electron and hole transfer from DNA base radicals to oxidized products of guanine in DNA. Radiation Research. 2003;159:411–419. doi: 10.1667/0033-7587(2003)159[0411:eahtfd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Cai Z, Sevilla MD. Studies of Excess Electron and Hole Transfer in DNA at Low Temperatures. In: Schuster GB, editor. Long-Range Charge Transfer in DNA II. Berlin/Heidelberg: Springer; 2004. pp. 103–128. [Google Scholar]
- Charlton DE, Booz J. A Monte Carlo treatment of the decay of 125I. Radiation Research. 1981;87:10–23. [PubMed] [Google Scholar]
- Conwell E. Polarons and Transport in DNA. In: Schuster GB, editor. Long-Range Charge Transfer in DNA II. Berlin/Heidelberg: Springer; 2004. pp. 73–102. [Google Scholar]
- Datta K, Neumann RD, Winters TA. Characterization of complex apurinic/apyrimidinic-site clustering associated with an authentic site-specific radiation-induced DNA double-strand break. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10569–10574. doi: 10.1073/pnas.0503975102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debije MG, Strickler MD, Bernhard WA. On the efficiency of hole and electron transfer from the hydration layer to DNA: An EPR study of crystalline DNA X-irradiated at 4 K. Radiation Research. 2000;154:163–170. doi: 10.1667/0033-7587(2000)154[0163:oteoha]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese B. Long-distance charge transport in DNA: the hopping mechanism. Accounts of Chemical Research. 2000;33:631–636. doi: 10.1021/ar990040b. [DOI] [PubMed] [Google Scholar]
- Giese B. Electron transfer in DNA. Current Opinion in Chemical Biology. 2002;6:612–618. doi: 10.1016/s1367-5931(02)00364-2. [DOI] [PubMed] [Google Scholar]
- Giese B, Biland A. Recent developments of charge injection and charge transfer in DNA. Chemical Communications. 2002;7:667–672. doi: 10.1039/b111044f. [DOI] [PubMed] [Google Scholar]
- Giese B, Wessely S. The significance of proton migration during hole hopping through DNA. Chemical Communications. 2001;20:2108–2109. doi: 10.1039/b106059g. [DOI] [PubMed] [Google Scholar]
- Jortner J, Bixon M, Langenbacher T, Michel-Beyerle ME. Charge transfer and transport in DNA. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:12759–12765. doi: 10.1073/pnas.95.22.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanvah S, Schuster GB. The sacrificial role of easily oxidizable sites in the protection of DNA from damage. Nucleic Acids Research. 2005;33:5133–5138. doi: 10.1093/nar/gki801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WB, Friedland W, Jacob P, Panyutin IG, Paretzke HG. Simulation of (125)I decay in a synthetic oligodeoxynucleotide with normal and distorted geometry and the role of radiation and non-radiation actions. Radiation and Environmental Biophysics. 2004;43:23–33. doi: 10.1007/s00411-004-0231-1. [DOI] [PubMed] [Google Scholar]
- Linz U, Stocklin G. Chemical and biological consequences of the radioactive decay of iodine-125 in plasmid DNA. Radiation Research. 1985;101:262–278. [PubMed] [Google Scholar]
- Lobachevsky PN, Martin RF. Iodine-125 decay in a synthetic oligodeoxynucleotide. I. Fragment size distribution and evaluation of breakage probability. Radiation Research. 2000a;153:263–270. doi: 10.1667/0033-7587(2000)153[0263:idiaso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lobachevsky PN, Martin RF. Iodine-125 decay in a synthetic oligodeoxynucleotide. II. The role of auger electron irradiation compared to charge neutralization in DNA breakage. Radiation Research. 2000b;153:271–278. doi: 10.1667/0033-7587(2000)153[0271:idiaso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Martin RF, Haseltine WA. Range of radiochemical damage to DNA with decay of iodine-125. Science. 1981;213:896–898. doi: 10.1126/science.7256283. [DOI] [PubMed] [Google Scholar]
- Ndlebe T, Panyutin I, Neumann R. Analysis of the contribution of charge transport in iodine-125-induced DNA damage. Radiation Research. 2010;173:98–109. doi: 10.1667/RR1865.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyutin IG, Neumann RD. Radioprobing of DNA: distribution of DNA breaks produced by decay of 125I incorporated into a triplex-forming oligonucleotide correlates with geometry of the triplex. Nucleic Acids Research. 1997;25:883–887. doi: 10.1093/nar/25.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razskazovskiy Y, Debije MG, Bernhard WA. Direct radiation damage to crystalline DNA: what is the source of unaltered base release? Radiation Research. 2000;153:436–441. doi: 10.1667/0033-7587(2000)153[0436:drdtcd]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roginskaya M, Bernhard WA, Razskazovskiy Y. Diffusion Approach to Long Distance Charge Migration in DNA: Time-Dependent and Steady-State Analytical Solutions for the Product Yields. The Journal of Physical Chemistry. B. 2004;108:2432–2437. doi: 10.1021/jp0353340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster GB. Long-range charge transfer in DNA: transient structural distortions control the distance dependence. Accounts of Chemical Research. 2000;33:253–260. doi: 10.1021/ar980059z. [DOI] [PubMed] [Google Scholar]
- Schuster GB, Gasper SM. Intramolecular Photoinduced Electron Transfer to Anthraquinones Linked to Duplex DNA: The Effect of Gaps and Traps on Long-Range Radical Cation Migration. Journal of the American Chemical Society. 1997;119:12762–12771. [Google Scholar]