Abstract
Repeated administration of antipsychotic drugs induces a sensitization-like or tolerance-like effect in many behavioral tasks, including the conditioned avoidance response (CAR) and the phencyclidine (PCP)-induced hyperlocomotion, two rodent models with high predictive validity for antipsychotic activity. This study investigated the impacts of contextual and behavioral variables on the expression of clozapine tolerance using a recently validated across-model transfer paradigm (Zhang and Li, 2012). Male Sprague-Dawley rats were first repeatedly treated with clozapine (2.5–10.0 mg/kg, sc) in the CAR model or PCP (1.6 mg/kg, sc)-induced hyperlocomotion model for five consecutive days. They were then tested for the expression of clozapine tolerance in another model for another five days. Finally, all rats were switched back to the original model and tested again for the expression of clozapine tolerance. When tested in the PCP model, rats previously treated with clozapine in the CAR model did not show an immediate weaker inhibition of PCP-induced hyperlocomotion than those treated with clozapine for the first time, but showed a significantly weaker inhibition over time. In contrast, when tested in the CAR model, rats previously treated with clozapine in the PCP model showed an immediate weaker disruption of avoidance response than those treated with clozapine for the first time, but this weaker effect diminished over time. These results suggest that the expression of clozapine tolerance is strongly modulated by the test environment and/or selected behavioral response. Clozapine tolerance and its situational specificity may be related to the drug’s low extrapyramidal motor side effect, its superior therapeutic efficacy and/or emergence of clozapine withdrawal syndrome.
Keywords: Clozapine, Conditioned avoidance response, Phencyclidine, Motor activity, Tolerance, Contextual control
1. Introduction
Clozapine (CLZ) is the prototypical atypical antipsychotic drug with superior efficacy in treating the negative symptoms (e.g. social withdrawal, anhedonia) and cognitive deficits (e.g. attentional deficits) associated with schizophrenia, and patients who respond poorly to other antipsychotic medications [2–5]. Animal studies find that CLZ differs from other antipsychotics in many ways. For example, repeated treatment of haloperidol elicits dopamine supersensitivity (up-regulation of D2 High receptors), whereas repeated treatment of CLZ has little effect on D2 High up-regulation [6, 7]. Neuroanatomically, CLZ, but not haloperidol, shows greater selectivity for the mesolimbic dopamine system than for the nigrostriatal dopamine system [8, 9]. Behaviorally, repeated treatment of haloperidol, olanzapine and risperidone tends to cause a sensitization [1, 10–12], whereas repeated CLZ causes a tolerance [11, 13]. For instance, in a motor function and attention test, Stanford and Fowler [14] reported that CLZ-treated rats exhibited tolerance to the drug’s suppressive effect on the amount of time that rats were in contact with a force-sensing target disk. In contrast, haloperidol-treated rats displayed little tolerance on this measure. In a fixed ratio 5 lever pressing test, Trevitt et al. [15] found that both haloperidol and CLZ significantly suppressed lever pressing. However, with repeated injections, haloperidol enhanced its suppression, while CLZ decreased it. In a lever pressing for food reward task, Varvel et al. [16] and Villanueva and Porter [17] also found that repeated haloperidol produced a significant increase in response duration, while repeated CLZ produced a decrease. CLZ-induced tolerance has also been observed in a drug discrimination task [18, 19].
The conditioned avoidance response (CAR) and phencyclidine (PCP)-induced hyperlocomotion are two tests commonly used to study antipsychotic drugs. Both tests have high predictive validity for antipsychotic efficacy, as acute administration of antipsychotic drugs, but not anxiolytics or antidepressants selectively disrupts avoidance response, and suppresses PCP-induced hyperlocomotion [20–25]. In recent years, we have examined the long-term consequences of repeated CLZ treatment in both tests. First, rats are repeatedly treated with different doses of CLZ for 5 days then their avoidance responses and PCP-induced hyperlocomotion are recorded. This period is termed the induction phase. Two days later, all rats are given a challenge dose of CLZ (5.0 mg/kg) to assess the expression of CLZ tolerance (termed the expression phase). In the CAR model [11, 13], we found that although repeated administration of CLZ produces an inhibition of avoidance responses persistently during the induction phase (no apparent tolerance), in the expression phase when all rats are challenged with a low dose of CLZ, rats previously treated with CLZ made significantly more avoidances than those who are treated with this drug for the first time, indicative of CLZ tolerance. Similarly, in the PCP hyperlocomotion model, repeated CLZ treatment dose-dependently and persistently inhibits PCP-induced hyperlocomotion in the induction phase (no apparent tolerance) [23], but exhibits a tolerance effect in the expression phase as rats previously treated with CLZ have a weaker inhibition of PCP-induced hyperlocomotion than those treated with CLZ for the first time. The overall pattern of CLZ effect in both models is in sharp contrast to the effects of haloperidol, olanzapine and risperidone which cause a sensitization [1, 11, 13].
Despite being widely demonstrated, experimental conditions that govern the expression of CLZ tolerance are still poorly understood. For example, the extent to which the expression of CLZ tolerance is modulated by contextual cues and behavioral variables is not known. How CLZ’s action on avoidance responses and on PCP-induced hyperlocomotion, in turn, influences the rate and extent of its tolerance development is also not known. In this study, we examined how the environmental cues and behavioral responses affect the expression of CLZ tolerance using a novel across-model transfer paradigm [1, 26]. Our general approach was to repeatedly treat animals with CLZ in one model to induce a tolerance process, then to test its expression in another model, and finally to retest its expression back in the original model. We recently used this paradigm and determined that the expression of haloperidol and olanzapine sensitization in the CAR and PCP models is strongly influenced by test environment and/or selected behavioral response [1]. This study extended this line of research into CLZ. If CLZ tolerance results from inevitable neurobiological adaptations produced by the direct pharmacological actions of the drug, it should persist into another model. However, if the contextual cues and behavioral responses associated with different models have a powerful control on the expression of CLZ tolerance, it should not be detectable when the model is changed.
2. Materials and Methods
2.1. Animals
Adult male Sprague-Dawley rats (226–250g upon arrival, Charles River, Portage, MI) were used. They were housed two per cage, in 48.3 cm × 26.7 cm × 20.3 cm transparent polycarbonate cages under 12-hr light/dark conditions (light on between 6:30 am and 6:30 pm). Room temperature was maintained at 22±1°C with a relative humidity of 45–60%. Food and water was available ad libitum. Animals were allowed at least one week of habituation to the animal facility before being used in experiments. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Nebraska-Lincoln.
2.2. Drugs
Clozapine (CLZ, gift from NIMH drug supply program) was dissolved in 1.2% glacial acetic acid in distilled sterile water [11, 27]. Phencyclidine hydrochloride (PCP, gift from the NIDA Chemical synthesis and Drug Supply Program) was dissolved in 0.9% saline. All drugs were administrated subcutaneously in a volume of 1.0 ml/kg body weight. In the first experiment (from CAR to PCP), we tested three doses of CLZ (2.5, 5.0, 10.0 mg/kg) to assess the possible dose-dependent nature of CLZ tolerance. These doses of CLZ produce a reliable disruption on avoidance responding and inhibit the PCP-induced hyperlocomotion [23, 24]. Furthermore, CLZ at 5.0 and 10.0 mg/kg gives rise to a clinically comparable range (40%–60%) of striatal dopamine D2 occupancy [27]. Based on findings from the first experiment and our previous work [23], we tested CLZ at 5.0 mg/kg in the second experiment (from PCP to CAR). The dose of PCP (1.6 mg/kg, sc) was based on our own study [1] and others [28].
2.3. Apparatus
2.3.1. Two-way avoidance conditioning apparatus
Eight identical two-way shuttle boxes custom designed and manufactured by Med Associates (St. Albans, VT) were used. Each box was housed in a ventilated, sound-insulated isolation cubicle (96.52 cm W × 35.56 cm D × 63.5 cm H). Each box was 64 cm long, 30 cm high (from grid floor), and 24 cm wide, and was divided into two equal-sized compartments by a partition with an arch style doorway (15 cm high × 9 cm wide at base). A barrier (4 cm high) was placed between the two compartments, so the rats had to jump from one compartment to the other. The grid floor consisted of 40 stainless-steel rods with a diameter of 0.48 cm, spaced 1.6 cm apart center to center, through which a scrambled footshock (Unconditioned stimulus, US, 0.8 mA, maximum duration: 5 s) was delivered by a constant current shock generator (Model ENV-410B) and scrambler (Model ENV-412). The rat location and crossings between compartments were monitored by a set of 16 photobeams (ENV-256-8P) affixed at the bottom of the box (3.5 cm above the grid floor). Illumination was provided by two houselights mounted at the top of each compartment. The conditioned stimulus (CS, i.e. 76 dB white noise) was produced by a speaker (ENV 224 AMX) mounted on the ceiling of the cubicle, centered above the shuttle box. Background noise (approximately 74 dB) was provided by a ventilation fan affixed at the top corner of each isolation cubicle. All training and testing procedures were controlled by Med Associates programs running on a computer.
2.3.2. Motor activity monitoring apparatus
Sixteen activity boxes were housed in a quiet room. The boxes were 48.3 cm × 26.7 cm × 20.3 cm transparent polycarbonate cages, which were similar to the home cages but were each equipped with a row of 6 photocell beams (7.8 cm between two adjacent photobeams) placed 3.2 cm above the floor of the cage. A computer detected the disruption of the photocell beams and recorded the number of beam breaks. All experiments were run during the light cycle.
2.4. Experiments
2.4.1. Experiment 1: Transferability of CLZ tolerance from the CAR model to the PCP hyperlocomotion model and back to the CAR model
This experiment examined whether the tolerance induced by repeated CLZ treatment in the CAR model expressed in the PCP-induced hyperlocomotion model. The experiment comprised the following three phases: Avoidance training/repeated CLZ treatment in the CAR, tolerance expression test in the PCP hyperlocomotion model, and tolerance expression retest back in the CAR model.
Avoidance training/repeated CLZ treatment in the CAR
Eighty-eight rats (in 2 batches) were first handled and habituated to the CAR boxes for 2 days (20 min/day). They were then trained for conditioned avoidance responding for a total of 10 sessions over a 2-week period. Each session consisted of 30 trials, with inter-trial intervals randomly varying between 30 and 60 s. Every trial started with a presentation of a white noise (CS) for 10 s, followed by a continuous scrambled foot shock (0.8 mA, US, maximum duration = 5 s) on the grid floor. If a subject crossed from one compartment into the other within the 10 s of CS presentation, it avoided the shock and this shuttling response was recorded as avoidance. If the rat remained in the same compartment for more than 10 s and made a crossing only after receiving the footshock, this response was recorded as escape. If the rat did not switch compartments during the entire 5 s presentation of the shock, the trial was terminated and escape failure was recorded. The total number of avoidance responses was recorded for each session.
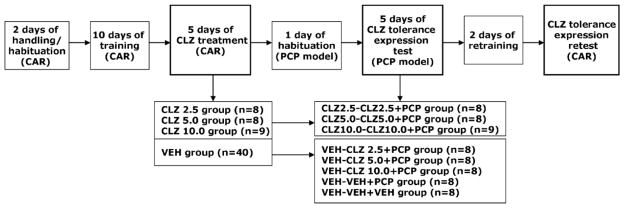
At the end of the training session, 65 rats reached the training criterion (>70% avoidance in each of the last 2 sessions). They were first matched on avoidance performance on the last training day (pre-drug) to create blocks of rats that were approximately equal in performance. Within each block, they were then randomly assigned to one of four groups: CLZ 2.5 mg/kg (n = 8), CLZ 5.0 mg/kg (n = 8), CLZ 10.0 mg/kg (n = 9), and vehicle (n = 40), and tested daily under the CS-only (no shock, 30 trials per session) condition for 5 consecutive days. The CS-only condition was used to control the possible confound of number of shocks received and to exclude any possible relearning effect caused by the presence of the US, as employed in our previous studies [1, 11]. On each test day, rats were first injected with CLZ or vehicle (sterile water), and tested 1 h later.
Tolerance expression test in the PCP hyperlocomotion model
One day after the last CAR drug test, rats were placed in the motor activity testing boxes for 30 min for habituation. On Day 2, rats that were previously treated with vehicle (n = 40) during the CAR tests were randomly assigned to five groups: three groups (n=8) received CLZ followed by PCP (VEH-CLZ 2.5+PCP; VEH-CLZ 5.0+PCP; and VEH-CLZ 10.0+PCP), and two groups (n=8) received sterile water followed by PCP or saline (VEH-VEH+PCP and VEH-VEH+VEH). Rats that had been previously treated with CLZ in the CAR model received the same CLZ treatment as before followed by PCP (CLZ 2.5-CLZ 2.5+PCP; CLZ 5.0-CLZ 5.0+PCP and CLZ 10.0-CLZ 10.0+PCP). During each motor activity test session, rats were first injected with CLZ or sterile water. Immediately after injection, they were placed in the boxes for 30 min. At the end of the 30-min period, rats were taken out and injected with either 0.9% saline or PCP (1.6 mg/kg, sc) and placed back in the boxes for another 60 min. Motor activity was measured in 5 min intervals throughout the entire 90-min testing session. This procedure was repeated for another 4 days (a total of 5 test days).
Tolerance expression retest in the CAR
One day after the last PCP hyperlocomotion test, all rats were brought back to the CAR boxes and retrained drug-free under the CS-only condition for one session and under the CS-US condition for another session to bring their avoidance back to the pre-drug level. A final challenge test for the expression of CLZ tolerance originally induced in the CAR model (e.g. CLZ 5.0-CLZ 5.0+PCP group) and newly induced in the PCP model (e.g. VEH-CLZ 5.0+PCP group) was conducted 24 h after the retraining session during which all rats were injected with CLZ 5.0 mg/kg and tested for avoidance performance in the CS-only condition (30 trails) 1 h later. Table 1 summarizes the experimental procedure and groups at different phases of the experiment.
Table 1.
A schematic depiction of the experimental procedure and groups in Experiment 1.
|
CAR: conditioned avoidance response; CLZ: clozapine; PCP: phencyclidine; VEH: vehicle.
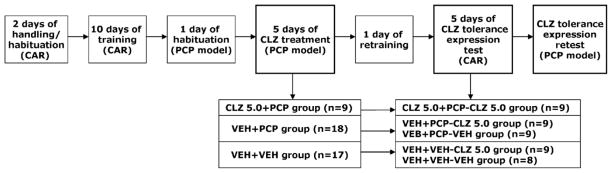
2.4.2. Experiment 2: Transferability of CLZ-induced tolerance effect from the PCP hyperlocomotion model to the CAR model and back to the PCP model
This experiment was a mirror experiment to Experiment 1 in the sense that it examined the opposite direction of CLZ tolerance transfer. Specifically, we examined whether CLZ tolerance effect induced in the PCP hyperlocomotion model extended into the CAR model and back to the original PCP model. The entire experiment was comprised of the following three phases: Avoidance training and repeated CLZ treatment in the PCP hyperlocomotion model, tolerance test in the CAR, and tolerance retest back in the PCP hyperlocomotion model.
Avoidance training and repeated CLZ treatment in the PCP hyperlocomotion model
Fifty rats were first handled and habituated to the avoidance conditioning apparatus for two days (20 min/day), and then trained for a total of ten sessions over a two-week period to acquire conditioned avoidance response. At the end of the training phase, 44 rats that had reached the training criterion (>70% avoidance in each of the last 2 sessions) were used in the PCP test. They were randomly assigned to the following three groups: CLZ 5.0 mg/kg (n = 9), PCP (n = 18) and vehicle (n = 17). On Day 1, rats were habituated in the motor activity boxes for 30 min. On Day 2, rats were first injected with CLZ 5.0 mg/kg or sterile water and then immediately placed in the boxes for 30 min. At the end of the 30-min period, they were taken out and injected with PCP (1.6 mg/kg) or saline and placed back in the boxes for another 60 min. This procedure was repeated for another 4 days (a total of 5 test days).
Tolerance expression test in the CAR
One day after the last (5th) PCP hyperlocomotion test, all rats were given a CAR retraining under the CS-US condition session to bring their avoidance back to the pre-drug level. One day later, the tolerance expression tests were conducted. During each test session, rats that were previously treated with CLZ in the PCP tests were still treated with CLZ 5.0 mg/kg (CLZ 5.0+PCP-CLZ 5.0, n = 9). Rats in the PCP or VEH group were randomly split into two groups injected with either CLZ (5.0 mg/kg, sc) or sterile water, respectively. Thus four new groups were formed: VEH+PCP-VEH (n = 9), VEH+PCP-CLZ 5.0 (n = 9), VEH+VEH-VEH (n = 8) and VEH+VEH-CLZ 5.0 (n = 9). All rats were tested for avoidance performance in the CS-only condition for 30 trials 1 h after injection. This procedure was repeated for another 4 days.
Tolerance expression retest in the PCP hyperlocomotion model
One day after the tolerance assessment in the CAR, a final tolerance expression retest was conducted. All rats were first injected with CLZ 5.0 mg/kg and then immediately placed in the motor activity boxes for 30 min. At the end of the 30-min period, rats were taken out and injected with PCP (1.6 mg/kg) and placed back in the boxes for another 60 min. Motor activity was measured in 5 min intervals throughout the entire 90-min testing session. Table 2 summarizes the experimental procedure and groups at different phases of the experiment.
Table 2.
A schematic depiction of the experimental procedure and groups in Experiment 2.
|
CAR: conditioned avoidance response; CLZ: clozapine; PCP: phencyclidine; VEH: vehicle.
2.5. Statistical Analysis
All data were expressed as mean ± SEM. Data from the five drug test sessions (e.g. avoidance response and PCP-induced motor activity) were analyzed using a split-plot analysis of variance (ANOVA) with the between-subjects factor being drug group and the within-subjects factor being test day. One-way ANOVA followed by post hoc Tukey’s HSD tests was used to compare group difference on specific days when more than three groups were involved, and planned samples t tests were used to compare two groups. For all comparisons, significant difference was assumed at p < 0.05, and all data was analyzed using SPSS version 19.
3. Results
3.1. Experiment 1: Transferability of CLZ-induced tolerance from the CAR model to the PCP hyperlocomotion model and back to the CAR model
Repeated CLZ treatment disrupted avoidance response in a dose-dependent fashion
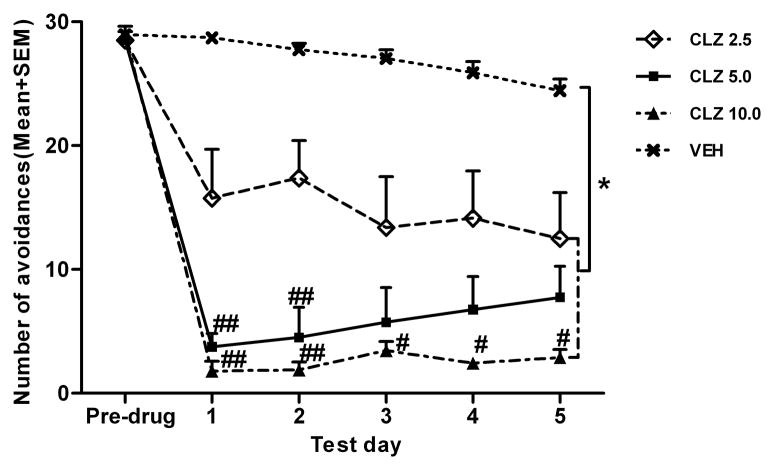
Figure 1 shows the mean number of avoidance responses made by rats in the four groups during the five drug test sessions. A spilt-spot ANOVA revealed a main effect of group, F (3, 61) = 90.369, p < 0.001, and a significant group × test day interaction, F (12, 244) = 3.218, p < 0.001. Post hoc Tukey’s HSD tests show that all three CLZ groups made significantly fewer avoidance responses than the VEH group, ps < 0.001, and the CLZ 5.0 and CLZ 10.0 groups also had significantly lower avoidance than the CLZ 2.5 group, all ps ≤ 0.05. One-way ANOVAs on each test day revealed that all three CLZ groups had significantly lower avoidance than the VEH group on all 5 days, ps < 0.001. In addition, the CLZ 10.0 group had significantly lower avoidance than the CLZ 2.5 group on all 5 days, ps < 0.05, and CLZ 5.0 group also had significantly lower avoidance than the CLZ 2.5 group on the first two days, ps < 0.05. It appears that the high dose CLZ (10.0 mg/kg) and the low dose CLZ (2.5 mg/kg) groups maintained their disruption over time, and the medium dose group (5.0 mg/kg) showed a progressive across-session decrease in its disruption. However, repeated measures ANOVA on the CLZ 5 group did not find this change over time to be statistically significant, p = 0.061.
Figure 1.
Effect of repeated CLZ treatment (2.5, 5.0 and 10.0mg/kg, sc, −60min) on conditioned avoidance response. Number of avoidance responses made by the rats in the four groups on the last training day and during the five drug test sessions. *p < 0.001 in comparison to the VEH group; #p < 0.05, ##p ≤ 0.001 in comparison to the CLZ 2.5 group.
CLZ tolerance did not manifest in the PCP hyperlocomotion model on the first day, but emerged after 5 days of testing
On the habituation day, all rats were placed in the motor activity boxes for 30 min with no drug treatment. One-way ANOVA showed no main effect of group, F (7, 57) = 0.960, p = 0.469, indicating that all groups had the equal level of spontaneous motor activity (data not shown).
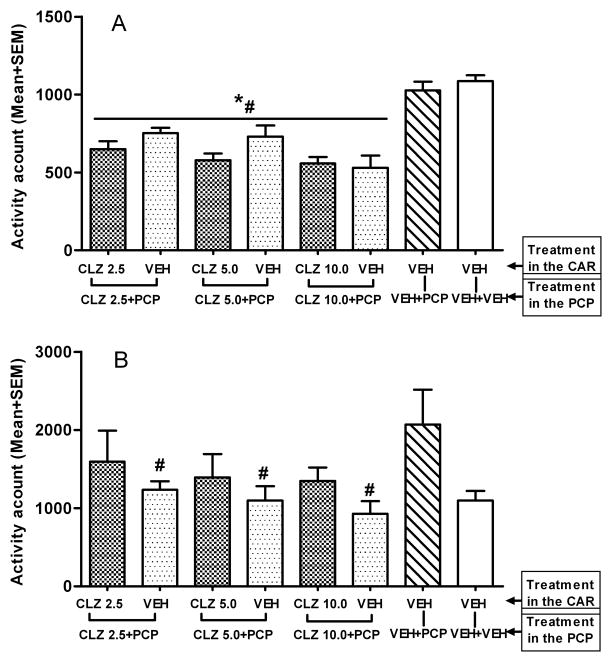
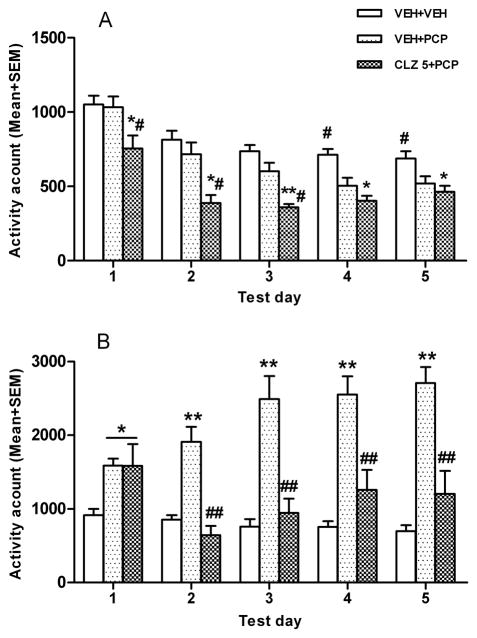
On the first day of the PCP hyperlocomotion test, CLZ decreased the spontaneous motor activity (as measured in the first 30 min after CLZ but before PCP injection). One-way ANOVA revealed a main effect of group, F (7, 57) = 15.699, p < 0.001. Post hoc tests showed that 6 CLZ groups had consistently lower motor activity than the two VEH groups, ps < 0.05 (Fig. 2A). In the 60 min after PCP injection, CLZ decreased the PCP-induced increase in motor activity as the three groups that received CLZ for the first time in this model showed significantly lower motor activity than the VEH-VEH+PCP group, ps < 0.05 (Fig. 2B). More importantly, when each pair of CLZ groups at the three dosage levels was compared (e.g. CLZ 5.0-CLZ 5.0+PCP group vs. VEH-CLZ 5.0+PCP group based), no significant difference was found, ps > 0.05, indicating no expression of CLZ tolerance on the first day in the PCP-induced hyperlocomotion model.
Figure 2.
Effect of prior CLZ treatment in the CAR model on the PCP-induced hyperlocomotion. (A) Motor activity was measured for the 30 min after CLZ or vehicle injection and expressed as mean number of photobeam breaks for each group. (B) Motor activity was measured for 60 min after vehicle or PCP (1.6 mg/kg) injection and expressed as mean number of photobeam breaks for each group. *p < 0.05 in comparison to the VEH-VEH+VEH group; #p < 0.05 in comparison to the VEH-VEH+PCP group.
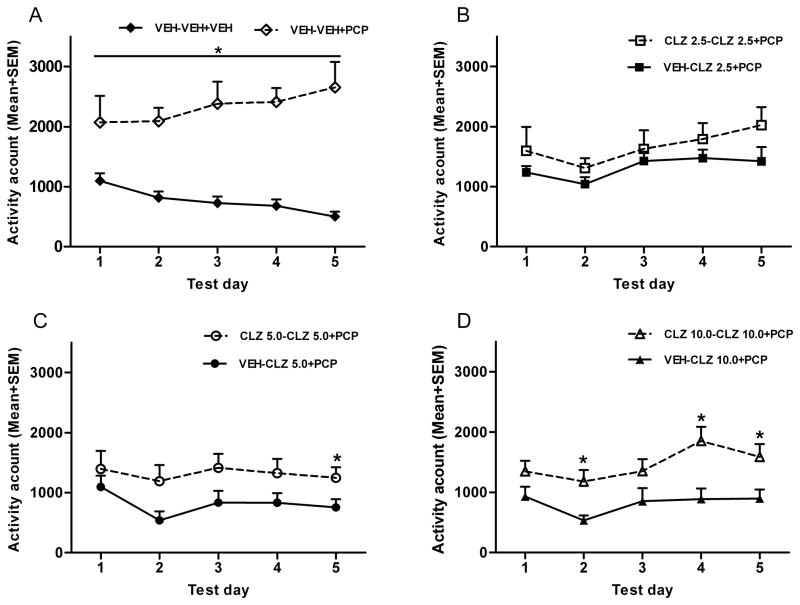
Throughout the 5 test days, all 6 CLZ groups consistently showed significantly lower motor activity in the first 30 min period than the two VEH groups, ps < 0.05 (data not shown). To examine the time course of the expression of CLZ tolerance in the PCP hyperlocomotion model, we analyzed the 60-min motor activity data using a split-plot ANOVA with drug dose (2.5, 5.0, 10.0 mg/kg) and prior treatment (CLZ or VEH in the CAR) as two between-subjects factors, and test day as the within-subjects factor. This analysis revealed a main effect of drug dose, F (2, 43) = 3.877, p = 0.028, and a main effect of prior treatment condition, F (1, 43) = 13.784, p = 0.001, and a main effect of day, F (4, 172) = 6.433, p < 0.001, indicating that prior CLZ treatment in the CAR model made CLZ less effective in inhibiting the PCP-induced hyperlocomotion, a clear sign of tolerance. We further compared each pair of CLZ groups separately to determine the dose-dependence of CLZ tolerance (Fig. 3B–3D). At 10.0 mg/kg, rats previously treated with CLZ in the CAR (i.e. CLZ 10.0-CLZ 10.0+PCP) had significantly higher motor activity than those treated with CLZ for the first time in this model (i.e. VEH-CLZ 10.0+PCP). There was a main effect of group, F (1, 15) = 11.153, p = 0.004, a main effect of test day, F (4, 60) = 3.481, p = 0.013, but no significant interaction between the two, F (4, 60) = 1.052, p = 0.388. Planned two group comparisons on each test day showed that the CLZ 10.0-CLZ 10.0+PCP group had a significantly higher level of motor activity than the VEH-CLZ 10.0+PCP group on day 2, p = 0.010, day 4, p = 0.006, and day 5, p = 0.020, suggesting a clear manifestation of tolerance at this dose. In contrast, the tolerance effect by CLZ at 2.5 and 5.0 mg/kg in the CAR was less evident. Under both conditions, the main effects of group were not significant (F (1, 14) = 4.016, p = 0.065 for CLZ 5.0 mg/kg; F (1, 14) = 1.901, p = 0.190 for CLZ 2.5 mg/kg), nor did the interaction between the group and test day, all ps > 0.723. However, planned two group comparisons on each test day did show that the CLZ 5.0-CLZ 5.0+PCP group had higher motor activity than the VEH-CLZ 5.0+PCP group on day 5, p = 0.045, indicating an even slower emergence of CLZ tolerance at this dose.
Figure 3.
Effect of prior CLZ treatment in the CAR model on the PCP-induced hyperlocomotion. Motor activity was measured for the 60 min after vehicle or PCP (1.6 mg/kg) injection and expressed as mean number of photobeam breaks for each group across the five CLZ tolerance expression test days. (A) Two group comparison between the PCP and vehicle groups. (B) Two group comparison between the two low dose CLZ groups (2.5 mg/kg). (C) Two group comparison between the two medium CLZ dose groups (5.0 mg/kg). (D) Two group comparison between the two high dose CLZ groups (10.0 mg/kg). *p < 0.05 in comparison to the corresponding groups.
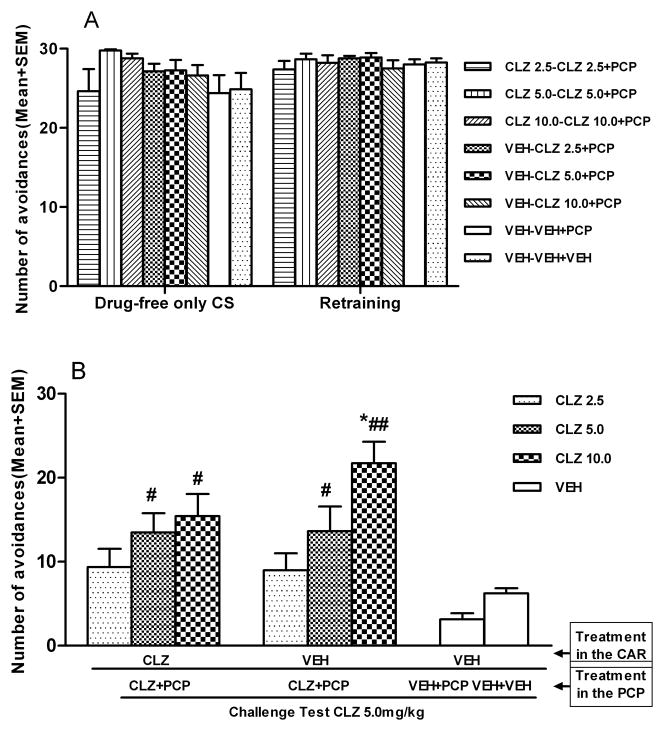
The original CLZ tolerance was dose-dependently expressed in the CAR model
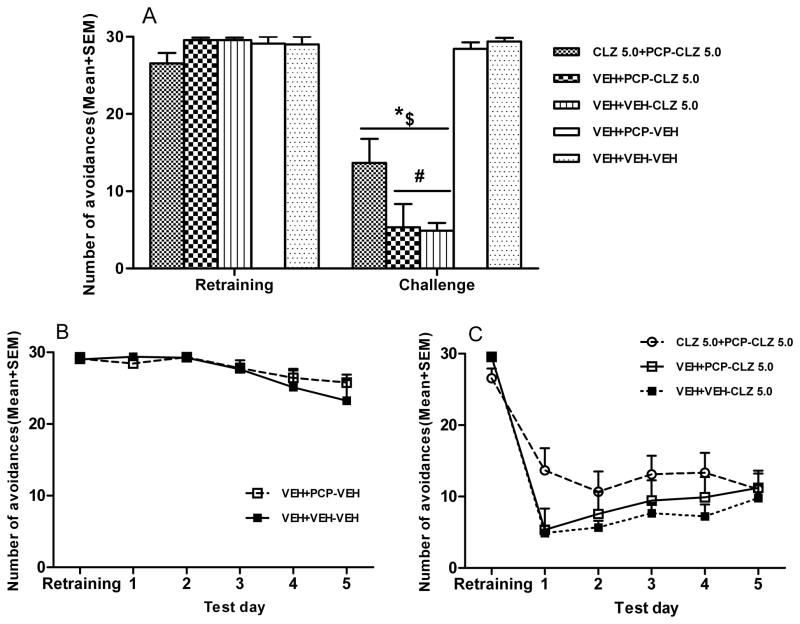
Figure 4 shows the number of avoidance responses during the CS-only, retraining, and tolerance retest sessions in the CAR. No main effect of group was found on the CS-only session, F (7, 57) = 1.448, p = 0.205, or on the retraining session, F (7, 57) = 0.482, p = 0.844 (Fig. 4A). One-way ANOVA on the tolerance retest session showed a main effect of group, F (7, 57) = 7.125, p < 0.001 (Fig. 4B). Post hoc tests indicated that the two CLZ 5.0 and CLZ 10.0 groups (i.e. one treated with CLZ in both CAR and PCP models and one treated with CLZ only in the PCP model) made significantly more avoidances than the corresponding CLZ-naïve group (VEH-VEH+PCP group), p < 0.05, confirming the CLZ tolerance.
Figure 4.
Effect of CLZ (5.0mg/kg, sc, −60min) challenge on conditioned avoidance response in the CLZ tolerance expression retest. (A) Number of avoidance responses made by rats during the CS-only and retraining sessions. (B) Number of avoidance responses made by the rats in the CLZ tolerance expression retest. *p<0.05 in comparison to the VEH-VEH+VEH group; #p < 0.05, ##p < 0.001 in comparison to the VEH-VEH+PCP group.
3.2. Experiment 2: Transferability of CLZ-induced tolerance effect from the PCP hyperlocomotion model to the CAR model and back to the PCP model
Repeated CLZ treatment decreased PCP-induced hyperlocomotion
On the habituation day, all rats were placed in the motor activity boxes for 30 min with no drug treatment. One-way ANOVA showed no main effect of group, F (2, 41) = 0.089, p = 0.915, indicating there was no significant difference among the three groups on spontaneous motor activity (data not shown).
Figure 5A shows that motor activity recorded in the first 30 min test period (as measured after CLZ but before PCP injection). A split-plot ANOVA followed by post hoc tests showed that the CLZ 5+PCP group had significantly lower motor activity than the VEH+VEH group and the VEH+PCP group throughout the 5 test days, ps < 0.05.
Figure 5.
Effect of repeated CLZ treatment (5.0 mg/kg, sc) on the PCP-induced hyperlocomotion across the five drug test sessions. (A) Motor activity was measured for the 30 min after CLZ or vehicle injection and expressed as mean number of photobeam breaks for each group. (B) Motor activity was measured for the 60 min after vehicle or PCP (1.6 mg/kg) injection and expressed as mean number of photobeam breaks for each group. *p < 0.05, **p < 0.001 in comparison to the VEH+VEH group; #p < 0.05, ##p < 0.001 in comparison to the VEH+PCP group.
Figure 5B shows the mean motor activity of the rats that received CLZ 5.0 mg/kg or vehicle treatment during the 60 min test period after saline or PCP injection across the five drug conditioning days. Repeated PCP treatment (1.6 mg/kg, sc) progressively increased motor activity across the five days. CLZ did not inhibit PCP-induced hyperlocomotion until the second day of testing and maintained its inhibition throughout. A split-plot ANOVA revealed a main effect of group, F (2, 41) = 29.438, p < 0.001, a main effect of test day, F (4,164) = 4.031, p = 0.004, and a significant group × test day interaction, F (8,164) = 8.077, p < 0.001. Post hoc tests showed that the VEH+PCP group had a significantly higher motor activity than the other groups, ps < 0.001. The VEH+PCP group made more motor activity than the other two groups on every test day, ps < 0.001, except on day 1 when the CLZ 5.0+PCP and VEH+PCP groups did not differ significantly from each other, p = 1.000.
CLZ tolerance manifested itself in the CAR model on the first day, but diminished throughout the repeated test period
One day before the tolerance expression test in the CAR model, rats were given a CAR retraining session to bring their avoidance back to the pre-drug level. Figure 6A shows the mean number of avoidance responses during the retraining and the first day of tolerance expression test in the CAR. On the retraining day, all groups had a high level of avoidance responding and there was no significant group difference, F (4, 39) = 2.097, p = 0.100. On the first day of tolerance expression test, one-way ANOVA revealed a main effect of group, F (4, 39) = 32.976, p < 0.001. Post hoc tests revealed that all three groups that were treated with CLZ had significantly lower avoidances than the two groups treated with vehicle (i.e. the VEH+VEH+VEH group, ps < 0.001 and VEH+PCP+VEH group, ps < 0.001). More importantly, the group that had been previously treated with CLZ in the PCP model (i.e. CLZ 5.0+PCP-CLZ 5.0) made significantly more avoidances than the two groups that received CLZ for the first time (i.e. VEH+VEH-CLZ 5.0 and VEH+PCP-CLZ 5.0), ps < 0.05 indicating that prior CLZ treatment in the PCP model induced a tolerance which extended to the CAR model on the first day of testing.
Figure 6.
Effect of prior CLZ treatment in the PCP-induced hyperlocomotion model on avoidance response (tolerance expression test). Number of avoidance responses made by the rats were recorded after CLZ injection (5.0mg/kg, sc, −60min). (A) Number of avoidance responses made by the rats during the retraining and the first CLZ tolerance expression test day in the CAR model. $p<0.001 in comparison to the VEH+PCP-VEH group; *p<0.001 in comparison to the VEH+VEH-VEH group; #p<0.05 in comparison to the CLZ 5.0+PCP-CLZ 5.0 group. (B) Two group comparison between the vehicle group and PCP group across the five CLZ tolerance expression test days. (C) Three group comparison among the three CLZ groups across the five test days.
Throughout the 5 days of testing, the two vehicle groups showed a slight but significant decline in avoidance responding (Fig. 6B). A split-plot ANOVA revealed a main effect of test day, F (4, 60) = 7.529, p < 0.001, but no main effect of group, F (1, 15) = 0.203, p = 0.659. A split-plot ANOVA was also conducted to examine the possible group difference among the three CLZ treated groups (Fig. 6C). There was a main effect of test day, F (4, 96) = 4.023, p = 0.005, a significant test day × group interaction, F (4, 96) = 2.299, p = 0.027, but no main effect of group, F (2, 24) = 1.524, p = 0.238. One-way ANOVA on each test day showed no main effect of group except on test day 1, p = 0.039. These findings suggested that CLZ tolerance induced in the PCP hyperlocomotion model was manifested in the CAR model on the first day, but this effect diminished throughout the 5 test days.
The original CLZ tolerance was expressed in the PCP model, but the new tolerance induced in the CAR model did not express in the PCP hyperlocomotion model
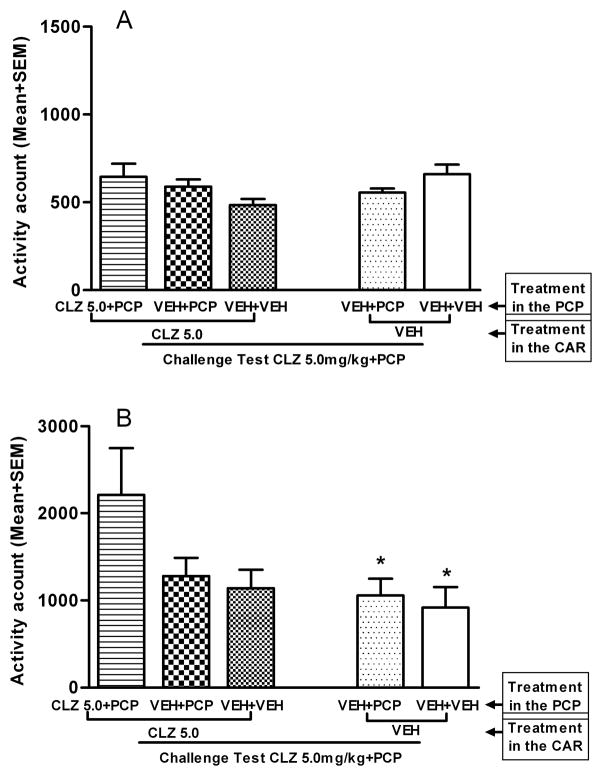
In the final tolerance expression retest in the PCP model, all rats received the first injection of CLZ 5.0 mg/kg followed by PCP 1.6 mg/kg injection 30 min later. Figure 7A shows that motor activity recorded in the first 30 min test period (as measured after CLZ but before PCP injection). One-way ANOVA showed no main effect of group, F (4, 39) = 2.165, p = 0.091. In the last 60 min of testing after PCP injection (Fig. 7B), rats that were previously treated with CLZ in the PCP model (i.e. CLZ 5.0+PCP-CLZ 5.0 group) exhibited a higher level of motor activity than the other groups. One-way ANOVA showed a main effect of group, F (4, 39) = 2.777, p = 0.040. Post hoc tests showed that the CLZ 5.0+PCP-CLZ 5.0 group differed significantly from the VEH+VEH-VEH group, ps < 0.046, and differed marginally from the VEH+PCP-VEH group, p = 0.077, indicating a CLZ tolerance effect. The findings that the VEH+PCP-CLZ 5.0 group did not differ from the VEH+PCP-VEH, p = 0.985, and the VEH+VEH-CLZ 5.0 group did not differ from the VEH+VEH-VEH, p = 0.987, strongly suggest that CLZ tolerance putatively induced in the CAR model did not extend immediately to the PCP hyperlocomotion model. This finding is consistent with the one reported in Experiment 1 (Fig. 2B).
Figure 7.
Effect of CLZ (5.0mg/kg, sc) challenge on the PCP-induced hyperlocomotion in the CLZ tolerance retest. (A) Motor activity was measured for the 30 min after CLZ or vehicle injection and expressed as mean number of photobeam breaks for each group. (B) Motor activity was measured for 60 min after vehicle or PCP (1.6 mg/kg) injection and expressed as mean number of photobeam breaks for each group. *p<0.05 in comparison to the CLZ 5.0+PCP-CLZ 5.0 group.
4. Discussion
The present study used an across-model transfer paradigm and examined the contextual and behavioral controls of the expression of CLZ tolerance. Table 3 summarizes the two patterns of CLZ tolerance expression in the CAR and PCP hyperlocomotion models.
Table 3.
A summary of the expression of clozapine tolerance in the across-model transfer paradigm.
| Start condition | Tolerance Test | Tolerance retest |
|---|---|---|
| CAR (5 days)→ | PCP (5 days)

|
CAR (1 day) |
| CLZ tolerance does not express on the first day but emerges over time | CLZ tolerance is detected | |
| PCP (5 days)→ | CAR (5 days)

|
PCP (1 days) |
| CLZ tolerance expresses on the first day but decreases over time | CLZ tolerance is detected |
CAR: conditioned avoidance response; CLZ: clozapine; PCP: phencyclidine.
Repeated CLZ treatment disrupted avoidance response in a dose-dependent fashion and decreased PCP-induced hyperlocomotion persistently throughout the drug treatment period (the induction phase) (Fig. 1 and 5B). These findings are consistent with our previous findings [11, 13, 23, 24] as well as others [20, 29, 30]. Our previous work also shows that repeated administration of haloperidol, olanzapine or risperidone induces a sensitization effect in both the induction and expression phases [1, 10, 11, 13, 23, 25, 31, 32]. CLZ differs from these antipsychotics in this regard, as prior CLZ exposure actually induced a tolerance effect in the original model where repeated CLZ took place, and this tolerance effect only manifested in the expression phase, but not in the induction phase. This finding suggests that the expression of CLZ tolerance does not necessarily require the manifestation of such a tolerance during the induction phase. The induction and expression of CLZ tolerance may involve different processes and mechanisms that affect ongoing behaviors differentially. A similar pattern has been reported for other psychotropic drugs [33]. Alternatively, the lack of sign of tolerance development in the induction phase may indicate that the experimental condition allowing its appearance is not optimal. In fact, our unpublished observation shows that CLZ tolerance does appear in the induction phase when rats are tested under the typical CS (sound)-US (shock) condition.
The present study focuses on the possible impact of environmental stimuli and behavioral variables on the expression of CLZ tolerance. To the best of our knowledge, there has ever only been one study that has implicitly addressed this issue. Sanger (1985) compared two groups of rats treated with CLZ (20 mg/kg, ip) in a CAR model. The first group was given CLZ 30 min before each of the four daily avoidance test sessions, while the second group was given CLZ immediately after the first three sessions and 30 min before the fourth session. He found that rats in the first group showed disrupted avoidance responding in the first two sessions, but no disruption in the last two sessions. Interestingly, rats in the second group also showed no disruption of avoidance when CLZ was given before the fourth session, despite the fact that they received CLZ in the first three sessions outside the CAR testing apparatus. This finding implies that CLZ tolerance is independent of context such that contextual cues and behavioral response have little impact on its induction and expression. Our results indicate that this conclusion is too simplistic and may be limited to the CAR model. In Experiment 2, we observed that rats previously treated with CLZ in the PCP model (i.e. CLZ 5.0+PCP-CLZ 5.0) made significantly more avoidances than rats that received CLZ for the first time (i.e. VEH+VEH-CLZ 5.0 and VEH+PCP-CLZ 5.0) (Fig. 6A), a sign of CLZ tolerance in agreement with that from Sanger (1985). However, results from Experiment 1 (Fig. 2 and 3) clearly show that it is not always the case: CLZ tolerance induced in the CAR model did not express immediately in the PCP-induced hyperlocomotion model and emerged only after several days of testing.
The present study significantly advances the study of CLZ tolerance by providing a novel approach to assess contextual and behavioral controls of this phenomenon. Studies on contextual controls of behavioral tolerance typically compare a “before” group (a group that receives drug injection in the test environment) with an “after” group (a group that receives vehicle injection in the test environment, and drug in the home cage) in a single model paradigm utilizing a “before-and-after” technique [34], as employed by Sanger (1985). The contextual controls of tolerance is indicated by the finding that it is expressed only in the “before” group but not in the “after” group [35]. The across-model transfer approach complements this traditional technique in testing both CLZ experienced and naïve groups (e.g. in the CAR model) were tested under the drug in the new model (e.g. in the PCP model) for the first time during the tolerance expression test. Because the across-model transfer paradigm utilizes two behavioral responses that are topographically different and appear in two distinct test environments, and encompasses changes in contextual cues and behavioral responses, this approach provides a more reliable assessment of contextual and behavioral controls of CLZ tolerance. Future work interested in the neurobiological mechanisms underlying the contextual and behavioral controls of CLZ tolerance could benefit from this approach.
One of the interesting findings was the different time courses of across-model expression of CLZ tolerance. CLZ tolerance induced in the CAR did not manifest itself in the PCP hyperlocomotion model on the first day, but emerged after 5 days of testing. In contrast, CLZ tolerance induced in the PCP hyperlocomotion model manifested itself in the CAR model on the first day, but decreased over the treatment period. Substantial evidence suggests that contextual cues, especially the environmental stimuli and interoceptive drug state can serve as conditioned stimuli (CS) and become associated with unconditional drug effects (as US) via a Pavlovian conditioning process after being repeatedly paired with a drug [36]. Anagnostaras and Robinson (1996) as well as others [37, 38] suggest that contextual cues and interoceptive drug states can also act as occasion-setters to modulate the expression of psychomotor sensitization. Based on these findings, we postulate that the distinct time courses reflect differential impacts of contextual cues and behaviors on the expression of CLZ tolerance. In addition, we suggest that contextual cues and different behavior responses may influence the rate at which CLZ tolerance develops, rather than affect the development of tolerance itself [34]. It is possible that distinct contextual cues (e.g. environmental stimuli, interoceptive drug cue, etc) and altered behavioral responses in each model may develop a different strength of association with pharmacological actions of CLZ via a Pavlovian conditioning process and thus acquire the ability to modulate its induction and expression [39–42]. Furthermore, the CS-elicited shuttling response in the CAR model may have a stronger influence (i.e. occasion-setting capacity) on CLZ tolerance development than PCP-induced locomotion in the test cages. This explains the findings that tolerance that developed in the PCP model extended immediately to the CAR model, whereas tolerance that developed in the CAR model did not immediately extend to the PCP model.
One fundamental issue is the exact neurobiological mechanisms responsible for CLZ tolerance and associated situational specificity. Our recent work on the neurochemical basis of CLZ tolerance in the CAR model implicates a role for dopamine D2/3 receptors [11]. We found that pretreatment of quinpirole, a selective D2/D3 dopaminergic receptor agonist, enhanced CLZ tolerance effect, suggesting that the induction of CLZ tolerance effect may be mediated by D2/D3 blockade-initiated neural processes. The involvement of dopamine receptor-mediated processes in CLZ tolerance is also supported by early findings showing that repeated CLZ treatment leads to a suppressed firing (i.e. depolarization inactivation) of dopamine neurons in the ventral tegmental area but not in the substantia nigra and a suppressed release of dopamine in the nucleus accumbens but not in the striatum [43, 44]. Since CLZ also acts on many other receptors (e.g. 5-HT2A/2C, D1, D3, α1-noradrenergic, etc), of which some have been implicated in CLZ tolerance [45–47], future work should systematically investigate the role of these receptors in the mediation of CLZ tolerance and its contextual and behavioral controls.
The present study illustrates the power of environmental and behavioral variables on the expression of CLZ tolerance. Given the poverty of our current understanding of CLZ tolerance and its contextual and behavioral controls, it is clearly premature to speculate on its clinical relevance. Nevertheless, as CLZis administered repeatedly to patients with schizophrenia for a prolonged period of time, CLZ tolerance seems to be an inevitable feature of the treatment. Previous work suggests that CLZ tolerance isre lated to the withdrawal symptoms seen with the discontinuation of CLZ use (e.g. nausea, vomiting, insomnia, diarrhoea, agitation, aggression, headache, etc) [48] and relapse to psychosis [49, 50]. Thus the environmental and behavioral controls of its induction and expression as evidenced in these two preclinical tests may indicate that the appearance of CLZ withdrawal syndromes is also subjected to the same modulation by various environmental and behavioral factors. If CLZ withdrawal syndrome and CLZ withdrawal-induced rapid relapse to psychosis were behavioral signs to be avoided in patients, understanding how these factors play a role in the induction and expression of CLZ tolerance may greatly enhance our understanding of its clinical treatment effects as well as possible side effects.
Research Highlights.
Repeated clozapine treatment caused a tolerance effect in the avoidance conditioning model.
Repeated clozapine treatment also caused a tolerance effect in the PCP hyperlocomotion model.
The transfer of clozapine tolerance from one model to another is situational specific and time-dependent.
Acknowledgments
This study was funded by the NIMH grant (R01MH085635) to Professor Ming Li.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang C, Li M. Contextual and behavioral control of antipsychotic sensitization induced by haloperidol and olanzapine. Behav Pharmacol. 2012;23:66–79. doi: 10.1097/FBP.0b013e32834ecac4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meltzer HY, Bastani B, Kwon KY, Ramirez LF, Burnett S, Sharpe J. A prospective study of clozapine in treatment-resistant schizophrenic patients. I. Preliminary report. Psychopharmacology (Berl) 1989;99 (Suppl):S68–72. doi: 10.1007/BF00442563. [DOI] [PubMed] [Google Scholar]
- 3.Chakos M, Lieberman J, Hoffman E, Bradford D, Sheitman B. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry. 2001;158:518–26. doi: 10.1176/appi.ajp.158.4.518. [DOI] [PubMed] [Google Scholar]
- 4.Nasrallah HA, Meyer JM, Goff DC, McEvoy JP, Davis SM, Stroup TS, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86:15–22. doi: 10.1016/j.schres.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–96. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 6.Seeman P, Schwarz J, Chen JF, Szechtman H, Perreault M, McKnight GS, et al. Psychosis pathways converge via D2high dopamine receptors. Synapse. 2006;60:319–46. doi: 10.1002/syn.20303. [DOI] [PubMed] [Google Scholar]
- 7.Rebec GV, Peirson EE, McPherson FA, Brugge K. Differential sensitivity to amphetamine following long-term treatment with clozapine or haloperidol. Psychopharmacology (Berl) 1982;77:360–6. doi: 10.1007/BF00432771. [DOI] [PubMed] [Google Scholar]
- 8.Moghaddam B, Bunney BS. Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: an in vivo microdialysis study. J Neurochem. 1990;54:1755–60. doi: 10.1111/j.1471-4159.1990.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 9.Robertson GS, Fibiger HC. Neuroleptics increase c-fos expression in the forebrain: contrasting effects of haloperidol and clozapine. Neuroscience. 1992;46:315–28. doi: 10.1016/0306-4522(92)90054-6. [DOI] [PubMed] [Google Scholar]
- 10.Mead A, Li M. Avoidance-suppressing effect of antipsychotic drugs is progressively potentiated after repeated administration: an interoceptive drug state mechanism. J Psychopharmacol. 2010;24:1045–53. doi: 10.1177/0269881109102546. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Sun T, Zhang C, Hu G. Distinct neural mechanisms underlying acute and repeated administration of antipsychotic drugs in rat avoidance conditioning. Psychopharmacology (Berl) 2010;212:45–57. doi: 10.1007/s00213-010-1925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swalve N, Li M. Parametric studies of antipsychotic-induced sensitization in the conditioned avoidance response model: roles of number of drug exposure, drug dose, and test-retest interval. Behav Pharmacol. 2012 doi: 10.1097/FBP.0b013e32835651ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Sun T, Mead A. Clozapine, but not olanzapine, disrupts conditioned avoidance response in rats by antagonizing 5-HT(2A/2C) receptors. J Neural Transm. 2012;119:497–505. doi: 10.1007/s00702-011-0722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanford JA, Fowler SC. Subchronic effects of clozapine and haloperidol on rats’ forelimb force and duration during a press-while-licking task. Psychopharmacology (Berl) 1997;130:249–53. doi: 10.1007/s002130050236. [DOI] [PubMed] [Google Scholar]
- 15.Trevitt J, Atherton A, Aberman J, Salamone JD. Effects of subchronic administration of clozapine, thioridazine and haloperidol on tests related to extrapyramidal motor function in the rat. Psychopharmacology (Berl) 1998;137:61–6. doi: 10.1007/s002130050593. [DOI] [PubMed] [Google Scholar]
- 16.Varvel SA, Vann RE, Wise LE, Philibin SD, Porter JH. Effects of antipsychotic drugs on operant responding after acute and repeated administration. Psychopharmacology (Berl) 2002;160:182–91. doi: 10.1007/s00213-001-0969-y. [DOI] [PubMed] [Google Scholar]
- 17.Villanueva HF, Porter JH. Differential tolerance to the behavioral effects of chronic pimozide and clozapine on multiple random interval responding in rats. Behav Pharmacol. 1993;4:201–8. [PubMed] [Google Scholar]
- 18.Goudie AJ, Cooper GD, Cole JC, Sumnall HR. Cyproheptadine resembles clozapine in vivo following both acute and chronic administration in rats. J Psychopharmacol. 2007;21:179–90. doi: 10.1177/0269881107067076. [DOI] [PubMed] [Google Scholar]
- 19.Goudie AJ, Cole JC, Sumnall HR. Olanzapine and JL13 induce cross-tolerance to the clozapine discriminative stimulus in rats. Behav Pharmacol. 2007;18:9–17. doi: 10.1097/FBP.0b013e328014138d. [DOI] [PubMed] [Google Scholar]
- 20.Gleason SD, Shannon HE. Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacology (Berl) 1997;129:79–84. doi: 10.1007/s002130050165. [DOI] [PubMed] [Google Scholar]
- 21.Natesan S, Reckless GE, Nobrega JN, Fletcher PJ, Kapur S. Dissociation between In Vivo Occupancy and Functional Antagonism of Dopamine D(2) Receptors: Comparing Aripiprazole to Other Antipsychotics in Animal Models. Neuropsychopharmacology. 2005 doi: 10.1038/sj.npp.1300983. [DOI] [PubMed] [Google Scholar]
- 22.Wadenberg ML, Hicks PB. The conditioned avoidance response test re-evaluated: is it a sensitive test for the detection of potentially atypical antipsychotics? Neurosci Biobehav Rev. 1999;23:851–62. doi: 10.1016/s0149-7634(99)00037-8. [DOI] [PubMed] [Google Scholar]
- 23.Sun T, Hu G, Li M. Repeated antipsychotic treatment progressively potentiates inhibition on phencyclidine-induced hyperlocomotion, but attenuates inhibition on amphetamine-induced hyperlocomotion: relevance to animal models of antipsychotic drugs. Eur J Pharmacol. 2009;602:334–42. doi: 10.1016/j.ejphar.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Parkes J, Fletcher PJ, Kapur S. Evaluation of the motor initiation hypothesis of APD-induced conditioned avoidance decreases. Pharmacol Biochem Behav. 2004;78:811–9. doi: 10.1016/j.pbb.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Li M, He W, Mead A. An investigation of the behavioral mechanisms of antipsychotic action using a drug-drug conditioning paradigm. Behav Pharmacol. 2009;20:184–94. doi: 10.1097/FBP.0b013e32832a8f66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith JB. Situational specificity of tolerance to decreased operant responding by morphine and l-nantradol. Psychopharmacology (Berl) 1991;103:115–20. doi: 10.1007/BF02244085. [DOI] [PubMed] [Google Scholar]
- 27.Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305:625–31. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- 28.Kalinichev M, Robbins MJ, Hartfield EM, Maycox PR, Moore SH, Savage KM, et al. Comparison between intraperitoneal and subcutaneous phencyclidine administration in Sprague-Dawley rats: A locomotor activity and gene induction study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:414–22. doi: 10.1016/j.pnpbp.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Arnt J. Pharmacological specificity of conditioned avoidance response inhibition in rats: inhibition by neuroleptics and correlation to dopamine receptor blockade. Acta Pharmacol Toxicol (Copenh) 1982;51:321–9. doi: 10.1111/j.1600-0773.1982.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 30.Sanger DJ. The effects of clozapine on shuttle-box avoidance responding in rats: comparisons with haloperidol and chlordiazepoxide. Pharmacol Biochem Behav. 1985;23:231–6. doi: 10.1016/0091-3057(85)90562-3. [DOI] [PubMed] [Google Scholar]
- 31.Mead A, Li M. Avoidance-suppressing effect of antipsychotic drugs is progressively potentiated after repeated administration: an interoceptive drug state mechanism. J Psychopharmacol. 2009 doi: 10.1177/0269881109102546. [DOI] [PubMed] [Google Scholar]
- 32.Li M, Fletcher PJ, Kapur S. Time course of the antipsychotic effect and the underlying behavioral mechanisms. Neuropsychopharmacology. 2007;32:263–72. doi: 10.1038/sj.npp.1301110. [DOI] [PubMed] [Google Scholar]
- 33.Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- 34.Corfield-Sumner PK, Stolerman IP. Behavioral tolerance. In: Blackman DE, Sanger DJ, editors. Contemporary Research in Behavioral Pharmacology. New York: Plenum Press; 1978. pp. 391–448. [Google Scholar]
- 35.Chen CS. A study of the alcohol-tolerance effect and an indtroduction of a new behavioural technique. Psychopharmacologia. 1968;12:433–40. doi: 10.1007/BF00401349. [DOI] [PubMed] [Google Scholar]
- 36.Siegel S, Baptista MA, Kim JA, McDonald RV, Weise-Kelly L. Pavlovian psychopharmacology: the associative basis of tolerance. Exp Clin Psychopharmacol. 2000;8:276–93. doi: 10.1037//1064-1297.8.3.276. [DOI] [PubMed] [Google Scholar]
- 37.Sripada S, Gaytan O, Swann A, Dafny N. The role of MK-801 in sensitization to stimulants. Brain Res Brain Res Rev. 2001;35:97–114. doi: 10.1016/s0165-0173(00)00046-1. [DOI] [PubMed] [Google Scholar]
- 38.Lanis A, Schmidt WJ. NMDA receptor antagonists do not block the development of sensitization of catalepsy, but make its expression state-dependent. Behav Pharmacol. 2001;12:143–9. doi: 10.1097/00008877-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Anagnostaras SG, Schallert T, Robinson TE. Memory processes governing amphetamine-induced psychomotor sensitization. Neuropsychopharmacology. 2002;26:703–15. doi: 10.1016/S0893-133X(01)00402-X. [DOI] [PubMed] [Google Scholar]
- 40.Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–54. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- 41.Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- 42.Browman KE, Badiani A, Robinson TE. Modulatory effect of environmental stimuli on the susceptibility to amphetamine sensitization: a dose-effect study in rats. J Pharmacol Exp Ther. 1998;287:1007–14. [PubMed] [Google Scholar]
- 43.Chiodo LA, Bunney BS. Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci. 1983;3:1607–19. doi: 10.1523/JNEUROSCI.03-08-01607.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiodo LA, Bunney BS. Possible mechanisms by which repeated clozapine administration differentially affects the activity of two subpopulations of midbrain dopamine neurons. J Neurosci. 1985;5:2539–44. doi: 10.1523/JNEUROSCI.05-09-02539.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coward DM. General pharmacology of clozapine. Br J Psychiatry Suppl. 1992:5–11. [PubMed] [Google Scholar]
- 46.Menon MK, Gordon LI, Fitten J. Interaction between clozapine and a lipophilic alpha 1-adrenergic agonist. Life Sci. 1988;43:1791–804. doi: 10.1016/0024-3205(88)90278-0. [DOI] [PubMed] [Google Scholar]
- 47.Millan MJ, Audinot V, Melon C, Newman-Tancredi A. Evidence that dopamine D3 receptors participate in clozapine-induced hypothermia. Eur J Pharmacol. 1995;280:225–9. doi: 10.1016/0014-2999(95)00250-o. [DOI] [PubMed] [Google Scholar]
- 48.Touyz SW, Saayman GS, Zabow T. A psychophysiological investigation of the long-term effects of clozapine upon sleep patterns of normal young adults. Psychopharmacology (Berl) 1978;56:69–73. doi: 10.1007/BF00571411. [DOI] [PubMed] [Google Scholar]
- 49.Seppala N, Kovio C, Leinonen E. Effect of anticholinergics in preventing acute deterioration in patients undergoing abrupt clozapine withdrawal. CNS Drugs. 2005;19:1049–55. doi: 10.2165/00023210-200519120-00006. [DOI] [PubMed] [Google Scholar]
- 50.Goudie AJ, Smith JA, Robertson A, Cavanagh C. Clozapine as a drug of dependence. Psychopharmacology (Berl) 1999;142:369–74. doi: 10.1007/s002130050901. [DOI] [PubMed] [Google Scholar]