Summary
Axon regeneration allows neurons to repair circuits after trauma, but most of the molecular players remain to be identified. As microtubule rearrangements have been observed in injured neurons, we tested whether microtubule severing proteins might play a role in axon regeneration. We found that axon regeneration is extremely sensitive to levels of the microtubule severing protein spastin. While microtubule behavior in uninjured neurons was not perturbed in animals heterozygous for a spastin null allele, axon regeneration was severely disrupted in this background. Two types of axon regeneration, regeneration of an axon from a dendrite after proximal axotomy and regeneration of an axon from the stump after distal axotomy, were defective in Drosophila with one mutant copy of the spastin gene. Other types of axon and dendrite outgrowth, including regrowth of dendrites after pruning, were normal in heterozygotes. We conclude that regenerative axon growth is uniquely sensitive to spastin gene dosage.
Introduction
Most neurons function for the lifetime of an animal. During the weeks, years, or decades that these cells survive, they may be damaged at some point. If this damage occurs on the single axon that most neurons possess, the cell will no longer be able to send signals, and will thus be rendered non-functional. The ability of the neuron to regrow, or regenerate, its axon is thus extremely important.
Many neurons have a tremendous capacity for axon regeneration. This is particularly true of neurons in invertebrates and lower vertebrates (Macagno et al., 1985; Wang and Jin, 2011), as well as peripheral neurons in higher vertebrates (Chen et al., 2007; Navarro et al., 2007). Central nervous system neurons in higher vertebrates seem to have a more limited capacity for regeneration (Huebner and Strittmatter, 2009; Liu et al., 2011).
When axons are completely severed at a distance of 50 microns or more from the cell body, the distal axon is rapidly cleared by Wallerian degeneration, and new growth initiates from the axon stump. New growth from the tip of the axon stump is seen by 24h in mouse spinal cord (Kerschensteiner et al., 2005). If axons are severed very close to the cell body, the axon stump is not competent for regeneration and new processes sprout from dendrites (Hall and Cohen, 1983; Hall et al., 1989; Rose et al., 2001). These new processes acquire molecular features of axons (Gomis-Ruth et al., 2008; Stone et al., 2010), and can become functional axons (Gomis-Ruth et al., 2008).
While axon regeneration in the periphery allows patients to regain feeling and motor control distal to a nerve transection site, little is known about the molecular players that are required. It is clear that transcriptional profiles of regenerating neurons are altered (Schmitt et al., 2003; Tanabe et al., 2003; Veldman et al., 2007; Yang et al., 2006), but it is not clear when and where most of the gene products function in the axon outgrowth process.
Live imaging studies of injured neurons have suggested that microtubule rearrangements might be important for initiation of regeneration (Erez et al., 2007; Stone et al., 2010). Changes in both microtubule dynamics and/or polarity seem to be required for growth of a new axon from the axon stump (Erez et al., 2007) or from a dendrite (Stone et al., 2010). Several different types of proteins, including kinesins, +TIPs and microtubule severing proteins can regulate microtubule behavior. In the current study we investigate the role of severing proteins in damaged neurons.
Microtubule severing is a key regulator of microtubule behavior in mitosis (Roll-Mecak and McNally, 2010). AAA ATPase family severing proteins, including spastin, katanin and fidgetin, control different aspects of microtubule behavior in the spindle (Zhang et al., 2007). In neurons, microtubule severing proteins play a role in axon branching (Yu et al., 2008), dendrite architecture (Jinushi-Nakao et al., 2007), dendrite pruning (Lee et al., 2009), axon outgrowth (Wood et al., 2006) and synaptic bouton formation (Sherwood et al., 2004; Trotta et al., 2004).
To determine whether microtubule rearrangements involved in axon regeneration might be mediated by severing proteins, we used several models of axon regeneration in Drosophila. We find that microtubule severing proteins are required in two different cell types for regeneration of an axon from a dendrite and regeneration of the axon from the axon stump. Most surprisingly, loss of a single copy of spastin, but not other severing protein genes, dramatically reduces axon regeneration in all cases. This requirement is specific for axon regeneration, as normal axon outgrowth is not affected by loss of a single copy of spastin, and dendrite regrowth after pruning is similarly unaffected by loss of one or two copies of spastin. We have thus found that spastin is a key regulator of axon regeneration, and initiation of regeneration is extremely sensitive to spastin copy number.
Results
Microtubule severing proteins are required for regeneration of an axon from a dendrite
We found previously that proximal axotomy of Drosophila dendritic arborization (da) sensory neurons increases the number of growing microtubules and then results in reversal of microtubule polarity in a dendrite. In these neurons, axons and dendrites normally have opposite microtubule polarity, with axons having uniform plus-end-out microtubule arrays and dendrites having minus-end-out microtubules (Stone et al., 2008). After polarity reversal, the dendrite with axonal polarity initiates tip growth and appears to be a regenerating axon (Stone et al., 2010). This is consistent with growth of new axons from dendrite tips after axotomy close to the cell body in leech and rodent neurons (Gomis-Ruth et al., 2008; Hall et al., 1989). Since microtubules are so dramatically rearranged after proximal axotomy, we wished to determine whether microtubule severing proteins might play a role in this type of axon regeneration.
To test microtubule severing proteins for a role in axon regeneration from a dendrite, we targeted them by RNAi in a subset of neurons. RNAi in Drosophila is performed by expressing large (several hundred nucleotide) hairpin RNAs in specific cell types using a binary Gal4-UAS expression system. The hairpins are then processed into dozens of different short double-stranded RNAs that can initiate RNAi. This leads to quite specific and effective RNAi in specific subsets of cells in vivo (Dietzl et al., 2007). In this case, we used a class I neuron-specific Gal4 (221-Gal4) to drive dicer2, hairpin RNAs and EB1-GFP in sensory neurons, including ddaE, with relatively simple dendrite arbors (Grueber et al., 2002). Because the ddaE dendrite arbor is simple and stereotyped, it is straightforward to track initiation of axon outgrowth from dendrites after proximal axotomy.
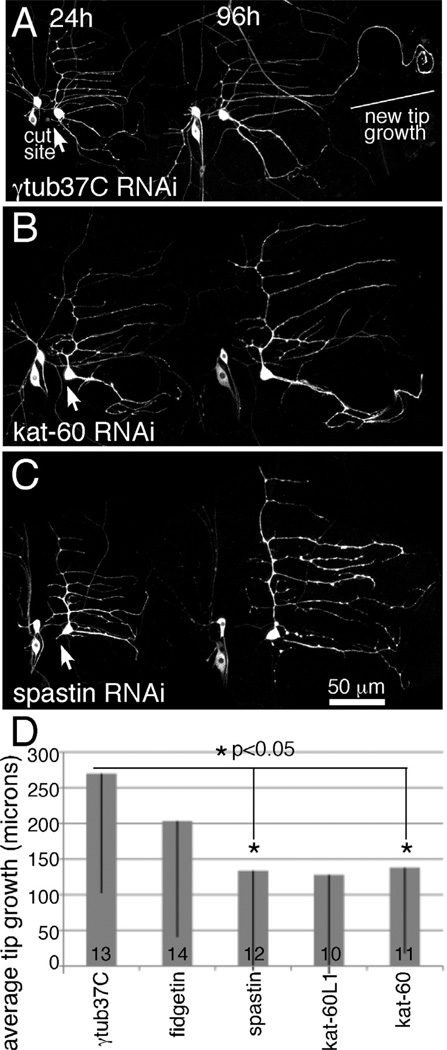
When a maternal protein (γtub37C), which is not thought to be present at significant levels after initial development (Wiese, 2008; Wilson and Borisy, 1998), was targeted by RNAi, outgrowth from a dendrite tip was observed in most cases after proximal axotomy (Figure 1A). This behavior was similar to previous results in neurons that did not express dicer2 or hairpin RNAs (Stone et al., 2010), and so we used this RNA hairpin as a control. We tracked growth from a dendrite tip over 96h after proximal axotomy. During this time the body size of the larva increased, and the sensory neurons on the body wall increased their dendrite arbors to match growth of the animal. This growth occurs over the whole length of the dendrites, while the overall shape remains constant. Overall growth is very different from new tip growth of an axon from a dendrite. To quantitate tip growth, we measured the length of the dendrite branch that initiated growth at 0h and 96h after axon severing. We also measured the growth of a different dendrite branch, and normalized growth of the growing dendrite to this one to account for developmental body wall growth. On average, γtub37C RNAi (control) neurons grew about 250 microns from the tip of a dendrite after proximal axotomy (Figure 1). As in previous control experiments, the amount of growth is somewhat variable and not all neurons initiate outgrowth (Stone et al., 2010). This means that the standard deviation of the amount of growth (shown on graphs as error bars) is quite large. This variability is normal so we show the standard deviations to represent this aspect of the growth response.
Figure 1. Regeneration of an axon from a dendrite is sensitive to levels of microtubule severing proteins.
EB1-GFP, dicer2 and hairpin RNAs were expressed in class I sensory neurons with 221-Gal4. The axon of the ddaE neuron was severed close to the cell body with a pulsed UV laser at 0h. Animals were remounted for imaging at 24h intervals. The 24h and 96h timepoints are shown. At 24h the axon is completely gone. A. At 96h in control neurons expressing a hairpin targeting γtub37C, a neurite can be seen to extend from beyond the normal territory covered by the dendrite. Comparison of the 24h and 96h images makes it clear that one of the dendrite tips has grown between the two time points. B and C. When either kat-60 or spastin was targeted by RNAi many cells did not extend their dendrites as in these examples. D. The length of outgrowth from dendrite tips was measured in neurons expressing different hairpin RNAs. Numbers in the columns indicate the number of animals of each genotype tested. Error bars show standard deviation; as these were fairly large the bar is shown in only one direction. Only part of the error bar is shown for kat-60L1; the standard deviation was 181 microns. Statistical significance was calculated with a student’s t-test.
In Drosophila there are four potential microtubule severing proteins, spastin, fidgetin, katanin-60 (kat-60) and katanin-60L1 (kat-60L1). Homozygous spastin mutants have synaptic bouton defects (Sherwood et al., 2004; Trotta et al., 2004), and loss of one copy of spastin affects the pattern of dendrite arborization in the most complex sensory neurons (class IV), but not simpler class I neurons like ddaE (Jinushi-Nakao et al., 2007). Kat-60L1 is required for pruning of class IV dendrites during metamorphosis (Lee et al., 2009) and establishment of normal class IV dendrite arbors (Stewart et al., 2012). Neuronal functions of the other two proteins have not been described. To determine whether any of these potential severing proteins were required for regeneration of an axon from a dendrite, we targeted each by RNAi and assayed axon regeneration after proximal severing. Reduction of either spastin or kat-60 resulted in a significant defect in tip growth (Figure 1). Both of these proteins are likely to act cell-autonomously in neurons as the Gal4 driver used to express the RNA hairpins is expressed in class I neurons and not surrounding tissue. The kat-60L1 RNAi phenotype was extremely variable, and this precluded any conclusions about its role in regeneration.
Regeneration of an axon from a dendrite is blocked by loss of one copy of the spastin gene
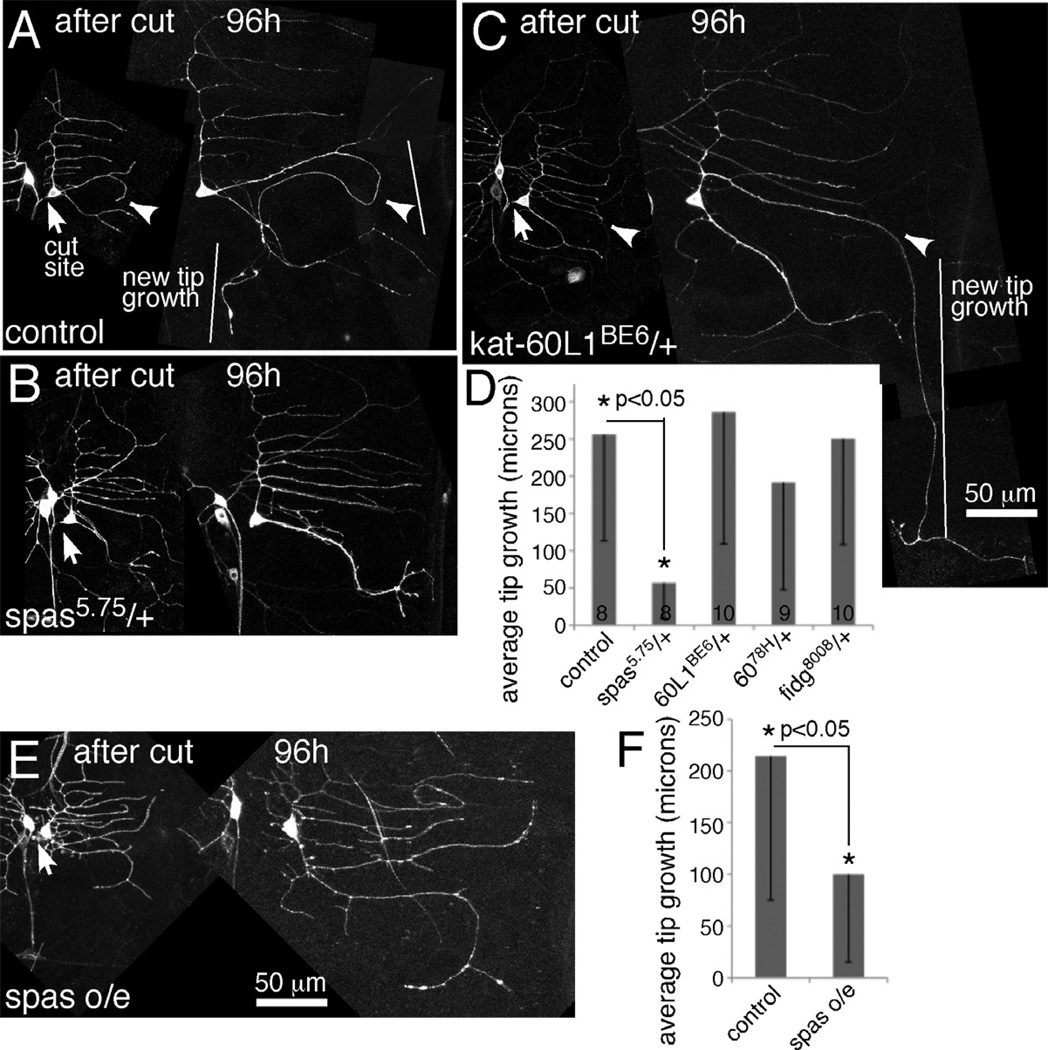
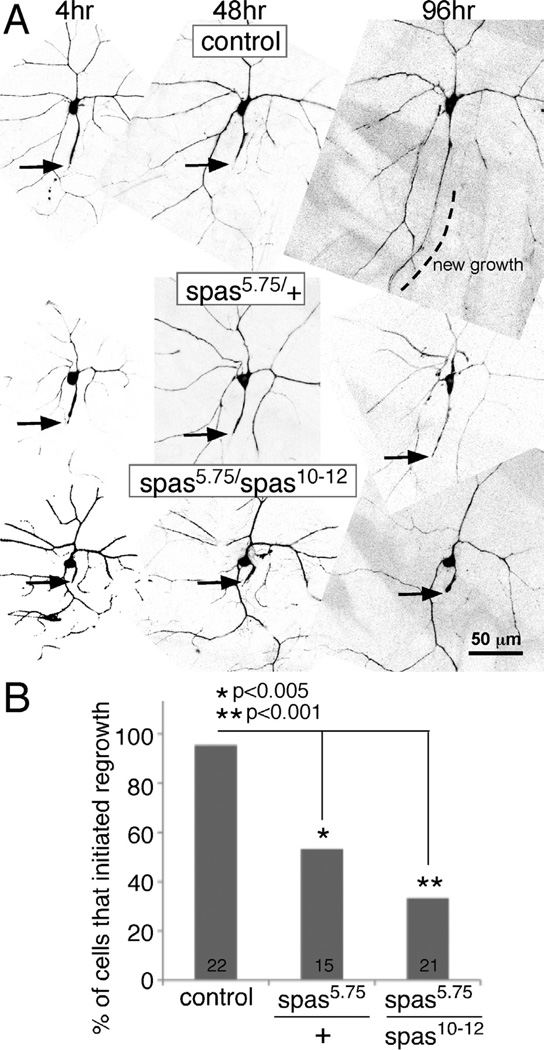
To determine which of the putative severing proteins was most strongly required for regeneration, we analyzed animals heterozygous for mutations in spastin, kat-60 and kat-60L1 and animals heterozygous for a small deficiency that removes the fidgetin gene (Df(2L)Exel8008). Flies containing null alleles of each gene or the deficiency were crossed to flies with the class I Gal4 driver 221 and EB1-GFP. Null mutants in the Drosophila spastin gene have been previously described (Sherwood et al., 2004). The kat-60L1BE6 allele is a null mutant generated by imprecise excision of a transposon (Stewart et al., 2012). A deletion allele, kat-6078H (Sherwood, unpublished), was also used for kat-60. To our surprise, loss of a single copy of spastin resulted in an almost complete failure of regeneration of an axon from a dendrite (Figure 2). A single mutant copy of kat-60 or kat-60L1 or loss of one copy of the fidgetin gene did not impair this type of axon regeneration (Figure 2C and D). Regeneration of an axon from a dendrite is thus extremely sensitive to spastin gene dosage, but not as sensitive to dosage of the other severing proteins. Since the requirement for spastin in axon regeneration was strong, and we obtained similar phenotypes with RNAi and mutant strategies, we focused our analysis on this severing protein.
Figure 2. Too little or too much spastin reduces regeneration of an axon from a dendrite.
Proximal axotomy was performed as in Figure 1. A–C. Images from animals immediately after ddaE axon severing are shown together with the same cell 96 hours later. Arrows indicate the site of severing, and arrowheads in A and C point to the dendrite that initiates tip growth. D. The length of new growth from a dendrite tip was quantitated. Numbers in the columns indicate the number of animals of each genotype tested. Error bars show the standard deviation, and this is shown in one direction only to keep the graph compact. Statistical significance was calculated with a t-test. E and F. Neurons expressing EB1-GFP and either mCD8-RFP (control) or spastin-CFP (spas o/e) were subjected to proximal axotomy. Tip growth at 96h after injury was quantitated as in D, error bars and significance are also the same as in D. An example of a spastin overexpressing ddaE cell is shown in E.
As it seemed important to have two copies of the spastin gene for successful axon regeneration after proximal axotomy, we considered that additional spastin might lead to increased regeneration. To test this hypothesis we crossed in a transgene that expresses CFP-tagged spastin under UAS control (Du et al., 2010). Control neurons that expressed a different transgene (mCD8-RFP) regrew more than 200 microns on average after proximal axotomy (Figure 2F) while spastin overexpressing (o/e) neurons regrew only about 100 microns (Figure 2E and F). Rather than promoting regeneration, extra spastin reduced regeneration. Thus regeneration is extremely sensitive to spastin dosage, and too little or too much spastin reduced the capacity of the neuron to regrow an axon from a dendrite.
Loss of one copy of spastin does not affect microtubule polarity in injured or uninjured neurons
We have shown that regeneration of an axon from a dendrite is preceded by reversal of microtubule polarity from minus-end-out to plus-end-out in a single dendrite (Stone et al., 2010). Under conditions when uniform plus-end-out polarity cannot be attained, regeneration fails (Mattie et al., 2010). We therefore hypothesized that failure of regeneration in spastin heterozygous mutant animals could result from disruption of microtubule polarity. To test this hypothesis we first assayed microtubule polarity in uninjured ddaE neurons with only one copy of the spastin gene. We used direction of EB1-GFP movement to assay microtubule polarity in living neurons in vivo. EB1, and family member EB3, bind to growing microtubule plus ends, and have been used to analyze direction of microtubule growth in mammalian neurons (Stepanova et al., 2003) and Drosophila neurons (Stone et al., 2008) and seem to represent microtubule polarity well (Baas and Lin, 2011) as long as they are used at low expression level (Mattie et al., 2010).
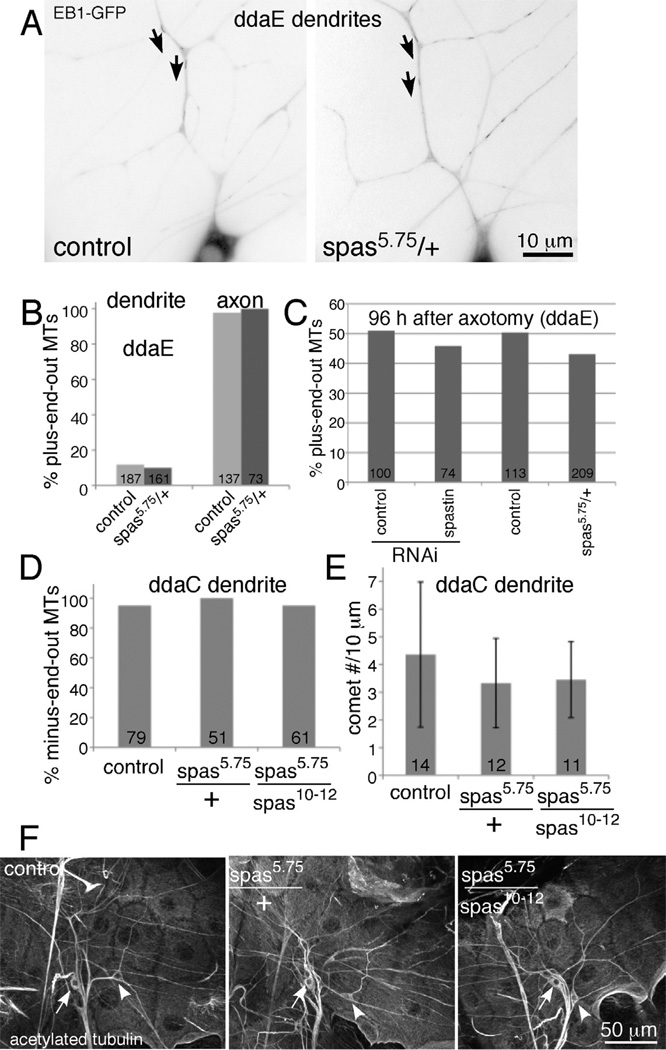
Microtubule polarity in the main trunk of the ddaE comb-like dorsal dendrite was the same in control and spas5.75/+ neurons (Figure 3A and B and Movie 1). Overall morphology of the ddaE cell appeared similar in spastin heterozygous and control animals (compare cells at 24h timepoint in Figure 2A and 2B), similar to previous reports that spastin RNAi does not disrupt ddaE morphology (Jinushi-Nakao et al., 2007). Thus failure of regeneration cannot be explained by global morphological defects or disruption of microtubule polarity before axotomy in the ddaE neuron.
Figure 3. Microtubule polarity and dynamics are not detectably altered in uninjured spastin mutant neurons.
A and B. Microtubule polarity in ddaE was quantitated in movies of EB1-GFP comets in control animals (221-Gal4, UAS-EB1-GFP in yw background) and spastin heterozygotes. Example images from each genotype are shown. Each image is a maximum z projection of 6 frames from the movies presented in Movie 1. Dendrite microtubule polarity was quantitated based on direction of comet movement in the main trunk of ddaE (arrows are placed beside examples of comets moving towards the cell body in the main trunk and point in the direction of movement). Axonal polarity was quantitated in the proximal axon based on similar movies. Numbers on the graph indicate numbers of comets counted in each condition. C. EB1-GFP was expressed in ddaE with 221-Gal4. Timelapse movies of EB1-GFP comet movement were used to determine the number of plus and minus-end-out microtubules in dendrites and axons. Proximal axotomy of ddaE was performed as in Figure 1. At the 96 h timepoint, the total number of plus and minus-end-out microtubules in all dendrites was counted. Spastin heterozygotes added similar numbers of plus-end-out microtubules as control animals to dendrites. n’s indicate total number of microtubules scored in all the animals tested. The same animals in which tip growth was measured for previous figures were used. D and E. EB1-GFP was expressed in class IV neurons including ddaC with the 477-Gal4 driver in control, spastin heterozygous animals or animals with two mutant copies of the spastin gene. At least 10 animals from each genotype were analyzed. For D, EB1-GFP comets were scored as moving towards or away from the cell body in movies of ddaC neurons. Numbers on the bars are the total number of comets analyzed. For E, the number of EB1-GFP comets in a 10 micron stretch of a ddaC dendrite was counted in a 200 frame timeseries. The average number of comets is shown in the graph and the error bars show the standard deviation. Numbers on the bars indicate the number of cells analyzed. F. Animals of the same genotypes used in D and E were dissected and stained for aceylated tubulin. Representative images for each genotype are shown.
As we also carried out regeneration experiments in the class IV ddaC neuron, we also assayed baseline microtubule behavior in ddaC neurons with different spastin mutations. As for the ddaE neuron, we assayed microtubule polarity in dendrites of heterozygous mutant neurons with EB1-GFP (Figure 3D). In addition we analyzed polarity with a combination of the null 5.75 allele and a hypomorphic 10–12 allele as these genotypes were used in experiments with the ddaC neuron below. In all cases dendrites remained minus-end-out. To determine whether spastin loss might affect some other aspect of microtubule behavior we also assayed the number of growing microtubules in mutant dendrites (Figure 3E) and stained for stable microtubules (Figure 3F). In no cases did we see any difference from control neurons, suggesting that spastin is not a global regulator of microtubule polarity or dynamics in either ddaE or ddaC neurons, but instead is likely to play a more specific role in time or space. We therefore also examined microtubule behavior after injury.
In order for a dendrite to be converted to a growing axon, plus-end-out microtubules must be added (Stone et al., 2010). Our next hypothesis about the function of spastin in regeneration was that it might be required for this step of microtubule rearrangement. To determine whether spastin contributed to switching a minus-end-out dendrite to a plus-end-out process capable of tip growth, we tracked microtubule polarity in dendrites after proximal axotomy. We found that plus-end-out microtubules could still be added to dendrites in neurons with reduced spastin. At 96h after axotomy, almost half of microtubules in dendrites of spastin heterozygotes were plus-end-out (Figure 3C), compared to only 10% in uninjured neurons (Figure 3B).
We conclude that the failure of spastin-depleted neurons to regenerate does not result from general disruption of microtubule polarity or dynamics, or failure to alter polarity in response to injury. Thus the function of spastin in regeneration of an axon from a dendrite seems to be at a step after polarity switching, but before extensive tip growth. We therefore hypothesized that spastin might be required for the initiation of tip growth or tip growth itself. If this was the case, then spastin might be generally required for axon regeneration, and not just regeneration of an axon from a dendrite.
Regeneration of an axon from the axon stump is robust in Drosophila sensory neurons
To test whether spastin played a role in regeneration of axons from the axon stump, we needed to develop an assay for this type of regeneration in Drosophila. The class I ddaE neurons that we use for assaying regeneration of an axon from a dendrite are not ideal for examining axon regeneration from an axon stump as multiple GFP-labeled axons bundle together soon after they emerge from the cell body. Fewer class IV dendritic arborization neurons are present in the larval body wall, and Gal4 drivers that are quite specific for these neurons are available. We therefore used the class IV neuron ddaC to study axon regeneration after distal axotomy.
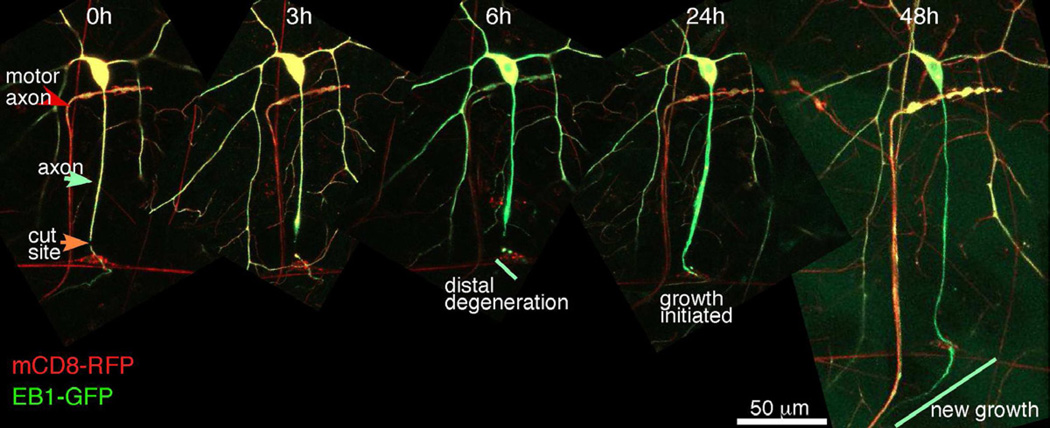
As for proximal axotomy of the ddaE neuron, we used a pulsed UV laser to sever ddaC neurons. Using the class IV driver 477-Gal4, we expressed EB1-GFP to label microtubules and mCD8-RFP to label membranes. Axons were severed more than 50 microns from the cell body, near the bipolar cell, which was also faintly labeled by 477-Gal4. At 3 hours after severing, slight swelling of the axon stump could be seen, but the distal axon was still smooth. By 6 hours the distal axon had initiated degeneration and was beaded. By 24 hours growth had initiated from the tip of the axon stump, and at 48 hours extensive growth was observed (Figure 4). Note, though, that the initiation of axon outgrowth was quite variable, and generally occurred between 24 and 72 hours. The axons of dendritic arborization neurons bundle together with one another and with motor axons. The nerve connects with the ventral ganglion in the central nervous system (CNS). The 477-Gal4 also frequently labels a motor neuron that synapses onto the muscle below the cell body of ddaC (Figure 4). The regenerating axon can be seen rejoining the nerve labeled with this motor axon (Figure 4, right panel). Thus distal axotomy of the ddaC cell can be used to monitor regeneration of axons from the axon stump, and this regeneration is comparable to that studied in other systems.
Figure 4. Axons regenerate from the axon stump after distal axotomy.
Transgenes were expressed in class IV da neurons with 477-Gal4. Axons of ddaC neurons were severed close to the bipolar cell (red cell with horizontal neurites visible in left panel), and neurons were tracked over time. Major events after distal axotomy were tracked in cells labeled with both a membrane marker (mCD8-RFP), and a microtubule marker (EB1-GFP). The axon of a motor neuron is often visible in this genetic background (red arrow).
Spastin is required for regeneration of axons from the axon stump
To determine whether spastin has a general role in axon outgrowth during regeneration, we tested heterozygous and homozygous animals in the ddaC distal axotomy model. Almost all control animals (21/22) initiated growth from the axon stump after distal axotomy (Figure 5). In contrast only about half of the spastin heterozygotes initiated axon outgrowth (Figure 5). Since this block in regeneration was not as complete as that seen in the proximal axotomy assay, we tested whether further reduction of spastin would result in a stronger regeneration block. We therefore used a trans-heterozygote of the spastin null allele (5.75) over a hypomorph (10–12) in which the 5’ end of the coding region is removed (Sherwood et al., 2004). This combination resulted in a stronger block of growth initiation (Figure 5), as expected if the lack of regeneration in the spas5.75/+ animals results from the mutation in spastin and not a second site defect. We conclude that reliable axon regeneration from the stump requires normal levels of spastin, and that loss of one copy of the gene impairs this process. This result is consistent with a role for spastin in an aspect of regeneration shared between growth of a new axon from a dendrite and regrowth from the axon stump, perhaps in initiation of tip growth.
Figure 5. Spastin is required for regeneration of an axon from the axon stump in the ddaC neuron.
ddaC neurons were labeled by expressing EB1-GFP with 477-Gal4, and axons were severed greater than 50 µm from the cell body at time 0. A. Example images of the response to distal axotomy in different genetic backgrounds are shown. Arrows indicate tips of axon stumps. Dotted lines indicate areas of new axon growth. B. Axons were scored for tip growth 96h after distal axotomy; cells were scored as initiating growth as in the control image shown in A, or not as in the two spastin mutant genotypes shown. Numbers of animals assayed are shown on the columns. Statistical significance was determined with a Fisher’s exact test.
Spastin is not required for dendrite outgrowth after pruning
Our results indicated that spastin was important for different types of axon regeneration. We therefore wished to test whether other types of neuron outgrowth were equally sensitive to spastin dosage.
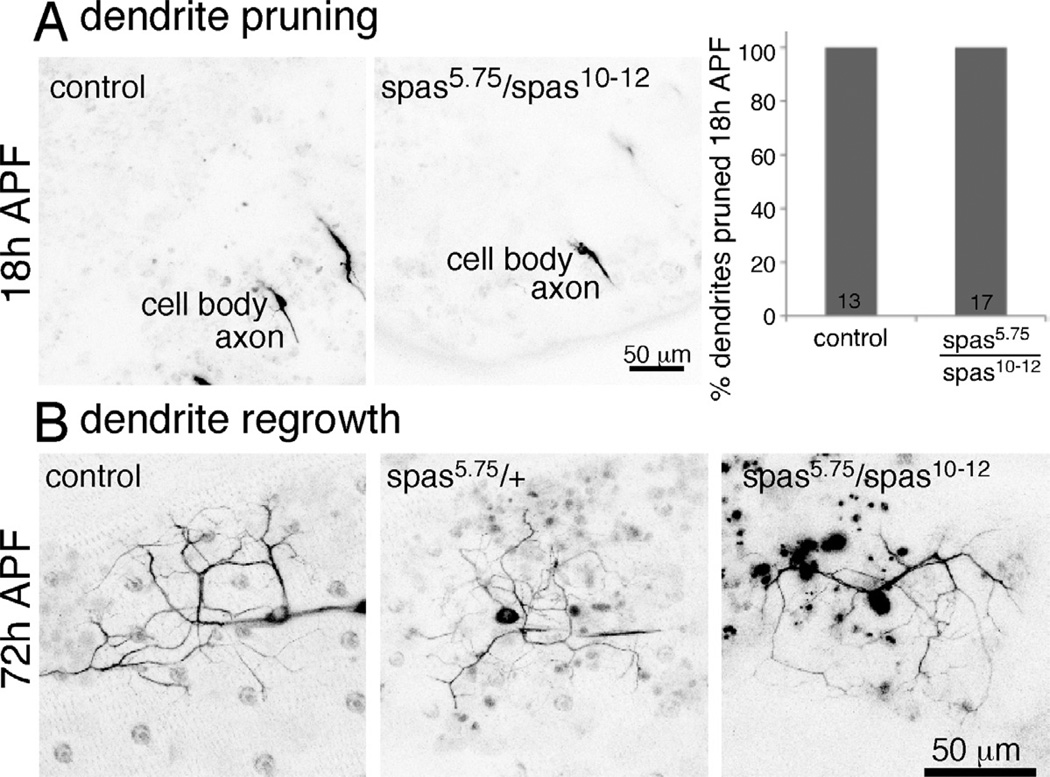
The regeneration assays were performed in larval ddaE and ddaC cells. Shortly after the 96 hour timepoint in the regeneration assays, larvae emerge from their food and initiate metamorphosis. During metamorphosis the body wall is rebuilt, and so the dendritic arborization neurons that innervate the body wall also need to be remodeled. Some of these neurons die, but both ddaE and ddaC prune their dendrite arbors, so that only the axon and cell body remain 18 hours after puparium formation (APF). They then regrow new dendrites into the body wall (Shimono et al., 2009). As this type of outgrowth is similar to regeneration in that it is reinitation of a growth program in a previously stable cell, we wished to determine whether it would also be sensitive to spastin dosage. First, though, we had to make sure that dendrites were normally pruned in spastin mutants.
To assay pruning in ddaC we mounted pupae for imaging 18h APF. At this time dendrites of control animals are almost always pruned (Figure 6A and (Lee et al., 2009; Tao and Rolls, 2011). Similarly, dendrites of ddaC in spas5.75/spas10–12 trans-heterozygotes were also all pruned 18h APF. Thus there was no defect in dendrite pruning in spastin mutants. To determine whether spastin was required for regrowth of ddaE and ddaC dendrites after pruning, we assayed their dendrites at 72 hours APF. At this time dendrites from both cells are labeled with 477-Gal4 and they lie on top of one another in the same region of the body wall (Shimono et al., 2009). Control neurons expressing EB1-GFP driven by 477-Gal4 had dendrites with several branches extending just over 50 microns. Dendrite outgrowth at 72h APF appeared similar to control in spastin heterozygous animals (spas5.75/+). All four animals examined had dendrites that were similar to those in the five control animals (Figure 6B). To make sure that this type of neuron outgrowth was less sensitive to spastin level than axon regeneration, we also examined it in animals in which both copies of spastin were mutant (spas5.75/spas10–12). Again, we observed no difference from control animals at 72 hours APF (Figure 6B). Four animals of this genotype were examined. We conclude that dendrites can regrow normally when spastin levels are reduced, even to levels below those in which axon regeneration is severely impaired. Moreover, dendrite regrowth was successful in the same cell types in which axon regeneration was assayed.
Figure 6. Dendrite pruning and regrowth are normal in spastin mutants.
A. Pupae expressing EB1-GFP under control of 477-Gal4 were imaged 18h APF to assay for presence of da neuron dendrites. Control animals had the Gal4 and GFP transgenes in a yw background. For spastin mutants, we used the null allele with hypomorph to examine string reduction in spastin levels. Even in this background all da neurons pruned their dendrites as normal. B. Animals of the indicated genotypes expressed EB1-GFP under control of 477-Gal4. Dendrite regrowth was assayed in living pupae at 72h APF. In all cases dendrites were present. The only feature that distinguished the appearance of animals of different genotypes was the presence of autofluorescent (black in these inverted images) blobs in background tissue of spastin mutants. At least four animals were examined for each genotype.
Spastin is not required for normal axon outgrowth during development
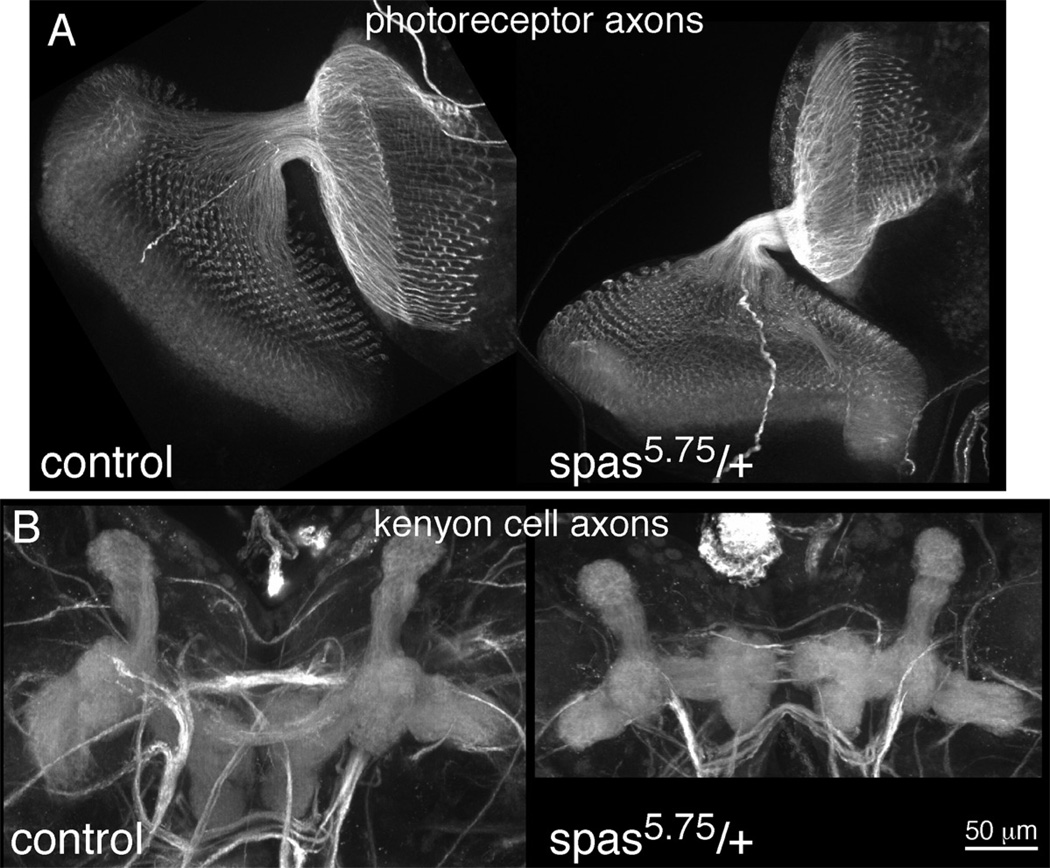
To further test whether spastin was generally required for neurite outgrowth, or specifically required for outgrowth during regeneration, we examined normal axon development in photoreceptor neurons and in kenyon cells that make up the mushroom body. We chose cell types that grow axons post-embryonically to make sure that during axon growth spastin levels in these cells would be comparable to or lower than levels in da neurons used in regeneration assays. Larval brains and developing eyes were dissected and stained for axon markers. Photoreceptor axons could be seen to emerge from the eye and project to the brain in both wild-type and spastin heterozygous brains (Figure 7A). The pattern of axon termini in the brain was identical in both genotypes. Similarly, the dorsal and medial lobes of the mushroom body, which contain the axon projections of the kenyon cells, were similar in control and spastin heterozygous larval brains (Figure 7B). Thus no defects in axon outgrowth were detectable in either cell type when spastin dosage were reduced. Thus developmental outgrowth of axons is not as sensitive to reduction of spastin as regenerative axon growth.
Figure 7. Axon outgrowth is normal in spastin heterozygotes.
Axons of photoreceptor neurons and kenyon cells were stained in dissected larval brains. A. Axon projections from the developing eye (left/ bottom) to the brain (top/ right) are shown. B. Axon projections of kenyon cells in the central brain are shown. Dorsal axon projections point to the top of the image, and medial projections are the tri-lobed structures pointing towards the middle of the image. Axons were stained with a cocktail of monoclonal antibodies recognizing Dac, FasII and Trio.
Discussion
We have shown that spastin is required for two different types of axon regeneration: regeneration of an axon from a dendrite after proximal axotomy, and regeneration of an axon from the axon stump after distal axotomy. Moreover, both types of regeneration are sensitive to spastin dosage. Loss of a single copy of the spastin gene dramatically impaired new axon outgrowth in both types of regeneration. Moreover overexpressed spastin also reduced regeneration. We showed that this sensitivity to spastin dosage is specific for regenerative growth of axons. Outgrowth of dendrites after pruning, or developmental outgrowth of axons, was unaffected by loss of one copy of the spastin gene. We therefore propose that spastin has a key role in axon outgrowth during regeneration.
Since spastin is required for both axon regeneration from a dendrite and from the axon stump, it is most likely involved in a process common to both. Spastin could play a role in local microtubule rearrangements that allow formation of a new growth cone. Local microtubule polarity reversals precede growth cone formation in cultured aplysia neurons in which the axon has been severed, and these seem to facilitate outgrowth from the axon stump (Erez et al., 2007). Alternately, microtubule severing by spastin could locally generate microtubule seeds that could serve as substrates for microtubule extension into the new axon. This type of function has been proposed for spastin in the spindle and synaptic bouton (Roll-Mecak and Vale, 2006), and during axon branch formation (Yu et al., 2008). Either way, our data support a role for spastin in the outgrowth of a new axon after axon injury.
The sensitivity of axon regeneration to spastin levels is interesting to consider in conjunction with the etiology of hereditary spastic paraplegia (HSP). HSP is often inherited in a dominant manner, and patients typically experience weakening and spasticity of their legs beginning at ages 20–40. This is due to degeneration of the upper motor neurons leading from the brain to the lower motor neurons that control the legs at the bottom of the spinal cord (Depienne et al., 2007). The upper motor neurons for the legs are the CNS neurons with longest axons. Mutations in SPG4, the gene that encodes spastin in people, are the most common cause of HSP, but the adult onset of degeneration is difficult to explain based on studies of spastin function. Most phenotypes that have been identified in model organisms or cultured neurons with reduced levels of spastin are developmental. For example, knock down of spastin in zebrafish embryos led to axon outgrowth defects (Wood et al., 2006), knock down in mammalian cultured neurons leads to axon branching defects (Yu et al., 2008), and Drosophila mutants have synaptic defects at the neuromuscular junction (Sherwood et al., 2004; Trotta et al., 2004) and dendrite branching defects (Jinushi-Nakao et al., 2007). However, in human disease, developmental defects do not seem to be present in many cases. Instead disease onset occurs after several asymptomatic decades of life. Mouse mutants do develop axon swellings that may be due to axon transport defects in homozygous and heterozygous animals, however the cause of these swellings is not totally clear (Tarrade et al., 2006). In the current study we identify a post-developmental function for spastin in axon regeneration. This function could explain the adult onset of HSP disease better than previously identified spastin functions.
The sensitivity of regeneration to spastin dosage is also consistent with this being the disease-relevant function. Most human disease caused by SPG4 mutations is inherited in a dominant fashion, meaning that only one copy of the gene is mutant. The fact that many spastin phenotypes do not manifest when spastin levels are mildly reduced has led to investigation of the idea that some of the spastin point mutants in disease patients may have dominant negative function. While this is likely in some cases (Du et al., 2010; Solowska et al., 2010), our data also suggests that loss of only one copy of functional spastin could lead to strong regeneration defects without needing to invoke dominant negative activity of mutant spastin.
Based on the sensitivity of axon regeneration to spastin dosage, it is possible that this contributes to HSP. Degeneration may manifest in the longest axons of the spinal cord after repeated attempts at repair or regeneration fail. One caveat to this model is that corticospinal (CST) neurons are thought to have very limited potential for axon regeneration (Liu et al., 2011). However, this idea is based on studies of large-scale injury, and perhaps some regrowth is possible after smaller wear-and-tear type injuries. Indeed, in a mouse model of multiple sclerosis, CST neurons did show extensive sprouting after localized inflammation, and were able to form functional connections that allowed the circuit to bypass the damaged region (Kerschensteiner et al., 2004). Why repeated failure of regeneration would not also affect peripheral neurons is unclear. However, peripheral neurons are generally more successful at regeneration (Chen et al., 2007; Huebner and Strittmatter, 2009), and so may be less sensitive to slightly reduced spastin levels. It is also possible that because peripheral neurons are damaged more and thus need to regenerate more, they constitutively express more spastin, and so may have enough residual function when one copy of the gene is lost. In our system this logic could explain why we see stronger regeneration defects in ddaE than ddaC cells. DdaC cells express higher levels of spastin than ddaE cells (Jinushi-Nakao et al., 2007), and so may have enough spastin expressed from one copy of the gene to initiate regeneration some of the time in heterozygotes. It is also possible that other severing proteins could functionally substitute for spastin in some neurons.
Our results also suggest that function of two other putative severing proteins kat-60 and kat-60L1, may be required for successful axon regeneration. However, regeneration can still proceed when one functional copy of either of these genes is present. It will be interesting to determine whether these other severing proteins function at the same step of regeneration as spastin and whether they could functionally substitute for spastin in some neurons.
Axon regeneration represents a profound change in neuronal behavior. Neurons that are relatively static must reinitiate a massive growth program. One possibility is that axon regeneration involves returning to a state similar to that during initial developmental outgrowth of axons. Indeed some genes, including GAP-43, that are associated with developmental neuronal growth are re-expressed during regenerative growth (Navarro et al., 2007). The difference in sensitivity to spastin gene dosage of developmental neurite outgrowth and regenerative growth suggests, however, that these two growth programs are not executed in the same way. Understanding how the regenerative growth program differs from developmental neurite outgrowth may provide new ideas for enhancing axon regeneration after nerve injury.
Experimental Procedures
Drosophila Stocks
Tester line for RNAi experiments was: (1)UAS-dicer2; 221-Gal4, UAS- EB1-GFP (2) 477 Gal4. Tester lines for mutant analysis experiments were: (1) 221 Gal4, UAS-EB1-GFP (2) 477 Gal4, UAS EB1-GFP. For experiments in which both copies of spastin were mutant, one of the mutant alleles was crossed into these tester backgrounds first.
RNAi experiments were done by crossing the tester lines to the following RNAi lines from the Vienna Drosophila RNAi center (VDRC, Vienna, Austria): UAS-γtubulin identification number 24746), UAS-katanin 60- RNAi (VDRC identification number 38368), UAS-katanin-p60L1-RNAi (VDRC identification number 31598), and UAS-spastin-RNAi (VDRC identification number 33110).
Mutant analysis experiments were done by crossing the tester lines to the spastin null (spastin5.75), spastin hypomorph (spastin10–12), and kat-60L1BE6 lines. For homozygous spastin mutant genotypes, lines were generated that contained the Gal4 driver and GFP transgene on the second chromosome with spastin5.75 on the third chromosome balanced with TM6(Tb). These lines were crossed to spastin10–12 flies balanced with TM6(Tb) and non-Tb progeny were analyzed. The deficiency line that removes the fidgetin gene, Df(2L)Exel8008/CyO, was obtained from the Bloomington Drosophila Stock Center and was rebalanced with CyO-actinGFP; a TM6 chromosome was present in some flies and progeny analyzed. YW flies were crossed to tester lines as a control. For all experiments animals of either sex were used.
RNAi screen of microtubule severing proteins and proximal axotomy experiments
The requirement for microtubule severing proteins in axon regeneration was tested using the proximal axotomy injury system and imaging conditions previously described (Stone et al., 2010). For expression of RNA hairpins, the tester line UAS-dicer2; 221-Gal4, UAS-EB1-GFP was crossed to RNAi lines obtained from the VDRC. The 221-Gal4 is a class I neuron specific Gal4 that will drive expression of dicer 2, EB1-GFP, and hairpin RNAs in the class I da neurons. For mutant analysis, the tester line 221-Gal4, UAS-EB1-GFP was crossed to spastin, katanin-60 and katanin-60L1 mutant lines. Embryos were collected overnight at room temperature and then incubated at 25C for 48 hours. Using whole, live larvae, axons of ddaE were severed close to the cell body using a pulsed Micropoint UV laser (Photonic Instruments, St. Charles, IL). After severing, larvae were immediately imaged using an LSM510 confocal microscope (Carl Zeiss, Thornwood, NY) and then recovered to Drosophila media. Larvae were then imaged every 24 or 48 hours over a 96 hour time period. Tip growth was quantitated by manually measuring the length of the dendrite that initiated tip growth immediately following severing and again at 96 hours using ImageJ software. A different dendrite branch (one that did not initiate tip growth) was also measured and used to determine the amount of normal developmental growth. Tip growth beyond normal developmental growth was then calculated.
Distal axotomy assay in ddaE
Two-day old control (221-Gal4, UAS-EB1-GFP x YW progeny) and mutant (221-Gal4, UAS-EB1-GFP x spas 5.75//TM6(Tb) non-Tb progeny) Drosophila larvae were mounted for live imaging. A UV laser was used to sever the all axons that contained visible fluorescence (which includes axons from ddaE, ddaD and ddaC cells) at least 50 microns away from the cell bodies. Larvae were kept alive in food caps at 20° C and imaged every 24 hours up to 96 hours after severing. Imaging was performed using a LSM510 confocal microscope (Carl Zeiss, Thornwood, NY) and frames were acquired every 2 s. Regeneration from the axon stump was scored in axons from Class I neurons (ddaE and ddaD cells) only.
Assay of microtubule polarity in ddaE
To assay microtubule polarity we tracked the movement of individual EB1-GFP comets in the main trunk of the ddaE neuron in whole, live third instar larvae. EB1-GFP only binds to the growing plus end of the microtubule. Therefore, the direction of movement represents microtubule polarity. Live imaging was performed using a Zeiss Axio Imager light microscope (Carl Zeiss, Thornwood, NY). To image EB1-GFP comets, single frames were taken every two seconds. Images were analyzed using ImageJ software. In order to be counted, comets had to be tracked for a minimum of three consecutive frames.
Microtubule dynamics and polarity in ddaC neurons
To generate control animals, yw flies were crossed to a line containing 477-Gal4, UAS-EB1-GFP on the second chromosome. Embryos were collected overnight and then aged for three days at 25C before imaging. Heterozygous and homozygous mutant animals were collected in a similar manner. The cross to generate heterozygous larvae was: 477-Gal4; spas5.75/TM6(Tb) X yw, and non-Tb larvae were selected. The cross to generate homozygous mutant larvae was: 477-Gal4; spas5.75/TM6(Tb) X spas10–12/TM6(Tb) and again non-Tb larvae were selected. Movies of EB1-GFP in ddaC dendrites were acquired using a Zeiss Imager M2 microscope equipped with LED illumination.
For quantification of microtubule dynamics in uninjured neurons, a 10 micron length of the dendrite was chosen and EB1-GFP comets were counted for the entire duration of the movie (200 frames, 1 frame per second). For quantification of microtubule polarity in uninjured neurons, EB1-GFP comets moving towards and away from the cell body were counted throughout the dendrite in focus for the entire duration of the movie.
Immunostaining
Third-instar larvae from the same genetic crosses described in the previous section were cut open with dissecting scissors along the dorsal midline in Schneider’s medium and fixed in 4% paraformaldehyde. Preparations were then washed several times in Blocking solution (PBS, 1% BSA, 0.2% Triton X-100, 10 mM glycine) and treated with primary antibody (mouse anti-acetylated tubulin, Sigma T6793) at 4°C overnight. The next day, primary antibody was removed and washes were performed in blocking solution followed by treatment with Rhodamine-Red-X coupled secondary antibody (Jackson ImmunoResearch) for approximately two hours at room temperatue. After a final few washes with blocking solution, larval preparations were stored at 4°C in 85% glycerol/ 50mM Tris (pH 8) until mounting. Images were acquired using the Olympus Fluoview 1000 and analyzed using ImageJ software (http://rsb.info.nih.gov/ij/; NIH).
Assay to study regeneration after distal axotomy in ddaC
In order to assay regeneration from the axon stump, axons were severed at least 50 µm from the cell body. The class IV specific neuronal driver 477 was used to express EB1-GFP or mCD8-RFP in the class IV ddaC neuron. Embryos were collected overnight at room temperature and then incubated at 25C for 48 hours. Axons of whole, live larvae were severed near the bipolar cell with a pulsed Micropoint UV laser (Photonic Instruments, St. Charles, IL). Cells were immediately imaged using an LSM510 confocal microscope (Carl Zeiss, Thornwood, NY) and then recovered to Drosophila media. Larvae were imaged at 4h, 6h or 12h after cutting to make sure axons were completely severed. They were then imaged every 24h to determine the time of growth initiation from the axon stump.
A candidate screen for microtubule regulators was performed in this sytstem using the tester line 477-Gal4, UAS-EB1-GFP; UAS-dicer2 and crossing it to RNAi lines obtained from the VDRC. Mutant analysis was done by crossing the tester line 477-Gal4, UAS-EB1-GFP to the spastin null mutant. Axons were then severed as described above and time of growth initiation was assayed.
Dendrite pruning and regrowth experiments
During metamorphosis the dendrites of the ddaE and ddaC cells are pruned and then regrow when the body wall is rebuilt. To assay this process in control and spastin mutant pupae, pupal cases were removed from animals 18 hours APF. Whole, live pupae were then placed on a microscope slide and imaged using an LSM510 confocal microscope (Carl Zeiss, Thornwood, NY). Dendrite regrowth was assayed by removing pupal cases of animals 72h APF and imaging.
Analysis of photoreceptor and kenyon cell axons
Larval brains were dissected from non-balancer progeny of spastin5.75/TM6B crossed to w1118, CantonS (WCS) flies, and compared to WCS larval brains. Brains were fixed in 4% paraformaldehyde (EM Sciences) for 30 min, rinsed in PBS with 0.2% TritonX-100 (PBT), and blocked in PBT with 5% normal goat serum, 0.01% BSA, and 0.02% sodium azide. Brains were incubated overnight at 4 deg C in block containing a primary antibody cocktail of monoclonals anti-Dac, anti-FasII (1D4 concentrate), and anti-Trio, each diluted 1:100. Subsequent secondary antibody incubation used goat anti-mouse Alexa-Fluor488 at 1:250, overnight at 4 degrees C. Brains were mounted in Vectashield and imaged on a Zeiss LSM 510.
Supplementary Material
Whole living larvae expressing EB1-GFP in the ddaE neuron were mounted for imaging. 200 frame movies were acquired with a widefield LED microscope. Images were acquired every 2s. A complete movie of a wildtype neuron containing one copy each of 221-Gal4 and UAS-EB1-GFP is shown on the left. A neuron from a spas5.75/+ animal is shown on the right. EB1-GFP comets can be seen moving in the dendrites. The majority of comets move towards the cell body, which can be seen at the bottom of the frame.
Highlights.
-
-
animals with one copy of the spastin gene have major deficits in axon regeneration
-
-
the three other severing proteins are not haploinsufficient for regeneration
-
-
developmental axon and dendrite outgrowth is normal in spastin mutants
-
-
the sensitivity of axon regeneration to spastin levels suggests a new idea for HSP
Acknowledgements
We are extremely grateful to the Vienna Drosophila RNAi Center (VDRC) for the RNAi lines used in this study. The Bloomington Drosophila Stock Center and Developmental Studies Hybridoma Bank are also invaluable resources. Wendy Hanna-Rose graciously shared her pulsed UV laser for all the experiments. We would like to thank Rolls lab members for discussions of the ideas, particularly Li Chen. We would also like to thank Li Chen and Michelle Nguyen for help collecting animals for the experiments. Funding sources include the Spastic Paraplegia Foundation, NINDS (R21 NS066216 and R01 NS63896) and NIGMS (R01 GM085115). MMR is a Pew Scholar in the Biomedical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baas PW, Lin S. Hooks and comets: The story of microtubule polarity orientation in the neuron. Dev Neurobiol. 2011;71:403–418. doi: 10.1002/dneu.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Depienne C, Stevanin G, Brice A, Durr A. Hereditary spastic paraplegias: an update. Curr Opin Neurol. 2007;20:674–680. doi: 10.1097/WCO.0b013e3282f190ba. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Du F, Ozdowski EF, Kotowski IK, Marchuk DA, Sherwood NT. Functional conservation of human Spastin in a Drosophila model of autosomal dominant-hereditary spastic paraplegia. Hum Mol Genet. 2010;19:1883–1896. doi: 10.1093/hmg/ddq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez H, Malkinson G, Prager-Khoutorsky M, De Zeeuw CI, Hoogenraad CC, Spira ME. Formation of microtubule-based traps controls the sorting and concentration of vesicles to restricted sites of regenerating neurons after axotomy. J Cell Biol. 2007;176:497–507. doi: 10.1083/jcb.200607098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis-Ruth S, Wierenga CJ, Bradke F. Plasticity of polarization: changing dendrites into axons in neurons integrated in neuronal circuits. Curr Biol. 2008;18:992–1000. doi: 10.1016/j.cub.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Hall GF, Cohen MJ. Extensive dendritic sprouting induced by close axotomy of central neurons in the lamprey. Science. 1983;222:518–521. doi: 10.1126/science.6623092. [DOI] [PubMed] [Google Scholar]
- Hall GF, Poulos A, Cohen MJ. Sprouts emerging from the dendrites of axotomized lamprey central neurons have axonlike ultrastructure. J Neurosci. 1989;9:588–599. doi: 10.1523/JNEUROSCI.09-02-00588.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner EA, Strittmatter SM. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ. 2009;48:339–351. doi: 10.1007/400_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinushi-Nakao S, Arvind R, Amikura R, Kinameri E, Liu AW, Moore AW. Knot/Collier and cut control different aspects of dendrite cytoskeleton and synergize to define final arbor shape. Neuron. 2007;56:963–978. doi: 10.1016/j.neuron.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Bareyre FM, Buddeberg BS, Merkler D, Stadelmann C, Bruck W, Misgeld T, Schwab ME. Remodeling of axonal connections contributes to recovery in an animal model of multiple sclerosis. J Exp Med. 2004;200:1027–1038. doi: 10.1084/jem.20040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- Lee HH, Jan LY, Jan YN. Drosophila IKK-related kinase Ik2 and Katanin p60-like 1 regulate dendrite pruning of sensory neuron during metamorphosis. Proc Natl Acad Sci U S A. 2009;106:6363–6368. doi: 10.1073/pnas.0902051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Tedeschi A, Park KK, He Z. Neuronal Intrinsic Mechanisms of Axon Regeneration. Annu Rev Neurosci. 2011 doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- Macagno ER, Muller KJ, DeRiemer SA. Regeneration of axons and synaptic connections by touch sensory neurons in the leech central nervous system. J Neurosci. 1985;5:2510–2521. doi: 10.1523/JNEUROSCI.05-09-02510.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattie FJ, Stackpole MM, Stone MC, Clippard JR, Rudnick DA, Qiu Y, Tao J, Allender DL, Parmar M, Rolls MM. Directed Microtubule Growth, +TIPs, and Kinesin-2 Are Required for Uniform Microtubule Polarity in Dendrites. Curr Biol. 2010;20:2169–2177. doi: 10.1016/j.cub.2010.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A, McNally FJ. Microtubule-severing enzymes. Curr Opin Cell Biol. 2010;22:96–103. doi: 10.1016/j.ceb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A, Vale RD. Making more microtubules by severing: a common theme of noncentrosomal microtubule arrays? J Cell Biol. 2006;175:849–851. doi: 10.1083/jcb.200611149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose PK, MacDermid V, Joshi M, Neuber-Hess M. Emergence of axons from distal dendrites of adult mammalian neurons following a permanent axotomy. Eur J Neurosci. 2001;13:1166–1176. doi: 10.1046/j.0953-816x.2001.1490.x. [DOI] [PubMed] [Google Scholar]
- Schmitt AB, Breuer S, Liman J, Buss A, Schlangen C, Pech K, Hol EM, Brook GA, Noth J, Schwaiger FW. Identification of regeneration-associated genes after central and peripheral nerve injury in the adult rat. BMC Neurosci. 2003;4:8. doi: 10.1186/1471-2202-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NT, Sun Q, Xue M, Zhang B, Zinn K. Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS Biol. 2004;2:e429. doi: 10.1371/journal.pbio.0020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono K, Fujimoto A, Tsuyama T, Yamamoto-Kochi M, Sato M, Hattori Y, Sugimura K, Usui T, Kimura K, Uemura T. Multidendritic sensory neurons in the adult Drosophila abdomen: origins, dendritic morphology, and segment- and age-dependent programmed cell death. Neural Dev. 2009;4:37. doi: 10.1186/1749-8104-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowska JM, Garbern JY, Baas PW. Evaluation of loss of function as an explanation for SPG4-based hereditary spastic paraplegia. Hum Mol Genet. 2010;19:2767–2779. doi: 10.1093/hmg/ddq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein) J Neurosci. 2003;23:2655–2664. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Tsubouchi A, Rolls MM, Tracey WD, Sherwood NT. Katanin p60-like1 Promotes Microtubule Growth and Terminal Dendrite Stability in the Larval Class IV Sensory Neurons of Drosophila. J Neurosci. 2012;32:11631–11642. doi: 10.1523/JNEUROSCI.0729-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone MC, Nguyen MM, Tao J, Allender DL, Rolls MM. Global up-regulation** of microtubule dynamics and polarity reversal during regeneration of an axon from a dendrite. Mol Biol Cell. 2010;21:767–777. doi: 10.1091/mbc.E09-11-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone MC, Roegiers F, Rolls MM. Microtubules Have Opposite Orientation in Axons and Dendrites of Drosophila Neurons. Mol Biol Cell. 2008;19:4122–4129. doi: 10.1091/mbc.E07-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K, Bonilla I, Winkles JA, Strittmatter SM. Fibroblast growth factor-inducible-14 is induced in axotomized neurons and promotes neurite outgrowth. J Neurosci. 2003;23:9675–9686. doi: 10.1523/JNEUROSCI.23-29-09675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Rolls MM. Dendrites have a rapid program of injury-induced degeneration that is molecularly distinct from developmental pruning. J Neurosci. 2011;31:5398–5405. doi: 10.1523/JNEUROSCI.3826-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrade A, Fassier C, Courageot S, Charvin D, Vitte J, Peris L, Thorel A, Mouisel E, Fonknechten N, Roblot N, et al. A mutation of spastin is responsible for swellings and impairment of transport in a region of axon characterized by changes in microtubule composition. Hum Mol Genet. 2006;15:3544–3558. doi: 10.1093/hmg/ddl431. [DOI] [PubMed] [Google Scholar]
- Trotta N, Orso G, Rossetto MG, Daga A, Broadie K. The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Curr Biol. 2004;14:1135–1147. doi: 10.1016/j.cub.2004.06.058. [DOI] [PubMed] [Google Scholar]
- Veldman MB, Bemben MA, Thompson RC, Goldman D. Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration. Dev Biol. 2007;312:596–612. doi: 10.1016/j.ydbio.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Wang Z, Jin Y. Genetic dissection of axon regeneration. Curr Opin Neurobiol. 2011;21:189–196. doi: 10.1016/j.conb.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese C. Distinct Dgrip84 isoforms correlate with distinct gamma-tubulins in Drosophila. Mol Biol Cell. 2008;19:368–377. doi: 10.1091/mbc.E07-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PG, Borisy GG. Maternally expressed gamma Tub37CD in Drosophila is differentially required for female meiosis and embryonic mitosis. Dev Biol. 1998;199:273–290. doi: 10.1006/dbio.1998.8900. [DOI] [PubMed] [Google Scholar]
- Wood JD, Landers JA, Bingley M, McDermott CJ, Thomas-McArthur V, Gleadall LJ, Shaw PJ, Cunliffe VT. The microtubule-severing protein Spastin is essential for axon outgrowth in the zebrafish embryo. Hum Mol Genet. 2006;15:2763–2771. doi: 10.1093/hmg/ddl212. [DOI] [PubMed] [Google Scholar]
- Yang Y, Xie Y, Chai H, Fan M, Liu S, Liu H, Bruce I, Wu W. Microarray analysis of gene expression patterns in adult spinal motoneurons after different types of axonal injuries. Brain Res. 2006;1075:1–12. doi: 10.1016/j.brainres.2005.12.060. [DOI] [PubMed] [Google Scholar]
- Yu W, Qiang L, Solowska JM, Karabay A, Korulu S, Baas PW. The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol Biol Cell. 2008;19:1485–1498. doi: 10.1091/mbc.E07-09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Rogers GC, Buster DW, Sharp DJ. Three microtubule severing enzymes contribute to the "Pacman-flux" machinery that moves chromosomes. J Cell Biol. 2007;177:231–242. doi: 10.1083/jcb.200612011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Whole living larvae expressing EB1-GFP in the ddaE neuron were mounted for imaging. 200 frame movies were acquired with a widefield LED microscope. Images were acquired every 2s. A complete movie of a wildtype neuron containing one copy each of 221-Gal4 and UAS-EB1-GFP is shown on the left. A neuron from a spas5.75/+ animal is shown on the right. EB1-GFP comets can be seen moving in the dendrites. The majority of comets move towards the cell body, which can be seen at the bottom of the frame.