Abstract
The incidence of type 2 diabetes and metabolic disease is rapidly increasing, but effective therapies for their prevention and treatment have been poorly tolerated or minimally effective. In this study, chronic administration of kudzu root extract (8 months, 0.2% w/w in diet) decreased baseline fasting plasma glucose (183±14 vs 148±11 mg/dl) and improved glucose and insulin tolerance in C57BL/6J ob/ob mice (1.67±0.17 ng/ml [kudzu treated] vs. 2.35±0.63 ng/ml [control]), but such treatment did not alter these parameters in lean control mice. Among the mice on the kudzu supplementation, plasma levels of isoflavone metabolites were significantly higher in ob/ob versus lean control mice, and unmetabolized puerarin (11.50±5.63 ng/gram) was found in adipose tissue only in the treated mice. Together, these data demonstrate that a puerarin containing kudzu diet improves glucose and insulin responsiveness in ob/ob mice, suggesting that puerarin may be a beneficial adjuvant for treating metabolic disease.
Keywords: Kudzu, puerarin, glucose metabolism, lipids
Introduction
The metabolic syndrome affects nearly one-fourth of US adults (~47 million people), and a prominent feature of this syndrome is impaired glucose regulation (Ford et al., 2002, Park et al., 2003). In the metabolic syndrome, insulin resistance, hypertension, obesity and hypercholesterolemia synergize to accelerate the development of cardiovascular disease, stroke and type 2 diabetes (Kraja et al., 2008). Elevated circulating free fatty acids (FFA), tumor necrosis factor-alpha, (TNF-α) and leptin are associated with obesity and may contribute to insulin resistance (Cohen et al., 1996, Segal et al., 1996, Muller et al., 1997, Summers, 2006). When their storage capacities are exceeded, the excess fat begins to accumulate in adipocytes, which can lead to the formation of specific metabolites that can inhibit insulin signal transduction. Among the fats that accumulate, non-esterified fatty acids, triglyceride, diacylglycerol and ceramide are all associated with insulin resistance. Sphingolipid ceramide is a putative intermediate linking excess nutrients (i.e. saturated fatty acids) and inflammatory cytokines (e.g., TNFα) to insulin resistance. Moreover, ceramide has been shown to be toxic in a variety of different cell types (e.g. pancreatic beta-cells, cardiomyocytes, etc. (Summers, 2006, Teruel et al., 2001).
Recently, there has been a growing interest in the ability of dietary polyphenols to reduce these interacting factors (Cefalu et al., 2008). In rats, we and others have shown that several dietary polyphenolic compounds can decrease blood pressure and serum total cholesterol and improve insulin signaling (Boue et al., 2003, Hsu et al., 2003, Meezan et al., 2005, Peng et al., 2005, Carlson et al., 2008). Kudzu root (Radix pueraria from Pueraria lobota), which has recently become commercially available in Western dietary supplements, is widely used in traditional Chinese medicine, and it is a rich source of isoflavone glucosides. Common isoflavones of kudzu root include puerarin (daidzein 8-C-glucoside), daidzin (daidzein 7-O-glucoside), daidzein, genistein and formononetin. These isoflavones have been associated with antioxidant, anti-dipsotropic and other pharmacological effects (Lee, 2004, Keung and Vallee, 1998, Zhang et al., 2010). Among the isoflavones in kudzu root, puerarin is the most abundant (~23% w/w) and has attracted considerable attention, because of its putative ability to protect against metabolic disorders (Meezan et al., 2005, Xu et al., 2005). However, the mechanisms underlying the beneficial actions of kudzu isoflavones are not clear.
The present study tested the effects of dietary kudzu supplementation on glucose and lipid metabolism in genetically obese animals. ESI-MS and MS/MS strategies were established to profile lipid molecular species in adipose tissue, so as to determine lipid changes that occur in obese and lean animals after chronic administration of kudzu. The results obtained from these studies provide insights into potential mechanisms by which kudzu extract, (likely via puerarin) can alter the course of metabolic disease.
Materials and methods
Reagents
Methanol (HPLC grade) and chloroform were from Fisher Scientific (Fair Lawn, NJ). Lipid standards were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Puerarin root extract was obtained in the powder form from AMAX NutraSource, Eugene, OR.
Diet Preparation
The experimental diet was made by adding powdered Pueraria root extract (0.2% w/w) to AIN-93M, a phytoestrogen-free diet (see Reeves et al, 1993 for a full description of this diet). The combination was blended overnight in a rotating mixer and pelleted (Test Diet, Richmond, IN). The control diet was a similarly pelleted AIN-93M diet with no additions (Test Diet, Richmond, IN). The diets were tested monthly to insure consistency of isoflavone content. The kudzu root extract concentration in the diet was chosen based on our previous studies (Peng et al., 2009).
Animal experiments
Male obese mice (C57BL/6J-ob/ob) and their lean controls (C57Bl/6J-+/+) were purchased from the Jackson Laboratory (Bar Harbor, ME) at 4weeks of age. They were housed three per cage at constant humidity (65 ± 5%), temperature (24 ± 1 °C), and light-dark cycle (0600–1800 h, lights on), and allowed ad libitum access to tap water and diet throughout the experimental protocols. All experimental procedures were conducted under the oversight and approval of the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham and in accordance with National Institutes of Health guidelines and the Guide to the Care and Use of Laboratory Animals.
Kudzu administration, general lipid/fat assessments, blood pressure and heart rate glycemic control and insulin sensitivity
To investigate whether long-term exposure to kudzu root extract alters plasma glucose and insulin tolerance, mice were maintained on a polyphenol-free AIN 93M diet with or without the addition of 0.2% kudzu root extract for 8 months (9 mice per diet group per strain). Blood pressure and heart rate were measured indirectly, using a Hatteras tail cuff system (MC 4000) once every two months.
After 7 months on the diets, in vivo body composition (total body fat and lean tissue) of mice was determined using an EchoMRI™ 3-in-1 quantitative magnetic resonance (QMR) machine (Echo Medical Systems, Houston, TX). A system test was performed using a known fat standard prior to the measurements being taken. Mice were weighed and then placed into a clear holding tube capped with a stopper that restricted vertical movement, but allowed constant airflow. The tube was inserted into the machine and the mouse scanned using the Normal Precision mode.
After the 8 month on the diets, glucose tolerance was tested. Mice were fasted overnight and blood glucose measurements were performed the following morning. Mice were lightly anesthetized (isoflurane), and a tail blood micro sample was taken for a baseline blood glucose concentration measurement using a blood glucose monitoring system (Prestige Smart System, CVS). Mice then received an oral glucose gavage (2 grams glucose/kg body weight). On an alternate day, to determine whether chronic dietary kudzu extract alters insulin sensitivity, mice received a bolus injection of insulin (0.75 U/kg, ip., bovine insulin, Sigma, St Louis, MO). Blood glucose was measured at 15, 30, 45, 60, 90 and 120 minutes after the glucose/insulin challenge.
At the end of the study, the mice were euthanized by cervical dislocation under isoflurane anesthesia, and blood was collected via heart puncture using heparin as anticoagulant. Adipose tissue was isolated and immediately frozen in liquid nitrogen and stored at −20 °C until used.
Plasma was isolated by centrifugation of the blood at 3,000 × g for 5 min at stored at −20 °C. Plasma lipids were analyzed using Vitros DT60 [Ektachem] analyzer, (Ortho-Clinical Diagnostics, Rochester, NY). Adipose tissue samples (wet weight 33–35 mg) were added to (1 ml) methanol containing 1% acetic acid and homogenized in a mortar. The samples were vortex mixed and centrifuged at 3,000 × g for 10 min and the supernatant was removed and dried under air and reconstituted in 80% methanol in water (200 μl).
For individual lipid assessments, lipids were extracted following Bleigh Dyer method (21). Briefly, adipose tissue (30–35 mg) was homogenized in a mortar filled with liquid nitrogen and extracted with 1 ml water, 2.5 ml of methanol and 1.25 ml of chloroform. The mixture was sonicated with 4 bursts, and added 1ml of water, and 1.25 ml of chloroform. After vigorous shaking and centrifugation at 3,000 × g for 5 min, the supernatant was removed and the bottom aqueous layer was collected. Supernatant was extracted again with 1.25 ml of chloroform. The extracted solution was evaporated to dryness and reconstitute with 100 μl of chloroform:methanol (1:1 v/v).
Lipids were analyzed by ESI-MS/MS using an API 4000 (Applied Biosystems/MDS Sciex, Concord, Ontario, Canada) triple quadrupole mass spectrometer. Samples (5 μl) were directly infused into the electrospray source using a Shimadzu Prominence HPLC with auto sampler (Shimadzu Scientific Instruments, Inc. Columbia, MD). The samples were infused into the mass spectrometer using a solvent mixture of chloroform-methanol (1:2, v/v) containing 0.1% formic acid. The analysis was performed in positive ion mode electrospray ion (ESI-MS) source and precursor ion scans m/z 264 and 282 and multiple reaction ion monitoring (MRM) mass transition m/z 564/282 (ceramides), and precursor ion scan m/z 184 lysophosphocholine (LPC), were used.
MSMS parameters were optimized to obtain best sensitivity. The declustering potential and temperature were set at 35 V and 250 °C, respectively. The collision energy was optimized and set at 35 V. The MS/MS system was controlled by BioAnalyst 1.4.2 software.
Plasma isoflavone analysis
Isoflavone analysis was performed following our published method (Prasain et al., 2010). Briefly, each serum sample (200 μl) was subjected to enzymatic hydrolysis of the isoflavone glucuronides and sulfates for the total isoflavone determination and extracted into diethyl ether. After concentrating samples to dryness, they were dissolved in methanol-water (80:20, v/v) prior to LC-MS/MS analysis. Sample preparation and quantification of puerarin in biological samples were performed as described before (Prasain et al., 2007).
Statistical analysis
All experimental data were evaluated by two-way analysis of variance (ANOVA) followed by post hoc Tukey’s test to determine the source of main effects and interactions (SPSS, Chicago, IL). The significance criterion for all experiments was p < 0.05. All data are reported in the results section as mean ± standard error.
Results
General health of mice in each group
Kudzu did not significantly affect the body weights of the mice in the study; however, as expected the ob/ob mice weighed nearly twice as much as the lean control mice (Table 2). Dietary kudzu supplementation did not affect food consumption in either lean control or ob/ob mice (data not shown). Further, QMR analysis at the end of the study demonstrated no significant differences between groups in fat or lean mass (Table 2). Plasma total cholesterol concentrations were not significantly different between groups; total cholesterol was highest in the ob/ob mice on kudzu (Table 2). Plasma triglycerides were also not different between groups (Table 2). Blood pressure and heart rate were lower in the ob/ob than lean control mice, but kudzu affected neither index (Table 2).
Table 2.
General physiological characteristics of ob/ob (OB) and lean control (C) mice on either a phytoestrogen-free diet (NK) or a diet supplemented with 0.2% (w/w) kudzu root extract.
| OB-NK | OB-K | C-NK | C-K | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BW (g) | 70.9 | 2.8 | 72.7 | 2.5 | 37.3 | 1.1 | 37.1 | 1.3 | p < 0.05 OB vs. C |
| Fat mass (%; QMR) | 34.6 | 2.3 | 36.2 | 2.7 | 31.3 | 1.5 | 32.7 | 1.9 | ns |
| Lean mass (%; QMR) | 22.5 | 1.1 | 23.2 | 1.7 | 24.1 | 1.8 | 25.8 | 1.8 | ns |
| Total Cholesterol (mg/dl) | 212 | 19 | 245 | 30 | 180 | 31 | 172 | 29 | ns |
| Triglycerides (mg/dl) | 83 | 6 | 91 | 5 | 86 | 6 | 87 | 5 | ns |
| BP (mm Hg; systolic) | 116 | 2 | 114 | 4 | 126 | 2 | 129 | 3 | p < 0.05 OB vs. C |
| HR (beats per minute) | 619 | 36 | 572 | 37 | 692 | 21 | 680 | 21 | p < 0.05 OB vs. C |
The data are mean ± SE. Significant differences between groups (post hoc after significant 1-way ANOVA) are noted to the right of the table.
BW= Body Weight; Fat and lean mass by QMR; Cholesterol, HDL, LDL and triglycerides; systolic Blood Pressure (BP) and heart rate (HR) measured by tail cuff plethysmography.
Glucose metabolism
In the present study in ob/ob mice, chronic dietary supplementation with kudu root extract (containing ~23% puerarin, w/w) significantly decreased baseline fasting glucose (183±14 vs. 148±11 mg/dl) and improved glucose tolerance and insulin sensitivity. On control diet, the ob/ob (compared to the lean control) mice displayed significant impairment in glucose tolerance, especially during the second half of the tolerance curve (after 45 m; Fig. 1). Ob/ob mice on the kudzu dietary supplementation showed significantly improved glucose tolerance, reaching the response levels of control mice by 45 minutes after glucose administration. In lean control mice, the kudzu supplementation modestly improved glucose tolerance at most time points.
Fig. 1.
Chronic administration of dietary kudzu root extract improves oral glucose tolerance in C57BL/6J-ob/ob mice. Values are given as means ± SEM.# p < 0.05 for both ob/ob groups compared to both lean control groups; * p < 0.05 for ob/ob on control diet compared to all other groups; p<0.05 for kudzu fed, compared to control diet fed, lean control mice for all time points except 30 min. Initial plasma glucose levels were significantly different for all four groups. OB-NK, ob/ob mice on control diet, OB-K, ob/ob mice on kudzu diet, Lean-NK, lean mice on control diet.
Kudzu supplementation reduced plasma insulin concentration in ob/ob mice (1.67±0.17 ng/ml [kudzu treated] vs. 2.35±0.63 ng/ml [control]). To determine whether dietary kudzu supplementation affected insulin-induced glucose clearance, insulin tolerance tests (ITT) were performed on kudzu-treated ob/ob and lean control mice. Insulin challenge (0.75 u/kg) significantly decreased blood glucose levels in all groups. However, in ob/ob mice, but not in lean mice, dietary kudzu root extract caused a significantly greater decrease in plasma glucose concentration (Fig. 2). Further, for the initial 45 minutes after the insulin challenge, kudzu treated ob/ob mice responded nearly identically to lean control mice (Fig. 2); however, by 60 m after insulin challenge, the ob/ob mice that were treated with dietary kudzu returned to the levels of the non-kudzu diet ob/ob mice, indicating that insulin sensitivity was largely but not completely restored by the kudzu supplementation.
Fig. 2.
Chronic administration of kudzu root extract improves insulin tolerance in C57BL/6J-ob/ob mice, but not lean control mice. Mice received a bolus injection of insulin (0.75 U/kg, ip) and blood glucose was measured at 15, 30, 45, 60, 90 and 120 minutes after insulin challenge. Values are given as means ± SEM. * p < 0.05 for ob/ob on control diet compared to all other groups. # p < 0.05 for ob/ob groups compared to lean control groups. Initial plasma glucose levels were significantly higher in ob/ob on the control diet versus all other groups for all four groups. OB-NK, ob/ob mice on control diet, OB-K, ob/ob mice on kudzu diet, Lean-NK, lean mice on control diet.
Lipid metabolism
In ob/ob mice, kudzu treatment had no significant effect on plasma adiponectin levels (13.79±0.52 μg/ml, vs. 12.84±0.66 μg/ml control), and the concentrations were similar to those in lean mice on the control diet (Fig. 3). Electrospray tandem mass spectrometry (ESI-MS/MS) analysis of ceramides and lysophosphocholine (LPC) in adipose tissues from kudzu treated and control animals was performed. Since ceramides in positive ion mode MS/MS generate characteristic product ions of m/z 264 and 282, precursor ion scans of m/z 264 and m/z 282 were used for detecting ceramides in the adipose tissue. Fig. 4A shows the precursor ion chromatogram with m/z 282 and the ion m/z 564 (C18:1) is the major ceramide in the extract. To increase the sensitivity and specificity, MRM analysis with different mass transitions was performed (data not shown). Although not conclusive, chronic dietary kudzu supplementation reduced elevated adipose C18:1 ceramide in obese animals (Fig. 4B). Precursor ion scan of m/z 184 in positive ion mode demonstrated no significant difference in lysophosphocholine (LPC) in adipose tissue of ob/ob and lean mice, irrespective of their diet (Fig. 5).
Fig. 3.
Plasma adiponectin concentration was not significantly different in the three groups tested (ob/ob control diet (OB-NK), ob/ob on kudzu root extract diet (OB-K) and lean mice on control diet (Lean-NK) mice.
Fig. 4.
Representative precursor ion scan m/z 282 [A] of adipocyte lipid extract. MRM chromatograms [B] showing adipocyte ceramide C18:1 in ob/ob mice on control diet [OB-NK], ob/ob mice on kudzu diet [OB-K] and lean control mice on control diet [Lean-NK]. Mass transition m/z 564/282 was used. Note the differences in the Y-axes (Intensity) scales.
Fig. 5.
Lysophosphocholines in adipose tissue of ob/ob mice on kudzu diet [OB-K], ob/ob mice on control diet [OB-NK] and lean control mice on control diet [lean-NK]. Precursor ion scan m/z184 was used to detected molecular species of LPC. Major LPC m/z 496 (16:0), m/z 520 (18:20), m/z 524 (18:0), and m/z 538 (20:0) were identified.
Bioavailability of isoflavones in obese and lean animals
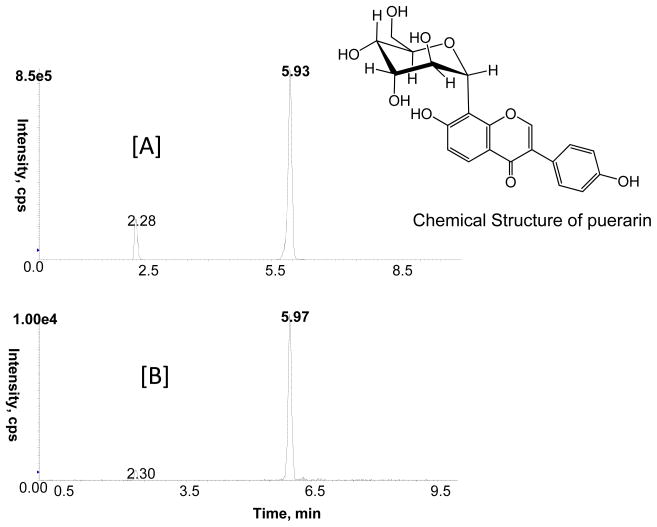
We previously reported a HPLC fingerprint analysis of Pueraria root extract used in this study (Peng et al., 2009). Based on this study, puerarin was the most abundant isoflavone (25.3%) followed by daidzin (7.07%) and daidzein (0.8%) in the root extract. The bioavailability of kudzu isoflavones and their metabolites in plasma and adipose tissue (LC-MS/MS analysis) was significantly higher in obese than lean control mice (Table 1). In contrast to the results of acute administration of puerarin, in which puerarin is the dominate isoflavone, in mice treated chronically with kudzu root extract, equol was the most abundant plasma metabolite 29,300±3,457 nM, followed by daidzein (883±185 nM) and dihydrodaidzein (751±269 nM; Table 1). The plasma concentration of unmetabolized puerarin in the treated group was very low (10.44±2.5 nM). No isoflavone metabolites were detected in the control groups. Intact puerarin (Fig. 6) was present in adipose tissue (11.50±5.63 ng/gram), potentially protecting the tissue against reactive lipids.
Table 1.
Mean ± SEM concentration for kudzu isoflavone levels in rat plasma samples (nM)
| Equol | daidzein | puerarin | DHD | formononetin | |
|---|---|---|---|---|---|
| OB-K | 29300±3457 | 883±185 | 10.44±2.56 | 751±269 | 143±68 |
| Lean-K | 8286±430 | 208±61 | 4.57±1.40 | 65±36 | ND |
DHD = dihydrodaidzein
ND = not detected
Fig. 6.
MRM ion chromatogram for puerarin (retention time 5.93 min)with mass transition m/z 416/267 at 1 μg/ml [A]; methanolic extract of adipose tissue chronically treated with kudzu extract in the diet [B].
Discussion
This current data demonstrate that chronic kudzu isoflavones can improve glucose and lipid homeostasis and insulin sensitivity in ob/ob mice, an animal model of type 2 diabetes mellitus. This extends our previous studies, which demonstrate that acute administration of puerarin significantly improves glucose tolerance in ob/ob mice (Meezan et al., 2005). It is notable that the kudzu supplementation significantly altered glucose metabolism, but it did not appear to affect cardiovascular control, or bodyweight or body fat/lean mass. This is in contrast to the situation in the rat, in which body weight and cardiovascular function is improved by the kudzu supplementation (Peng, et al., 2009). The lack of these latter effects of kudzu supplementation in the ob/ob is not entirely unexpected, since the driving force in physiology of the ob/ob mouse is the lack a functional leptin system, a system that the kudzu root extract or puerarin appear to have little influence over. Total plasma cholesterol and HDL were increased and LDL was lowered by the kudzu supplementation in the ob/ob but not lean control mice. Despite kudzu root extract’s apparent lack of beneficial action on the leptin pathway, the supplement clearly has beneficial effects on glucose/insulin regulation and lipids in the ob/ob, and thus, these findings, paired with our previous reports in the rat, suggest the effectiveness of kudzu polyphenols in different models of glucose/insulin dysregualtion.
Kudzu root extract contains both puerarin and daidzin. We have shown that acute administration of daidzin has an adverse effect on glucose tolerance, whereas acute administration of either kudzu root extract or puerarin improves glucose tolerance (Meezan et al., 2005). The present results demonstrate that chronic feeding with the whole kudzu root extract improves glucose tolerance to about the same extent as previously observed following acute treatment with either puerarin or kudzu root extract. Thus, any adverse effects of dietary daidzin on glucose handling appear minimal in mice fed kudzu root extract. The role that circulating equol and daidzien have in the chronic effects of kudzu root extract supplementation requires further study.
It is noteworthy that in the lean mice the kudzu root extract significantly lowered baseline plasma glucose concentration, but it did not significantly affect the glucose tolerance curve (Fig. 1). Insulin challenge resulted in a larger decrease in blood glucose in kudzu-treated compared to the control diet ob/ob mice, but the treatment had no effect on lean control mice (Fig. 2). Thus, the chronic supplementation improves both glucose tolerance and insulin sensitivity in ob/ob mice, as it also does in the rat model (24). The failure of the supplementation to improve insulin tolerance in lean mice is likely the result of a “floor” effect. Plasma insulin levels in ob/ob mice were lowered by kudzu treatment, suggesting that kudzu supplementation both improves glucose tolerance and reduces peripheral insulin resistance, without increasing circulating insulin.
The finding that kudzu root extract can favorably decrease plasma ceramide concentration in ob/ob, despite its lack of effect on total cholesterol is potentially an important mechanism in its ability to improve glucose/insulin regulation. Previous in vitro studies have demonstrated that some lysophosphatidylcholine (LPC) species activate adipocyte glucose uptake and lower blood glucose levels in murine models of diabetes (Yea et al., 2009), and LPC appears to have pathophysiological functions including inflammation (Oestvang et al., 2011). We therefore, analyzed adipose samples for LPC by tandem mass spectrometry in positive ion mode. Precursor ion scan m/z 184 is very specific for identifying choline-containing phospholipids in positive ion mode. In this study, ion intensities of LPC molecular species in adipose tissue of lean and obese were not significantly different, irrespective of diet (Fig. 5).
Insulin resistance is associated with dysregulation of lipid metabolites including triglyceride, ceramides and fatty acids. Excessive accumulation of ceramide contributes to metabolic dysfunction, whereas adiponectin improves metabolic function (Holland and Scherer, 2009). The present study tested the hypothesis that dietary kudzu supplementation increases plasma adiponetin (the major adipokine circulating in plasma in different isoforms) and decreases ceramide in adipocytes. The current data demonstrate no effect on of the dietary extract on circulating adiponectin; although not conclusive quantitatively, kudzu supplementation decreases C18:1 ceramide levels in adipose tissue. Ceramide can directly inhibit the insulin receptor subunit 1 (IRS1; 28), and thus, the ability of dietary kudzu root extract to improve glucose and insulin responses may directly relate to its actions on ceramide.
Although drug absorption is not directly affected by obesity, obesity alters the distribution, protein binding, metabolism and renal excretion of drugs (Blouin et al., 1987). In the present study, circulating isoflavones were much higher in ob/ob compared to lean control mice. There has been almost no documentation specifically addressing the impact of obesity on isoflavone metabolism. Following acute administration of puerarin or kudzu root extract, the puerarin is rapidly absorbed into the blood with plasma levels peaking about 45 min after administration (Prasain et al., 2007, Prasain et al., 2004). Thereafter, the circulating puerarin is rapidly excreted in the urine and only low concentrations of daidzein and equal remain present in the blood. In our previous studies in rats, oral administration of puerarin produced a high concentration of intact puerarin in the urine collected within the first 4 h (Prasain et al., 2004). This indicates that puerarin is rapidly absorbed in the small intestines. After 4 h, the concentration of intact puerarin decreased substantially while the concentrations of daidzein, dihydrodaidzein, and equol were increased in the urine samples. By 72 h equol was the most abundant metabolite in the urine (Prasain et al., 2004). Since equol is produced after reductive metabolism of daidzeinby intestinal bacteria, these results suggest that puerarin is slowly hydrolyzed to daidzein by bacterial enzymes in the large intestine and subsequently reduced to dihydrodaidzein and equol. Chronic feeding studies with puerarin versus kudzu root extract will assist in understanding which of these mechanisms is primarily responsible for the high circulating equol concentrations in mice. Further, our previous studies indicate that puerarin is in relatively high concentration in some organs after both acute and chronic feeding (e.g., lung, brain, heart, and kidney) (Prasain et al., 2009). It will be important in the future to determine if the effects of kudzu root extract and puerarin are, at least in part, due to the concentration/sequestration of puerarin into these organs. The current results open a new discussion into the mechanisms by which isoflavone metabolisms have long- and short-term effects on insulin resistance, obesity and other physiological characteristics.
Conclusion
Despite our incomplete understanding of mechanisms involved in beneficial effects of kudzu isoflavones, the results of this study clearly demonstrate that chronic feeding of puerarin containing kudzu root extract improves glucose and insulin responsiveness in ob/ob mice. Further, this study clearly demonstrates that compared to lean controls, insulin resistant obese animals display significantly higher circulating isoflavones concentrations and administration of a complex botanical (i.e., kudzu root extract) leads to different mixtures of circulating isoflavones, depending on the post-administration time that is tested. Thus, the assessment of the active mechanisms of complex botanicals must consider differential actions due to these changes in circulating isoflavones.
Acknowledgments
These studies were supported in part by grants from the National Center for Complementary and Alternative Medicine and the National Institutes of Health Office of Dietary Supplements (5P50 AT-00477 – to the Purdue University-UAB Botanicals Center for Age-Related Disease, Connie Weaver, PI) and the NIH Neuroscience Blueprint Mouse Phenotyping Core at UAB (P30 NS-057098; JMW). Thanks go to the UAB Targeted Metabolomics and Proteomics Laboratory for mass spectrometry analysis. The mass spectrometer was purchased by funds from a NIH/NCRR Shared Instrumentation Grant (S10 RR19231) and from this institution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blouin RA, Kolpek JH, Mann HJ. Influence of obesity on drug disposition. Clin Pharm. 1987;6:706–14. [PubMed] [Google Scholar]
- Boue SM, Wiese TE, Nehls S, Burow ME, Elliott S, Carter-Wientjes CH, Shih BY, McLachlan JA, Cleveland TE. Evaluation of the estrogenic effects of legume extracts containing phytoestrogens. J Agric Food Chem. 2003;51:2193–2199. doi: 10.1021/jf021114s. [DOI] [PubMed] [Google Scholar]
- Carlson S, Peng N, Prasain JK, Wyss JM. Effects of botanical dietary supplements on cardiovascular, cognitive, and metabolic function in males and females. Gend Med. 2008;5(Suppl A):S76–S90. doi: 10.1016/j.genm.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cefalu WT, Ye J, Zuberi A, Ribnicky DM, Raskin I, Liu Z, Wang ZQ, Brantley PJ, Howard L, Lefevre M. Botanicals and the metabolic syndrome. Am J Clin Nutr. 2008;87:481S–487S. doi: 10.1093/ajcn/87.2.481S. [DOI] [PubMed] [Google Scholar]
- Cohen B, Novick D, Rubinstein M. Modulation of insulin activities by leptin. Science. 1996;274:1185–8. doi: 10.1126/science.274.5290.1185. [DOI] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC, Cheng JT. Antihyperglycemic effect of puerarin in streptozotocin-induced diabetic rats. J Nat Prod. 2003;66:788–792. doi: 10.1021/np0203887. [DOI] [PubMed] [Google Scholar]
- Keung WM, Vallee BL. Kudzu root: an ancient Chinese source of modern antidipsotropic agents. Phytochemistry. 1998;47:499–506. doi: 10.1016/s0031-9422(97)00723-1. [DOI] [PubMed] [Google Scholar]
- Kraja AT, Province MA, Huang P, Jarvis JP, Rice T, Cheverud JM, Rao DC. Trends in metabolic syndrome and gene networks in human and rodent models. Endocr Metab Immune Disord Drug Targets. 2008;8:198–207. doi: 10.2174/187153008785700145. [DOI] [PubMed] [Google Scholar]
- Lee JS. Supplementation of Pueraria radix water extract on changes of antioxidant enzymes and lipid profile in ethanol-treated rats. Clin Chim Acta. 2004;347:121–128. doi: 10.1016/j.cccn.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Meezan E, Meezan EM, Jones K, Moore R, Barnes S, Prasain JK. Contrasting effects of puerarin and daidzin on glucose homeostasis in mice. J Agric Food Chem. 2005;53:8760–8767. doi: 10.1021/jf058105e. [DOI] [PubMed] [Google Scholar]
- Miura A, Kajita K, Ishizawa M, et al. Inhibitory effect of ceramide on insulin-induced protein kinase Czeta translocation in rat adipocytes. Metabolism. 2003;52:19–24. doi: 10.1053/meta.2003.50011. [DOI] [PubMed] [Google Scholar]
- Muller G, Ertl J, Gerl M, Preibisch G. Leptin impairs metabolic actions of insulin in isolated rat adipocytes. J Biol Chem. 1997;272:10585–10593. doi: 10.1074/jbc.272.16.10585. [DOI] [PubMed] [Google Scholar]
- Oestvang J, Anthonsen MW, Johansen B. LysoPC and PAF Trigger Arachidonic Acid Release by Divergent Signaling Mechanisms in Monocytes. J Lipids. 2011:532145–532156. doi: 10.1155/2011/532145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Scherer PE. PAQ receptors: a counteracting force to ceramides? Mol Pharmacology. 2009;75:740–3. doi: 10.1124/mol.109.054817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng N, Clark JT, Prasain J, Kim H, White CR, Wyss JM. Antihypertensive and cognitive effects of grape polyphenols in estrogen-depleted, female, spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R771–R775. doi: 10.1152/ajpregu.00147.2005. [DOI] [PubMed] [Google Scholar]
- Peng N, Prasain JK, Dai Y, Carlson SH, Wyss JM. Dietary kudzu root extract supplementation improves glucose tolerance and plasma lipid profiles in Spontaneously Hypertensive Rats (SHR) fed a basal, but not high, NaCl diet. FASEB J. 2008;22:948. (abs.) [Google Scholar]
- Peng N, Prasain JK, Dai Y, Moore R, Arabshahi A, Barnes S, Carlson S, Wyss JM. Chronic dietary kudzu isoflavones improve components of metabolic syndrome in stroke-prone spontaneously hypertensive rats. J Agric Food Chem. 2009;57:7268–73. doi: 10.1021/jf901169y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasain JK, Arabshahi A, Moore R, Greendale G, Wyss JM, Barnes S. Simultaneous determination of eleven phytoestrogens in human serum using two minutes liquid chromatography/tandem mass spectrometry method. J Chrom B. 2010;878:994–1002. doi: 10.1016/j.jchromb.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasain JK, Jones K, Brissie N, Moore DR, II, Wyss JM, Barnes S. Identification of puerarin and its metabolites in rats by liquid chromatography-tandem mass spectrometry. J Agric Food Chem. 2004;52:3708–3712. doi: 10.1021/jf040037t. [DOI] [PubMed] [Google Scholar]
- Prasain JK, Peng N, Acosta E, Moore R, Arabshahi A, Meezan E, Barnes S, Wyss JM. Pharmacokinetic study of puerarin in rat serum by liquid chromatography tandem mass spectrometry. Biomed Chromatogr. 2007;21:410–4. doi: 10.1002/bmc.772. [DOI] [PubMed] [Google Scholar]
- Prasain JK, Peng N, Moore R, Arabshahi A, Barnes S, Wyss JM. Tissue distribution of puerarin and its conjugated metabolites in rats assessed by liquid chromatography-tandem mass spectrometry. Phytomedicine. 2009;16:65–71. doi: 10.1016/j.phymed.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC. AIM-93 purified diet for laboratory rodents: final report of the American Institute of Nutrition ad hoc Writing Committee on the reformulation of the AIN-76A diet. J Nutrition. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Segal K, Landt M, Klein S. Relationship between insulin sensitivity and plasma leptin concentration in lean and obese men. Diabetes. 1996;45:987–991. doi: 10.2337/diab.45.7.988. [DOI] [PubMed] [Google Scholar]
- Summers SA. Ceramides in insulin resistance and lipotoxicity. Progress in Lipid Research. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes. 2001;50:2563–2571. doi: 10.2337/diabetes.50.11.2563. [DOI] [PubMed] [Google Scholar]
- Xu ME, Xiao SZ, Sun YH, Zheng XX, Ou-Yang Y, Guan C. The study of anti-metabolic syndrome effect of puerarin in vitro. Life Sci. 2005;77:3183–96. doi: 10.1016/j.lfs.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Yea K, Kim J, Yoon JH, Kwon T, Kim JH, Lee BD, Lee HJ, Lee SJ, Kim JI, Lee TG, Baek MC, Park HS, Park KS, Ohba M, Suh PG, Ryu SH. Lysophosphatidylcholine activates adipocyte glucose uptake and lowers blood glucose levels in murine models of diabetes. J Biol Chem. 2009;284:33833–40. doi: 10.1074/jbc.M109.024869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Liu CQ, Wang PW, Sun SY, Su WJ, Zhang HJ, Li XJ, Yang SY. Puerarin improves insulin resistance and modulates adipokine expression in rats fed a high-fat diet. Eur J Pharmacol. 2010;649:398–402. doi: 10.1016/j.ejphar.2010.09.054. [DOI] [PubMed] [Google Scholar]