Abstract
Background Context
No clinical trial of spinal manipulation for chronic neck pain, either for single or multiple intervention session(s), has employed an effective sham-manipulation control group.
Purpose
Validate a practical sham cervical high velocity, low amplitude (HVLA) spinal manipulation.
Study design/Setting
Randomized, experimental validation study in an institutional clinical research laboratory
Patient Sample
Eligible subjects were males and females, 18–60 years of age with mechanical neck pain (as defined by the International Association for the Study of Pain Classification) of at least 3 months duration. Subjects with arm pain, any pathologic cause of neck pain or any contra-indication to spinal manipulation were excluded.
Outcome Measures
The primary outcome was the patient’s self-report or “registration” of group allocation following treatment. Secondary outcomes were NRS-101 for neck pain, range of motion (by goniometer), tenderness (by pressure algometry).
Methods
Eligible subjects were randomly allocated to one of two groups: “real” or sham cervical manipulation (RM or SM). All subjects were given two procedures in sequence, either RM+SM or SM+SM. Immediately following the two procedures, subjects were asked to register any pain experienced during the procedures and to identify their treatment group allocation. Force-time profiles were recorded during all procedures. Secondary clinical outcome measures were obtained at baseline, 5 and 15 minutes after the intervention including range of motion, self-report of pain and local spinous process tenderness. Data for each variable were summarized and tested for normality in distribution. Summary statistics were obtained for each variable and statistically tested. Funding for this study was obtained from the National Institutes of Health (NCCAM: R21 AT004396-01A1) and the Canadian Institutes of Health Research (BMT91926). No conflicts of interest exist in this study.
Results
Sixty-seven subjects were randomized. Data from 64 subjects (32 per group) were available for analysis. There were no significant differences between the groups at baseline. One adverse event occurred in the “real” group which was a mild post- treatment pain reaction lasting < 24 hours. In the RM group, 50% of subjects incorrectly registered their treatment allocation; in the sham group, 53% did so. For the SM group, none of the procedures resulted in cavitation while in the RM group, 87% of procedures resulted in cavitation. There were no significant changes between groups on pain, tenderness or ROM. Force-time profiles of the RM and SM procedures demonstrated fidelity with significant differences between components as intended.
Conclusions
The novel sham procedure has been shown to be effective in masking subjects to group allocation and to be clinically inert with respect to common outcomes in the immediate post-treatment stage. Further research on serial applications and for multiple operators is warranted.
Keywords: cervical, manipulation, sham, clinical study, neck pain
INTRODUCTION
Neck pain is a very common problem, second only to low back pain in its frequency both within the general population [1–6] and in musculoskeletal practice [7–9]. Approximately 15% of females and 10% of men suffer with chronic neck pain at any one time [4–6]. Chronic neck pain produces a high level of morbidity by affecting occupational and avocational activities of daily living and by affecting quality of life [10–18].
A variety of conservative treatments are available for chronic mechanical neck pain. One commonly used treatment, spinal manipulation (SM), is recommended by several evidenced-based guidelines for patients without severe or progressive neurological deficits [19–26]. There is a wide range of terms often grouped under the heading of SM that currently show limited differences with respect to clinical effectiveness, but are mechanically distinct. One classification system of procedures provides four clusters based on mechanisms of treatment delivery and include: continuous passive motion, mobilization, high-velocity low-amplitude (HVLA) and mechanically assisted [27]. The differences in procedures rely upon cyclical repetition versus single impulse loading with user variability on frequency, force and postural displacement amplitudes. Mechanically assisted procedures couple use of segmented tables with moving sections that guide patient body segments in controlled cyclical ranges of motion or that drop under force control to assist in developing an impulse load during manual force applications. Other applications include hand held probes that direct a brief impulse to a target site.
Recently, Vernon et al. [28] identified clinical trials that had utilized control procedures. Twenty-one trials were identified, four of which employed some form of placebo or sham control [29–32]. None of the trials of manipulation for neck pain or for headache [28–32], employed an effective manual sham manipulation procedure. Sham/placebo/mimic procedures have been attempted, with limited success, for treatment in the lumbar spine [33–36].
Strong placebogenic effects have been hypothesized for manipulation [37–40] resulting from manual contact, personal attention and provider enthusiasm. Placebo or sham- controlled studies are necessary to determine whether results of treatment are related to the degree that the clinical outcomes are attributable to the intervention as an active factor rather than to non-specific effects. A valid sham manipulation procedure is critical for future studies in the efficacy of spinal manipulation through randomized clinical trials. We hypothesized that a novel sham cervical manipulation based on core elements of the patient experience during a procedure, would result in blinding of subjects so that their ability to accurately detect their group assignment would be no greater than chance.
METHODS
The study was conducted in the Biomechanics and Elastography Laboratory of the Canadian Memorial Chiropractic College utilizing a randomized, single-blind, repeated measures experimental design with independent assessors. Subjects were recruited from the population of patients consulting for care of chronic mechanical neck pain (NP) at an outpatient teaching clinic and by local area advertisements.
Patient recruitment and random allocation
Table 1 lists the Inclusion/Exclusion criteria. For the purposes of this study, neck pain was defined as being; 1) located anywhere in the bilateral area from the posterior skull (nuchal ridge), inferiorly to the spine of the scapula, 2) determined clinically to be of mechanical origin following the criteria of the International Association for the Study of Pain (IASP) [42]. IASP criteria include specifying the affected cervical segment, aggravation of pain by selectively stressing that segment and no pain upon stressing adjacent segments.
Table 1.
Inclusion / Exclusion Criteria
Inclusion:
|
Exclusion:
|
Candidates were given an orientation to the experiment and provided written informed consent, approved by the institutional ethics board, prior to proceeding with any intake assessments. During consent, subjects were told they would receive one of two kinds of cervical spinal manipulation treatments and that there was no scientific evidence as to whether one is superior to the other. This was done in an effort to minimize participant expectation bias. The Research Clinician (RC) then performed a clinical screen for eligibility and a manual examination of the neck. The target segment for tenderness measurement and for manipulation was identified by palpation (See Table 1). On clearance for participation, the patient was randomly assigned to the intervention group. Randomization was accomplished prospectively using block allocation to ensure equal numbers in each group. The random allocation was concealed using sealed individual numbered envelopes sequestered from the assessors in the study.
Treatment Interventions
For purposes of this study, the conceptualization of the sham sought to provide a similar sensory experience for the subject as would be provided during treatment with HVLA. To achieve this, four sensory cues were mimicked: touch near the target region, positioning of the head and neck, movement and sound timed with treatment delivery. As described below, the touch and positioning are readily achieved manually. To achieve movement and sound, mimicking the events during application of force and frequently associated joint cavitations, a table drop-assist approach was taken. Drop segments for treatment tables are widely available and used with HVLA in isolation [43] or combined with continuous passive motion [44]. Reported as the third most common procedure type [43] by survey of practitioners, drop table mechanisms provide short interval movement associated with noise that involves the head-neck unit in the targeted region.
Each group was given treatment by a practising chiropractor, the RC, with 35 years of clinical experience, who was responsible for carrying out all interventions. Once the intervention procedure commenced, verbal communication with the participant was limited to positioning instructions. The spinal site targeted for the study intervention was the most locally tender/restricted level [45–47] determined during the screening evaluation. Following pre-positioning, the RC performed the assigned treatment, either a real (RM) or sham (SM) manipulation. Each of these treatments included two procedures: the real treatment consisted of one RM on the side of the lesion followed by one SM on the opposite side, separated by a 60-second rest period. The sham treatment consisted of an SM procedure on the side of the lesion, followed by a second SM to the opposite side, again, after a 60-second rest interval. This dual treatment approach is consistent with the common practice where more than one procedure is often applied to the neck in treatment of neck pain [18] complaints while simultaneously making operator intent for the individual procedure more ambiguous to the subject.
The RM procedure was accomplished following the protocol described by Petersen and Bergmann [48]. With the patient supine the head lifted into mild flexion, rotated (≤ 40°) [49] to the side away from the lesion and allowed to rest on the RC’s forearm (Figure 1) which, in turn, rested on the head piece. The head rest support consisted of a mechanical cam mechanism set to trip with 20 lb downward force, dropping the support a distance of 3/8 inch, with associated sound of impact. Local joint preload, in the direction of the intended treatment load, was applied by pressure over the target lesion site only, not by additional head motion. A combination of minimal (<10 degree) [49] rotary/cephalad axial motion to the head with manipulative impulse load to the lesion site was administered. Simultaneously, a downward pressure was given by the head- supporting arm onto the head-piece activating the cam-drop mechanism. The participant’s head/neck was then returned to the neutral position. Joint cavitation within the cervical spine, commonly associated with RM, was expected to occur during this procedure [50–54].
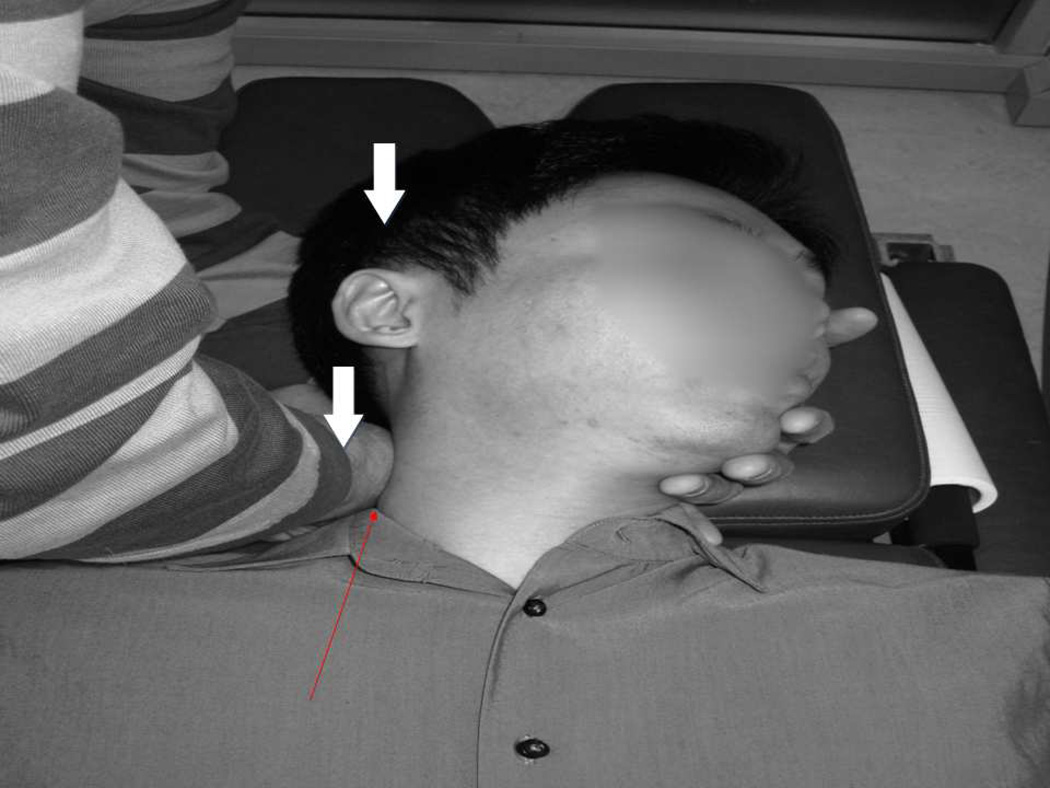
Figure 1.
Head and operator arm orientations for the SM. Wide arrows show the direction of the downward force to trip the cam mechanism. The thin arrow shows the skin contact between the arm and patient’s neck.
The SM procedure differed from the RM by eliminating the joint preload and thrust component, following the preliminary work of Vernon et al. [37]. It consisted of the same degree of head rotation and manual support to the head and neck as in the RM with light touching of the skin by the back of the RC’s hand over the target segment, but no thrusting force applied through that site. A rapid application of motion was created only through the drop action of the head-piece cam mechanism with associated sharp sound.
Immediately following the intervention, the participant was asked by the Project Manager to 1) identify their group assignment using the question “Did you receive a real chiropractic treatment to your neck (YES/NO)?”, and, 2) indicate if there was any new pain or exacerbation of pre-treatment pain during the procedure. The term “treatment” was used to avoid technical jargon associated with manipulation that might cue subjects previously experienced with these procedures and because of its generalizability to any therapeutic encounter. Approximately twenty-four hours after completion of the testing, the participant was contacted again to determine if any late adverse events had occurred. At that time, they were also informed of their group allocation.
Outcome Measures
The primary outcome measure used to evaluate the study hypothesis was the rate of successful identification of group allocation through registering the subject’s response at de-briefing after the procedure was administered. The proportion of subjects who correctly characterized the procedure was compared with the proportion incorrectly identifying their group allocation.
Local tenderness of the spinous process of the target vertebra, neck range of motion (ROM) and NRS-101[41] pain scale obtained at baseline 5 and 15 minutes post-treatment served to evaluate for objective symptomatic change. An electronic pressure algometer was constructed from a uniaxial load cell (Futek, 10 Thomas, Irvine, CA, 92618 USA), sampling force levels at 50 Hz, and a probe ending in a 0.79 cm2 flat rubber tip. Tenderness was determined by a single application of the probe to the spinous process of the lesioned cervical segment from the posterior direction. The patient was seated with the head held by the assessor in slight flexion. The probe pressure was increased at 1 kg force per second until the participant signalled onset of tenderness by pressing an event marker. Range of motion was measured in seated position using a CROM [55–57] device for range in all three cardinal planes. The presence of audible or palpable cavitation of cervical joints arising during the administration of the procedure was recorded by the RC.
Force-time profiles were monitored during administration of each procedure. The loads passing through the neck during the manipulation procedures were estimated using inverse statics [49]. A treatment table (Leader Health Technologies 900 Z– series, Port Orchard, WA) was modified with a cam-drop headpiece isolated on its own support separate from the upper torso support system which was instrumented by a force plate (AMTI OR6 Series, Watertown, MA) capable of sensing reaction forces and moments in three planes. The lower body was also supported by an independent platform. The separate support infrastructure to the table pieces permitted isolation of the force plate from redundant loads that would confound the measurements. Using anthropometric measures from the subject (height, weight, treatment site) inverse dynamic calculations provided estimates of the loads passing through the neck at the target site. Samples were obtained at 1000 Hz over the 5 second window during which the treatment manoeuvres were performed and post-processed with the anthropometric data.
Data Analysis
According to Bang et al. [58] the ideal assumption in typical clinical trials is that 50% of subjects in both control and treatment groups would correctly identify their group allocation at de-briefing, a rate equal to chance alone. We employed the assumptions from Bang et al.[58], the data from Vernon et al. [37] and clinical judgment to derive a sample size estimate of 68 subjects (alpha = 0.05, beta = 0.80, PO = 0.73, P1 = 0.50, PHI = 0.02). A difference in proportions test was applied to test this primary outcome.
The tenderness scores, NRS-101 and ROM, were assessed for normality in distribution and analyzed in separate repeated-measures ANCOVA’s . Post-hoc means testing was performed using appropriate parametric or non-parametric procedures. In order to develop hypotheses for future studies, a secondary analysis of the pain scores was conducted to determine the proportion of subjects in each group who achieved pain reduction at or above a minimally clinically important difference (MCID) of 20% [59]. Once the number of subjects achieving this MCID was determined, the proportion of those subjects who correctly identified their group assignment was determined. Chi Square analysis of these data was performed. Peak values from force-time profiles of the treatment procedures were compared by Student-t test, adjusted for multiple measures, to physically characterize the effective difference between sham and real procedures.
RESULTS
A total of 67 subjects were recruited. The demographic and clinical baseline measures are shown in Table 2. No group differences were observed in the data prior to intervention. The mean age of participants was 38 with a mean duration of neck pain lasting 40 months. In the SM group, one subject aborted the test for unrelated health reasons and failed to return to complete the study. Two protocol violations (one per group) occurred when the cam-drop mechanism failed to engage during the treatment procedure. In both instances, the data was eliminated from final analysis.
Table 2.
Baseline Characteristics – Sham and Real groups
| VARIABLE | SHAM | REAL | TOTAL |
|---|---|---|---|
| Number enrolled | 34 | 33 | 67 |
| Session Success | 97% (1 drop out;1 protocol violation) |
97% (1 protocol violation) |
|
| Number analyzed | 32 | 32 | 64 |
| M/F | 12/20 | 18/14 | 30/34 |
| Age | 38.8 (11.3) | 38.3 (9.9) | p = 0.46 |
| Height (in; M/F) | 68.6(3.8)/63.3(2.3) | 69.8(3.4)/62.4(4.6) | p = 0.27 |
| Weight (lbs; M/F) | 179.2(19.5)/158(3.2) | 185(39.1)/142.9(31.2) | p = 0.90 |
| Duration (Months) | 31.4 (43.6) | 48.9 (82.6) | p = 0.16 |
| NRS | 49.5 (17.6) | 44.4 (15.3) | p = 0.09 |
| Tenderness | 58.8 (31.4) | 65.7 (32.4) | p = 0.19 |
At de-briefing, 50% of those subjects who were in the RM treatment group correctly reported that they had received a real treatment. In the SM group, 47% correctly stated they had received a sham treatment. There was no statistical difference between the subject perceptions by group (X2=0.06, df = 1, p=0.80).
In the SM group, 9% of subjects of subjects indicated that the intervention was slightly painful while 12% of the RM reported similarly (p=0.69). Only 1 subject, who was a member of the RM group, had slight mild pain lasting to the 24-hour follow-up which then resolved uneventfully. Cavitation was obtained during the procedure from 87% of the RM and in none of the SM subjects. Measures of tenderness obtained by pressure pain threshold remained unchanged for both the sham and treatment groups from baseline to 15 minutes post-treatment. Pain scores from the NRS-101, on the other hand, showed a trend (p= 0.049), decreasing for both groups over time.
Pain change scores (Table 4) between time points were calculated. The analysis of the proportion of subjects whose NRS-101 change scores exceeded the 20% threshold showedno difference between groups (38% in RM vs 28% in SM). When the factor of group registration for the sub-sets of subjects achieving at least 20% change in pain was examined, it appeared that 83% of RM ‘pain improvers’ (10/12) guessed that they had received the “real” treatment, while, in the SM group, only 22% (2/9) correctly guessed their group allocation.
Table 4.
Change in pain pressure threshold and NRS101 pain scores.
| Pressure Pain Threshold | NRS101 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham | Rea Manipulation | Sham | Real Manipulation | |||||||||
| Correct | Incorrect | P | Correct | Incorrect | P | Correct | Incorrect | P | Correct | Incorrect | P | |
| N | 15 | 17 | 16 | 16 | 15 | 17 | 16 | 16 | ||||
| 5 min- Baseline | 4.7 (4.1) | −4.6 (3.1) | 0.08 | −4.8 (4.1) | −8.8 (3.6) | 0.47 | −4.3 (1.7) | −5.0 (2.9) | 0.85 | −12.2 (4.7) | −1.9 (1.9) | 0.05 |
| 15 min- Baseline | 0.6 (5.4) | 2.9 (6.6) | 0.79 | −2.5 (4.6) | −6.9 (3.4) | 5.5 | −5.5 (2.3) | −8.2 (3.2) | 0.49 | −14.7 (5.1) | −1.6 (1.5) | 0.02 |
Cervical ROM remained unchanged across all three times of measurement regardless of group (F = 0.4, p = 0.96).
Force-time histories were compared for each component of treatment force applied during all SM and RM procedures. Results were centered on the force magnitude and the value of force for each directional component (transverse, axial or antero-posterior) determined at that point. The cam-drop mechanism threshold (20 Lb), activated in both RM and SM procedures, accounted for essentially all of the antero-posterior force, as would be expected. Statistical comparisons were conducted on the remaining components taken as the effective treatment forces (Table 5). All components of the mean sham procedures ranged between 10% and 50% (0.055 < p < 0.001) of the real manipulations. Only the procedures delivered by the operator’s left hand failed to be significantly different between SM and RM, only trending with a p<0.055.
Table 5.
Peak force components of Sham and Real maneuvers defined in terms of percent of subject body weight. Negative signs denote direction opposite positive values.
| Sham Maneuver | Real Maneuver | ||||
|---|---|---|---|---|---|
| mean | sd | mean | sd | P | |
| Left Transverse | −0.1 | 3.5 | 2.5 | 3.7 | 0.01 |
| Right Transverse | 0.4 | 3.1 | −3.8 | 5.2 | 0.0006 |
| Left Axial | −1.2 | 3.1 | 2.4 | 9.4 | 0.055 |
| Right Axial | −1.2 | 3.6 | 7.3 | 8.4 | 0.0001 |
Discussion
To our knowledge, this is the first study to demonstrate the validity of a sham cervical manipulation procedure. The critical features of this procedure can be divided into two categories: intrinsic and extrinsic. The intrinsic features include: systematic accounting for multiple sensations that the subject may experience during treatment including auditory, movement and skin surface loading cues as well as the consistent application of procedural forces that are quantitatively different between groups. The extrinsic feature is that the sham manoeuvre was performed twice, once on each side in the SM group (double placebo manoeuvre). Both features are believed to act in concert to influence the subject’s ability to identify treatment allocation.
Machado et al. [60] described the ideal attributes of a placebo treatment as having two components: inertness, meaning no known or substantiated therapeutic mechanism; and, mimicry of the index treatment. An inert placebo which mimics the index treatment in all aspects, including the replication of any common side effects is termed “indistinguishable”. When side effects are absent, the placebo is said to have “structural equivalence”, where, at minimum, the registration of group allocation is no better than chance. When these criteria are applied to the sham treatment in this study, the lack of change in measures of pressure pain threshold, spontaneous pain by NRS101 (Table 2) and range of motion after a single treatment application suggest that the sham procedure is clinically inert. More patients reporting improvement in pain following treatment were in the RM group (38% vs 28% in the sham group); however, this study was not designed for nor did it have sufficient power for this comparison.
With regard to masking of group registration, the results are consistent with Machado et al.’s qualification of a “structurally equivalent” [60] placebo. This is especially noteworthy given that almost all participants had had prior experience with cervical manipulation, with many having current treatment. The use of a “double” administration of treatment that paired sham with real or sham with sham in a dual maneuver mimics the common occurrence in practice where more than one procedure may be applied to the neck. At the same time it holds a potential advantage of increasing the ambiguity in the subject’s experience as to which element the operator intends to be therapeutic. While the sensory input to the subject was comparable with respect to manual touch, head positioning, movement and sudden noise the force-time history of the applied loads demonstrate clearly the successful dampening of loads (Table 4) to a range of 10% to 50% of the treatment loads. The sole larger force in the antero-posterior direction matches the force necessary to trip the cam-drop mechanism and is independent of the treatment forces.
An obvious advantage of our procedure is that it is a manual sham maneuver making it a desirable comparison to other manual methods. Previous randomized clinical trials of manipulation have employed other forms of placebo or sham control [28–32]. Two studies employed a de-tuned therapy instrument [31, 32] whereas Sloop et al. [29] employed a manipulation under anamnestic valium administration, attempting to avoid all sensory cues. Only Vernon et al.’s trial [30] for manipulation and tension-type headache attempted a manual sham manipulation procedure [37], but this was in conjunction with a placebo version of a medication, creating a double-placebo condition that did not permit the identification of the separate effect of the manual sham.
An observation noted on secondary analysis, is the tendency of subjects, when their clinical findings improve to a minimum clinically important difference of 20%, to identify their group allocation as being RM, regardless of their actual group assignment. This happened in 83% and 23% of the subset of subjects who achieved a minimum clinically important improvement in the real and sham treatment groups, respectively. This may provide a means in future studies to begin understanding those who are sometimes called placebo responders [35–38, 40, 59].
The most important limitation of this study is that the findings apply only to a single treatment over a 24-hour investigative interval. It is not known if these procedures can sustain similar levels of blinding and, in the case of the sham manipulation procedure, the same levels of inertness over a series of treatments over intervals of many days. This is a critical topic for future research. Future studies should also address subject’s baseline clinical expectations as well as the reason(s) they give for their post- intervention group registration.
Conclusions
The double-treatment method of pairing real-sham and sham-sham procedures using carefully selected physical components that systematically account for patient experience during manipulation provides an effective and inert sham/placebo for manual manipulation of the cervical spine.
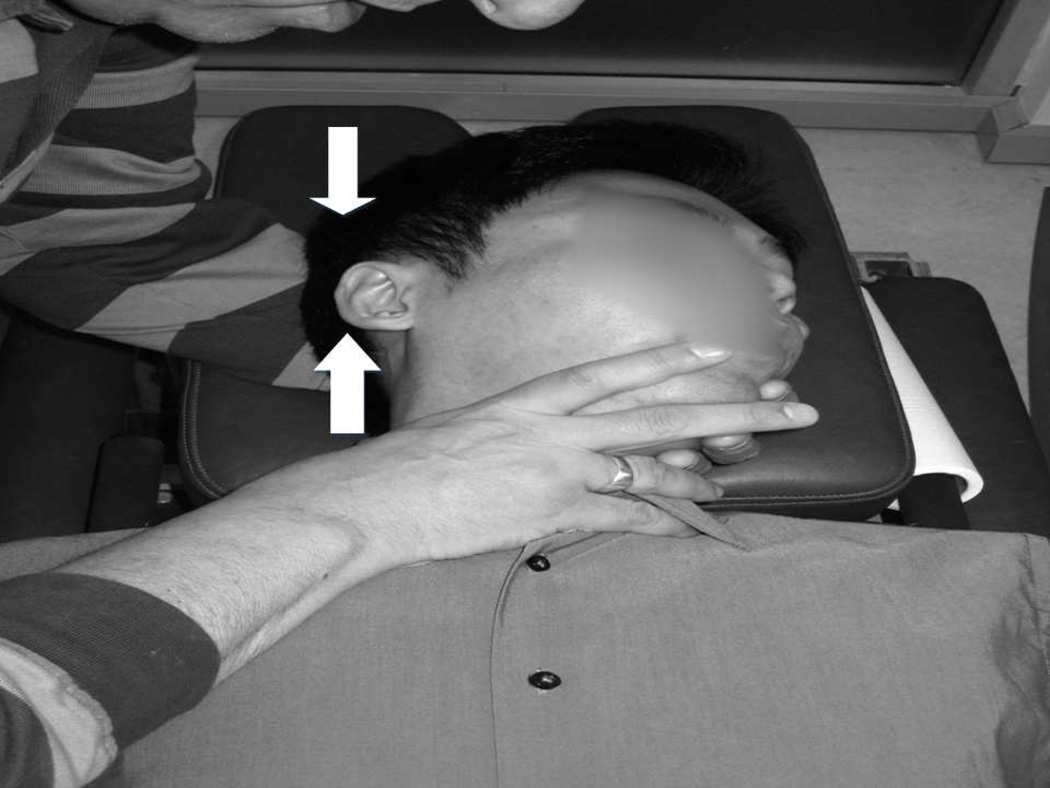
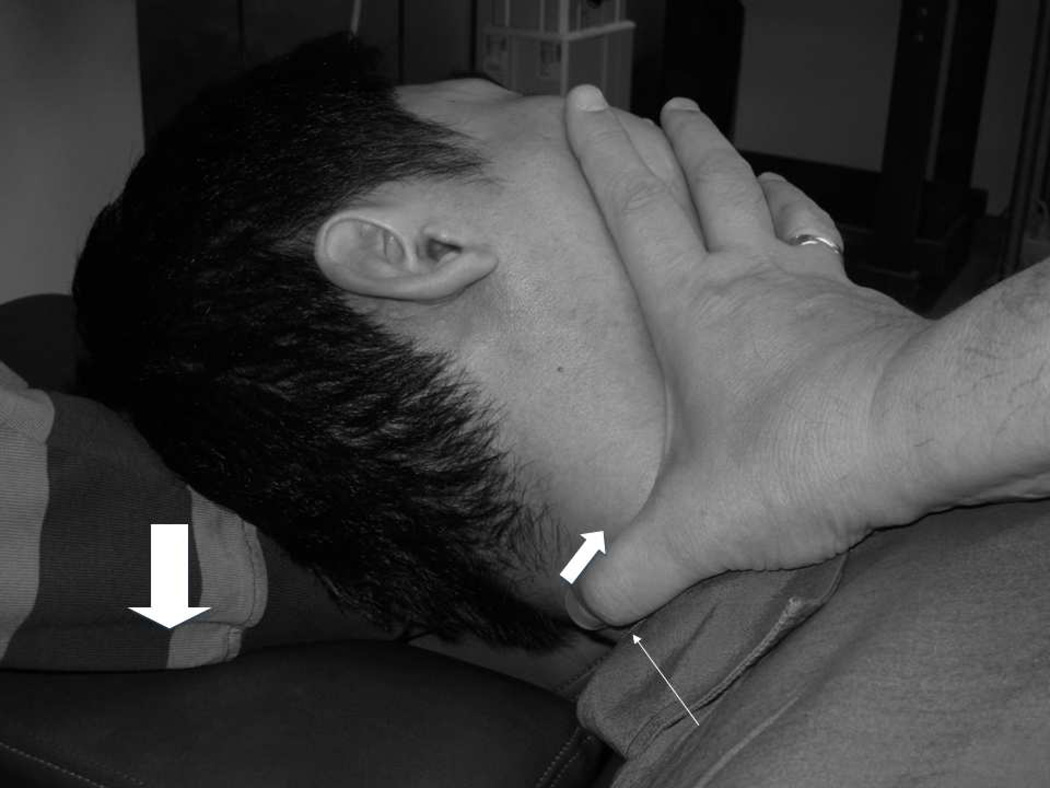
Figure 2.
RM configuration; the wide arrow shows the force to trip the cam and the thinner arrow gives one component of the intended treatment force.
RM configuration; the wide arrow as in 2a; the manual contact with the operator’s thumb (thin arrow) and direction of second intended component (thinnest arrow) are shown (head is over-rotated to show thumb contact).
Table 3.
Intervention and post-intervention outcomes
| SM Group (Correct/Incorrect) |
RM Group (Correct/Incorrect) |
Significance | |
|---|---|---|---|
| Treatment Registration | 15/17 | 16/16 | p=0.80 |
| Procedure painful | 3/32 mild | 4/32 mild | p = 0.689 |
| Reaction at follow-up | 0/32 | 1/32 mild | |
|
Cavitation (procedure 1/procedure 2) |
0%/0% | 87%/0% | Not tested |
|
Pressure Pain Threshold (Kilopascals) Baseline 5 min post 15 min post |
405.4 (216.5) 404 (227.5) 418.5(278.5) |
456.4(226.1) 408.8(211.6) 408.8(211.6) |
p= 0.166 |
|
NRS-101 Pain Score Baseline 5 min post 15 min post |
50.4(17.5) 45.7(20.1) 43.4 (20.9) |
44.3(15.5) 37.2 (16.7) 36.1 (17.4) |
For Time only (both groups), P = 0.049 |
Acknowledgments
Financial disclosure
This study was funded by the NIH-NCCAM #R21 AT004396-01A1 and the CIHR BMT91926. The authors declare no financial interests associated with this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nachemson A, Waddell G, Norlund AI. Epidemiology of neck and back pain. In Neck and Back Pain: The Scientific Evidence of Causes. In: Nachemson A, Jonsson E, editors. Diagnosis and Treatment. Philadelphia, PA: Lippincott Williams and Wilkins; 2000. pp. 165–187. [Google Scholar]
- 2.Wolsko PM, Eisenberg DM, Davis RB, Kessler R, Phillips RS. Patterns and perceptions of care for treatment of back and neck pain: results of a national survey. Spine. 2003;28:292–298. doi: 10.1097/01.BRS.0000042225.88095.7C. [DOI] [PubMed] [Google Scholar]
- 3.Webb R, Brammah T, Lunt M, Unwin M, Allison T, Symmons D. Prevalence and predictors of intense, chronic and disabling neck and back pain in the UK general population. Spine. 2003;28:1195–1202. doi: 10.1097/01.BRS.0000067430.49169.01. [DOI] [PubMed] [Google Scholar]
- 4.Guez M, Hildingsson C, Nilsson M, Toolanen G. The prevalence of neck pain. A population-based study from northern Sweden. Acta Orthop Scand. 2002;73:455–459. doi: 10.1080/00016470216329. [DOI] [PubMed] [Google Scholar]
- 5.Makela M, Heliovaara M, Sievers K, Impivaara O, Knecht P, Aromaa A. Prevalence, determinants and consequences of chronic neck pain in Finland. Am J Epidemiol. 1991;134:1356–1367. doi: 10.1093/oxfordjournals.aje.a116038. [DOI] [PubMed] [Google Scholar]
- 6.Bovim G, Schrader H, Sand T. Neck pain in the general population. Spine. 1994;19:1307–1309. doi: 10.1097/00007632-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Waalen DP, White TP, Waalen JK. Demographic and clinical characteristics of chiropractic patients: a five-year study of patients treated at the Canadian Memorial Chiropractic College. J Can Chirop Assoc. 1994;38:75–82. [Google Scholar]
- 8.Hagberg M, Wegman DH. Prevalence rates and odds ratios of shoulder-neck diseases in different occupational groups. Br J Ind Med. 1987;44:602–610. doi: 10.1136/oem.44.9.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westgaard RH, Jenssen C, Hansen K. Individualized work-related risk factors associated with symptoms of musculoskeletal complaints. Inter Arch Occup Environ Health. 1993;64:405–413. doi: 10.1007/BF00517946. [DOI] [PubMed] [Google Scholar]
- 10.Cote P, Cassidy JD, Carroll L. The Saskatchewan Health and Back Pain Survey: the prevalence of neck pain and related disability. Spine. 1998;23:1689–1698. doi: 10.1097/00007632-199808010-00015. [DOI] [PubMed] [Google Scholar]
- 11.Daffner SD, Hilibrand AS, Anscom BS, Brislin BT, Vaccaro AR, Albert TJ. Impact of neck and arm pain on overall health status. Spine. 2003;28:2030–2035. doi: 10.1097/01.BRS.0000083325.27357.39. [DOI] [PubMed] [Google Scholar]
- 12.Borghouts JAJ, Koes BW, Bouter LM. The clinical course and prognostic factors of non-specific neck pain: a systematic review. Pain. 1998;77:1013. doi: 10.1016/S0304-3959(98)00058-X. [DOI] [PubMed] [Google Scholar]
- 13.Gore DR, Sepic SB, Gardner GM, Murray MP. Neck pain: a long-term follow-up of 205 patients. Spine. 1987;12:1–5. doi: 10.1097/00007632-198701000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Berg M, Sanden A, Torell G, Jarvholm B. Persistence of musculoskeletal symptoms: a longitudinal study. Ergonomics. 1988;31:1281–1285. doi: 10.1080/00140138808966767. [DOI] [PubMed] [Google Scholar]
- 15.Rossignol M, Suissa S, Abenhaim L. Working disability due to occupational back pain: three-year follow-up of 2,300 compensated workers in Quebec. J Occup Environ Med. 1988;30:502–505. [PubMed] [Google Scholar]
- 16.Abenhaim I, Suissa S, Rossignol M. Risk of recurrence of occupational back pain over three years follow-up. Occup Environ Med. 1988;45:829–833. doi: 10.1136/oem.45.12.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holm LW, Carroll LJ, Cassidy JD, Hogg-Johnson S, Cote P, Guzman J, Peloso P, Nordin M, Hurwitz E, van der Velde G, Carragee E, Haldeman S. The burden and determinants of neck pain in Whiplash-Associated Disorders: Results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and its Associated Disorders. Spine. 2008;33(Suppl 4S):S52–S59. doi: 10.1097/BRS.0b013e3181643ece. [DOI] [PubMed] [Google Scholar]
- 18.Bronfort G. Effectiveness of spinal manipulation and adjustments. In: Haldeman S, editor. Principles and Practice of Chiropractic. Norwalk, CN: Appleton and Lange; 1992. pp. 415–441. [Google Scholar]
- 19.Gross AR, Kay TM, Kennedy C, Gasner D, Hurley L, Yardley K, Hendry L, McLaughlin L. Clinical practice guideline on the use of manipulation or mobilization in the treatment of adults with mechanical neck disorders. Man Ther. 2002;7:193–205. doi: 10.1054/math.2002.0477. [DOI] [PubMed] [Google Scholar]
- 20.Philadelphia Panel Evidence-Based Clinical Practice Guidelines on Selected Rehabilitation Interventions for Neck Pain. Phys Ther. 2001;81:1701–1717. [PubMed] [Google Scholar]
- 21.Gross AR, Hoving JL, Haines TA, Goldsmith CH, Kay T, Aker P, Bronfort G Cervical Overview Group. A Cochrane review of manipulation and mobilization for mechanical neck disorders. Spine. 2004 Jul 15;29(14):1541–1548. doi: 10.1097/01.brs.0000131218.35875.ed. [DOI] [PubMed] [Google Scholar]
- 22.Gross AR, Hoving JL, Haines TA, Goldsmith CH, Kay T, Aker P, Bronfort G Cervical overview group. Manipulation and mobilisation for mechanical neck disorders. Cochrane Database of Systematic Reviews. 2006;(Issue 3) doi: 10.1002/14651858.CD004249.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Vernon HT, Humphreys BK, Hagino CA. A systematic review of conservative treatments for acute neck pain not due to whiplash. J Manip Physiol Ther. 2005;28:443–448. doi: 10.1016/j.jmpt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Vernon H, Humphreys BK. Manual therapy for neck pain: an overview of randomized clinical trials and systematic reviews. Eura Medicophys. 2007;43:91–118. [PubMed] [Google Scholar]
- 25.Miller J, Gross A, D'Sylva J, Burnie SJ, Goldsmith CH, Graham N, Haines T, Brønfort G, Hoving JL. Manual therapy and exercise for neck pain: a systematic review. Man Ther. 2010 Aug;15(4):334–354. [PubMed] [Google Scholar]
- 26.D'Sylva J, Miller J, Gross A, Burnie SJ, Goldsmith CH, Graham N, Haines T, Brønfort G, Hoving JL for the Cervical Overview Group. Manual therapy with or without physical medicine modalities for neck pain: a systematic review. Man Ther. 2010 Oct;15(5):415–433. doi: 10.1016/j.math.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Triano J. The theoretical basis for spinal manipulation. In: Haldeman S, editor. Principles and Practice of Chiropractic. 3rd Edition. New York, NY: McGraw-Hill; 2005. p. 374. Ed-in-Chief. [Google Scholar]
- 28.Vernon H, Puhl A, Reinhart C. Systematic review of clinical trials of cervical manipulation: control group procedures and outcomes. Chirop Osteop. 2011;19:3. doi: 10.1186/2045-709X-19-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sloop PR, Smith DS, Goldenberg E, Dore C. Manipulation for chronic neck pain: a double-blind controlled study. Spine. 1982;7(6):532–535. doi: 10.1097/00007632-198211000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Vernon H, Jansz G, Goldsmith CH, McDermaid C. A randomized, placebo-controlled clinical trial of chiropractic and medical prophylactic treatment of adults with tension-type headache: results from a stopped trial. J Manipulative Physiol Ther. 2009;32:344–351. doi: 10.1016/j.jmpt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Tuchin PJ, Pollard H, Bonello R. A randomized controlled trial of chiropractic spinal manipulative therapy for migraine. J Manipulative Physiol Ther. 2000;23(2):91–95. [PubMed] [Google Scholar]
- 32.Pikula JR. The effect of spinal manipulative therapy (SMT) on pain reduction and range of motion in patients with acute unilateral neck pain: a pilot study. J Can Chiropr Assoc. 1999;43(2):111–119. [Google Scholar]
- 33.Triano JJ, McGregor M, Hondras MA, Brennan PC. Manipulative therapy versus education programs in chronic low back pain. Spine. 1995 Apr 15;20(8):948–955. doi: 10.1097/00007632-199504150-00013. [DOI] [PubMed] [Google Scholar]
- 34.Hawk C, Long C. Use of a pilot to refine the design of a study to develop a manual placebo treatment. J Neuromusculoskel Sys. 2000;8:39–48. [Google Scholar]
- 35.Hawk C, Azad A, Phongphua C, Long C. Preliminary study of the effects of a placebo chiropractic treatment with sham adjustments. J Manip Physiol Therap. 1999;22:436–443. doi: 10.1016/s0161-4754(99)70031-x. [DOI] [PubMed] [Google Scholar]
- 36.Hawk C, Long C, Reiter R, Davis C, Cambron J, Evans R. Issues in planning a placebo-controlled trial of manual methods: results of a pilot study. J Am Chirop Med. 2002;8:21–32. doi: 10.1089/107555302753507159. [DOI] [PubMed] [Google Scholar]
- 37.Vernon H, MacAdam K, Marshall V, Pion M, Sadowska M. Validation of a sham manipulative procedure for the cervical spine for use in clinical trials. J Manip Physiol Ther. 2005;28:662–666. doi: 10.1016/j.jmpt.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Ernst E, Harkness E. Spinal manipulation: A systematic review of sham-controlled, double-blind, randomized clinical trials. J Pain Sympt Manage. 2001;22:879–889. doi: 10.1016/s0885-3924(01)00337-2. [DOI] [PubMed] [Google Scholar]
- 39.Hancock MJ, Maher CG, Latimer J, McCauley JH. Selecting an appropriate placebo for a trial of spinal manipulative therapy. Aust J Physiother. 2006;52:135–138. doi: 10.1016/s0004-9514(06)70049-6. [DOI] [PubMed] [Google Scholar]
- 40.Coulehan JL. Chiropractic and the clinical art. Soc Sci Med. 1985;21(4):383–390. doi: 10.1016/0277-9536(85)90218-7. [DOI] [PubMed] [Google Scholar]
- 41.Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37:378–381. doi: 10.1136/ard.37.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merskey H, Bogduk N, editors. Classification of Chronic Pain: Description of chronic pain syndromes and definitions of pain terms. Seattle, WA: IASP Press; 1994. [Google Scholar]
- 43.Mykietiuk C, Wambolt M, Pillipow T, Mallay C, Gleberzon B. Technique systems used by post-graduates of the Canadian Memorial Chiropractic College practicing in five Canadian provinces: a preliminary study. J Can Chirop Assoc. 2009;53(1):32–39. [PMC free article] [PubMed] [Google Scholar]
- 44.Triano J. The mechanics of spinal manipulation. In: Herzog W, editor. Clinical Biomechanics of Spinal Manipulation. Philadelphia, PA: Churchill Livingstone; 2000. pp. 92–90. [Google Scholar]
- 45.Zito G, Jull G, Story I. Clinical tests of musculoskeletal dysfunction in the diagnosis if cervicogenic headache. Man Ther. 2006 May;11(2):118–129. doi: 10.1016/j.math.2005.04.007. Epub 2005 Jul 18. [DOI] [PubMed] [Google Scholar]
- 46.Piva SR, Erhard RE, Childs JD, Browder DA. Inter-tester reliability of passive intervertebral and active movements of the cervical spine. Man Ther. 2006;11:321–330. doi: 10.1016/j.math.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Schops P, Pfingsten M, Siebert U. Reliability of manual medical examination techniques of the cervical spine. Study of quality assurance in manual diagnosis. Z Orthop Ihre Grenzgeb. 2000;138:2–7. doi: 10.1055/s-2000-10105. [DOI] [PubMed] [Google Scholar]
- 48.Peterson D, Bergmann T. Chiropractic Technique Principles and Procedure. St. Louis, MO: Mosby, Inc.; 2011. pp. 152–187. [Google Scholar]
- 49.Triano JJ, Schultz AB. Motions of the head and thorax during neck manipulations. J Manip Physiol Ther. 1994;17(9):573–583. [PubMed] [Google Scholar]
- 50.Herzog W, Zhang YT, Conway PJ, Kawchuk GN. Cavitation sounds during spinal manipulative treatments. J Manip Physiol Ther. 1993 Oct;16(8):523–526. [PubMed] [Google Scholar]
- 51.Brodeur R. The audible release associated with joint manipulation. J Manip Physiol Therap. 1995;18:155–164. [PubMed] [Google Scholar]
- 52.Ross JK, Bereznick DE, McGill SM. Determining cavitation location during lumbar and thoracic spinal manipulation: is spinal manipulation accurate and specific? Spine. 2004 Jul 1;29(13):1452–1457. doi: 10.1097/01.brs.0000129024.95630.57. [DOI] [PubMed] [Google Scholar]
- 53.Beffa R, Matthews R. Does the adjustment cavitate the targeted joint? An investigation into the location of cavitation sounds. J Manip Physiol Ther. 2004 Feb;27(2):e2. doi: 10.1016/j.jmpt.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 54.Reggars JW, Pollard HP. Analysis of zygapophyseal joint cracking during chiropractic manipulation. J Manip Physiol Ther. 1995 Feb;18(2):65–71. [PubMed] [Google Scholar]
- 55.Whitcroft KL, Massouh L, Amirfeyz R, Bannister G. Comparison of methods of measuring active cervical range of motion. Spine. 2010;35(19):E976–E980. doi: 10.1097/BRS.0b013e3181cd6176. [DOI] [PubMed] [Google Scholar]
- 56.de Koning CH, van den Heuvel SP, Staal JB, Smits-Engelsman BC, Hendriks EJ. Clinimetric evaluation of active range of motion measures in patients with non-specific neck pain: a systematic review. Eur Spine J. 2008 Jul;17(7):905–921. doi: 10.1007/s00586-008-0656-3. Epub 2008 Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Audette I, Dumas JP, Côté JN, De Serres SJ. Validity and between-day reliability of the cervical range of motion (CROM) device. J Orthop Sports Phys Ther. 2010 May;40(5):318–323. doi: 10.2519/jospt.2010.3180. [DOI] [PubMed] [Google Scholar]
- 58.Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials. Con Clin Trial. 2004;25:143–156. doi: 10.1016/j.cct.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 59.Vernon H, Humphreys BK, Hagino C. The outcome of control groups in clinical trials of conservative treatments for chronic neck pain: a systematic review. BMC Musculoskel Dis. 2006;7:58. doi: 10.1186/1471-2474-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Machado LAC, Kamper SJ, Herbert RD, Maher CG, McAuley JH. Imperfect placebos are common in low back pain trials: a systematic review of the literature. Eur J Spine. 2008;17:889–904. doi: 10.1007/s00586-008-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]