SUMMARY
Together with GTP and the initiator methionyl-tRNA, the translation initiation factor eIF2 forms a ternary complex that binds the 40S ribosome and then scans an mRNA to select the AUG start codon for protein synthesis. Here, we show that a human X-chromosomal neurological disorder characterized by intellectual disability and microcephaly is caused by a missense mutation in eIF2γ (encoded by EIF2S3), the core subunit of the heterotrimeric eIF2 complex. Biochemical studies of human cells overexpressing the eIF2γ mutant and of yeast eIF2γ with the analogous mutation revealed a defect in binding the eIF2β subunit to eIF2γ. Consistent with this loss of eIF2 integrity, the mutation in yeast eIF2γ impaired translation start codon selection and eIF2 function in vivo in a manner that was suppressed by overexpression of eIF2β. These findings directly link intellectual disability to impaired translation initiation, and provide a mechanistic basis for the human disease due to partial loss of eIF2 function.
INTRODUCTION
Translation initiation constitutes the first and rate-limiting step of protein synthesis (Jackson et al., 2010). A ternary complex of the eukaryotic translation initiation factor 2 (eIF2), GTP, and initiator methionyl-tRNA (Met-tRNAi Met) binds to the 40S ribosome forming a pre-initiation complex. This complex binds an mRNA at the 5’ end and scans to identify the translation start site, which in most cases is an AUG codon. The GTP-binding γ-subunit (eIF2γ) forms the core of the heterotrimeric eIF2 complex (Yatime et al., 2007). Following ribosomal scanning and selection of the AUG start site, hydrolysis of the eIF2-bound GTP is completed and inactive eIF2-GDP is released. In order for eIF2 to participate in further rounds of translation initiation, the GDP bound to eIF2 is exchanged for GTP in a reaction catalyzed by the heteropentamer eIF2B (Pavitt and Proud, 2009). While mutations affecting any of the five eIF2B subunits cause the neurological disease leukoencephalopathy with vanishing white matter (VWM) (van der Knaap et al., 2002; Bugiani et al., 2010), genetic defects of eIF2 are not known in mammals; and yeast with a deletion of the orthologous gene, GCD11, are not viable (Hannig et al., 1992). The identification of naturally occurring eIF2 mutations might allow deeper insights into the regulation of gene expression and protein translation in vivo.
RESULTS AND DISCUSSION
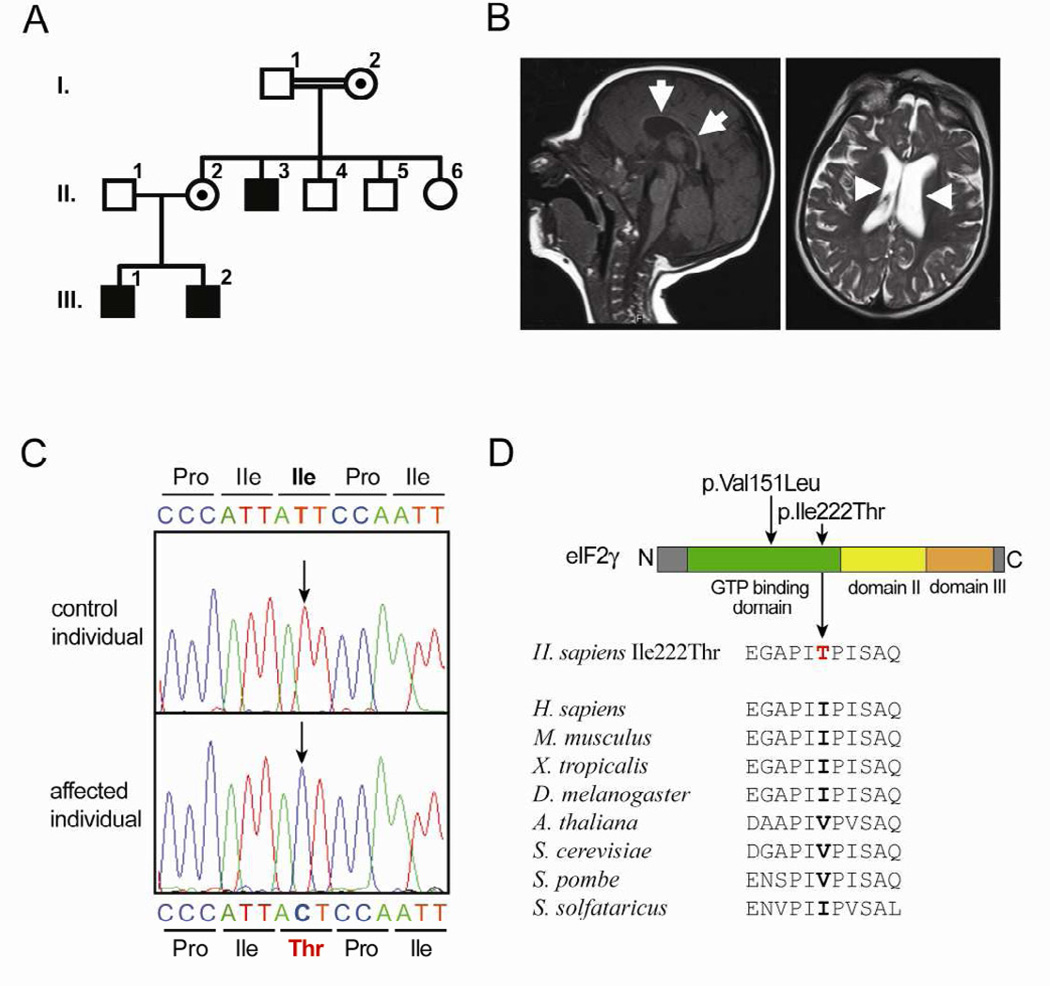
We ascertained a family of Morocco Jewish ancestry in the maternal line in which three male individuals were affected by an intellectual disability (ID) syndrome with apparent X-chromosomal inheritance (X-linked ID, XLID) (Figure 1A) (Gecz et al., 2009; Ropers, 2010). Clinical features included moderate-to-severe ID, microcephaly, short stature, and facial dysmorphic features (Figure S1). Additionally, each of the affected individuals had unique symptoms including cleft lip and palate and behavioral impairments (individual III:1), generalized seizures (III:2), and postpubertal microgenitalism and obesity (II:3) (see Supplemental Data). Brain magnetic resonance imaging revealed a thin corpus callosum and enlarged lateral ventricles (Figure 1B).
Figure 1. A mutation of EIF2S3/eIF2γ causes an intellectual disability syndrome.
(A) Pedigree of the family. Male family members affected by intellectual disability and microcephaly are shown as filled symbols; female carriers of the mutation are represented by a dot inside the circle; and the double line connecting I.1 and I.2 indicates consanguinity (I.1 and I.2 are first cousins).
(B) Brain magnetic resonance imaging (MRI) scans of patient III.2 performed at the age of one year. Midline sagittal image demonstrates a very thin corpus callosum (arrows) and microcephaly (left). Axial image shows mild enlargement and asymmetry (arrowheads) of the lateral ventricles (right).
(C) Identification of a hemizygous EIF2S3 missense variant c.665T>C (p.Ile222Thr). Sequence chromatograms showing a part of EIF2S3 exon 7 in an unaffected individual (top) and an affected family member (bottom).
(D) Protein diagram of eIF2γ with its functional domains (top). The positions of the p.Ile222Thr and p.Val151Leu mutations are indicated. Amino acid sequence alignments (bottom) surrounding Ile222 in human eIF2γ and its orthologs.
Targeted linkage analysis was performed and a candidate region spanning 10.7 megabases on the short arm of the X chromosome (Xp22.11-p21.1; Figure S2A) and containing 35 annotated genes (Figure S2B) was identified. After exclusion of mutations in the ID-associated genes ARX, IL1RAPL1 and PTCHD1, the exome of individual III:1 was sequenced to a mean coverage of 191x (for details see Supplemental Data). A single non-annotated variant was detected in the X-chromosomal candidate region that affected the gene EIF2S3 (GenBank accession number NM_001415.3). This variant was predicted to result in an isoleucine-to-threonine missense substitution in eIF2γ, the gene product of EIF2S3 (Gaspar et al., 1994). Capillary sequencing confirmed the c.665T>C (p.Ile222>Thr) mutation (Figure 1C) and showed that it cosegregated with the ID syndrome in the family. This variant was not detected in 188 X chromosomes from Morocco Jewish controls, nor was it present in dbSNP132, the 1000 Genomes data, or >8,000 alleles sequenced by the NHLBI Exome Sequencing Project. Ile222 is located in the GTP-binding (G) domain of eIF2γ (Figure 1D) and is strictly conserved in the animal kingdom, whereas it is conservatively replaced by valine in Arabidopsis thaliana, Saccharomyces cerevisiae, and Schizosaccharomyces pombe (Figure 1D and Figure S3).
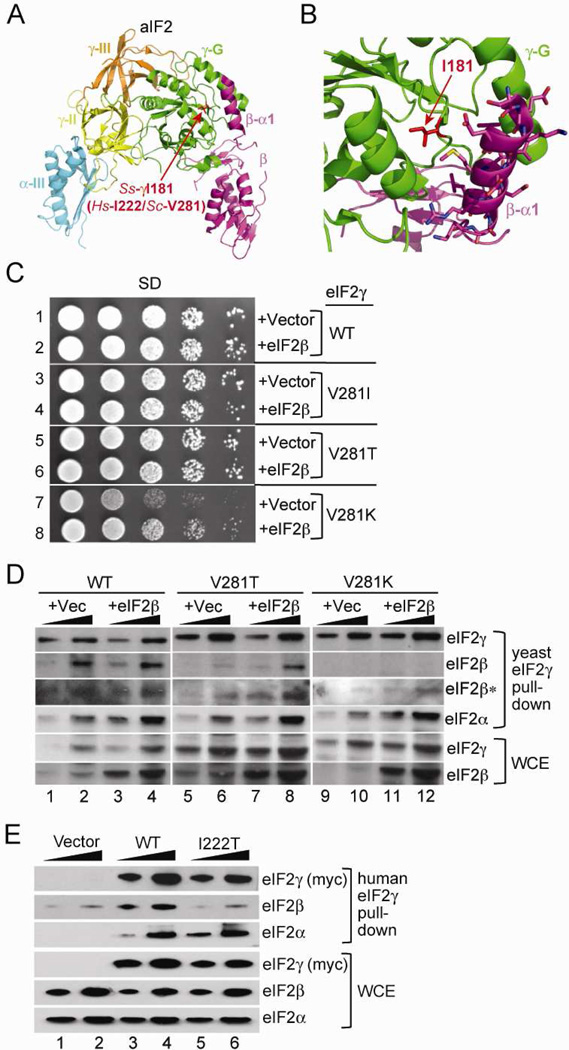
While no structures of eIF2γ or the eIF2 complex from eukaryotic sources are available, the crystal structure of the homologous archaeal aIF2 complex has been determined (Yatime et al., 2007; Stolboushkina et al., 2008). aIF2 can functionally replace eIF2 in binding Met-tRNAi Met to the ribosome and scanning (Dmitriev et al., 2011). The aIF2γ subunit, composed of a GTP-binding and two β-barrel domains, forms the core of the aIF2 complex with binding sites for both the aIF2α and aIF2β subunits (Figure 2A) (Yatime et al., 2007; Stolboushkina et al., 2008). The Ile181 residue in Sulfolobus solfataricus aIF2γ, which corresponds to the Ile222 mutation site in human eIF2γ and Val281 in S. cerevisiae eIF2γ, lies in a hydrophobic cleft on the backside of the GTP-binding domain (Figure 2A). Interestingly, this cleft forms the binding site for helix α1 of aIF2β (Figure 2B), suggesting that the eIF2γ-I222T mutation may interfere with eIF2 integrity and function by disrupting eIF2β binding to eIF2γ.
Figure 2. The eIF2γ mutation impairs yeast cell growth and eIF2β binding in yeast and human cells.
(A) Ribbon representation of S. solfataricus aIF2 complex (PDB code 2QMU) using PyMOL software (DeLano Scientific). The three domains of aIF2γ are colored green (G), yellow (II) and orange (III). aIF2β is colored magenta, and domain III of aIF2α is shown in cyan. The side chain of Ile181, corresponding to human Ile222 and yeast Val281, is shown in stick representation and colored red.
(B) Magnification of the aIF2γ – aIF2β helix α1 interface; colored as in panel A.
(C) Serial dilutions of yeast cells expressing the indicated eIF2γ mutants with or without overexpression of eIF2β were grown on minimal synthetic dextrose (SD) medium at 30 °C for 3 days.
(D) Whole cell extracts (WCEs) from yeast strains expressing the indicated His-tagged eIF2γ protein, or the same strains overexpressing eIF2β, were incubated with Ni2+ resin, and two different amounts of precipitated proteins were subject to immunoblot analysis using antisera specific for the indicated yeast eIF2 subunits. *Immunoblot analysis using increased amounts of anti-eIF2β antiserum.
(E) WCEs from HeLa cells transfected with empty vector or plasmids expressing the indicated myc- and His6-tagged human eIF2γ protein were incubated with Ni2+ resin, and two different amounts of precipitated proteins were subject to immunoblot analysis using anti-myc (eIF2γ) antiserum or antisera specific for human eIF2α or human eIF2β.
As it has not been possible to express recombinant forms of mammalian eIF2γ, or to obtain cells from the affected individuals, we mutated the corresponding Val281 residue in yeast eIF2γ for further functional studies. We examined the growth of yeast lacking the chromosomal GCD11 gene (encoding eIF2γ) and expressing His8-eIF2γ (WT), His8-eIF2γ-V281I, His8-eIF2γ-V281T or His8-eIF2γ-V281K as the sole source of eIF2γ. Mutating Val281 to Ile, the WT residue in humans, or to Thr, as found in the patients, had no impact on yeast cell growth (Figure 2C, rows 1,3,5). Reasoning that yeast eIF2γ may be more permissive than human eIF2γ to substitutions at this position, a more drastic mutation was introduced by substituting Lys in place of Val281. As shown in Figure 2C (row 7), the eIF2γ-V281K mutation substantially impaired yeast cell growth. Next, we examined the integrity of the eIF2 complex in strains expressing the eIF2γ mutants. Western analyses revealed that eIF2α and eIF2β readily co-precipitated with His8-eIF2γ on Ni2+ affinity resin (Figure 2D, lanes 1–2). In contrast, the eIF2γ-V281T mutation substantially impaired eIF2β binding (Figure 2D, lanes 5–6), indicating that a substantial loss of eIF2 integrity is tolerated in yeast without an affect on cell growth. The eIF2γ-V281K mutation nearly abolished eIF2β binding (Figure 2D, lanes 9–10), compatible with the observed growth defect. Importantly, the eIF2γ mutations did not affect the expression of eIF2γ or the binding of eIF2α (Figure 2D, 5th and 4th panel from top, respectively). These results indicate that the Val281 mutations in yeast eIF2γ disrupt eIF2β binding to eIF2γ. Accordingly, these results provide in vivo support for the structural studies mapping an aIF2β binding site on the backside of the aIF2γ G domain (Yatime et al., 2007; Stolboushkina et al., 2008).
If the growth defect of yeast expressing eIF2γ-V281K is due to impaired eIF2β binding, then overexpression of eIF2β might, through mass action, restore eIF2 integrity. Consistent with this hypothesis, introduction of a high copy-number plasmid expressing eIF2β partially suppressed the slow-growth phenotype of yeast expressing eIF2γ-V281K (Figure 2C, row 8 vs. 7). Moreover, overexpression of eIF2β also partially restored the binding of eIF2β to His8-eIF2γ-V281T and to His8-eIF2γ-V281K (Figure 2D, lanes 7–8, 11–12). Thus, consistent with the location of the corresponding Ile181 residue in the aIF2β binding site on aIF2γ, the Val281 mutations in yeast eIF2γ disrupt eIF2β binding to eIF2γ and provide a molecular rationale for how the I222T mutation in the patients impairs gene expression.
To directly test the impact of the eIF2γ mutation on human eIF2 complex integrity, C-terminally myc- and His6-tagged forms of wild type human eIF2γ and eIF2γ-I222T were expressed in HeLa cells and examined for the ability to interact with the endogenous eIF2α and eIF2β. Whereas eIF2α and eIF2β were co-precipitated with the tagged wild type eIF2γ on Ni2+ affinity resin (Figure 2E, lanes 3–4), substantially less eIF2β was pulled-down with eIF2γ-I222T (Figure 2E, lanes 5–6). As was observed in yeast, the I222T mutation in human eIF2γ did not impair eIF2α binding. Thus, consistent with the results of the Val281 mutations in yeast eIF2γ, the I222T mutation identified in the affected individuals disrupts eIF2 complex formation by specifically impairing the binding of eIF2β to human eIF2γ.
To provide further evidence that the Val281 mutations impair eIF2 function we performed additional genetic analyses in yeast. Whereas GCN4 expression in yeast is normally triggered by phosphorylation of eIF2α by the kinase GCN2, mutations that impair eIF2–GTP–Met-tRNAi Met ternary complex (TC) formation or TC binding to the ribosome confer a general-control derepressed (Gcd−) phenotype and stimulate GCN4 expression in cells lacking GCN2 (Hinnebusch, 2005). Reduced TC abundance enables reinitiating ribosomes on the GCN4 mRNA to bypass inhibitory upstream open reading frames and to translate GCN4. The V281T and V281K mutations in eIF2γ increased expression of a GCN4-lacZ reporter 2.5- and 40-fold, respectively (Table 1, panel A). This high-level GCN4-lacZ expression in cells expressing eIF2γ-V281K was partially suppressed in cells overexpressing eIF2β (data not shown). These results support the notion that the mutations reduced eIF2 integrity, thereby decreasing TC levels and leading to increased GCN4 expression.
Table 1. eIF2γ Mutations Impair Translation Initiation.
A GCN4-lacZ (A) or a dual AUG-Renilla, UUG-firefly luciferase (B) reporter construct was introduced into yeast strains expressing the indicated form of eIF2γ with or without overexpression of eIF2β. β-galactosidase activities and standard deviations (s.d.), or mean ratios and standard deviations of UUG and AUG luciferase reporters, were determined from at least three independent transformants.
| A GCN4-lacZ expression | |||
|---|---|---|---|
| eIF2γ |
GCN4-lacZ Expression(U) |
s.d. | p-value* |
| WT | 25.7 | 4.9 | |
| V281T | 63.3 | 20.8 | 0.002 |
| V281K | 1038.6 | 139.4 | 5 × 10−6 |
| B UUG/AUG initiation ratio | |||||
|---|---|---|---|---|---|
| eIF2γ | +High-Copy | UUG/AUG | s.d. | p-value* | p-value** |
| WT | Vector | 0.034 | 0.013 | ||
| WT | eIF2β | 0.029 | 0.009 | 0.503 | |
| V281T | Vector | 0.068 | 0.012 | 0.018 | |
| V281T | eIF2β | 0.027 | 0.013 | 0.007 | |
| V281K | Vector | 0.095 | 0.005 | 7.6 × 10−4 | |
| V281K | eIF2β | 0.072 | 0.017 | 0.077 | |
p-values calculated relative to the WT control using student’s t-test
p-values calculated comparing cells carrying an empty vector or overexpressing eIF2β.
Defects in eIF2 function, including eIF2 complex integrity, have also been linked to changes in the fidelity of translation start site selection. Whereas ribosomes typically initiate translation at an AUG codon, mutations that weaken Met-tRNAi Met binding to eIF2 or stimulate eIF2 GTPase activity have been found to enhance initiation at a UUG codon and confer a suppressor of initiation (Sui−) phenotype (Hinnebusch, 2011). In cells expressing WT eIF2γ, expression of a firefly luciferase reporter with a UUG start codon is much poorer than expression of Renilla luciferase with an AUG start site resulting in a low UUG/AUG initiation ratio (Table 1, panel B, top row). The V281T and V281K mutations in eIF2γ increased the UUG/AUG initiation ratio by 2–3 fold (Table 1, panel B). Interestingly, while overexpression of eIF2β did not affect the initiation ratio in cells expressing WT eIF2γ, it partially (V281K) or fully (V281T) restored the WT ratio in cells expressing the eIF2γ mutants (Table 1, panel B). These results demonstrate that disruption of eIF2 complex integrity lowers the fidelity of translation start site selection, and they complement findings of increased translation initiation at non-AUG codons conferred by mutations in yeast eIF2β helix α1 that impair binding to eIF2γ (Hashimoto et al., 2002). Taken together, we conclude that the Val281 mutations in yeast eIF2γ, and by analogy the I222T mutation in human eIF2γ, impair eIF2 complex integrity leading to defects in translation initiation including impaired TC function and decreased fidelity in start site selection.
In a search for supportive evidence linking impaired eIF2 function to ID in humans we did not identify additional mutations in 60 affected individuals from families with unspecific (and potentially X-linked) ID by sequencing the 12 EIF2S3 exons (see Supplemental Information). This result is compatible with the large genetic heterogeneity of XLID with >80 underlying genes known to date and suggests that EIF2S3 mutations confer a specific phenotype. We noted that a distinct missense variant (c.451G>C, p.V151L) was previously reported by a large-scale resequencing project of X-chromosomal genes in families with XLID (Tarpey et al., 2009). The clinical phenotype and possible cosegregation of this variant with ID in the family was not reported in that study and no functional characterization of the mutation was provided, precluding an assessment of its pathogenicity. We note however that the p.V151L variant is absent from SNP databases and is predicted to be “probably damaging“ and “not tolerated“ by the bioinformatic prediction algorithms for missense variants Polyphen-2 and SIFT, respectively, making a causative role in ID plausible. Consistently, our analysis of the orthologous V210L mutation that lies near the Met-tRNAi Met binding site in yeast eIF2γ (Figure S4A) revealed defects in cell growth, GCN4 expression and translation start site selection (Figure S4B–C). As the growth defect of the eIF2γ-V210L mutant strain was substantially suppressed by overexpression of tRNAi Met (IMT4 gene in yeast), this mutation may impair Met-tRNAi Met binding to eIF2.
The link reported here between eIF2 mutations and ID is supported by previous studies that revealed a connection between eIF2α phosphorylation and learning and memory (Gkogkas et al., 2010). Phosphorylation of eIF2α inhibits protein synthesis indirectly by converting eIF2 into an inhibitor of its guanine nucleotide exchange factor eIF2B and thereby decreasing eIF2–GTP–Met-tRNAi Met TC levels. Mice lacking the eIF2α kinases GCN2 or PKR, or expressing a non-phosphorylatable form of eIF2α, have altered synaptic plasticity and memory (Costa-Mattioli et al., 2005; Costa-Mattioli et al., 2007; Zhu et al., 2011). Thus mutations that impair eIF2 function may likewise alter protein synthesis in the brain leading to ID.
It is interesting to contrast the phenotypes associated with mutations in eIF2 and eIF2B. Whereas we show that a hypomorphic eIF2γ mutation causes ID, eIF2B mutations are associated with VWM disease, which presents distinct symptoms compared to the ID syndrome reported here. Moreover, the patients with eIF2γ mutations do not display loss of white matter; conversely, mental development is normal or only mildly delayed in early stages in most forms of VWM disease (Bugiani et al., 2010). However, when analyzed in yeast both the eIF2γ mutations and VWM mutations in eIF2B impair cell growth and induce GCN4 expression indicating reduced eIF2 activity (Richardson et al., 2004; Pavitt and Proud, 2009). How two diseases associated with impaired eIF2 function display distinct phenotypes, and how mutation of a general translation factor found in all cells causes a disorder mainly affecting the brain will be important questions to address in future studies.
EXPERIMENTAL PROCEDURES
Research projects on the genetics of intellectual disability syndromes were performed with approval by the ethics committees of the Rabin Medical Center, Petah Tikva, Israel; and the University of Ulm, Ulm, Germany. Written informed consent was obtained from participating individuals or their parents. Following DNA extraction from blood samples by standard methods linkage analysis was performed after genotyping DNA of 10 family members for 18 X-chromosomal short tandem repeat markers. The exome of individual III:1 was sequenced on an Illumina HiSeq 2000 Sequencer using a paired-end 100 basepair (bp) protocol after enrichment of exonic and splice-site sequences with the Agilent SureSelect Human All Exon 50 Mb kit. Short sequence reads (>66 million) were mapped to the hg19 human reference genome. Approximately 95% of target sequences were covered at least 10-fold with a mean coverage of about 191x. Sequence data were filtered against dbSNP134, the 1000 Genomes Project data and our in-house database of exome variants (with data from >200 exomes of individuals affected by different disorders). Due to their impact on protein structure and function the analysis was focused on rare missense, nonsense, frameshift and splice-site variants. The 12 coding exons of EIF2S3 were PCR amplified and sequenced on an ABI 3730 sequencer (Applied Biosystems) using BigDye chemistry. Yeast strain J292 (MATα leu2–3,-112 ura3–52 his3, gcn2Δ::loxP, gcd11Δ::KanMX p[GCD11, URA3]) (Alone et al., 2008) was used for examination of eIF2γ mutants. Details on the construction of yeast mutant plasmids (Supplemental Experimental Procedures) are available upon request. GCN4-lacZ expression was determined as described previously(Hinnebusch, 1985). Dual luciferase reporters (Takacs et al., 2011) were used to measure UUG/AUG ratios essentially as described previously (Harger and Dinman, 2003) using the Dual-Luciferase Reporter Assay System (Promega). All assays were performed in triplicate at least three times.
Supplementary Material
HIGHLIGHTS.
X-linked intellectual disability (XLID) syndrome due to mutation in eIF2γ gene
Human eIF2γ mutation and analogous mutation in yeast eIF2γ impair binding of eIF2β
Yeast eIF2γ mutation impairs translation and enhances initiation at non-AUG codons
Findings link ID to loss of eIF2 complex integrity and impaired translation
ACKNOWLEDGMENTS
We wish to thank the family members for their invaluable participation and cooperation and Fatima Abidi for bioinformatic sequence analyses. This work was supported in part by the Deutsche Forschungsgemeinschaft (G.B.), the intramural research program of the National Institutes of Health (T.E.D.), the Israeli Ministry of Health Chief Scientist foundation (grant No 3-4963) and Israeli Science Foundation (grant No 09/558) (L.B.-V.), and by a grant from the South Carolina Department of Disabilities and Special Needs (C.E.S.). L.C.’s laboratory was supported in part by the Centre National de la Recherche Scientifique and the Fondation de France.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental information includes Supplemental Data, four figures, Supplemental Experimental Procedures, and Supplemental References.
REFERENCES
- Alone PV, Cao C, Dever TE. Translation initiation factor 2γ mutant alters start codon selection independent of Met-tRNA binding. Mol Cell Biol. 2008;28:6877–6888. doi: 10.1128/MCB.01147-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugiani M, Boor I, Powers JM, Scheper GC, van der Knaap MS. Leukoencephalopathy with vanishing white matter: a review. J Neuropathol Exp Neurol. 2010;69:987–996. doi: 10.1097/NEN.0b013e3181f2eafa. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Harding H, Herdy B, Azzi M, Bruno M, Bidinosti M, Ben Mamou C, Marcinkiewicz E, Yoshida M, et al. Translational control of hippocampal synaptic plasticity and memory by the eIF2a kinase GCN2. Nature. 2005;436:1166–1173. doi: 10.1038/nature03897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, et al. eIF2a phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev SE, Stolboushkina EA, Terenin IM, Andreev DE, Garber MB, Shatsky IN. Archaeal translation initiation factor aIF2 can substitute for eukaryotic eIF2 in ribosomal scanning during mammalian 48S complex formation. J Mol Biol. 2011;413:106–114. doi: 10.1016/j.jmb.2011.08.026. [DOI] [PubMed] [Google Scholar]
- Gaspar NJ, Kinzy TG, Scherer BJ, Humbelin M, Hershey JW, Merrick WC. Translation initiation factor eIF-2. Cloning and expression of the human cDNA encoding the γ-subunit. J Biol Chem. 1994;269:3415–3422. [PubMed] [Google Scholar]
- Gecz J, Shoubridge C, Corbett M. The genetic landscape of intellectual disability arising from chromosome X. Trends Genet. 2009;25:308–316. doi: 10.1016/j.tig.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Gkogkas C, Sonenberg N, Costa-Mattioli M. Translational control mechanisms in long-lasting synaptic plasticity and memory. J Biol Chem. 2010;285:31913–31917. doi: 10.1074/jbc.R110.154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannig EM, Cigan AM, Freeman BA, Kinzy TG. GCD11, a negative regulator of GCN4 expression, encodes the γ subunit of eIF-2 in Saccharomyces cerevisiae. Mol Cell Biol. 1992;13:506–520. doi: 10.1128/mcb.13.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harger JW, Dinman JD. An in vivo dual-luciferase assay system for studying translational recoding in the yeast Saccharomyces cerevisiae. RNA. 2003;9:1019–1024. doi: 10.1261/rna.5930803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto NN, Carnevalli LS, Castilho BA. Translation initiation at non- AUG codons mediated by weakened association of eukaryotic initiation factor (eIF) 2 subunits. Biochem J. 2002;367:359–368. doi: 10.1042/BJ20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG. A hierarchy of trans-acting factors modulate translation of an activator of amino acid biosynthetic genes in Saccharomyces cerevisiae. Mol Cell Biol. 1985;5:2349–2360. doi: 10.1128/mcb.5.9.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG. Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol Mol Biol Rev. 2011;75:434–467. doi: 10.1128/MMBR.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavitt GD, Proud CG. Protein synthesis and its control in neuronal cells with a focus on vanishing white matter disease. Biochem Soc Trans. 2009;37:1298–1310. doi: 10.1042/BST0371298. [DOI] [PubMed] [Google Scholar]
- Richardson JP, Mohammad SS, Pavitt GD. Mutations causing childhood ataxia with central nervous system hypomyelination reduce eukaryotic initiation factor 2B complex formation and activity. Mol Cell Biol. 2004;24:2352–2363. doi: 10.1128/MCB.24.6.2352-2363.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropers HH. Genetics of early onset cognitive impairment. Annu Rev Genomics Hum Genet. 2010;11:161–187. doi: 10.1146/annurev-genom-082509-141640. [DOI] [PubMed] [Google Scholar]
- Stolboushkina E, Nikonov S, Nikulin A, Blasi U, Manstein DJ, Fedorov R, Garber M, Nikonov O. Crystal structure of the intact archaeal translation initiation factor 2 demonstrates very high conformational flexibility in the a- and b-subunits. J Mol Biol. 2008;382:680–691. doi: 10.1016/j.jmb.2008.07.039. [DOI] [PubMed] [Google Scholar]
- Takacs JE, Neary TB, Ingolia NT, Saini AK, Martin-Marcos P, Pelletier J, Hinnebusch AG, Lorsch JR. Identification of compounds that decrease the fidelity of start codon recognition by the eukaryotic translational machinery. RNA. 2011;17:439–452. doi: 10.1261/rna.2475211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpey PS, Smith R, Pleasance E, Whibley A, Edkins S, Hardy C, O'Meara S, Latimer C, Dicks E, Menzies A, et al. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat Genet. 2009;41:535–543. doi: 10.1038/ng.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap MS, Leegwater PA, Konst AA, Visser A, Naidu S, Oudejans CB, Schutgens RB, Pronk JC. Mutations in each of the five subunits of translation initiation factor eIF2B can cause leukoencephalopathy with vanishing white matter. Ann Neurol. 2002;51:264–270. doi: 10.1002/ana.10112. [DOI] [PubMed] [Google Scholar]
- Yatime L, Mechulam Y, Blanquet S, Schmitt E. Structure of an archaeal heterotrimeric initiation factor 2 reveals a nucleotide state between the GTP and the GDP states. Proc Natl Acad Sci U S A. 2007;104:18445–18450. doi: 10.1073/pnas.0706784104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Huang W, Kalikulov D, Yoo JW, Placzek AN, Stoica L, Zhou H, Bell JC, Friedlander MJ, Krnjevic K, et al. Suppression of PKR promotes network excitability and enhanced cognition by interferon-gamma-mediated disinhibition. Cell. 2011;147:1384–1396. doi: 10.1016/j.cell.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.