Abstract
Prior studies have demonstrated associations between β-adrenergic receptor polymorphisms and left ventricular dysfunction—an important cause of allograft non-utilization for transplantation. We hypothesized that βAR polymorphisms predispose donor hearts to LV dysfunction after brain death. 1,043 organ donors managed from 2001-2006 were initially studied. The following βAR single nucleotide polymorphisms were genotyped: β1AR 1165C/G (Arg389Gly), β1AR 145A/G (Ser49Gly), β2AR 46G/A (Gly16Arg), and β2AR 79C/G (Gln27Glu). In multivariable regression analyses, the β2AR46 SNP was significantly associated with LV systolic dysfunction, with each minor allele additively decreasing the odds for LV ejection fraction<50%. The β1AR1165 and β2AR46 SNPs were associated with higher dopamine requirement during the donor management period: donors with the GG and AA genotypes had ORs of 2.64 (95% CI 1.52-4.57) and 2.70 (1.07-2.74) respectively for requiring >10 mcg/kg/min of dopamine compared to those with the CC and GG genotypes. However, no significant associations were found between βAR SNPs and cardiac dysfunction in 364 donors managed from 2007-2008, perhaps due to changes in donor management, lack of power in this validation cohort, or the absence of a true association. βAR polymorphisms may be associated with cardiac dysfunction after brain death, but these relationships require further study in independent donor cohorts.
Keywords: allograft function, brain death, cardiac allograft, cardiac transplant, cohort study, donor evaluation, donor management, donor outcomes, genetic polymorphism, genotyping
INTRODUCTION
Left ventricular (LV) systolic dysfunction has been well-described in the setting of brain death,(1) and is likely a multi-factorial process resulting from activation of the sympathetic nervous system, diffuse loss of vasomotor tone, endothelial dysfunction, and hormone depletion.(2, 3) Classic baboon studies have demonstrated that the initial Cushing reaction that accompanies brainstem herniation results in direct myocardial injury. Within minutes after brain death, an “autonomic storm” occurs(4) in which serum epinephrine levels increase by 1,100%, norepinephrine by 300%, and dopamine by 200%.(5, 6) Endomyocardial biopsy specimens have shown contraction band necrosis—histologic evidence of microinfarction secondary to catecholamine-mediated calcium overload. This initial period of heightened sympathetic activity may then be followed by a period of parasympathetic dominance characterized by reduced catecholamine sensitivity.(7)
It is unknown why only a subgroup of people develops LV dysfunction after brain death. This phenomenon, however, appears to affect acceptance of allografts for cardiac transplantation, leading to refusal of donor organs in approximately 25% of cases,(8) even for organs from young, otherwise healthy donors.(4) The beta-adrenergic receptors (βAR) are major mediators of catecholamine effects on the heart, and βAR polymorphism genotypes are associated with adverse cardiovascular outcomes in the general population.(9-14) We hypothesized that specific βAR polymorphism genotypes increase cardiac sensitivity to circulating catecholamines in the setting of brain death, and are therefore associated with a higher prevalence of left ventricular dysfunction in potential organ donors for cardiac transplantation.
MATERIALS and METHODS
Subjects
This is a retrospective genetics study utilizing a cohort of 2,048 consecutive organ donors managed by the California Transplant Donor Network (CTDN, Oakland, CA) from 2001-2008. The inclusion criteria included availability of stored DNA samples from brain dead organ donors and consent for at least one organ to be donated (n=1624). Our discovery cohort was comprised of 1,043 donors managed from 2001-2006; we then studied an additional 364 donors managed from 2007-2008 as a validation cohort, recognizing that changes in donor management protocols had been implemented after 2006. CTDN is the largest organ procurement agency in Northern California and supplies donor organs mainly to transplant centers in northern and central California and occasionally to neighboring states. Potential brain dead organ donors were identified by treating physicians at hospitals throughout the region, and consent for organ donation was obtained from family members or next-of-kin. Management of the organ donor was subsequently assumed by CTDN staff, and consent was obtained from the donor's family to collect patient data and biological samples. This study was approved by CTDN, the Committee on Human Research at the University of California San Francisco, and by the Research Compliance Office at Stanford University.
Measurement of Phenotypic and Echocardiographic Parameters
Upon assumption of donor management, comprehensive data on donor-level variables were recorded by CTDN staff in a standardized fashion, including demographic variables, cause of death, health-related behaviors, and past medical history. Data on comprehensive laboratory testing were also recorded, including serologies; hematologic, hepatic, and renal function indices; and cardiac troponin assays. Standard testing for potential donors who were not immediately ruled-out for cardiac graft donation (due to known coronary artery disease treated with percutaneous stents or bypass surgery, prior cardiac valve surgery, age>65 years, lack of consent for donation, or coroner exclusion) included an electrocardiogram; one or more echocardiograms; and a coronary angiogram for male donors over 40 years and female donors over 45 years. All cardiac testing was performed and interpreted at the donor hospital and results were recorded by CTDN personnel. Data on vital signs, invasive hemodynamics, and medications were also recorded.
Donor data were extracted from the medical records and were entered into the study database by three CTDN study investigators (VH, RLM, and JN) who were blinded to outcomes. A subsequent quality-assessment review of 5% of the medical records was performed (KK, JZ, RLM), reviewing 177 fields per donor chart, and demonstrated 97% accuracy of data collection.
Polymorphism Selection and Genotyping
Four β-adrenergic receptor polymorphisms previously reported to be functionally significant were chosen for analysis (Table 1). The uncommon β2AR 491C/T (Thr164Ile) SNP was also genotyped, but was not chosen for analysis due to the very low frequency of the T allele (1.7%) at this locus. Genomic DNA was isolated from the blood or spleen of deceased donors using the salting out method or commercial product (QIAmp DNA kits, Valencia, CA) at the Immunogenetics and Transplantation Laboratory at the University of California, San Francisco. Polymorphisms were genotyped by template-directed dye-terminator incorporation assay with fluorescence polarization detection (FP-TDI), using the AcycloPrime-FP kit (Perkin-Elmer),(15) as previously described.(14) β1AR 1165C/G (Arg389Gly) was genotyped using the TaqMan SNP Genotyping Assay (Applied Biosystems, Assay ID: C_8898494_10) per the manufacturer's protocol. Positive (duplicates) and negative controls were included in all plates. The genotyping call rates were as follows: β1AR 1165=98.7%, β1AR 145=93.7%, β2AR 46=95.3%, β2AR 79=96.9%. Genotyping was performed by investigators blinded to clinical variables.
Table 1.
Rationale for Polymorphism Selection
| Gene | Variant | dbSNP ID | Functional Rationale | Clinical Rationale | References |
|---|---|---|---|---|---|
| β1AR (ADRB1) | 1165 C>G (Arg 389Gly) | rs 1801252 | C allele:↑ response to β-agonist stimulation | CC genotype associated with ↑ risk of AMI | 11, 13, 21 |
| CC genotype associated with ↑ risk of HF | |||||
| 145 A>G (Ser49Gly) | rs1801253 | G allele: ↓ response to β-agonist stimulation | GG or AG genotype associated with improved survival in HF | 22, 28 | |
| β2AR (ARDB2) | 46 G>A (Gly16Arg) | rs1042713 | G allele: alters agonist-promoted downregulation | GG genotype associated with ↑ left ventricular ejection fraction | 9, 10, 12 |
| 79 C>G Gln27Glu | rs1042714 | G allele: alters agonist-promoted downregulation | CC genotype associated with ↑ risk of CAD events | 9, 10, 12, 18 |
AMI: acute myocardial infarction; HF: heart failure; CAD: coronary artery disease
Statistical Analysis
Statistical analyses were performed with R (version 2.13). The primary outcome variable in this study was donor left ventricular ejection fraction (LVEF), which was treated as a dichotomous variable (LVEF < or ≥ 50%). Secondary outcome variables included any left ventricular regional wall motion abnormalities (RWMA) and maximum dopamine requirement during the donor management period (≤ or > 10 mcg/kg/min). The threshold values for dichotomization of LVEF and dopamine requirement were determined a priori, based on the collective clinical experience of the study investigators with respect to donor management and organ allocation.(8, 16, 17) Each polymorphism was tested for Hardy-Weinberg equilibrium (HWE) by χ2 test within each of the four main racial/ethnic groups (Caucasian, African-American, Hispanic, and Asian). A racial group was dropped from analysis for that SNP if the HWE p-value was less than 0.001.
Multivariable logistic regression models were fit to estimate the association between the polymorphisms in each gene and the outcome of interest. Genotypes were coded as additive effects (0-1-2) with each level indicating the presence of an additional minor allele. Models were adjusted for donor age, sex, race (treated as a categorical variable, with Caucasians as the reference group), and cause of death (dichotomized into death due to intracranial hemorrhage/stroke versus all other causes). Interactions between SNPs in the same gene were also explored. Individuals with missing covariates or genotypes were dropped from the analysis. Sensitivity analyses were then performed, restricting the cohort to the largest racial-ethnic subgroup (Caucasians), to investigate potential population stratification effects.
For those genes and outcomes that passed the nominal significance threshold in the additive model (p < .05), we also present results from a co-dominant model treating genotypes as a categorical variable to allow for non-linear effects and to further explore the patterns of association (Supplementary Table 1). This strategy is analogous to performing an ANOVA and then follow-up t-tests only if the overall association is significant. No correction for multiple testing was performed.
The above-described models were initially fit for donors managed by CTDN from 2001 to 2006. We then refit the models for donors managed from 2007 to 2008 as a validation cohort. Finally, we calculated our power to replicate associations in the validation cohort empirically, assuming an alpha level of 0.05 and 10,000 simulations using the effect estimates, along with the upper and lower boundaries of their 95% confidence intervals.
RESULTS
Subjects
A total of 1407 of the 2048 potential organ donors managed by CTDN from 2001 and 2008 had stored DNA and complete genotyping available for analysis and defined the two study cohorts: a discovery cohort of 1043 donors from 2001-2006 and a validation cohort of 364 donors managed in 2007 and 2008. Eight hundred ninety four donors (75%) in the discovery cohort and 288 (67%) in the validation cohort had an echocardiogram. Donor characteristics, by cohort, are summarized in Table 2. Median values and the associated inter-quartile ranges (IQR) are shown for continuous variables and percentages for dichotomous variables. P-values were calculated by Wilcoxon rank-sum and chi-square tests respectively. Differences in donor management protocols between the two study cohorts included use of higher doses of phenylephrine and corticosteroids, and greater use of thyroid hormone in the latter cohort. The clinical characteristics of the genetics study cohorts did not differ significantly from the entire CTDN donor cohort (data not shown).
Table 2.
Donor characteristics, by study cohort
| Discovery cohort 2001-2006 | Validation cohort 2007-2008 | p-value* | |
|---|---|---|---|
| Donors, n | 1043 | 364 | |
| Age (years) | 42 (24, 52) | 41 (25.5, 51) | 0.85 |
| Female | 41% | 37% | 0.13 |
| Body mass index (kg/m2) | 25.5 (22.2, 29.4) | 26.4 (23.0 , 30.7) | 0.0025 |
| Race/Ethnicity | 0.9300 | ||
| Caucasian | 55% | 53% | |
| African-American | 10% | 11% | |
| Hispanic | 25% | 26% | |
| Asian | 8% | 8% | |
| Other | 2% | 2% | |
| Cause of death | <0.0001 | ||
| Anoxia | 11% | 19% | |
| Cerebrovascular Accident/Stroke | 48% | 42% | |
| Head Trauma | 40% | 38% | |
| CNS | 0.3% | 0.5% | |
| Other | 0.6% | 0.5% | |
| Medical history | |||
| Hypertension | 28% | 30% | 0.16 |
| Diabetes | 7% | 11% | 0.01 |
| Smoking | 51% | 52% | 0.35 |
| History of coronary artery disease | 4% | 6% | 0.32 |
| Drug use (cocaine, methamphetamines, inhaled drugs) | 41% | 38% | 0.41 |
| Hemodynamics | |||
| Peak heart rate (bpm) | 124 (110, 137) | 122 (110, 136) | 0.12 |
| Final heart rate (bpm) | 100 (90, 111) | 100 (88, 110) | 0.11 |
| Peak systolic blood pressure (mmHg) | 157 (144, 178) | 161 (149, 180) | 0.01 |
| Final systolic blood pressure (mmHg) | 124 (110, 139) | 125 (114, 139) | 0.10 |
| Peak diastolic blood pressure (mmHg) | 90 (78, 100) | 89 (76, 100) | 0.97 |
| Final diastolic blood pressure (mmHg) | 68 (60, 77) | 69 (60, 76) | 0.52 |
| Peak central venous pressure (mmHg) | 11 (9, 13) | 12 (10, 15) | < 0.001 |
| Final central venous pressure (mmHg) | 7 (6, 9) | 9 (7, 10) | < 0.001 |
| Laboratory values | |||
| Peak sodium (mmol/L) | 153 (148, 160) | 155 (149, 161) | 0.0007 |
| Peak creatinine (mg/dL) | 1.3 (1.0, 1.6) | 1.4 (1.1, 2.0) | < 0.001 |
| Peak ALT (U/L) | 44 (28, 84) | 59 (36, 133.5) | < 0.001 |
| Peak AST (U/L) | 66 (40, 128) | 82 (46, 204.5) | < 0.001 |
| Lowest hemoglobin (g/dL) | 9.8 (8.4, 11.6) | 9.1 (8.1, 10.7) | < 0.001 |
| Peak troponin (ng/mL) | 0.38 (0.10, 1.84) | 0.29 (0.05, 1.69) | 0.04 |
| Peak CPK-MB (ng/mL) | 1.6 (0.8, 3.1) | 0.07 (0.04, 1.3) | 0.12 |
| Echocardiogram | |||
| Echocardiogram performed, % | 76%% | 71.0% | 0.06 |
| Median LVEF, % | 65 (57, 70) | 64 (55, 70) | 0.07 |
| EF<50%, % | 11% | 15%% | 0.05 |
| Regional wall motion abnormalities, % | 20% | 20% | 0.96 |
| Left ventricular hypertrophy†, % | 11% | 9%% | 0.35 |
| Vasoactive Medications | |||
| Dopamine (%) | 83% | 65% | <0.001 |
| Peak dopamine dose, mcg/kg/min | 5.2 (3.0, 10.0) | 5.0 (3.9, 8.8) | 0.87 |
| Peak dopamine>10 mcg/kg/min, % | 18% | 14% | 0.18 |
| Phenylephrine (%) | 74% | 78% | 0.13 |
| Peak phenylephrine dose, mcg/min | 67 (8, 140) | 109 (50, 200) | <0.0001 |
| Epinephrine (%) | 6% | 7% | 0.57 |
| Esmolol (%) | 17% | 17% | 0.99 |
| Hormone replacement | |||
| Steroids | 98% | 95% | 0.0012 |
| Solumedrol dose over 24 hrs (grams) | 2.0 (1.0, 2.0) | 3.0 (2.0, 3.0) | <0.0001 |
| Thyroid hormone (T4), % | 14% | 54% | <0.0001 |
| Cardiac allograft acceptance for transplantation | 47% | 39% | 0.0062 |
Wilcoxon Test for continuous variables (median with interquartile range), chi2 test for categorical variables
defined as septal or posterior wall thickness > 1.1 cm; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK-MB, creatine phosphokinase, myocardial fraction; LVEF, left ventricular ejection fraction; EF, ejection fraction
Genotype frequencies of the four adrenergic receptor polymorphisms, by cohort and racial group, are shown in Table 3. Genotype distributions differed across the racial groups but were consistent with Hardy-Weinberg equilibrium within racial groups, except for the β2AR79 SNP in African-Americans in the validation cohort (2007-2008 donors, p<0.0003); this group was dropped from further analysis. Within each racial group, genotype frequencies were similar across the two study cohorts.
Table 3.
Beta Adrenergic Receptor Polymorphism Genotype Frequency, by Study Cohort and Race
| βAR Receptor Polymorphism | Discovery cohort 2001-2006 | Validation cohort 2007-2008 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Caucasian | Hispanic | African-American | Asian | Overall | Caucasian | Hispanic | African-American | Asian | |
| B1AR 1165 (Arg389Gly) | ||||||||||
| CC | 59% | 56% | 70% | 44% | 61% | 63% | 63% | 73% | 39% | 61% |
| CG | 34% | 37% | 25% | 42% | 32% | 32% | 33% | 24% | 41% | 36% |
| GG | 7% | 7% | 5% | 14% | 7% | 6% | 5% | 3% | 20% | 3% |
| B1AR 145 (Ser49Gly) | ||||||||||
| AA | 71% | 78% | 56% | 63% | 74% | 67% | 77% | 46% | 64% | 71% |
| AG | 25% | 20% | 35% | 29% | 21% | 28% | 22% | 45% | 26% | 21% |
| GG | 4% | 1% | 9% | 8% | 5% | 5% | 1% | 10% | 10% | 9% |
| B2AR 46 (Gly16Arg) | ||||||||||
| GG | 36% | 39% | 36% | 25% | 27% | 40% | 40% | 37% | 43% | 27% |
| AG | 46% | 45% | 47% | 54% | 45% | 38% | 39% | 40% | 36% | 39% |
| AA | 18% | 15% | 18% | 21% | 28% | 22% | 21% | 24% | 21% | 33% |
| B2AR 79 (Gln27Glu) | ||||||||||
| CC | 50% | 37% | 63% | 66% | 69% | 50% | 35% | 65% | 64% | 73% |
| CG | 38% | 43% | 32% | 29% | 29% | 37% | 48% | 27% | 18% | 20% |
| GG | 13% | 20% | 5% | 5% | 2% | 14% | 17% | 8% | 18% | 7% |
Association of Adrenergic Receptor Genotypes with Outcomes
Table 4 summarizes associations between specific βAR genotypes and cardiac outcomes, by study cohort. No significant interactions were identified between SNPs in the same gene or across genes.
Table 4.
Associations Between Beta Adrenergic Receptor Polymorphisms and Cardiac Allograft Dysfunction, by Study Cohort
| Discovery cohort 2001-2006 | Validation cohort 2007-2008 | |||
|---|---|---|---|---|
| LV Ejection Fraction < 50% | OR (95% CI) | p-value | OR (95% CI) | p-value |
| N=778 | N=249 | |||
| B1AR 1165G | 1.14 (0.77 – 1.71) | 0.51 | 1.04 (0.55 – 1.95) | 0.91 |
| β1AR 145G | 1.04 (0.65 – 1.66) | 0.86 | 2.00 (0.86 – 4.70) | 0.11 |
| β2AR 46A | 0.60 (0.40, 0.89) | 0.012 | 1.68 (0.89 – 3.17) | 0.11 |
| β2AR 79G | 1.04 (0.67 – 1.63) | 0.86 | 1.17 (0.63 – 2.19) | 0.62 |
| LV Regional Wall Motion Abnormalities | OR (95% CI) | p-value | OR (95% CI) | p-value |
| N=776 | N=261 | |||
| B1AR 1165G | 0.72 (0.53 – 0.99) | 0.046 | 0.86 (0.48 – 1.51) | 0.59 |
| β1AR 145G | 0.92 (0.65 – 1.30) | 0.64 | 0.75 (0.39 – 1.44) | 0.38 |
| β2AR 46A | 1.06 (0.78 – 1.43) | 0.71 | 1.03 (0.60 – 1.77) | 0.90 |
| β2AR 79G | 0.73 (0.53 – 1.02) | 0.06 | 1.22 (0.71 – 2.10) | 0.48 |
| Peak Dopamine > 10 mcg/kg/min | OR (95% CI) | p-value | OR (95% CI) | p-value |
| N=977 | N=234 | |||
| B1AR 1165G | 1.37 (1.05 – 1.78) | 0.020 | 0.45 (0.19 – 1.07) | 0.07 |
| β1AR 145G | 0.80 (0.57 – 1.14) | 0.23 | 0.87 (0.42 – 1.82) | 0.72 |
| β2AR 46A | 1.39 (1.04 – 1.85) | 0.026 | 1.03 (0.56 – 1.89) | 0.92 |
| β2AR 79G | 1.15 (0.85 – 1.57) | 0.36 | 1.21 (0.63 – 2.33) | 0.57 |
Multivariable regression models assuming additivity in minor allele adjusted for donor age, sex, race, cause of death, and the other polymorphism (SNP) within the same gene.
The point estimate is the odds ratio for each additional minor allele.
LV: left ventricular
Discovery cohort: 2001-2006 donors
Left Ventricular Ejection Fraction
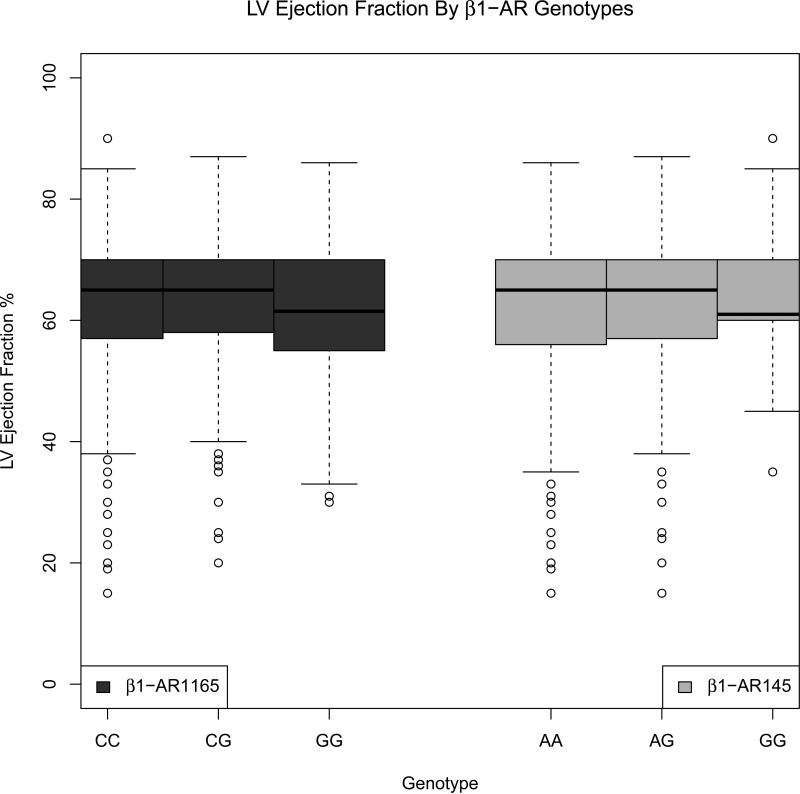
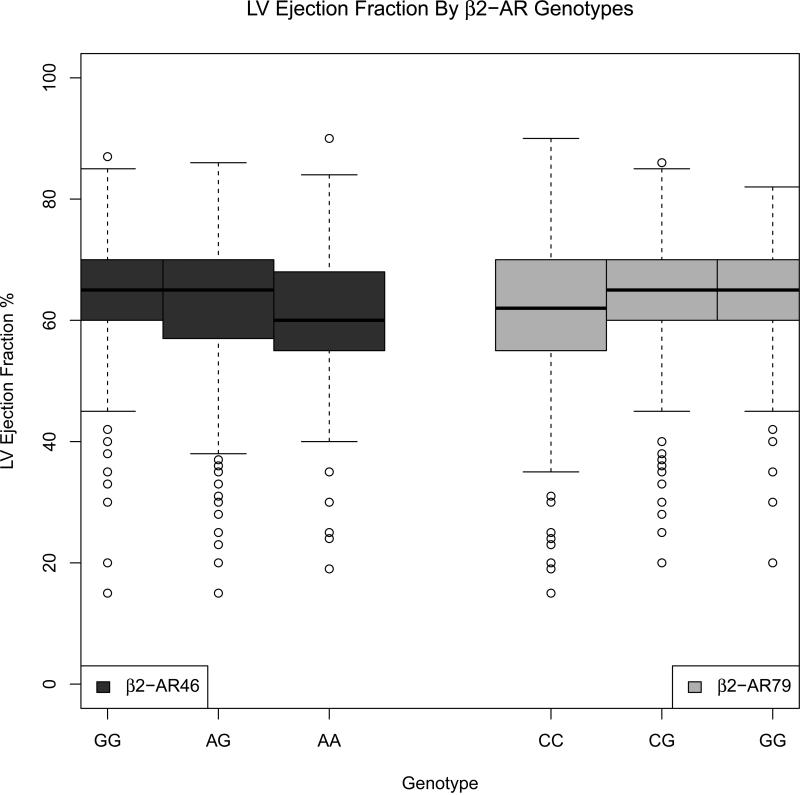
Seven hundred seventy eight donors had an echocardiogram that included measurement of LVEF and complete covariate information. The median LVEF was 65%, with 10% of donors having an LVEF less than 50%. Figures 1 and 2 show boxplots for the β1AR and β2AR genotypes. Donors who were homozygous for the minor G allele for both β1AR SNPs had lower LVEF compared to heterozygotes and major allele homozygous groups. Similarly, for the β2AR46 SNP, the AA genotype group had a lower median LVEF than heterozygotes or major allele homozygous groups. Conversely, for the β2AR79 SNP, the CC genotype group had a lower median LVEF than heterozygotes or minor allele homozygous groups.
Figure 1.
LV ejection fraction, by β1-adrenergic receptor genotypes, in CTDN donors (2001-2006 cohort)
Figure 2.
LV ejection fraction, by β2-adrenergic receptor genotypes, in CTDN donors (2001-2006 cohort)
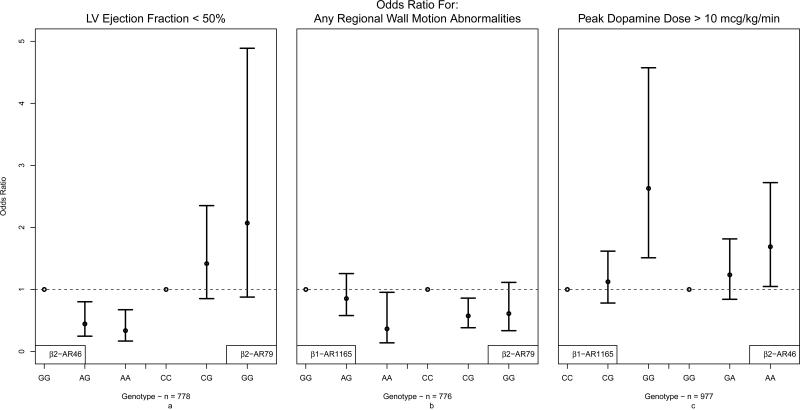
In multivariable analysis, the β2AR46 SNP was significantly associated with donor left ventricular systolic dysfunction, defined as having LVEF < 50%, after adjusting for the effects of age, sex, race and cause of death. Assuming an additive model, each additional minor allele A of β2AR46 (Gly16Arg) decreased the odds of LVEF<50% by 40% (p=0.012, Table 4). Figure 3a shows the results of the co-dominant model for each of the β2AR SNPs (without the presence of the other). Compared to the β2AR46 GG genotype group, the odds ratio (OR) for LVEF<50% was 0.45 (95% CI 0.25-0.80) for the AG genotype and 0.34 (95% CI 0.17-0.67) for the AA genotype group, consistent with an additive genetic model. The β2AR79 (Gln27Glu) SNP also suggests an additive relationship with LV dysfunction, with ORs for the CG and GG alleles being 1.42 (95% CI 0.85-2.35) and 2.07 (95% CI 0.88-4.89), though this association is not significant in the presence of the β2AR46 SNP (Table 4). The β1AR SNPs did not show a significant association with LVEF. Restricting the cohort to Caucasians yielded similar results (Supplementary Table 2).
Figure 3.
Odds ratio for LV ejection fraction<50%, regional wall motion abnormalities, and peak dopamine requirement>10 mcg/kg/min, by donor β-adrenergic receptor in CTDN donors (2001-2006 cohort)
Left Ventricular Regional Wall Motion Abnormalities
Seven hundred seventy six donors had echocardiograms with RWMA data as well as complete covariate information. Twenty percent of donors exhibited left ventricular RWMA. The β1AR1165 SNP (p-value < 0.05) showed a marginal association with each minor allele associated with a 28% decrease in the odds of having RWMA (p=0.046, Table 4). Figure 3b shows that the association of β1AR1165 genotypes and RWMA follows an additive model whereas a dominant model might be a better fit for the β2AR79 SNP, as one additional minor allele has the same protective effect as having both alleles.
Peak Dopamine Requirement
Dopamine dosing and complete covariate information was available for 977 donors. The median maximum dopamine dose required during the donor management period was 5.2 (IQR: 3.0, 10.0) mcg/kg/min. A donor dopamine requirement of >10 mcg/kg/min is often considered a contraindication for cardiac allograft acceptance for transplantation, and 18% of donors managed from 2001-2006 exhibited this high dopamine requirement. SNPs from both β1AR and β2AR genes showed significant association with peak dopamine dose (Table 4). Compared to the β1AR 1165CC genotype, the GG genotype group shows a significant association with peak dopamine dose>10 mcg/kg/min [OR = 2.63 (95% CI 1.51-4.57)], while the CG genotype was not associated [OR = 1.12 (95% CI 0.78-1.62)], suggestive a recessive model. The β2AR46 SNP also shows a recessive effect, with the AA (but not GA) genotype significantly associated with peak dopamine requirement [OR = 2.63 (95% CI 1.51-4.57)] compared to the GG genotype group.
Validation cohort: 2007-2008 donors
After 2006, significant staffing changes occurred at CTDN, including the hiring of many new coordinators responsible for donor management. Several major changes in donor management strategies ensued. Donors in this latter cohort had a higher incidence of left ventricular dysfunction, higher peak central venous pressure, worse renal and hepatic function, and lower rates of cardiac allograft acceptance for transplantation (Table 2).
No significant associations were found between β-adrenergic receptor polymorphisms and cardiac allograft function in the 2007-2008 cohort (Table 4). A full model including donors from all years showed a significant interaction between the cohort and the SNPs significantly associated with outcome in the 2001-2006 cohort. In order to investigate whether specific changes in donor management strategies may have accounted for the lack of replication found in the latter cohort, we constructed additional multivariable regression models adjusting for inotrope/vasopressor doses, doses of steroid and thyroid hormone, and hemodynamic differences, among other relevant covariates. We also tested for interactions between the individual βAR polymorphisms and medications administered. Neither strategy accounted for the lack of replication in this validation cohort. Post-hoc power estimates for the replication of the five significant associations observed in the discovery cohort ranged from 20%-36%, below the standard 80% threshold for type II error (Supplementary Table 3).
DISCUSSION
In our discovery cohort of 1,043 organ donors managed between 2001 and 2006 we identified several significant associations between β-adrenergic receptor polymorphisms and the presence of left ventricular dysfunction after brain death. Specifically, genotypes known to be associated with increased sensitivity to circulating catecholamines were associated with a higher risk of LV dysfunction during the donor management period.
The β-adrenergic receptors are located at the cell membrane of cardiomyocytes and mediate the effects of circulating catecholamines.(18) The β1-adrenergic receptor is the predominant β-adrenergic receptor expressed on the cardiomyocyte and is responsive to circulating epinephrine and to local norepinephrine derived from cardiac sympathetic nerves.(13) In rodents, transgenic cardiac overexpression of β1-adrenergic receptors causes progressive cardiomyopathy and heart failure.(19, 20) We therefore considered the β1AR 1165C/G (Arg389Gly) and 145A/G (Ser49Gly) polymorphisms as potential risk factors for donor LV dysfunction, as they affect the sensitivity and response of β1-adrenergic receptors to circulating catecholamines,(21, 22) which are present at very high levels early after brain death.(5, 23) Similarly, the β2-adrenergic receptors are also present in human myocardium, as well as in vascular smooth muscle beds. Stimulation of β2-adrenergic receptors mediates cardiac inotropic and chronotropic effects,(24) induces cardiomyocyte apoptosis,(25) and causes vascular smooth muscle relaxation and vasodilation in response to sympathetic tone.(26) We therefore evaluated the associations between the β2-adrenergic receptor polymorphisms 46A/G (Gly16Arg) and 79C/G (Gln27Glu) and cardiac injury after brain death. These polymorphisms in the β1- and β2-adrenergic receptors are well-studied and have previously been associated with altered response to sympathetic stimulation,(27) resting heart rate,(22) risk of coronary events,(10) arrhythmias,(12) vascular reactivity,(9) and survival in patients with heart failure.(28)
The initial “catecholamine storm”(23) that occurs after brain death is often followed by a period of functional denervation, during which there is a dominance of vagal parasympathetic or inhibitory effects.(7) This theory is supported by the observed excessive activity of the inhibitory G protein Giα in brain-dead organ donors with LV dysfunction.(29) These dramatic physiologic changes after brain death may mediate the relationship between the “catecholamine insensitive” β1AR 1165GG genotype and high dopamine requirements during the subsequent donor management period.
Our initial provocative findings were then studied in a second cohort of organ donors managed between 2007 and 2008. The original study findings did not replicate in the latter cohort, and there are several potential explanations for this discrepancy. First, as discussed previously, changes in staffing and donor management strategies may have masked the influence of βAR genetic variation on allograft function. For example, significantly fewer donors in the 2007-2008 cohort were treated with dopamine (a β-receptor agonist), and higher doses of phenylephrine (an α-receptor agonist) were used. This change in inotrope/vasopressor support during donor management may have overshadowed the role of βAR signaling on cardiac function. In addition, there was a dramatic increase in the use of thyroid hormone supplementation between the 2001-2006 cohort (14%) and the 2007-2008 cohort (54%). As thyroid hormone can increase cardiac contractility, this strategic change in donor management could have also masked the relationship seen between βAR genotype and cardiac function in our discovery cohort. Second, there were notable differences in laboratory values, hemodynamics, and allograft function, suggesting that the 2007-2008 cohort was comprised of a “sicker” donor population. This observation may account for the decrease in allograft acceptance for transplantation in the latter cohort. Third, it is possible that unrecognized or unmeasured differences between study cohorts may have accounted for lack of replication. Finally, the smaller sample size in the second cohort (364 versus 1,043 donors) impacted the power to replicate our initial findings (20-36%). Thus, it is possible that our initial findings may have been false positive results, or may represent true associations, but our study was underpowered to replicate.
Understanding the pathophysiology of LV dysfunction after brain death plays a crucial role in the graft selection process for heart transplantation. Currently, approximately 60% of available cardiac grafts are discarded due to stringent acceptance criteria,(17) leading to a great discrepancy between the number of critically-ill patients on the waiting list compared to the number of available grafts for transplantation.(30) While non-utilization of donor hearts is a multi-factorial problem, encompassing diverse donor characteristics and logistical issues, LV dysfunction is the most frequently cited reason for non-utilization.(8, 31) Left ventricular dysfunction in a cardiac donor raises the specter of irreversible cardiac injury which may lead to clinically significant graft dysfunction and graft failure in the transplant recipient. However, animal and human studies now support the hypothesis that catecholamine toxicity plays a central role in reducing myocardial contractility after brain death(29, 32) and that cardiac dysfunction is often reversible in organ donors.(16) Supporting this hypothesis are our discovery cohort findings of significant associations between βAR polymorphisms that mediate myocardial catecholamine sensitivity and LV dysfunction after brain death. Similarly, many transplant centers consider an allograft to be unsuitable if inotrope requirements are high during the donor management period. Our results suggest that high inotrope requirements may be associated with βAR genotypes that confer insensitivity to circulating catecholamines.
This study has significant strengths and limitations that deserve discussion. First and foremost, this represents the largest existing research database of detailed, rigorously adjudicated clinical and genetic data on over 1,400 potential organ donors managed in the current era. Second, this study represents a unique approach to the study of genetic influences in organ transplantation. We chose to study candidate gene polymorphisms in organ donors, and their influences on allograft function. Most genetic studies to-date in organ transplantation have examined associations between recipient genetic variation and post-transplant outcomes. Finally, we studied functional βAR polymorphisms that were previously shown to be associated with adverse cardiac outcomes in the general population as a means to study the pathogenesis of cardiac dysfunction after brain death, utilizing a very unique organ donor population. Limitations of this study include non-replication of initial findings in the validation cohort, which we were unable to account for by adjusting for recognized (and measurable) differences in donor management strategies during the study period. This is further exacerbated by the fact that donors were managed at a variety of local hospitals that may have had different medical management strategies prior to assumption of donor management by CTDN staff. Furthermore, characteristics and outcomes of donors managed by CTDN may not be equivalent to donor outcomes in other regions of the country, due to nationwide variations in donor management strategies. We also recognize the possible influence of uncontrolled confounding or population admixture on our genetic analyses. Although we did see consistent results when repeating our analyses in the sub-population of Caucasian donors, subtle population substructure may still be present within this racial group. Finally, complete phenotypic data could not be obtained for every donor, due to the retrospective nature of the data collection.
In conclusion, β-adrenergic receptor polymorphisms may contribute to the development of cardiac dysfunction after brain death. While we initially identified several compelling associations between the βAR SNPs of interest and cardiac function, our findings did not replicate in the validation cohort and there are several potential explanations for these discrepant results, as described above. Additional studies are therefore needed to examine the influence of donor genetic variants on post-transplant outcomes, and to assess for interactions between donor and recipient genetic modifiers in organ transplantation.
Supplementary Material
Acknowledgments
We thank the California Transplant Donor Network for access to the donor data required for this study.
Funding Sources: This study was supported by grants from the American Heart Association (0865249F) and the National Heart, Lung, and Blood Institute (K23HL091143).
Abbreviations
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- CTDN
California Transplant Donor Network
- RWMA
Regional Wall Motion Abnormalities
- βAR
Beta Adrenergic Receptor
- SNP
Single Nucleotide Polymorphism
- HWE
Hardy Weinberg Equilibrium
Footnotes
Disclosures: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Contributor Information
K.K. Khush, Division of Cardiovascular Medicine, Department of Medicine, Stanford University School of Medicine, Stanford, California.
L. Pawlikowska, Department of Anesthesia and Perioperative Care and Institute for Human Genetics, University of California, San Francisco.
R.L. Menza, Graduate School of Nursing, Midwifery and Health, Victoria University, Wellington, New Zealand.
B.A. Goldstein, Quantitative Sciences Unit, Department of Medicine, Stanford University School of Medicine, Stanford, California.
V. Hayden, California Transplant Donor Network, Oakland, California.
J. Nguyen, California Transplant Donor Network, Oakland, California.
H. Kim, Departments of Anesthesia and Perioperative Care, and Epidemiology and Biostatistics, Institute for Human Genetics, University of California, San Francisco.
A. Poon, Cardiovascular Research Institute, University of California, San Francisco.
A. Sapru, Department of Pediatrics, University of California, San Francisco.
M.A. Matthay, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of California, San Francisco.
P.Y. Kwok, Cardiovascular Research Institute and Institute for Human Genetics, University of California, San Francisco.
W.L. Young, Department of Anesthesia and Perioperative Care, University of California, San Francisco.
L.A. Baxter-Lowe, Immunogenetics and Transplantation Laboratory, University of California, San Francisco.
J.G. Zaroff, Kaiser Northern California Division of Research, Oakland, California.
REFERENCES
- 1.Goel R, Johnson F, Mehra MR. Brain injury and ventricular dysfunction: insights into reversible heart failure. Congest Heart Fail. 2005;11(2):99–101. doi: 10.1111/j.1527-5299.2005.04178.x. [DOI] [PubMed] [Google Scholar]
- 2.Allman FD, Herold W, Bosso FJ, Pilati CF. Time-dependent changes in norepinephrine-induced left ventricular dysfunction and histopathologic condition. J Heart Lung Transplant. 1998;17(10):991–997. [PubMed] [Google Scholar]
- 3.Lang SA, Maron MB, Bosso FJ, Pilati CF. Temporal changes in left ventricular function after massive sympathetic nervous system activation. Can J Physiol Pharmacol. 1994;72(6):693–700. doi: 10.1139/y94-098. [DOI] [PubMed] [Google Scholar]
- 4.Audibert G, Charpentier C, Seguin-Devaux C, Charretier PA, Gregoire H, Devaux Y, et al. Improvement of donor myocardial function after treatment of autonomic storm during brain death. Transplantation. 2006;82(8):1031–1036. doi: 10.1097/01.tp.0000235825.97538.d5. [DOI] [PubMed] [Google Scholar]
- 5.Novitzky D, Horak A, Cooper DK, Rose AG. Electrocardiographic and histopathologic changes developing during experimental brain death in the baboon. Transplant Proc. 1989;21(1 Pt 3):2567–2569. [PubMed] [Google Scholar]
- 6.Novitzky D, Wicomb WN, Cooper DK, Rose AG, Reichart B. Prevention of myocardial injury during brain death by total cardiac sympathectomy in the Chacma baboon. Ann Thorac Surg. 1986;41(5):520–524. doi: 10.1016/s0003-4975(10)63032-9. [DOI] [PubMed] [Google Scholar]
- 7.Samuels MA. The brain-heart connection. Circulation. 2007;116(1):77–84. doi: 10.1161/CIRCULATIONAHA.106.678995. [DOI] [PubMed] [Google Scholar]
- 8.Zaroff JG, Babcock WD, Shiboski SC. The impact of left ventricular dysfunction on cardiac donor transplant rates. J Heart Lung Transplant. 2003;22(3):334–337. doi: 10.1016/s1053-2498(02)00554-5. [DOI] [PubMed] [Google Scholar]
- 9.Cockcroft JR, Gazis AG, Cross DJ, Wheatley A, Dewar J, Hall IP, et al. Beta(2)-adrenoceptor polymorphism determines vascular reactivity in humans. Hypertension. 2000;36(3):371–375. doi: 10.1161/01.hyp.36.3.371. [DOI] [PubMed] [Google Scholar]
- 10.Heckbert SR, Hindorff LA, Edwards KL, Psaty BM, Lumley T, Siscovick DS, et al. Beta2-adrenergic receptor polymorphisms and risk of incident cardiovascular events in the elderly. Circulation. 2003;107(15):2021–2024. doi: 10.1161/01.CIR.0000065231.07729.92. [DOI] [PubMed] [Google Scholar]
- 11.Iwai C, Akita H, Kanazawa K, Shiga N, Terashima M, Matsuda Y, et al. Arg389Gly polymorphism of the human beta1-adrenergic receptor in patients with nonfatal acute myocardial infarction. Am Heart J. 2003;146(1):106–109. doi: 10.1016/S0002-8703(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 12.Kanki H, Yang P, Xie HG, Kim RB, George AL, Jr., Roden DM. Polymorphisms in beta-adrenergic receptor genes in the acquired long QT syndrome. J Cardiovasc Electrophysiol. 2002;13(3):252–256. doi: 10.1046/j.1540-8167.2002.00252.x. [DOI] [PubMed] [Google Scholar]
- 13.Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med. 2002;347(15):1135–1142. doi: 10.1056/NEJMoa020803. [DOI] [PubMed] [Google Scholar]
- 14.Zaroff JG, Pawlikowska L, Miss JC, Yarlagadda S, Ha C, Achrol A, et al. Adrenoceptor polymorphisms and the risk of cardiac injury and dysfunction after subarachnoid hemorrhage. Stroke. 2006;37(7):1680–1685. doi: 10.1161/01.STR.0000226461.52423.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu TM, Kwok PY. Homogeneous primer extension assay with fluorescence polarization detection. Methods Mol Biol. 2003;212:177–187. doi: 10.1385/1-59259-327-5:177. [DOI] [PubMed] [Google Scholar]
- 16.Zaroff JG, Babcock WD, Shiboski SC, Solinger LL, Rosengard BR. Temporal changes in left ventricular systolic function in heart donors: results of serial echocardiography. J Heart Lung Transplant. 2003;22(4):383–388. doi: 10.1016/s1053-2498(02)00561-2. [DOI] [PubMed] [Google Scholar]
- 17.Zaroff JG, Rosengard BR, Armstrong WF, Babcock WD, D'Alessandro A, Dec GW, et al. Circulation; Consensus conference report: maximizing use of organs recovered from the cadaver donor: cardiac recommendations; Crystal City, Va. March 28-29, 2001; 2002. pp. 836–841. [DOI] [PubMed] [Google Scholar]
- 18.Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human beta 2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33(32):9414–9419. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- 19.Bisognano JD, Weinberger HD, Bohlmeyer TJ, Pende A, Raynolds MV, Sastravaha A, et al. Myocardial-directed overexpression of the human beta(1)-adrenergic receptor in transgenic mice. J Mol Cell Cardiol. 2000;32(5):817–830. doi: 10.1006/jmcc.2000.1123. [DOI] [PubMed] [Google Scholar]
- 20.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci U S A. 1999;96(12):7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason DA, Moore JD, Green SA, Liggett SB. A gain-of-function polymorphism in a G-protein coupling domain of the human beta1-adrenergic receptor. J Biol Chem. 1999;274(18):12670–12674. doi: 10.1074/jbc.274.18.12670. [DOI] [PubMed] [Google Scholar]
- 22.Ranade K, Jorgenson E, Sheu WH, Pei D, Hsiung CA, Chiang FT, et al. A polymorphism in the beta1 adrenergic receptor is associated with resting heart rate. Am J Hum Genet. 2002;70(4):935–942. doi: 10.1086/339621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hachinski VC, Smith KE, Silver MD, Gibson CJ, Ciriello J. Acute myocardial and plasma catecholamine changes in experimental stroke. Stroke. 1986;17(3):387–390. doi: 10.1161/01.str.17.3.387. [DOI] [PubMed] [Google Scholar]
- 24.Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev. 1999;51(4):651–690. [PubMed] [Google Scholar]
- 25.Menon B, Singh M, Ross RS, Johnson JN, Singh K. beta-Adrenergic receptor-stimulated apoptosis in adult cardiac myocytes involves MMP-2-mediated disruption of beta1 integrin signaling and mitochondrial pathway. Am J Physiol Cell Physiol. 2006;290(1):C254–261. doi: 10.1152/ajpcell.00235.2005. [DOI] [PubMed] [Google Scholar]
- 26.Feldman RD. Beta-adrenergic receptor alterations in hypertension--physiological and molecular correlates. Can J Physiol Pharmacol. 1987;65(8):1666–1672. doi: 10.1139/y87-261. [DOI] [PubMed] [Google Scholar]
- 27.Dishy V, Sofowora GG, Xie HG, Kim RB, Byrne DW, Stein CM, et al. The effect of common polymorphisms of the beta2-adrenergic receptor on agonist-mediated vascular desensitization. N Engl J Med. 2001;345(14):1030–1035. doi: 10.1056/NEJMoa010819. [DOI] [PubMed] [Google Scholar]
- 28.Borjesson M, Magnusson Y, Hjalmarson A, Andersson B. A novel polymorphism in the gene coding for the beta(1)-adrenergic receptor associated with survival in patients with heart failure. Eur Heart J. 2000;21(22):1853–1858. doi: 10.1053/euhj.1999.1994. [DOI] [PubMed] [Google Scholar]
- 29.Owen VJ, Burton PB, Michel MC, Zolk O, Bohm M, Pepper JR, et al. Myocardial dysfunction in donor hearts. A possible etiology. Circulation. 1999;99(19):2565–2570. doi: 10.1161/01.cir.99.19.2565. [DOI] [PubMed] [Google Scholar]
- 30.Gridelli B, Remuzzi G. Strategies for making more organs available for transplantation. N Engl J Med. 2000;343(6):404–410. doi: 10.1056/NEJM200008103430606. [DOI] [PubMed] [Google Scholar]
- 31.Hornby K, Ross H, Keshavjee S, Rao V, Shemie SD. Non-utilization of hearts and lungs after consent for donation: a Canadian multicentre study. Can J Anaesth. 2006;53(8):831–837. doi: 10.1007/BF03022801. [DOI] [PubMed] [Google Scholar]
- 32.White M, Wiechmann RJ, Roden RL, Hagan MB, Wollmering MM, Port JD, et al. Cardiac beta-adrenergic neuroeffector systems in acute myocardial dysfunction related to brain injury. Evidence for catecholamine-mediated myocardial damage. Circulation. 1995;92(8):2183–2189. doi: 10.1161/01.cir.92.8.2183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.