Abstract
Evidence suggests that stress increases alcohol drinking and promotes relapse in humans. Animal models that assess related behaviors include the sipper tube ethanol self-administration and the stress-induced reinstatement paradigms. While selectively bred for the same high-ethanol-drinking behavior, alcohol-preferring P rats appear to show greater sensitivity to ethanol reinforcement than high-alcohol-drinking HAD rats. The present experiment tested the effects of the pharmacological stressor, yohimbine, on the motivation to seek and consume ethanol implementing a combined sipper tube/reinstatement model using male P and HAD-2 rats. Following training to self-administer ethanol using the sipper tube procedure, rats were tested for the effects of yohimbine (0.625-2.5 mg/kg) on ethanol drinking. Subsequently, rats were tested for the effects of 1.25 mg/kg yohimbine on reinstatement of ethanol seeking. Yohimbine (0.625 and 1.25 mg/kg) increased ethanol self-administration, and the latter dose also decreased latency to complete the response requirement. Yohimbine elicited reinstatement of ethanol seeking in both lines. HAD-2 rats drank more ethanol, but showed similar responding on the ethanol-associated lever compared to P rats. These findings extend both the reinstatement and sipper tube models and justify further exploration of this unique combined paradigm. Despite prior evidence suggesting that P rats are more motivated to seek and consume ethanol, differences in these behaviors between P and HAD-2 rats were not systematic in the present experiment. Further investigation may elucidate whether either selected line may be more sensitive than other selectively bred or outbred rats to stress-related changes in ethanol's reinforcing effects.
Keywords: Stress, motivation, reinstatement, relapse, binge drinking
1. Introduction
The extent to which stress contributes to alcohol drinking has been the focus of numerous empirical studies and reviews [1-3]. At the neurobiological level, the relationship between stress and alcohol abuse is unified through overlapping reward and stress pathways [4]. Like stress, acute alcohol administration elevates ACTH and corticosteroids in rodents [5, 6] and humans [7, 8]. These stress hormones have both direct and indirect effects on mesolimbic dopamine signaling and implicate integration of multiple brain systems that adapt to changing behavioral and drug contingencies in the addiction process [9].
Theories of addiction have posited that both positive and negative reinforcement can contribute to the escalation of drug and alcohol use [10, 11]. While both reward and relief may account for stress-related craving [12, 13], several lines of research converge on the notion that negative reinforcement is highly implicated in mediating stress-related alcohol drinking due to its tension- and anxiety-reducing effects [14, 15]. Negative reinforcement may be at play during early stages of problem alcohol use, such as binge drinking. As outlined by the NIAAA, binge drinking is defined by reaching a blood ethanol concentration of 80 mg% within two hours, and is a risk factor for future problems with alcohol [16-18]. Studies examining the relationship between stress and binge drinking have found that escape drinking, rather than social drinking, directly predicts binge drinking in college students [19], and that the likelihood of binge drinking is increased on days individuals experiences more stress [20]. However, findings from studies conducted in animal models of binge-like ethanol consumption have shown variable responses to stress. When given limited access, ethanol intake was increased following restraint stress [21, 22], and injection with the pharmacological stressor, yohimbine [23, 24], but not following social defeat stress [25]. Stress has also been shown to stimulate craving for ethanol as evidenced by self-report in humans [26-28] and stress-induced reinstatement procedures in rodents [24, 29].
Yohimbine is an ! -2 adrenoreceptor antagonist that increases norepinephrine release in several brain areas [30], elevates glucocorticoid levels in both rats [31, 32] and humans [33, 34]) and elicits anxiety in both rodents [35, 36] and humans [37, 38]. In healthy human subjects, ethanol and yohimbine have additive effects on subjective reports of anxiety and intoxication, and on cortisol levels [37]. In recently detoxified alcoholic subjects, yohimbine increases anxiety and cortisol, but fails to substitute for ethanol or increase craving [39]. However, a recent study [40] has shown that yohimbine increases craving for ethanol, but not anxiety in treatment-seeking alcoholics. In animal models of the stress-alcohol interaction, yohimbine has been used extensively in stress-induced reinstatement paradigms [23, 29, 31, 41-44]. Importantly, yohimbine induces the expression of c-fos and CRF mRNA in limbic brain areas associated with reward to a similar degree as footshock, a commonly used physical stressor [45]. Taken together, these findings demonstrate the efficacy of yohimbine as a stressor on both the behavioral and neurobiological level.
While the reinstatement procedure is one paradigm for exploring ethanol reinforcement, another useful method is the sipper tube model [46]. This model procedurally separates ethanol seeking from consumption by imposing a single response requirement that the animal must emit before gaining 20-minute access to ethanol, during which time rats typically engage in binge-like drinking (e.g., [47]). Using this procedure, the operant (seeking) behavior is not impinged upon by the effects of the reinforcer. Further, if a non-reinforced session is imposed, it is possible to assess appetitive aspects of ethanol reinforcement while not under the pharmacological effects of ethanol. While the effects of several treatment compounds aimed at reducing ethanol reinforcement have been tested using this model [47-49], application of an agent – such as stress – predicted to increase ethanol seeking and self-administration has yet to be explored.
Two lines of rats selectively bred for high alcohol intake [50, 51], that voluntarily consume in excess of 6 g/kg of ethanol per day when given free access, appear to differ in the motivation to seek ethanol. For example, P rats show resistance to increasing fixed ratio schedules of reinforcement [52], greater appetitive responding during non-reinforced sessions, and higher breakpoint values [53] than HAD rats. In addition, P rats robustly express the alcohol deprivation effect (ADE), another proposed model of craving [54, 55] after a single deprivation period [55, 56], while HAD rats fail to show an ADE unless multiple deprivation periods are imposed [57, 58]. Important to the present investigation, P and HAD rats also differ with regard to stress- and anxiety-related behaviors, with P rats showing greater anxiety-like behavior in a number of paradigms [59], and more acute elevated post-restraint stress-induced alcohol drinking [60] compared to their non-preferring (NP) counterparts. However, recent studies have shown P and NP rats to not differ in anxiety-like behavior [61], suggesting further evaluation of the relationship between anxiety-like behavior and excessive alcohol drinking is warranted. In contrast, HAD rats tend not to differ from low-alcohol-drinking LAD rats in measures of anxiety-like behavior [62, 63], and show post-stress increases in ethanol drinking after footshock stress [64], but not restraint stress [60].
The goal of the present investigation was to determine the role of stress in altering ethanol seeking and self-administration in P and HAD-2 rats using yohimbine as a pharmacological stressor. The sipper tube model was implemented using its established protocol, but was also modified to measure reinstatement, effectively assessing “craving”-like behavior using a different methodology. The effects of a range of doses of yohimbine (0.625-2.5 mg/kg) on ethanol consumption were first investigated, and subsequently a single dose of yohimbine (1.25 mg/kg) was tested for its effects on reinstatement. It was predicted that yohimbine would increase ethanol self-administration and seeking. While HAD-2 rats tend to drink more ethanol than P rats under free-choice conditions [65], P rats appear to show a greater motivation to seek ethanol, as described above; as such, it was predicted that the lines would differ in ethanol self-administration and seeking.
2. Material and Methods
2.1 Subjects
Male P (68th generation) and HAD-2 (60th generation) rats aged approximately 6 weeks upon arrival were used in the present experiment. Rats were singly housed in plastic shoebox cages in a temperature- and humidity-controlled vivarium maintained on a regular light/dark cycle (lights on at 7:00am). Water and food were available ad libitum throughout the experiment except where noted. Rats were given a week to acclimate to the vivarium during which time they were weighed, handled, and habituated to transport. The experimental protocol was approved by the Indiana University School of Medicine Animal Institutional Animal Care and Use Committee and was conducted in accordance with NIH guidelines (Guide for the Care and Use of Laboratory Animals; NIH Guide 1996).
2.2 Apparatus
Operant sessions were conducted in Med-Associates chambers (St. Albans, VT, USA; 30 ! 30 ! 24.5 cm) equipped with a houselight, retractable levers, and a retractable sipper tube. The sipper tube was a graduated cylinder fitted with a rubber stopper and a stainless steel spout with double ball bearings to prevent leakage. The levers were located on the wall opposite to the sipper tube. Operant chambers were housed in sound-attenuated cubicles equipped with exhaust fans to mask external noise. Electrical inputs and outputs of each chamber were controlled using Med-Associates software (Med-Associates).
2.3 Drugs
Ethanol solutions were prepared using 95% ethanol diluted with water to a 10% v/v concentration. Ethanol/sucrose solutions were prepared using the appropriate concentration of sucrose as the solute. Yohimbine HCl (Sigma-Aldrich, St. Louis, MO) was prepared with sterile water at doses of 0.625, 1.25, or 2.5 mg/kg body weight in a 1.0 mg/ml injection volume.
2.4 Operant Ethanol Self-Administration Training
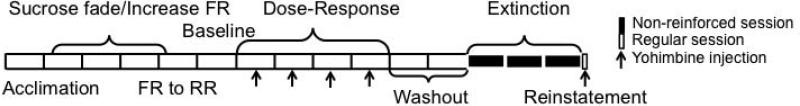
During the initial operant training sessions, rats were water restricted in their home cages and were shaped in 1-hour operant sessions on a fixed ratio (FR)1 schedule for 60-second access to a sipper tube containing a 10% sucrose solution. Following this, the session length was reduced to 30 minutes and the sipper access time was reduced to 30, then 15 seconds. Prior to each session, the house light was off and the levers and sipper tube were retracted in the operant chamber until the session began. A modified sucrose-fading procedure [66] was used to initiate ethanol consumption and to maintain consistency with previous studies conducted in non-selected rats using this model. Rats received 1-2 sessions on an FR1 schedule at each of the following concentrations: 10% sucrose/2% ethanol; 10% sucrose/5% ethanol; 10% sucrose/10% ethanol; 5% sucrose/10% ethanol; 2% sucrose/10% ethanol; and finally 10% ethanol. The response requirement increased on successive sessions from an FR1 to an FR4. Next, a response requirement (RR) was implemented in which a number of responses were required to gain uninterrupted access to the sipper tube for 20 minutes. This requirement increased from an RR4 to an RR10. The entire training procedure took 3-4 weeks to complete. For a timeline of training and testing procedures, please refer to Figure 1.
Figure 1.
Timeline for experimental procedures. Each block represents one week of time. White blocks represent reinforced sessions, while black blocks represent non-reinforced sessions. Arrows indicate sessions prior to which test injections of yohimbine or vehicle were given.
2.5 Procedure
A total of 24 male P and 24 male HAD-2 rats were trained in the present experiment. Operant sessions were conducted 5 days/week during the light cycle and consisted of a response period that lasted until the RR was met (up to 20 minutes), followed by a 20-minute drinking period. After one week of RR training, a second, inactive lever was introduced upon which responses were recorded but elicited no programmed consequences. After reaching the maximal response requirement (RR10), rats were then given an additional week of baseline sessions. Then, during the dose-response phase, injections of vehicle or one of three doses of yohimbine (0.625, 1.25, or 2.5 mg/kg; -15 min, IP) were given once each week in a balanced design (injections preceded Wednesday sessions). Rats were then given 2 weeks of non-injection reinforced sessions before the reinstatement phase began. During extinction sessions, conditions were identical to those during the training and dose-response phases with the exception that responses on the active lever did not result in sipper tube access. A retracted sipper tube containing a small amount of ethanol was present to ensure that visual and olfactory cues present during self-administration were also present during extinction. The extinction criterion was set at ≤10 active lever presses for at least two of the last three consecutive extinction sessions, which was met within 14 extinction sessions by all rats included in the analysis. Rats were then assigned to either the yohimbine or vehicle group balanced for ethanol intake during the reinforced sessions just prior to extinction and also for responding on the active lever during the first five days of extinction. On the 15th session (reinstatement), rats were injected with either 1.25 mg/kg of yohimbine or vehicle (-15min IP) prior to a non-reinforced session.
2.6 Statistical Analyses
Mixed factorial ANOVAs with line (P vs. HAD-2) as the between-subjects factor and yohimbine dose (vehicle, 0.625, 1.25, or 2.5 mg/kg) as the within-subjects factor were conducted for ethanol intake (g/kg), licks, latency to complete the RR, and inactive lever presses for the dose-response phase. For the extinction/reinstatement phase, a mixed factorial ANOVA with line (P vs. HAD-2) as the between-subjects factor and extinction day (1-14) as the within-subjects factor, using Bonferroni post-hoc comparisons, was conducted for active lever presses during extinction. Repeated-measures ANOVAs with line (P vs. HAD-2) and yohimbine dose (1.25 mg/kg vs. vehicle) as between-subjects factors and phase (extinction vs. reinstatement) as the within-subjects factor were conducted for active and inactive lever presses during reinstatement. Body weights collected on the last day of baseline were compared between P and HAD-2 rats using independent-samples t-tests. Fisher's LSD post-hoc comparisons were made where appropriate. The significance level was set at p < 0.05 in all analyses.
3. Results
3.1 Yohimbine Dose-Response
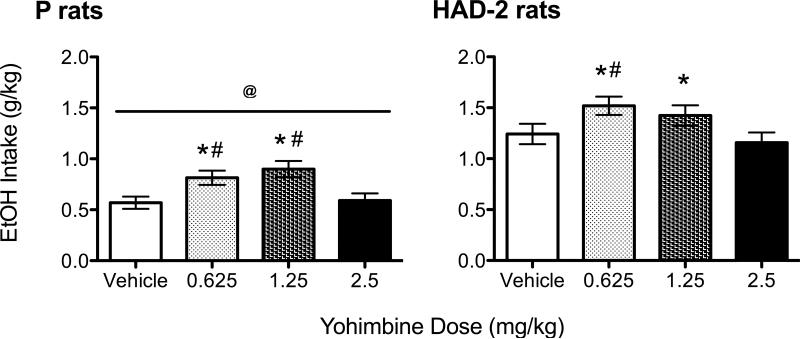
Three P rats and 2 HAD-2 rats did not complete the response requirement on at least one test day (n = 4 following 2.5 mg/kg yohimbine, n = 1 following vehicle) and therefore were excluded from the analysis. The final sample sizes were n = 21 for P rats and n = 22 for HAD-2 rats. An independent-samples t-test revealed that P rats weighed significantly more (455.6±42 g) than HAD-2 rats (247.7±27 g; [t(41) = 19.17, p < 0.001]) on the final day of the baseline period. A significant main effect of line [F(3,123) = 70.43, p < 0.001] was found for ethanol intake, with HAD-2 rats drinking more ethanol overall than P rats (Figure 2). A significant main effect of dose [F(3,123) = 9.13, p < 0.001] was also found, with post-hoc comparisons indicating that ethanol intake was increased following administration of the 0.625 and 1.25 mg/kg doses of yohimbine compared to vehicle and the 2.5 mg/kg dose (Figure 2). Significant main effects of dose were also found for licks [F(3,123) = 10.98, p < 0.001] and latency to complete the response requirement [F(3,123) = 2.91, p = 0.037] (Table 1). Post-hoc comparisons revealed that licks were increased following administration of the 0.625 and 1.25 mg/kg doses of yohimbine compared to vehicle and the 2.5 mg/kg dose. However, latency to complete the response requirement was shorter following injection of the 1.25 mg/kg dose compared to vehicle only. Inactive lever presses did not differ as a function of yohimbine dose or line. No interactions were found among factors for any measure.
Figure 2.
Ethanol intake (g/kg) in P (a) and HAD-2 (b) rats injected with vehicle, 0.625, 1.25, and 2.5 mg/kg yohimbine in operant sessions separated by one week using the sipper tube model. * = p < 0.05 vs. vehicle; # = p < 0.05 vs. 2.5 mg/kg; @ = p < 0.05 vs. HAD-2
Table 1.
Inactive lever presses (LPs), licks, and latency to complete the response requirement in rats injected with vehicle, 0.625, 1.25, and 2.5 mg/kg yohimbine in operant sessions separated by one week using the sipper tube model. As there were no main effects of or interactions involving line for any of these outcome measures, these data are collapsed across line.
| Dose | Inactive LPs | Licks | Latency |
|---|---|---|---|
| Vehicle | 0.18(0.10) | 584.56(48.01) | 49.91(12.33) |
| 0.625 mg/kg | 0.02(0.02) | 792.93(44.25)*# | 25.60(2.90) |
| 1.25 mg/kg | 0.51(0.30) | 738.40(43.20)*# | 23.21(3.39)* |
| 2.5 mg/kg | 0.16(0.09) | 545.09(37.22) | 70.35(23.69) |
p < 0.05 vs. vehicle
p < 0.05 vs. 2.5 mg/kg
3.2 Extinction and Reinstatement
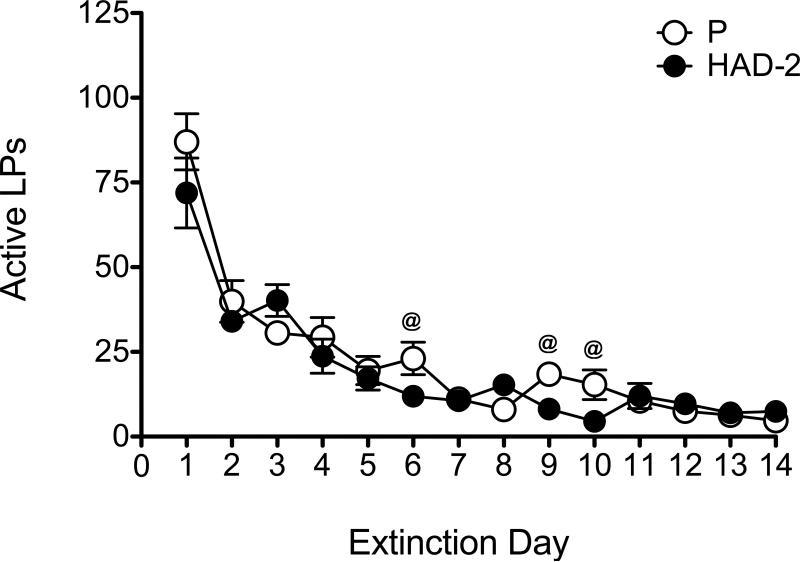
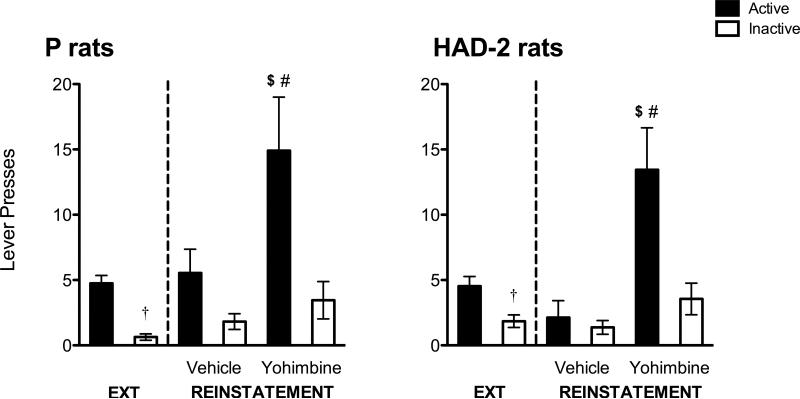
During extinction, a significant main effect of day [F(13,286) = 52.75, p < 0.001] was found for active lever presses, with post-hoc tests indicating the typical overall pattern of decreased responding as a function of day, and with responding during the first day of extinction being larger than all other days (Figure 3). There was no main effect of line, but a significant day by line interaction [F(13,286) = 1.86, p = 0.034] was found. Pairwise comparisons indicated greater responding by P rats on days 6, 9, and 10 of extinction compared to HAD-2 rats. One P rat and 4 HAD-2 rats did not meet extinction criteria; further, 1 P rat (yohimbine group) and 3 HAD-2 rats (n = 1 in the yohimbine group, n = 2 in the vehicle group) were considered to be outliers based on box plots and confirmed by outcome measures exceeding 2 standard deviations from the mean; therefore, these rats were excluded from the reinstatement analysis. Final sample sizes were n = 11 in both the yohimbine and vehicle groups for P rats, and n = 9 in the yohimbine group and n = 8 in the vehicle group for HAD-2 rats. No significant differences between P and HAD-2 rats were detected for active or inactive lever presses. On the active lever, there was a significant main effect of phase [F(1,35) = 8.98, p = 0.005], indicating a greater number of responses emitted on the day of reinstatement compared to the last day of extinction (Figure 4). There was also a significant main effect of yohimbine dose [F(1,35) = 12.29, p = 0.001], with rats injected with yohimbine pressing more than those injected with vehicle. The pattern of effects for the inactive lever was the same for phase [F(1,35) = 4.52, p = 0.041] and dose [F(1,35) = 6.8, p = 0.013]. However, there was only a significant phase by dose interaction for active lever presses [F(1,35) = 12.29, p = 0.002], which follow-up oneway ANOVAs conducted separately for the extinction and reinstatement phases revealed was due to yohimbine-treated rats showing an increase in responding during reinstatement only [F(1,35) = 13.02, p = 0.001].
Figure 3.
Active lever presses during the 14 days of extinction. White circles represent P rats and black circles represent HAD-2 rats. Responding decreased as a function of extinction day. While P and HAD-2 rats did not differ in responding overall, analyses following a day by line interaction showed that P rats pressed more on days 6, 9, and 10. @ = p < 0.05 vs. HAD-2
Figure 4.
Lever presses during extinction (EXT) and reinstatement in P (a) and HAD-2 (b) rats given either vehicle or yohimbine prior to the reinstatement session. Black bars represent responses on the active lever while white bars represent responses on the inactive lever. $ = p < 0.05 vs. EXT; # = p < 0.05 vs. vehicle; † = p < 0.05 vs. reinstatement.
4. Discussion
The results from the present experiment confirm the efficacy of yohimbine in increasing operant ethanol self-administration and eliciting reinstatement of ethanol seeking. Importantly, this effect was demonstrated in rats selectively bred for high ethanol intake and using the sipper tube model, which represents a novel approach to the stress-induced reinstatement procedure. Ethanol intake was increased following the 0.625 and 1.25 mg/kg doses of yohimbine, and the latter dose also decreased latency to complete the response requirement. This dose of yohimbine subsequently reinstated ethanol seeking. Taken together, these findings show that yohimbine increases the motivation to consume and seek ethanol in P and HAD-2 rats using the sipper tube model.
Yohimbine has previously been shown to increase operant self-administration of ethanol in outbred rats at all doses used in the present experiment. However, this effect is less consistently found following administration of the 2.5 mg/kg dose, as increased ethanol intake is evident in some studies [44] but not others [23]. Consistent with the most recent finding from Lê and colleagues, ethanol consumption following the 2.5 mg/kg dose of yohimbine did not differ from vehicle in the present experiment. This dose of yohimbine significantly increases anxiety-like behavior [35], and a similar dose (2.0 mg/kg) decreases locomotor activity [67]. Thus, it is possible that stress-related suppression in activity or freezing could contribute to the effects of the high dose of yohimbine on ethanol self-administration, and could underlie the failure to complete the response requirement by four rats in the present experiment. However, rats injected with 2.5 mg/kg yohimbine did not significantly differ from vehicle-injected rats in the amount of time it took to perform the response requirement in the present experiment with or without the non-responders removed from the analysis. In contrast, 1.25 mg/kg yohimbine increased ethanol intake and decreased latency to complete the response requirement relative to vehicle. There was no appreciable responding on the inactive lever during this phase of the experiment, and inactive lever responding did not differ among the doses, suggesting that yohimbine did not elicit dose-dependent changes in general locomotor activity. In addition, a recent study [68] showed that yohimbine increases nicotine self-administration and progressive ratio breakpoints, implicating motivational mechanisms in the effect of yohimbine on drug seeking and taking. Taken together, these findings suggest that motivational, rather than locomotor stimulating, effects of yohimbine are responsible for this effect.
During the reinstatement phase of the present experiment, rats injected with 1.25 mg/kg yohimbine demonstrated a robust increase in responding on the active lever and a modest, yet significant increase in responding on the inactive lever. However, responding on the inactive lever during the dose-response phase was not altered by any dose of yohimbine. This response pattern during reinstatement may reflect an increase in the rats’ response topography while under conditions similar to early extinction, during which time an increase in non-specific behaviors are executed in an attempt to gain access to the reinforcer [69]. Yohimbine produced interoceptive stimuli during reinstatement similar to the unconditioned stimuli present during reinforcer access, eliciting reinstatement of the conditioned response (lever pressing); however, when the rats were not reinforced, response variability increased and resulted in higher inactive lever presses than seen in rats not receiving those interoceptive cues. Increased responding on the inactive lever following yohimbine has been shown in some studies [31, 42, 70], but not others [44, 71]. It is not clear what effect prior exposure to yohimbine during the dose-response phase of the experiment may have on subsequent reinstatement performance. However, Kupferschmidt et al. [72] showed that compared to vehicle, repeated exposure to 1.25 mg/kg yohimbine given after extinction sessions did not alter later reinstatement of cocaine seeking, suggesting that experience with yohimbine does not necessarily affect yohimbine-induced reinstatement. Overall, the present experiment adds further evidence that yohimbine is a reliable pharmacological stressor and a useful tool in examining “craving”-like behavior, but also suggests that subsequent work examining the parameters of yohimbine administration, such as the dose and chronicity of its application, on the reinstatement of ethanol seeking is warranted.
Of particular interest, the findings from the present study extend both the stress-induced reinstatement and sipper tube models by merging the two methodologies. In traditional reinstatement procedures, animals are typically trained on a low fixed-ratio schedule in 30- or 60-minute sessions [31, 44, 71] prior to extinction and reinstatement. Adjusted for session length, this results in higher levels of responding during training and, therefore, during reinstatement, compared to the sipper tube model, in which the maximum number of responses during training is determined by the response requirement. Despite lower overall responding during reinstatement in the present experiment, the magnitude of yohimbine-stimulated responding compared to vehicle or to responding during extinction was roughly threefold, consistent with previous studies [42, 44, 71]. Further, responding on the last day of extinction was 6-10% of the first day, which is less than the 20% criterion typical of reinstatement studies [73]. This shows that setting the response criterion at ≤10 active lever presses (the response requirement during self-administration) for 2 of 3 consecutive sessions is an appropriate modification of the reinstatement procedure. These findings demonstrate that the sipper tube model can be used to measure reinstatement of ethanol seeking and provide a basis for future experiments.
Few studies have examined reinstatement models in rats selectively bred for high ethanol intake, and none, to our knowledge, have compared lines of alcohol-preferring rodents in stress-induced reinstatement. P rats have shown cue-induced reinstatement of both nicotine [74] and ethanol seeking [75], with response patterns being similar in the previous and present experiments. In addition, Marchigian Sardinian alcohol-preferring (msP) rats have shown a lower threshold for stress-induced reinstatement of ethanol seeking than outbred Wistar rats [76]. It is of interest, therefore, to extend these findings by comparing P and HAD rats to non-selected rats in stress-induced reinstatement procedures. Among P and HAD-2 rats, line differences in ethanol self-administration were evident as HAD-2 rats drank significantly more ethanol than P rats, consistent with previous studies [65]. However, P and HAD-2 rats did not exhibit systematic differences in responding during extinction or reinstatement, indicating that these lines do not appear to differ in the motivation to seek ethanol. This finding contrasts with the prediction that P rats would be more motivated to seek ethanol following stress exposure based on their propensity to adjust their responding when presented with an increased response demand [52, 53], demonstrate a robust ADE [55-58], and show greater anxiety-like behavior than their non-preferring counterparts [59]. However, a recent study found P, HAD-1, and HAD-2 rats to be remarkably similar in a variety of behaviors, including those related to anxiety [61]. Despite being selected for the same behavior (voluntary consumption of >5 g/kg of ethanol per day), the P and replicate HAD lines were generated from different progenitor stocks (Wistar and N/Nih rats, respectively; see Murphy et al. [65]); as such, inheritance of potentially trait-relevant (e.g., those associated with motivation, anxiety-like behavior) and non-relevant (e.g., those associated with body weight, coat color) alleles may account for phenotypic differences among these high-drinking rats. Further investigation is needed to determine whether these two lines selectively bred for high ethanol intake may represent distinct populations that respond differently to interventions aimed at reducing relapse.
In conclusion, the results from the present study demonstrate the efficacy of yohimbine as a stressor in eliciting increases in the seeking and binge-like self-administration of ethanol in P and HAD-2 rats using the sipper tube model combined with the reinstatement model. It would be of interest to examine treatments that may differentially modify ethanol seeking and self-administration in the sipper tube reinstatement model and to extend its utility by performing cue- and priming-induced reinstatement procedures, using alternate reinforcers, and using rodent populations with varied genetic backgrounds.
Research Highlights.
! ! Yohimbine increased ethanol self-administration and reinstated ethanol seeking
! ! P and HAD-2 do not differ in the effects of yohimbine on ethanol reinforcement
! ! The sipper tube model can be modified to test for reinstatement
Acknowledgments
Funded by T32AA007462, P60AA007611
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silberman Y, Bajo M, Chappell AM, Christian DT, Cruz M, Diaz MR, et al. Neurobiological mechanisms contributing to alcohol-stress-anxiety interactions. Alcohol. 2009;43:509–19. doi: 10.1016/j.alcohol.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mason BJ, Shaham Y, Weiss F, Le AD. Stress, alcohol craving, and relapse risk: mechanisms and viable treatment targets. Alcohol. 2009;43:541–3. doi: 10.1016/j.alcohol.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–59. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- 4.Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- 5.Guaza C, Torrellas A, Borrell S. Adrenocortical response to acute and chronic ethanol administration in rats. Psychopharmacology (Berl) 1983;79:173–6. doi: 10.1007/BF00427806. [DOI] [PubMed] [Google Scholar]
- 6.Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF). J Pharmacol Exp Ther. 1984;229:127–31. [PubMed] [Google Scholar]
- 7.Adinoff B, Ruether K, Krebaum S, Iranmanesh A, Williams MJ. Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcohol Clin Exp Res. 2003;27:1420–7. doi: 10.1097/01.ALC.0000087581.13912.64. [DOI] [PubMed] [Google Scholar]
- 8.Mendelson JH, Stein S. The definition of alcoholism. Int Psychiatry Clin. 1966;3:3–16. [PubMed] [Google Scholar]
- 9.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verheul R, van den Brink W, Geerlings P. A three-pathway psychobiological model of craving for alcohol. Alcohol Alcohol. 1999;34:197–222. doi: 10.1093/alcalc/34.2.197. [DOI] [PubMed] [Google Scholar]
- 11.Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinz A, Lober S, Georgi A, Wrase J, Hermann D, Rey ER, et al. Reward craving and withdrawal relief craving: assessment of different motivational pathways to alcohol intake. Alcohol Alcohol. 2003;38:35–9. doi: 10.1093/alcalc/agg005. [DOI] [PubMed] [Google Scholar]
- 13.Grusser SM, Morsen CP, Wolfling K, Flor H. The relationship of stress, coping, effect expectancies and craving. Eur Addict Res. 2007;13:31–8. doi: 10.1159/000095813. [DOI] [PubMed] [Google Scholar]
- 14.Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–45. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- 15.Cooper ML, Russell M, Skinner JB, Frone MR, Mudar P. Stress and alcohol use: moderating effects of gender, coping, and alcohol expectancies. J Abnorm Psychol. 1992;101:139–52. doi: 10.1037//0021-843x.101.1.139. [DOI] [PubMed] [Google Scholar]
- 16.Chassin L, Pitts SC, Prost J. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: predictors and substance abuse outcomes. J Consult Clin Psychol. 2002;70:67–78. [PubMed] [Google Scholar]
- 17.Dawson DA, Archer LD. Relative frequency of heavy drinking and the risk of alcohol dependence. Addiction. 1993;88:1509–18. doi: 10.1111/j.1360-0443.1993.tb03136.x. [DOI] [PubMed] [Google Scholar]
- 18.Hill KG, White HR, Chung IJ, Hawkins JD, Catalano RF. Early adult outcomes of adolescent binge drinking: person- and variable-centered analyses of binge drinking trajectories. Alcohol Clin Exp Res. 2000;24:892–901. [PMC free article] [PubMed] [Google Scholar]
- 19.Williams A, Clark D. Alcohol consumption in university students: the role of reasons for drinking, coping strategies, expectancies, and personality traits. Addict Behav. 1998;23:371–8. doi: 10.1016/s0306-4603(97)80066-4. [DOI] [PubMed] [Google Scholar]
- 20.Grzywacz JG, Almeida DM. Stress and Binge Drinking: A Daily Process Examination of Stressor Pile-up and Socioeconomic Status in Affect Regulation. Int J Stress Manag. 2008;15:364–80. doi: 10.1037/a0013368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chester JA, de Paula Barrenha G, DeMaria A, Finegan A. Different effects of stress on alcohol drinking behaviour in male and female mice selectively bred for high alcohol preference. Alcohol Alcohol. 2006;41:44–53. doi: 10.1093/alcalc/agh242. [DOI] [PubMed] [Google Scholar]
- 22.Lynch WJ, Kushner MG, Rawleigh JM, Fiszdon J, Carroll ME. The effects of restraint stress on voluntary ethanol consumption in rats. Exp Clin Psychopharmacol. 1999;7:318–23. doi: 10.1037//1064-1297.7.4.318. [DOI] [PubMed] [Google Scholar]
- 23.Le AD, Funk D, Harding S, Juzytsch W, Fletcher PJ. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology (Berl) 2009;204:477–88. doi: 10.1007/s00213-009-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 1998;135:169–74. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- 25.van Erp AM, Miczek KA. Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav. 2001;73:301–11. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- 26.Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- 27.Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 29.Richards JK, Simms JA, Bartlett SE. Conditioned cues and yohimbine induce reinstatement of beer and near-beer seeking in Long-Evans rats. Addict Biol. 2009;14:144–51. doi: 10.1111/j.1369-1600.2008.00139.x. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka M, Yoshida M, Emoto H, Ishii H. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol. 2000;405:397–406. doi: 10.1016/s0014-2999(00)00569-0. [DOI] [PubMed] [Google Scholar]
- 31.Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, et al. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–55. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- 32.Suemaru S, Dallman MF, Darlington DN, Cascio CS, Shinsako J. Role of alpha-adrenergic mechanism in effects of morphine on the hypothalamo-pituitary-adrenocortical and cardiovascular systems in the rat. Neuroendocrinology. 1989;49:181–90. doi: 10.1159/000125112. [DOI] [PubMed] [Google Scholar]
- 33.Vythilingam M, Anderson GM, Owens MJ, Halaszynski TM, Bremner JD, Carpenter LL, et al. Cerebrospinal fluid corticotropin-releasing hormone in healthy humans: effects of yohimbine and naloxone. J Clin Endocrinol Metab. 2000;85:4138–45. doi: 10.1210/jcem.85.11.6968. [DOI] [PubMed] [Google Scholar]
- 34.Mattila M, Seppala T, Mattila MJ. Anxiogenic effect of yohimbine in healthy subjects: comparison with caffeine and antagonism by clonidine and diazepam. Int Clin Psychopharmacol. 1988;3:215–29. doi: 10.1097/00004850-198807000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Johnston AL, File SE. Yohimbine's anxiogenic action: evidence for noradrenergic and dopaminergic sites. Pharmacol Biochem Behav. 1989;32:151–6. doi: 10.1016/0091-3057(89)90225-6. [DOI] [PubMed] [Google Scholar]
- 36.Pellow S, Chopin P, File SE. Are the anxiogenic effects of yohimbine mediated by its action at benzodiazepine receptors? Neurosci Lett. 1985;55:5–9. doi: 10.1016/0304-3940(85)90303-9. [DOI] [PubMed] [Google Scholar]
- 37.McDougle CJ, Krystal JH, Price LH, Heninger GR, Charney DS. Noradrenergic response to acute ethanol administration in healthy subjects: comparison with intravenous yohimbine. Psychopharmacology (Berl) 1995;118:127–35. doi: 10.1007/BF02245830. [DOI] [PubMed] [Google Scholar]
- 38.Charney DS, Woods SW, Heninger GR. Noradrenergic function in generalized anxiety disorder: effects of yohimbine in healthy subjects and patients with generalized anxiety disorder. Psychiatry Res. 1989;27:173–82. doi: 10.1016/0165-1781(89)90132-7. [DOI] [PubMed] [Google Scholar]
- 39.Krystal JH, Webb E, Cooney N, Kranzler HR, Charney DS. Specificity of ethanollike effects elicited by serotonergic and noradrenergic mechanisms. Arch Gen Psychiatry. 1994;51:898–911. doi: 10.1001/archpsyc.1994.03950110058008. [DOI] [PubMed] [Google Scholar]
- 40.Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, et al. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology. 2011;36:1178–86. doi: 10.1038/npp.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–93. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- 42.Simms JA, Haass-Koffler CL, Bito-Onon J, Li R, Bartlett SE. Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol-seeking. Neuropsychopharmacology. 2012;37:906–18. doi: 10.1038/npp.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, et al. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacology (Berl) 2010;208:417–26. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- 44.Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–73. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- 45.Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–43. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 46.Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcohol Clin Exp Res. 1998;22:1783–7. [PubMed] [Google Scholar]
- 47.Czachowski CL, Delory MJ. Acamprosate and naltrexone treatment effects on ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology (Berl) 2009;204:335–48. doi: 10.1007/s00213-009-1465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henderson AN, Czachowski CL. Neuropeptide Y (NPY) in the central nucleus of the amygdala (CeA) does not affect ethanol-reinforced responding in binge-drinking, nondependent rats. Pharmacol Biochem Behav. 2012;101:8–13. doi: 10.1016/j.pbb.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verplaetse TL, Rasmussen DD, Froehlich JC, Czachowski CL. Effects of Prazosin, an alpha(1) -Adrenergic Receptor Antagonist, on the Seeking and Intake of Alcohol and Sucrose in Alcohol-Preferring (P) Rats. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lumeng L, Hawkins TD, Li TK. New strains of rats with alcohol preference and non-preference. In: Thurman RG, Williamson JR, Drott H, Chance B, editors. Alcohol and Aldehyde Metabolizing Systems. Academic Press; New York: 1977. pp. 537–44. [Google Scholar]
- 51.Li TK, Lumeng L, Doolittle DP. Selective breeding for alcohol preference and associated responses. Behav Genet. 1993;23:163–70. doi: 10.1007/BF01067421. [DOI] [PubMed] [Google Scholar]
- 52.Files FJ, Samson HH, Denning CE, Marvin S. Comparison of alcohol-preferring and nonpreferring selectively bred rat lines. II. Operant self-administration in a continuous-access situation. Alcohol Clin Exp Res. 1998;22:2147–58. [PubMed] [Google Scholar]
- 53.Czachowski CL, Samson HH. Ethanol- and sucrose-reinforced appetitive and consummatory responding in HAD1, HAD2, and P rats. Alcohol Clin Exp Res. 2002;26:1653–61. doi: 10.1097/01.ALC.0000036284.74513.A5. [DOI] [PubMed] [Google Scholar]
- 54.Heyser CJ, Schulteis G, Koob GF. Increased ethanol self-administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcohol Clin Exp Res. 1997;21:784–91. [PubMed] [Google Scholar]
- 55.Sinclair JD, Li TK. Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. Alcohol. 1989;6:505–9. doi: 10.1016/0741-8329(89)90059-1. [DOI] [PubMed] [Google Scholar]
- 56.McKinzie DL, Nowak KL, Yorger L, McBride WJ, Murphy JM, Lumeng L, et al. The alcohol deprivation effect in the alcohol-preferring P rat under free-drinking and operant access conditions. Alcohol Clin Exp Res. 1998;22:1170–6. [PubMed] [Google Scholar]
- 57.Oster SM, Toalston JE, Kuc KA, Pommer TJ, Murphy JM, Lumeng L, et al. Effects of multiple alcohol deprivations on operant ethanol self-administration by high-alcohol-drinking replicate rat lines. Alcohol. 2006;38:155–64. doi: 10.1016/j.alcohol.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 58.Rodd-Henricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li TK. The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcohol Clin Exp Res. 2000;24:747–53. [PubMed] [Google Scholar]
- 59.Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- 60.Chester JA, Blose AM, Zweifel M, Froehlich JC. Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcohol Clin Exp Res. 2004;28:385–93. doi: 10.1097/01.alc.0000117830.54371.7a. [DOI] [PubMed] [Google Scholar]
- 61.Roman E, Stewart RB, Bertholomey ML, Jensen ML, Colombo G, Hyytia P, et al. Behavioral profiling of multiple pairs of rats selectively bred for high and low alcohol intake using the MCSF test. Addict Biology. 2011 doi: 10.1111/j.1369-1600.2011.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chester JA, Blose AM, Froehlich JC. Acoustic startle reactivity during acute alcohol withdrawal in rats that differ in genetic predisposition toward alcohol drinking: effect of stimulus characteristics. Alcohol Clin Exp Res. 2004;28:677–87. doi: 10.1097/01.alc.0000125345.19665.09. [DOI] [PubMed] [Google Scholar]
- 63.Badia-Elder NE, Stewart RB, Powrozek TA, Murphy JM, Li TK. Effects of neuropeptide Y on sucrose and ethanol intake and on anxiety-like behavior in high alcohol drinking (HAD) and low alcohol drinking (LAD) rats. Alcohol Clin Exp Res. 2003;27:894–9. doi: 10.1097/01.ALC.0000071929.17974.DA. [DOI] [PubMed] [Google Scholar]
- 64.Vengeliene V, Siegmund S, Singer MV, Sinclair JD, Li TK, Spanagel R. A comparative study on alcohol-preferring rat lines: Effects of deprivation and stress phases on voluntary alcohol intake. Alcohol Clin Exp Res. 2003;27:1048–54. doi: 10.1097/01.ALC.0000075829.81211.0C. [DOI] [PubMed] [Google Scholar]
- 65.Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–88. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- 66.Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–42. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- 67.Bowes MP, Peters RH, Kernan WJ, Jr., Hopper DL. Effects of yohimbine and idazoxan on motor behaviors in male rats. Pharmacol Biochem Behav. 1992;41:707–13. doi: 10.1016/0091-3057(92)90216-3. [DOI] [PubMed] [Google Scholar]
- 68.Li S, Zou S, Coen K, Funk D, Shram MJ, Le AD. Sex differences in yohimbine-induced increases in the reinforcing efficacy of nicotine in adolescent rats. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00473.x. [DOI] [PubMed] [Google Scholar]
- 69.Antonitis JJ. Response variability in the white rat during conditioning, extinction, and reconditioning. J Exp Psychol. 1951;42:273–81. doi: 10.1037/h0060407. [DOI] [PubMed] [Google Scholar]
- 70.Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, et al. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl) 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, et al. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–17. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kupferschmidt DA, Tribe E, Erb S. Effects of repeated yohimbine on the extinction and reinstatement of cocaine seeking. Pharmacol Biochem Behav. 2009;91:473–80. doi: 10.1016/j.pbb.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 73.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 74.Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26:1872–9. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–54. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, et al. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–41. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]