Abstract
Environmental compounds are known to promote epigenetic transgenerational inheritance of disease. The current study was designed to determine if a “pesticide mixture” (pesticide permethrin and insect repellent N,N-Diethyl-meta-toluamide, DEET) promotes epigenetic transgenerational inheritance of disease and associated DNA methylation epimutations in sperm. Gestating F0 generation female rats were exposed during fetal gonadal sex determination and the incidence of disease evaluated in F1 and F3 generations. There were significant increases in the incidence of total diseases in animals from pesticide lineage F1 and F3 generation animals. Pubertal abnormalities, testis disease, and ovarian disease (primordial follicle loss and polycystic ovarian disease) were increased in F3 generation animals. Analysis of the pesticide lineage F3 generation sperm epigenome identified 363 differential DNA methylation regions (DMR) termed epimutations. Observations demonstrate that a pesticide mixture (permethrin and DEET) can promote epigenetic transgenerational inheritance of adult onset disease and potential sperm epigenetic biomarkers for ancestral environmental exposures.
Keywords: Environmental Epigenetics, Transgenerational, Testis, Ovary, Puberty, DNA Methylation, Pesticide, Systems Biology
Introduction
Epigenetic transgenerational inheritance involves the transmission of a phenotypic alteration to offspring and subsequent generations (F3) through germline epimutations following ancestral environmental exposure of a gestating F0 generation female [1, 2]. Previous studies [2, 3] with the agricultural fungicide vinclozolin administered to gestating female rats during the gonadal sex determination period have been shown to promote a male germline epigenome reprogramming that induces transgenerational adult-onset disease. This germline epigenome modification occurs during the primordial germ cell developmental period of DNA demethylation followed by DNA remethylation during gonadal sex determination [1, 4]. The modified epigenetic programming of the male germ line (sperm) subsequently leads to all tissues propagated from this germline to have an altered epigenome and transcriptome that can influence development of adult-onset disease. The altered DNA methylation (epigenome) in the germline is transmitted through subsequent generations due to apparent permanent imprinted-like properties [3]. These germline-mediated epimutations enable epigenetic transgenerational inheritance of altered phenotypes.
Environmental chemicals such as vinclozolin and the pesticide methoxychlor [2] have been shown to promote epigenetic transgenerational inheritance of adult onset diseases. The current study was designed to investigate the actions of a “pesticide mixture” (pesticide permethrin and the insect repellent N,N-Diethyl-meta-toluamide, DEET). Permethrin and DEET are components of commonly used skin applications for scabies in humans. Human populations such as military personnel use this mixture to prevent insect bites and subsequent blood-borne parasitic diseases. Apart from mild symptoms, permethrin and DEET generally do not show adverse in vivo or in vitro toxic effects [5]. However, some reported toxicological effects of a mixture of permethrin and/or DEET include uterine enlargement, atrophy of male secondary sex organs [6], testicular cancer [7], neurological disorders and behavioral changes [8–10], oxidative stress in brain [11], DNA damage [12] and immunotoxicity [13]. Previous studies with permethrin used higher doses (10-800 mg/kg) [6] and only F0 generations were studied [14, 15]. In the current study a developmental exposure to pharmacological doses of permethrin and DEET (39% for permethrin and 2% of DEET of LD50 dose) were used. No toxicity in the F1 generation was expected to be produced by this developmental exposure. This study was designed to examine the potential of these compounds to influence epigenetic transgenerational inheritance and not designed to do risk analysis. The current study only examined the actions of the mixture as this is the typical use in humans, such that conclusions on the actions of the individual compounds can not be assessed. Currently there is no information about the pesticide mixture's potential to promote transgenerational disease.
Previous studies with vinclozolin have documented epigenetic transgenerational inheritance of adult-onset diseases in F3 generation progeny following ancestral developmental exposure [2, 16]. The current study examined the hypothesis that the exposure of a gestating female during the fetal gonadal sex determination period to the pesticide mixture can promote epigenetic transgenerational inheritance of adult onset disease. In the present study disease or abnormalities of testis, prostate, kidney, ovary, tumor development, abnormal pubertal onset and obesity were evaluated in 1-year old rats of F1 and F3 generations. Phenotypes observed in the F1 generation animals can be induced by a direct chemical exposure of the fetus and somatic cells. In contrast, the phenotypes observed in F3 generation animals are due to epigenetic transgenerational inheritance through the germline and not due to any direct effect of the chemical exposure [17]. Therefore, phenotypes or diseases observed in F1 and F3 generation animals are not due to the same mechanism and can be distinct. The current study was designed to evaluate the F1 generation due to direct exposure and compare disease phenotypes to the F3 generations to evaluate transgenerational effects. The objective was to investigate epigenetic transgenerational inheritance of disease after the pregnant ancestor (great-grandmother) was exposed to the pesticide mixture. Observations support the ability of environmental exposures to induce epigenetic transgenerational inheritance of adult-onset disease through sperm epimutations. A previous publication compared the actions of the pesticide mixture with a plastic compound mixture (BPA, DEHP, DBP), dioxin and a hydrocarbon mixture (jet fuel, JP8) on postnatal day 120-old (P120) rats [18]. Observations from this publication demonstrated transgenerational pubertal and ovarian disease phenotypes, as well as unique transgenerational sperm epimutations [18]. The current study extends these previous observations to examine the pesticide mixture's actions on 1-year old F1 and F3 generation animals and further investigates the specific transgenerational sperm epimutations.
Materials and Methods
Animal Studies
Experimental protocols with rats were pre-approved by the Institutional Animal Care and Use Committee. The University Department of Environmental Health and Safety approved the protocols for the use of environmental chemicals. Female and male rats of an outbred strain Hsd:Sprague Dawley SD (Harlan) at about 70 and 100 days of age were maintained in ventilated isolator cages containing Aspen Sani-chips. Rats were fed ad libitum with a standard rat diet and ad libitum tap water for drinking. During the injection and vaginal smear collection procedures rats were held in an animal transfer station. To obtain time-pregnant females the female rats in proestrus were pair-mated with male rats. The sperm-positive (day 0) rats were monitored for diestrus and body weight. On days embryonic day 8 (E8) through E14 of gestation [19], the gestating females were administered daily intraperitoneal injections of a pesticide mixture (permethrin 150 mg/kg BW/day and insect repellent DEET 40 mg/kg/BW/day) or dimethyl sulfoxide (DMSO) vehicle control with an equal volume of sesame oil (Sigma) to prevent irritation at the injection site. The gestating female rats treated with vehicle control or pesticide mixture were designated as the F0 generation. Exposure lineages are designated “control” and “pesticide” (permethrin and DEET mixture) lineages throughout the manuscript. All experimental protocols for the procedures with rats were approved by the Washington State University Animal Care and Use Committee (IACUC) (approval # 02568-026).
Breeding
The offspring of the F0 generation rats were the F1 generation. Non-littermate females and males aged 70–90 days from F1 generation control or pesticide lineages were bred to obtain F2 generation offspring. The F2 generation rats were bred to obtain F3 generation offspring. No sibling or cousin breeding was used to avoid any inbreeding artifacts. Suckling rats were weaned from their mothers at 21 days of age. It is important to note that only the F0 generation gestating female was exposed directly to the control vehicle or pesticide mixture and the F1–F3 generation animals were not subjected to any treatment. The breeding crosses were from the same lineage such that no conclusions can be made regarding male versus female transmission.
Tissue Harvest and Histology Processing
Rats were euthanized at 1-year of age by CO2 inhalation for tissue harvest. Body and organ weights were measured at dissection time. Testis, epididymis, prostate, seminal vesicle, ovaries, uterus and kidney were collected and fixed in Bouin's solution (Sigma) and 70% ethanol, then processed for paraffin embedding by standard procedures for histopathology examination. Five-micrometer tissue sections were made and were either unstained and used for TUNEL analysis or stained with hematoxylin and eosin and examined for histopathology. Blood samples were collected at the time of dissection, allowed to clot, centrifuged and serum samples stored for steroid hormone assays. The hormone radioimmunoassays (RIA) were performed by the WSU Center for Reproductive Biology Assay Core Laboratory.
Testicular Apoptotic Cell Analysis
Testis sections were examined by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay (In situ cell death detection kit, Fluorescein, Roche Diagnostics, Mannheim, Germany) as per the manufacturer's protocols. Sections were deparaffinized, rehydrated and then deproteinized by Proteinase K (20 mg/ml; Invitrogen, Carlsbad, CA) followed by washing in phosphate buffered saline (PBS). A volume of 25 μl of the enzyme-label solution mix was applied on the testis sections and incubated at 37°C for 90 min. After PBS washes slides were mounted and kept at 4°C until examination under a fluorescent microscope in dark field. Both testis sections of each slide were microscopically examined to identify and to count apoptotic germ cells by their bright green fluorescence.
Histopathology Examination and Disease Classification
Three different observers independently examined each unmarked tissue slide and identical criteria were applied to identify diseased tissue. A cut-off was established to declare a tissue `diseased' based on the mean number of histopathological abnormalities plus two standard deviations from the mean of control tissues by each of the three individual observers. This number was used to classify rats into those with and without disease in each lineage. A rat tissue section was declared `diseased' only when at least two of the three observers marked the same tissue section as such. Necropsy and histopathology examinations on rats that died prior to 1-year of age and pathology analysis of tissues sent with unknown or suspected diseases were performed by the WSU Washington Animal Disease Diagnostic Laboratory and these results were also included in the study. The proportion of rats with obesity or tumor development was obtained by accounting those that had these conditions out of all the animals evaluated. The number of animals per litter (litter representation) mean ± SEM used for a specific disease/abnormality for the F1 and F3 generation control versus pesticide lineages were found not to be statistically different (p>0.05). Therefore, the litter representation was the same between control and pesticide comparisons for each specific disease/abnormality comparison and litter bias was not detected.
A marked central portion of each prostate, kidney and testis section was microscopically examined under 200× magnification. An additional peripheral portion of each testis section was also examined. Testis histopathology criteria included the presence of a vacuole, azoospermic atretic seminiferous tubule and `other' abnormalities including sloughed spermatogenic cells in center of the tubule and a lack of a tubule lumen [18, 20]. Prostate histopathology criteria included the presence of vacuoles, atrophic epithelial layer of ducts and hyperplasia of prostatic duct epithelium. Kidney histopathology criteria included reduced size of glomerulus, thickened Bowman's capsule and the presence of proteinaceous fluid-filled cysts.
For ovarian histology every 30th section was stained with hematoxylin and eosin, then three stained sections (150 μm apart) through the central portion of the ovary with the largest cross section were evaluated. Ovary sections were assessed for two diseases, primordial follicle loss and polycystic ovarian disease. Primordial follicle loss was determined by microscopically counting the number of primordial follicles per ovary section. Primordial follicles had an oocyte surrounded by a single layer of either squamous or both squamous and cuboidal granulosa cells [21, 22]. Primordial follicle loss was considered present in the ovary when the primordial follicle number was less than the control mean minus two standard deviations. Polycystic ovaries were determined by microscopically counting the number of small and large cystic structures. Cysts were defined as fluid-filled structures of a specified size that were not filled with red blood cells and had no oocyte and negligible granulosa cells. A single layer of cells may line cysts. Small cysts were 50 to 250 μm in diameter measured from the inner cellular boundary across the longest axis. Histological data from the rat ovaries was analyzed differently than previously reported [23] to present disease incidence. The mean number of primordial follicles or cysts was calculated from three sections. Polycystic ovarian disease was considered present when the number of small cysts was more than the control mean plus two standard deviations. Follicles had to be non-atretic and showing an oocyte nucleus in order to be counted. Percentages of females with primordial follicle loss or polycystic ovarian disease were computed.
Onset of puberty was assessed in females by daily examination for vaginal opening from 30 days of age and in males by balano-preputial separation from 35 days of age. For identifying a rat with a pubertal abnormality (either an early onset or delayed onset of puberty) a mean from all the rats from the control lineage that were evaluated for pubertal onset was computed and its standard deviation calculated. A range of normal pubertal onset was chosen based on mean ± 2 standard deviations. Any rat with a pubertal onset below this range was considered to have had an early pubertal onset and any rat with a pubertal onset above this range was considered to have had a delayed pubertal onset and the proportion of rats with pubertal abnormality (early or late pubertal onset) was computed from the total number of rats evaluated for puberty onset.
A table of the incidence (percentage of animals with specific disease/abnormality) for individual diseases/abnormalities in rats from each lineage was created and the proportion of individual disease, total disease and multiple disease incidence (% disease) was computed from this table. For the individual disease, only those rats that showed a plus (presence of disease) or minus (absence of disease) in the table are included in the computation. For the total disease, a column with total disease is presented and the number of plus signs (indicating the presence of disease) were added up for each of the rats and the proportion was computed as the number of rats with one or more diseases (total disease) out of all listed rats. For the multiple disease, the proportion was computed as the number of rats with more than one disease (multiple disease) out of all of the listed rats. It should be noted that not all the rats were evaluated for all the diseases due to technical limitations. The computation of the percent incidence of disease data is limiting in this respect and the data presented represent only the minimal incidence of total or multiple disease. For example, if more animals in the current set had been evaluated for ovarian disease, there could have been a higher incidence of total or multiple disease.
Epididymal Sperm collection
The epididymis was dissected free of connective tissue, the fat pad, the muscles and the vas deferens and then a small cut was made to the cauda epididymis. The tissue was placed in 5 ml F12 culture medium containing 0.1% bovine serum albumin for 10 minutes at 37°C and then kept at 4°C to immobilize the sperm. The epididymal tissue in the buffer was put on a petri dish and minced with a blade to release the sperm then the buffer was aspirated with a pipette into a 1.5 ml centrifuge tube and centrifuged at 13, 000 × g to pellet the sperm. Sperm were stored in fresh NIM buffer (Nucleus Isolation Medium: 123.0 mmol/l KCl, 2.6 mmol/l NaCl, 7.8 mmol/l NaH2PO4, 1.4 mmol/l KH2PO4 and 3 mmol/l EDTA (disodium salt) at −20°C until processed further.
Sperm DNA isolation and methylated DNA immunoprecipitation (MeDIP)
Sperm heads were separated from tails through sonication following previously described protocol (without protease inhibitors) [24] and then purified using a series of washes and centrifugations [25] from a total of nine F3 generation rats per treatment (control or pesticide) that were 120 days of age. DNA extraction on the purified sperm heads was performed as previously described [3]. The same concentrations of DNA from individual sperm samples were then used to produce pools of DNA material. Three DNA pools were produced in total per treatment, each one containing the same amount of sperm DNA from three different animals. Therefore, a total of 18 animals were used for building three DNA pools per treatment (control or pesticide) making the following groups: C1–C3 and P1–P3. These DNA pools were then used for methylated DNA immunoprecipitation (MeDIP). MeDIP was performed as follows: 6 μg of genomic DNA was subjected to series of three 20 pulse sonications at 20% amplitude and the appropriate fragment size (200–1000 ng) was verified through 2% agarose gels; the sonicated genomic DNA was resuspended in 350 μl TE buffer and denatured for 10 min at 95°C and then immediately placed on ice for 5 min; 100 μl of 5× IP buffer (50 mM Na-phosphate pH 7, 700 mM NaCl, 0.25% Triton X-100) was added to the sonicated and denatured DNA. An overnight incubation of the DNA was performed with 5 μg of antibody anti-5-methylCytidine monoclonal from Diagenode (Denville, NJ) at 4°C on a rotating platform. Protein A/G beads from Santa Cruz were prewashed on PBS-BSA 0.1% and resuspended in 40 μl 1× IP buffer. Beads were then added to the DNA-antibody complex and incubated 2 h at 4°C on a rotating platform. Beads bound to DNA-antibody complex were washed 3 times with 1 ml 1× IP buffer; washes included incubation for 5 min at 4°C on a rotating platform and then centrifugation at 6000 rpm for 2 min. Beads-DNA-antibody complex were then resuspended in 250 μl digestion buffer (50 mM Tris HCl pH 8, 10 mM EDTA, 0.5% SDS) and 3.5 μl of proteinase K (20 mg/ml) was added to each sample and then incubated overnight at 55°C on a rotating platform. DNA purification was performed first with phenol and then with chloroform:isoamyl alcohol. Two washes were then performed with 70% ethanol, 1 M NaCl and glycogen. MeDIP selected DNA was then resuspended in 30 μl TE buffer.
Tiling Array MeDIP-Chip Analysis
Roche Nimblegen's Rat DNA Methylation 3x720K CpG Island Plus RefSeq Promoter Array was used, which contains three identical sub-arrays, with 720,000 probes per sub-array, scanning a total of 15,287 promoters (3,880 bp upstream and 970 bp downstream from transcription start site). Probe sizes range from 50–75 mer in length with the median probe spacing of 100 bp. Three different comparative (MeDIP vs. MeDIP) hybridization experiments were performed (3 sub-arrays) for pesticide lineage versus control, with each subarray encompassing DNA samples from 6 animals (3 each from pesticide and control). MeDIP DNA samples from experimental lineages were labeled with Cy3 and MeDIP DNA samples from the control lineage were labeled with Cy5.
Bioinformatic and Statistic Analyses of Chip Data
For each comparative hybridization experiment raw data from both the Cy3 and Cy5 channels were imported into R (R Development Core Team (2010), R: A language for statistical computing, R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org), checked for quality and converted to MA values (M = Cy5−Cy3; A = (Cy5+Cy3)/2). The following normalization procedure was conducted as previously described [18]. Within each array probes were separated into groups by GC content and each group was separately normalized between Cy3 and Cy5 using the LOESS normalization procedure [26]. After each array was normalized within array, the arrays were then normalized across arrays using the A quantile normalization procedure [27]. The LOESS and A quantile normalization were performed using the limma library [28]. Following normalization the probe's normalized M (and then A) values were replaced with the median value of all probe normalized M (and then A) values within a 600 bp window across all arrays [29–31], due to the size of DNA fragments analyzed. If the number of adjacent probes present in the window was less than 3 no value was assigned to that probe. Following normalization, the median intensity difference between pesticide F3 generation lineage and control F3 generation lineage of a 600 bp window was determined. Significance was assigned to probe differences between pesticide F3 generation lineage samples and control F3 generation lineage samples by calculating the median value of the intensity differences as compared to a normal distribution scaled to the experimental mean and standard deviation of the normalized M. A Z-score and P-value were computed for each probe from that distribution. The statistical analysis was performed in pairs of comparative IP hybridizations between pesticide (P) and controls (C) lineages (e.g. P1-C1 and P2-C2; P1-C1 and P3-C3; P2-C2 and P3-C3). In order to assure the reproducibility of the candidates obtained, only the candidates showing significant changes in all of the single paired comparisons were chosen as having a significant change in DNA methylation between each experimental lineage and control. This is a very stringent approach to select for changes, since it only considers repeated changes in all paired analysis. Clustered Regions of interest were then determined by combining consecutive probes within 600 bases of each other and based on whether their mean M values were positive or negative with significance P-values less than 10−5. The statistically significant differential DNA methylated regions (DMR) were identified and P-value associated with each region presented. Each region of interest was then annotated for gene and CpG content. This list was further reduced to those regions with an average intensity value exceeding 9.5 (log scale) and a CpG density ≥ 1 CpG/100 bp.
Statistical Analysis of Rat Organ and Disease Data
For statistical analysis all data on body and organ weights were used as input in the program GraphPad© Prism 5 statistical analysis program and t-tests were used to determine if the data from the pesticide lineage differ from those of control lineage. For the number of rats with disease a logistic regression analysis was used to analyze the data (control or pesticide and diseased or unaffected). Similar statistical results were obtained with Chi square analysis, but was less stringent than regression analysis. All treatment differences were considered significant if P value was less than 0.05. The number of animals per litter (litter representation) was statistically similar between the control and pesticide lineage analysis for each individual disease/abnormality examined, for both male and female F1 and F3 generation analysis.
Results
Transgenerational Adult-onset Disease Analysis
The experimental design involved exposure of gestating female rats to daily intraperitoneal injections of DMSO vehicle (control) or a “pesticide mixture” (permethrin and DEET), during embryonic day 8 to 14 of gestation. F1 generation rat offspring born to different exposed females were bred to obtain the F2 generation. No sibling or cousin breeding was used to avoid any inbreeding artifacts in generating the different lineages. F3 generation animals were obtained by breeding non-littermate females and males of F2 generation. Selected offspring of F1 and F3 generations were aged to one year and examined for disease. Testis, prostate, kidney and ovary were examined for histopathology as outlined in the Methods.
Potential toxicity from direct fetal exposure to pesticide in the rat F1 generation was determined and comparisons made to the F3 generation through analysis of body weight and organ weights of one-year old rats (Supplemental Table S1). There were no effects in F1 generation of pesticide lineage females on body and organ weights. In F3 generation females of the pesticide lineage compared to the control lineage body weight increased slightly and uterine weights decreased slightly. There were no effects on male F1 and F3 generation male body weight, as well as weights of the testis, prostate and seminal vesicle. Small decreases in epididymis and kidney were observed in the F1 generation males, but not F3 generation (Supplemental Table S1B). Therefore, no overt toxicity was observed in the F1 generation from direct exposure and the suitable changes observed in the F1 and F3 generations require further investigation regarding physiological significance. To assess endocrine alterations, serum sex steroid hormone concentrations were measured in F3 generation control and pesticide lineages. Serum testosterone concentrations in 1-year-old F3 generation male rats from pesticide lineage did not differ from that of control lineage. Serum estradiol concentrations in female rats during proestrus-estrus phase or diestrus phase were also not different between pesticide and control lineages (Supplementary Figure S1). Combined observations indicate that there were no major steroid hormone alterations or gross toxicity effects of the pesticide exposure at the doses administered.
One of the major disease phenotypes observed in F1 and F3 generation pesticide lineage males was testicular disease. There was a significant increase in transgenerational testis disease incidence in age-matched F1 and F3 generation pesticide lineage males aged to one year (Figure 1A). Testis histopathological abnormalities included azoospermic and seminiferous tubule defects, the presence of vacuoles in basal regions of the seminiferous tubules, sloughed spermatogenic cells in the center of seminiferous tubules and lack of seminiferous tubule lumen, as previously described [20] (Figures 1C and 1D). Further analysis of testis abnormalities examined the number of apoptotic spermatogenic cells within the testis of male rats in pesticide lineage using TUNEL analysis and no significant alterations from that of the control lineage in both F1 and F3 generations were observed (Supplemental Figure S2). All cells in the testis were examined for TUNEL staining with no differences determined. Therefore, spermatogenic defects that were previously observed in vinclozolin lineage F3 generation rats [2] were not observed in F3 generation rats from pesticide lineage. The F3 generation males from pesticide lineage did not show any significant increase in transgenerational prostate disease (Figure 1B), kidney disease (Figure 2B), tumor development or obesity (data not shown). A significant increase in pubertal abnormalities (comprising early or delayed onset of puberty) was documented in F3 generation male rats of pesticide lineage compared to control lineage (Figure 3). The F1 generation males of pesticide lineage had a total 28% incidence of pubertal abnormalities, with the majority of these being early pubertal onset. In the control lineage a total 18% incidence of pubertal abnormalities was observed with the majority being delayed pubertal onset. The F3 generation males of pesticide lineage had a total 18% incidence of pubertal abnormalities, with the majority being delayed onset of puberty. In the control lineage a total 8% incidence of pubertal abnormalities was observed with the majority being early pubertal onset. Therefore, ancestors exposed to pesticide transmitted testis disease and pubertal abnormalities to their unexposed F3 generation male descendants.
Figure 1.
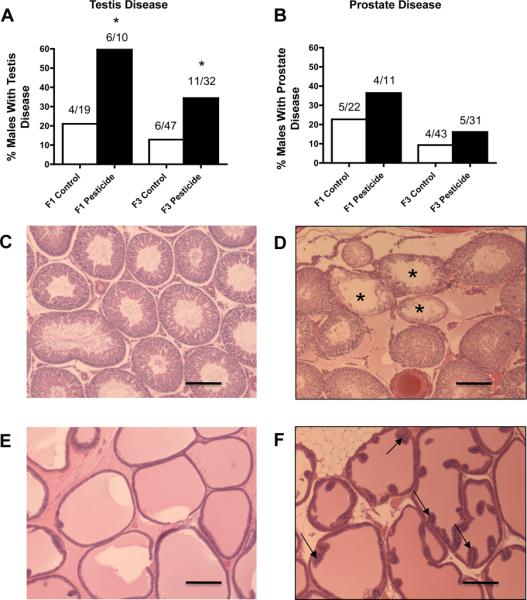
Ancestral (F0 generation female) exposure to a commonly used pesticide-insect repellent mixture (Permethrin + DEET) promoted adult-onset transgenerational testis disease, but no significant prostate disease. Percentages of males with testis (panel A) or prostate disease (panel B) in F1 and F3 generations are presented. The actual number of diseased rats / total number of rats in each lineage are shown above the respective bar graphs (* P<0.05). Representative micrographs (Scale bar = 200 μm) showing histopathology images of adult-onset transgenerational testis and prostate disease in pesticide lineage (panels D and F) compared to F3 generation control lineage (panels C and E). Testis sections from pesticide lineage showed histopathology including azoospermic and atretic seminiferous tubules, (marked by asterisks in panel D), presence of vacuoles in basal regions of seminiferous tubules, sloughed spermatogenic cells in center of seminiferous tubule and lack of seminiferous tubule lumen. Prostate sections showed hyperplastic ductular epithelium (marked by arrows in panel F).
Figure 2.
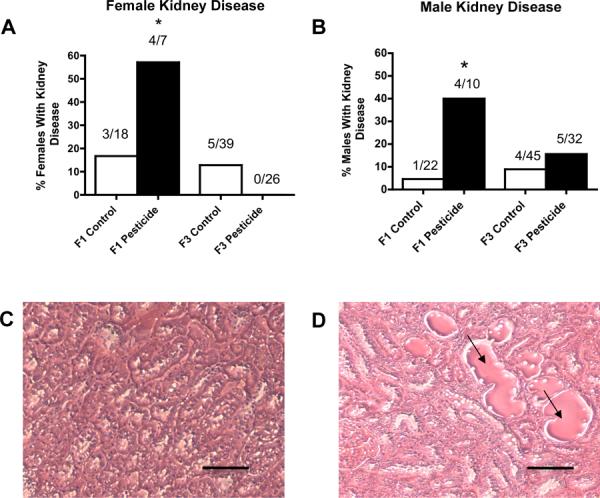
Ancestral (F0 generation female) exposure to a commonly used pesticide-insect repellent mixture (Permethrin + DEET) promoted no significant adult-onset transgenerational kidney disease in males or females. Percentages of females (panel A) and males (panel B) with kidney disease in F1 and F3 generations are presented. The actual number of diseased rats / total number of rats in each lineage are shown above the respective bar graphs (* P<0.05). Representative micrographs (Scale bar = 100 μm) showing histopathology images of adult-onset transgenerational kidney disease in pesticide lineage (panel D) compared to F3 control lineage (panel C). Kidney sections showed proteinaceous fluid filled cysts (marked by arrows in panel D).
Figure 3.
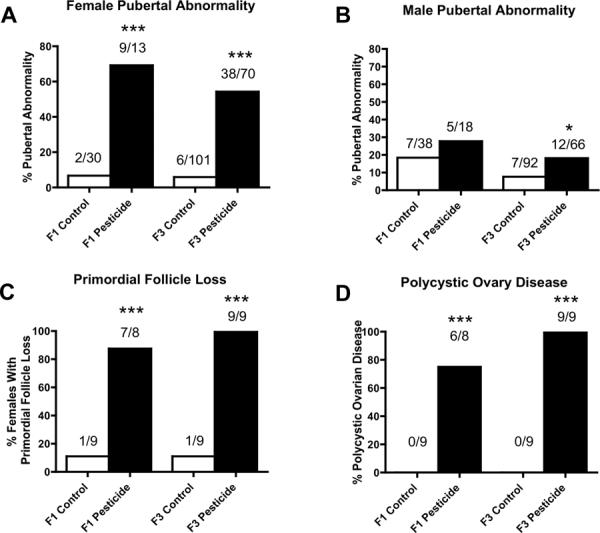
Ancestral (F0 generation female) exposure to a commonly used pesticide-insect repellent mixture (Permethrin + DEET) promoted transgenerational pubertal abnormality in females and males, adult-onset transgenerational primordial follicle loss and polycystic ovary disease in females and no significant tumor development in males or females. Percentages of females (panel A) and males (panel B) with pubertal abnormality or those with primordial follicle loss (panel C) or polycystic ovary disease (panel D) in F1 and F3 generations are presented. The actual number of diseased rats / total number of rats in each lineage are shown above the respective bar graphs (* P<0.05; *** P<0.001).
There were significant increases in the incidence of transgenerational diseases in F3 generation female rats of the pesticide lineage. They include pubertal abnormalities and ovarian disease as previously described [18, 23]. The ovarian disease was comprised of primordial follicle loss (Figure 3C), which was shown by a severe reduction in the number of primordial follicles per ovary section, and polycystic ovarian disease (Figure 3D), which was characterized by an increase in the number of cysts. The increase in the proportion of F3 generation females of pesticide lineage with ovarian disease was dramatic. In control lineage F1 and F3 generation females only 1 out of 9 had primordial follicle loss. The majority of F1 generation pesticide lineage females (87.5%, 7/8) and all of the F3 generation females of pesticide lineage (100%, 9/9) examined had a significant loss of primordial follicles, depleting the ovarian follicular reserve. This condition is a predecessor of primary ovarian insufficiency. In the control lineage none of the 9 females in each of F1 and F3 generations had polycystic ovarian disease. In animals that experienced a primordial follicle loss, the majority of F1 females (75%, 6/8) and all of the F3 females of pesticide lineage (100%, 9/9) showed a significant increase in number of cysts within the ovary. Polycystic ovarian disease is the most common ovarian disease prevalent in women of reproductive age. Therefore, the epigenetic transgenerational inheritance of ovarian disease following ancestral exposure to environmental compounds is a novel etiology to be considered [23].
The F1 generation females of pesticide lineage had a total 69% incidence of pubertal abnormalities, with the majority (89%) of these being delayed pubertal onset (Figure 3A). The control lineage had a total 7% incidence of pubertal abnormalities, all of them being delayed pubertal onset. In contrast, the F3 generation females of pesticide lineage had a total 54% incidence of pubertal abnormalities, with the majority (55%) of these being early onset of puberty (Figure 3A). The control lineage F3 generation females had a total 6% pubertal abnormalities, with the majority (83%) of these being early pubertal onset. The F3 generation females from pesticide lineage did not present any increase in adult onset kidney disease (Figure 2A), tumor development and obesity disease (data not shown). These combined observations indicate ancestral exposure to pesticide promotes transgenerational inheritance of pubertal abnormalities and ovarian disease to the F3 generation female descendants.
Additional less frequent diseases were observed in the F3 generation animals from the pesticide lineage. These “other diseases” include focal fat necrosis, mammary gland hyperplasia and white discharge from penis. Focal fat necrosis is usually a sign of inflammation due to contusions or constant sitting. Lack of activity from constant sitting may predispose the rat to obesity as well. Mammary gland hyperplasia was determined histologically by the Washington Animal Disease Diagnostic Laboratory. Mammary gland presence of milk in a female was also detected without being pregnant previously. This could arise due to a pituitary tumor secreting prolactin. Penile white discharge may indicate urethritis and a possible urinary infection. These various pathologies were infrequent, but were predominant in rats from the pesticide lineage and not observed in rats from the control lineage.
The presence or absence of diseases in individual rats in control and pesticide lineages is presented in Supplemental Tables S2A (F1 generation females), S2B (F1 generation males), S3A (F3 generation females) and S3B (F3 generation males). These tables list the occurrence of diseases for each rat. The incidence of total disease increased significantly in both the F1 and F3 generation females of the pesticide lineage (Figure 4A). In addition, the incidence of multiple disease increased significantly both in F1 and F3 generation females from the pesticide lineage (Figure 4C). The incidence (%) of total disease increased significantly in both the F1 and F3 generation males of the pesticide lineage (Figure 4B). The incidence of multiple disease increased significantly in the F1 generation males of the pesticide lineage. These observations demonstrate that ancestral exposure to pesticide increased the overall incidence of transgenerational adult onset diseases in both F1 and F3 generation females and males.
Figure 4.
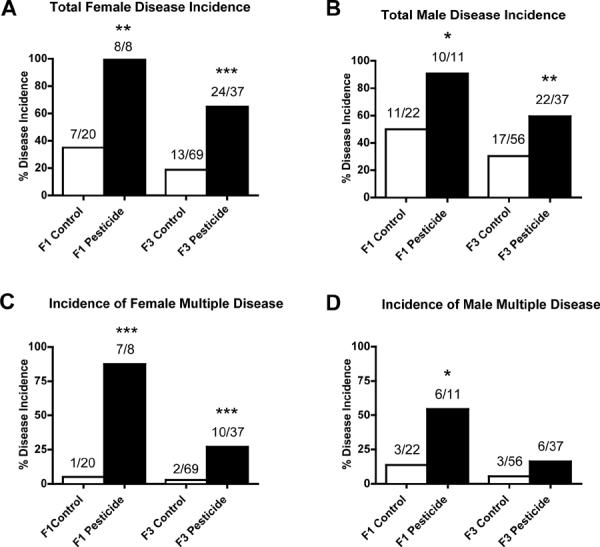
Ancestral (F0 generation female) exposure to a commonly used pesticide-insect repellent mixture (Permethrin + DEET) promoted adult-onset transgenerational diseases in rats. Incidences of total female disease (panel A), total male disease (panel B), female multiple disease (panel C) and male multiple disease (panel D) in F1 and F3 generations are presented. The actual number of diseased rats / total number of rats in each lineage are shown above the respective bar graphs (* P<0.05; ** P<0.01; *** P<0.001).
Transgenerational Effects on the Sperm Epigenome
Environmentally induced epigenetic transgenerational inheritance of adult-onset disease involves an altered germline (sperm) epigenome transmission between generations. The transgenerational F3 control and pesticide lineage sperm epigenomes were analyzed and compared using a methyl cytosine antibody methylated DNA immunoprecipitation (MeDIP) followed by a genome-wide promoter tiling array chip (MeDIP-Chip) assay [3]. A comparative hybridization with the MeDIP-Chip assay was performed as described in the Methods to identify differential DNA methylation between the control and pesticide lineage sperm DNA pools. This analysis identified statistically significant differential DNA methylation regions (DMR) in 363 different promoters with a range of 600–1800 bp in size, Table S4. The chromosomal locations of these differentially methylated regions are presented in Figure 5. The sperm DMR (termed epimutations) were distributed throughout the genome on all chromosomes examined. The functional gene categories of the genes containing the DMR are shown in Figure 6 and Table S4. Therefore, the exposure to a pesticide mixture induced a transgenerational sperm epigenome alteration.
Figure 5.
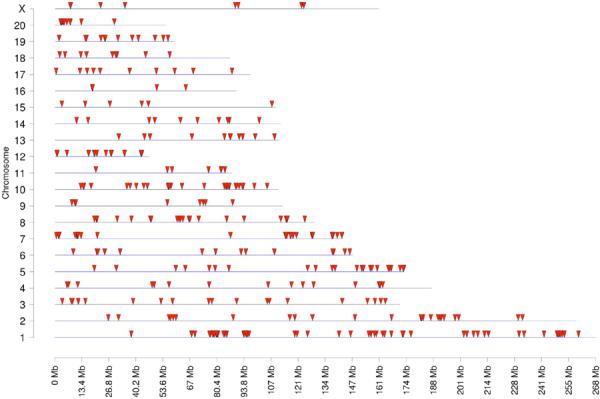
Regions presenting pesticide-induced transgenerational change in F3 generation sperm DNA methylation. Chromosomal locations for regions detected with MeDIP-Chip with transgenerational change in DNA methylation are indicated with arrowheads. There were 363 differentially methylated regions in sperm DNA from pesticide lineage compared to control lineage.
Figure 6.
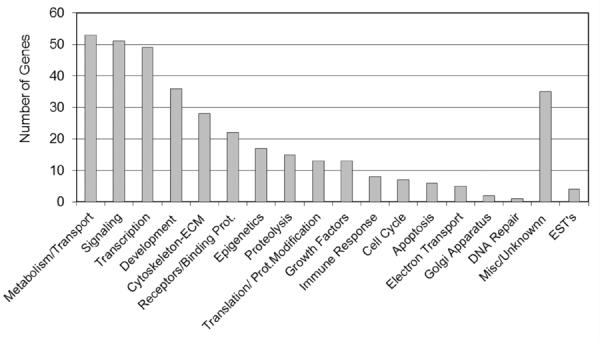
Functional gene categories associated with the F3 generation rat genes with altered DNA methylation (DMR) due to ancestral exposure to pesticide.
Analysis of the genes containing the DMR for potential highly represented cellular pathways and processes did not identify any pathways with a predominance of DMR, Table S5. The potential impact of the identified pathways are presented. A further gene network analysis was performed to identify a potential direct connection gene network associated with the DMR, Figure 7. The network contained a number of extracellular, membrane, cytoplasmic and nuclear localized genes associated with the DMR identified. The vascular endothelial growth factor A (VEGFA), matrix metalloprotein 2 (MMP2) and glial derived neurotrophic factor (GDNF) are the cellular signaling pathways and processes that appear to be predominant in the gene network identified. Therefore, pesticide induced a transgenerational sperm epigenome and the DMR epimutations potentially influence specific cellular pathways and a gene network.
Figure 7.
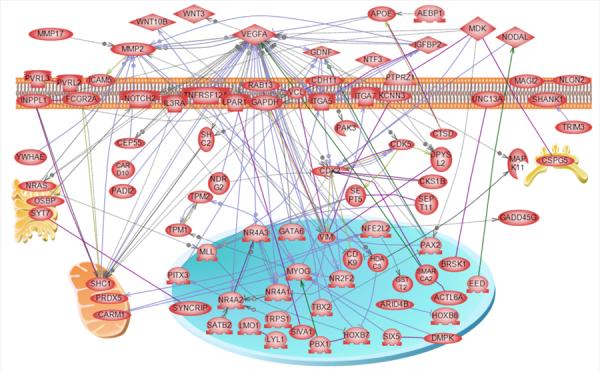
Direct connection gene network for list of F3 generation rat genes with altered DNA methylation. Direct connection genes are shown according to their location in the cell, the rest of genes are not connected and not shown. Node shapes code: oval and circle – protein; diamond – ligand; circle/oval on tripod platform – transcription factor; ice cream cone – receptor; crescent – kinase or protein kinase; irregular polygon - phosphatase. Arrows with plus sign show positive regulation/activation, arrows with minus sign – negative regulation/inhibition; grey arrows represent regulation, lilac - expression, purple – binding, green – promoter binding, and yellow – protein modification.
Discussion
The current study was designed to investigate the potential actions of a pesticide mixture to promote epigenetic transgenerational inheritance of adult-onset disease. Gestating female rats, designated F0 generation, were exposed to the pesticide mixture or DMSO vehicle daily intraperitoneal injections from embryonic day 8 (sperm positive day = day 0) through embryonic day 14. The F1 generation progeny were bred to obtain the F2 generation, which were then bred to generate the F3 generation. Only the F0 generation females and not the F1, F2 and F3 generation individuals were treated. The F1 and F3 generation animals were aged to one year of age and then euthanized. Histopathological examination included analysis of the testis, prostate, kidney and ovary. The transgenerational F3 generation sperm epigenome (DNA methylation) was examined by an MeDIP-Chip analysis and the chromosomal locations of the DMR identified.
Toxicological effects of the pesticide mixture has been documented in previous studies primarily using direct exposure studies. These studies generally used high toxic doses [32]. The documented toxicity of permethrin and DEET includes testicular histopathological damage [33], uterine enlargement, atrophy of male secondary sex organs [6], testicular cancer [7], liver damage [34], heart damage [35], neurological disorders and behavioral changes [8–10], oxidative stress in brain [11], DNA damage [12] and immunotoxicity [13]. The current study was designed to examine the actions of pharmacological doses of the pesticide mixture compounds on epigenetic transgenerational inheritance of adult-onset disease. The doses used were based on a fraction of the oral LD50 dose for permethrin (39%) and DEET (2%). Although no overt toxicity was observed in the F1 generation, the mode of administration and dose do not allow for risk assessment of these compounds from the current study. The objective of the study was to investigate if exposure to the pesticide mixture could potentially promote epigenetic transgenerational inheritance of a disease phenotype and not to assess risk of exposure to these compounds. Future studies with a more appropriate mode of administration and dose curves will be required for risk assessment. However, the current study demonstrates the potential of the mixture of the pesticide permethrin and insect repellent DEET to promote epigenetic transgenerational inheritance of disease.
Transgenerational disease phenotypes are unique in the sense that they are not caused directly by the exposure to the environmental chemical. As discussed above, toxicological effects of these compounds are generally seen in direct exposure studies. When the exposure occurs on a gestating female during the critical time of gonadal sex determination, not only the mother but also the developing fetus and its fetal germ cells are directly exposed. Therefore, pathology observed in F0, F1 (via fetal exposure) and F2 (via fetal germ cell exposure) might be caused at least in part by the direct effect on somatic cells. The F3 generation derived from the exposed F0 generation is the first generation to clearly demonstrate epigenetic transgenerational disease phenotypes [17]. In the current study we examined the pathology in the F1 generation to observe any direct exposure somatic cell epigenetic effects and in the F3 generation animals to observe the epigenetic transgenerational effects. Although similarities in phenotype can occur, the distinct mechanisms involved suggests differences in phenotypes are anticipated.
The primary epigenetic transgenerational disease phenotypes observed include testis disease, ovary disease, and pubertal abnormalities. Testicular disease incidence was significantly higher in F3 generation pesticide lineage males at one year of age. The spermatogenic cell apoptosis or sperm numbers and sperm motility in these males did not differ from those of F3 generation control lineage males. Therefore, the testis disease was not associated with a corresponding apoptosis of germ cells, as seen with other exposures [2, 18]. In recent years, there has been a trend of a gradual decline in sperm concentration in most human populations [36] and human male infertility is approaching 10% [37]. However, there are forms of idiopathic male infertility and testis disease that are not related to sperm number decline. The etiology of testicular disease and rise in infertility are suspected to be at least in part due to exposure to environmental chemicals including endocrine disruptor toxicants [38]. The potential role of environmentally induced epigenetic transgenerational inheritance of male infertility needs to be considered [1, 20]. The testicular disease in F3 generation pesticide lineage males provides support for a role of environmental epigenetics and ancestral exposures in male infertility.
Ovarian disease in the form of primordial follicle loss and polycystic ovarian disease was significantly increased in F3 generation pesticide lineage females at one year of age. The current human female population of the world are facing ovarian diseases of primary ovarian insufficiency, characterized by primordial follicle loss, and polycystic ovarian disease, characterized by the presence of anovulatory cystic structures [39, 40]. Similar to the testicular disease observed, ovarian disease phenotypes in the current study are the outcome of epigenetic transgenerational inheritance following environmental compound exposure to F0 generation ancestors [23]. It is important to note that this ovarian disease was observed at an increased frequency in both the directly exposed F1 generation and the transgenerational F3 generation. In a previous publication, it was shown that there was a significant transgenerational alteration in both the transcriptome and the epigenome of F3 generation vinclozolin-lineage granulosa cells [23]. Therefore, ancestral exposures may contribute to the development of these ovarian diseases. Observations suggest a new paradigm to be considered for the etiology of primary ovarian insufficiency and polycystic ovarian disease in women. Although the ovarian morphology is consistent with these ovarian diseases, other conditions associated such as anovulation, insulin resistance and abnormal adiposity remain to be evaluated [23]. Epigenetic mechanisms have been suggested to underlie the development of polycystic ovarian syndrome in women [41] and the primary ovarian insufficiency observed in prenatally androgenized rhesus monkeys [42]. Observations demonstrate germline mediated epigenetic mechanism allows the transmission of the ovarian disease to future generation offspring following ancestral exposure to a variety of toxicants [18].
Pubertal abnormalities in the form of early onset or delayed onset were significantly increased in both female and male F3 generation animals of the pesticide lineage. Puberty is a milestone in developmental physiology with the hypothalamus-pituitary-gonad axis showing progressive changes during adolescence [43]. In rats there are clear external genital changes that indicate pubertal onset such as vaginal opening and balano-preputial separation [44]. In this study, puberty checks were performed from postnatal day 30 in females and day 35 in males. In an earlier report [18] it was shown that F3 generation pesticide lineage females did not have a significant alteration of the pubertal onset (number of days to pubertal onset) compared to control females. The current study assessed the pubertal abnormalities differently using a puberty cutoff which was equal to the mean of controls ± 2 standard deviations and reports a significantly increased percent incidence of pubertal abnormalities of F3 generation pesticide lineage females and males. Pubertal abnormalities were examined because of the dramatic increase in pubertal abnormalities over the past several decades in human populations [43]. Importantly, the early and delayed onsets of puberty are forerunners to different adult onset disease. Early onset of puberty leads to accelerated bone mineralization and short adult height in girls and predisposes them to breast cancers [45]. Delayed onset of puberty leads to decreased bone mineralization, psychological stress and metabolic disease [45]. In the present study, F1 generation females of pesticide lineage had increased proportion of delayed pubertal onset, while males of pesticide lineage had increased proportion of early pubertal onset, indicating sexually dimorphic direct-exposure effects. In the F3 generation pesticide lineage females had an increased proportion of early onset of puberty while males of pesticide lineage manifested increased proportion of delayed onset of puberty. Therefore, the pubertal abnormalities were sexually dimorphic. The pubertal onset in the pesticide lineage rats is an example where direct exposure (F1) and transgenerational (F3) effects are very different. Previous studies have suggested early onset of puberty in girls is suspected to be caused by environmental exposure to endocrine disruptor [46]. One support for this suggestion is the early onset of puberty that resulted from exposure of mice to phytoestrogens [47]. Early onset of puberty in girls disrupts health by affecting brain development, endocrine organ systems and growth leading to later life increase in susceptibility to disease. Interestingly, the pubertal onset (an early developmental milestone) in the current study is associated with the epigenetic transgenerational inheritance of ovarian diseases. Therefore, ancestral exposure to the pesticide mixture (permethrin and DEET) results in pubertal abnormalities that may have an endocrine and complex association with adult onset ovarian diseases in the transgenerational phenotypes.
The molecular mechanism involved in the environmentally induced epigenetic transgenerational inheritance of adult-onset disease phenotypes involves reprogramming of the germline (sperm) epigenome during male gonadal sex determination [1, 4]. The modified sperm epigenome appears to be permanently reprogrammed and protected from DNA demethylation and reprogramming events during early embryonic development. This allows transgenerational transmission of the modified sperm epigenome that subsequently alters all somatic cell and tissue epigenomes and transcriptomes promoting epigenetic transgenerational inheritance of disease phenotypes [48]. Therefore, the current study examined the altered sperm epigenome and epimutations induced by the pesticide mixture.
Transgenerational alterations in sperm DNA methylation has been shown to be induced by vinclozolin [2, 3]. Similarly, a transgenerational change in the testis transcriptome is documented to be induced by vinclozolin [49]. In the current study, F3 generation rat sperm from pesticide and control lineages were used for genome wide promoter DNA methylation analysis by MeDIP-Chip analysis as previously described [3]. Differential DNA methylated regions (DMR) defined as epimutations and epigenetic biomarkers [18] were identified for pesticide lineage F3 generation sperm in comparison with control lineage F3 generation sperm. The list of DMR is presented in Supplemental Table S4. As previously described [18], the primary genomic feature identified in the F3 generation pesticide lineage sperm DMR was a CpG density less than 10 CpG/100bp (10%). The epigenetic analysis confirmed the development of epimutations in the sperm and a role in epigenetic transgenerational inheritance of the disease phenotypes observed. A gene network analysis for direct connections within the total gene set associated with DMR is provided in Figure 7. Several cellular signaling pathways and processes were identified within the gene network. Although these pathways were identified, the sperm epigenome will generate altered epigenomes in all the cells generated which will be distinct between cells [48]. The cascade of epigenetic and genetic (transcriptome) changes to generate an adult cell type will likely have negligible correlation with the specific epigenome and associated genes. As an example, the vinclozolin induced F3 generation granulosa cell epigenetic alterations were distinct from the F3 generation sperm [23]. Future studies on the various cells associated with disease phenotype will be required to determine the potential somatic epigenome changes and the correlations with the sperm DMR identified. However, the current study documents the altered germline epigenome that can transmit the transgenerational phenotype. Although the current study has focused on the transgenerational sperm, the potential the female germline also may be influenced remains a possibility that needs to be addressed. The transmission of genetic or epigenetic information between the generations requires the germline, such that the altered sperm epigenome appears to initiate a cascade of events in the epigenomes in a variety of organs that promote adult onset disease [48]. The specific cascade of events that provided the causal relationship between the altered germline epigenome and adult onset disease [23] remains to be elucidated.
Epigenetic transgenerational inheritance of diseases has been shown to be promoted by a variety of environmental compounds [18]. Vinclozolin exposure resulted in F3 generation testis disease, prostate disease, kidney disease, immune system abnormalities, tumors, uterine hemorrhage during pregnancy and polycystic ovary disease [2, 16, 19, 50]. Alterations in methylation patterns of sperm of F3 generation rats and mice have been reported following exposure of F0 generation females to vinclozolin [2, 3, 50, 51]. Exposure of F0 generation gestating rats to bisphenol-A (BPA) caused decreased fertility in F3 generation males [52]. Transgenerational decline in fertility in F3 generation mice was also documented following exposure to dioxin of gestating F0 generation females [53]. Recently, the ability of plastics (BPA and phthalates), dioxin, hydrocarbon mixture (jet fuel JP8), and the pesticide mixture of permethrin and DEET have been shown to promote epigenetic transgenerational inheritance of adult onset disease [18]. Other environmental factors such as nutrition [54] also can promote epigenetic transgenerational inheritance of disease phenotypes. Demonstration of epigenetic transgenerational inheritance in worms [55], flies [56], plants [57] and mammals [58–60] suggest this phenomenon will likely be critical in biology and disease etiology in mammals [1].
Combined observations demonstrate exposure of gestating females during the critical development period of gonadal sex determination to a pesticide mixture consisting of permethrin and DEET promotes epigenetic transgenerational inheritance of adult-onset disease including testis disease, ovary disease and pubertal abnormalities. All of the disease phenotypes have an impact on fertility and reproduction. The overall increase in total disease and multiple diseases in the F3 generation pesticide lineage is considerable. Associated with the occurrence of these transgenerational diseases are the epigenetic changes in the rat sperm DNA. These epimutations may be useful as early stage biomarkers of not only the compound exposure but also the adult onset disease. Although this study was not designed for risk assessment, observations have implications for the human population that is increasingly exposed to these compounds and is experiencing significant decline in fertility and incidence of adult-onset disease.
Supplementary Material
Highlights
First observation that a mixture of permethrin and DEET promotes epigenetic transgenerational inheritance of disease.
Ancestral environmental exposures promoted transgenerational sperm epimutations.
Suggestion ancestral exposures may be part of the etiology of testis and ovarian disease.
Provides additional support for the ability of environmental toxicants to promote epigenetic transgenerational inheritance of disease.
Acknowledgments
We thank the expert technical assistance of Dr. Eric Nilsson, Dr. Marina Savenkova, Ms. Shelby Weeks, Ms. Renee Espinosa Najera, Ms. Jessica Shiflett, Ms. Ginger Beiro, Ms. Chrystal Bailey, Ms. Colleen Johns, and Ms. Sean Leonard, as well as the assistance of Ms. Heather Johnson in preparation of the manuscript. We acknowledge the helpful advice of Dr. David Jackson and Dr. John Lewis, US Army Center for Environmental Health Research, Department of Defense (DOD), and Dr. Paul Nisson and the leadership at the DOD TATRC. This research was supported by DOD and NIH grants to MKS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21:214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerrero-Bosagna C, Settles M, Lucker B, Skinner M. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE 2010. 5:e13100. doi: 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitchen LW, Lawrence KL, Coleman RE. The role of the United States military in the development of vector control products, including insect repellents, insecticides, and bed nets. J Vector Ecol. 2009;34:50–61. doi: 10.1111/j.1948-7134.2009.00007.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim SS, Lee RD, Lim KJ, Kwack SJ, Rhee GS, Seok JH, Lee GS, An BS, Jeung EB, Park KL. Potential estrogenic and antiandrogenic effects of permethrin in rats. J Reprod Dev. 2005;51:201–210. doi: 10.1262/jrd.16060. [DOI] [PubMed] [Google Scholar]
- 7.Knoke JD, Gray GC, Garland FC. Testicular cancer and Persian Gulf War service. Epidemiology. 1998;9:648–653. [PubMed] [Google Scholar]
- 8.Abou-Donia MB, Wilmarth KR, Abdel-Rahman AA, Jensen KF, Oehme FW, Kurt TL. Increased neurotoxicity following concurrent exposure to pyridostigmine bromide, DEET, and chlorpyrifos. Fundam Appl Toxicol. 1996;34:201–222. doi: 10.1006/faat.1996.0190. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Donia MB, Wilmarth KR, Jensen KF, Oehme FW, Kurt TL. Neurotoxicity resulting from coexposure to pyridostigmine bromide, deet, and permethrin: implications of Gulf War chemical exposures. J Toxicol Environ Health. 1996;48:35–56. doi: 10.1080/009841096161456. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Rahman A, Shetty AK, Abou-Donia MB. Subchronic dermal application of N,N-diethyl m-toluamide (DEET) and permethrin to adult rats, alone or in combination, causes diffuse neuronal cell death and cytoskeletal abnormalities in the cerebral cortex and the hippocampus, and Purkinje neuron loss in the cerebellum. Exp Neurol. 2001;172:153–171. doi: 10.1006/exnr.2001.7807. [DOI] [PubMed] [Google Scholar]
- 11.Nasuti C, Gabbianelli R, Falcioni ML, Di Stefano A, Sozio P, Cantalamessa F. Dopaminergic system modulation, behavioral changes, and oxidative stress after neonatal administration of pyrethroids. Toxicology. 2007;229:194–205. doi: 10.1016/j.tox.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Gabbianelli R, Nasuti C, Falcioni G, Cantalamessa F. Lymphocyte DNA damage in rats exposed to pyrethroids: effect of supplementation with Vitamins E and C. Toxicology. 2004;203:17–26. doi: 10.1016/j.tox.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Peden-Adam MM, Eudaly J, Eudaly E, Dudley A, Zeigler J, Lee A, Robbs J, Gilkeson G, Keil DE. Evaluation of immunotoxicity induced by single or concurrent exposure to N,N-diethyl-m-toluamide (DEET), pyridostigmine bromide (PYR), and JP-8 jet fuel. Toxicol Ind Health. 2001;17:192–209. doi: 10.1191/0748233701th120oa. [DOI] [PubMed] [Google Scholar]
- 14.Abou-Donia MB, Goldstein LB, Jones KH, Abdel-Rahman AA, Damodaran TV, Dechkovskaia AM, Bullman SL, Amir BE, Khan WA. Locomotor and sensorimotor performance deficit in rats following exposure to pyridostigmine bromide, DEET, and permethrin, alone and in combination. Toxicol Sci. 2001;60:305–314. doi: 10.1093/toxsci/60.2.305. [DOI] [PubMed] [Google Scholar]
- 15.Issam C, Zohra H, Monia Z, Hassen BC. Effects of dermal sub-chronic exposure of pubescent male rats to permethrin (PRMT) on the histological structures of genital tract, testosterone and lipoperoxidation. Exp Toxicol Pathol. 2011;63:393–400. doi: 10.1016/j.etp.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Anway MD, Leathers C, Skinner MK. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology. 2006;147:5515–5523. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod Toxicol. 2008;25:2–6. doi: 10.1016/j.reprotox.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK. Transgenerational Actions of Environmental Compounds on Reproductive Disease and Epigenetic Biomarkers of Ancestral Exposures. PLoS ONE. 2012;7:e31901. doi: 10.1371/journal.pone.0031901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsson EE, Anway MD, Stanfield J, Skinner MK. Transgenerational epigenetic effects of the endocrine disruptor vinclozolin on pregnancies and female adult onset disease. Reproduction. 2008;135:713–721. doi: 10.1530/REP-07-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anway MD, Memon MA, Uzumcu M, Skinner MK. Transgenerational effect of the endocrine disruptor vinclozolin on male spermatogenesis. J Androl. 2006;27:868–879. doi: 10.2164/jandrol.106.000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meredith S, Dudenhoeffer G, Jackson K. Classification of small type B/C follicles as primordial follicles in mature rats. J Reprod Fertil. 2000;119:43–48. doi: 10.1530/jrf.0.1190043. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson EE, Schindler R, Savenkova MI, Skinner MK. Inhibitory actions of Anti-Mullerian Hormone (AMH) on ovarian primordial follicle assembly. PLoS ONE. 2011;6:e20087. doi: 10.1371/journal.pone.0020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson E, Larsen G, Manikkam M, Guerrero-Bosagna C, Savenkova M, Skinner M. Environmentally Induced Epigenetic Transgenerational Inheritance of Ovarian Disease. PLoS ONE. 2012;7:e36129. doi: 10.1371/journal.pone.0036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tateno H, Kimura Y, Yanagimachi R. Sonication per se is not as deleterious to sperm chromosomes as previously inferred. Biol Reprod. 2000;63:341–346. doi: 10.1095/biolreprod63.1.341. [DOI] [PubMed] [Google Scholar]
- 25.Ward WS, Kimura Y, Yanagimachi R. An intact sperm nuclear matrix may be necessary for the mouse paternal genome to participate in embryonic development. Biol Reprod. 1999;60:702–706. doi: 10.1095/biolreprod60.3.702. [DOI] [PubMed] [Google Scholar]
- 26.Smyth GK, Speed T. Normalization of cDNA microarray data. Methods. 2003;31:265–273. doi: 10.1016/s1046-2023(03)00155-5. [DOI] [PubMed] [Google Scholar]
- 27.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 28.Smyth GK. Limma: linear models for microarray data. In: Gentleman VC R, Dudoit S, Irizarry R, Huber W, editors. Bioinformatics and Computational Biology Solutions using R and Bioconductor. Springer; New York: 2005. pp. 397–420. [Google Scholar]
- 29.Tukey J. Reading. Addison-Wesley; MA: 1977. Exploratory data analysis. [Google Scholar]
- 30.Hardle W, Steiger W. Algorithm AS 296: Optimal median smoothing. Appl. Stat. 1977:258–264. [Google Scholar]
- 31.Toedling J, Skylar O, Krueger T, Fischer JJ, Sperling S, Huber W. Ringo--an R/Bioconductor package for analyzing ChIP-chip readouts. BMC Bioinformatics. 2007;8:221. doi: 10.1186/1471-2105-8-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCain WC, Lee R, Johnson MS, Whaley JE, Ferguson JW, Beall P, Leach G. Acute oral toxicity study of pyridostigmine bromide, permethrin, and DEET in the laboratory rat. J Toxicol Environ Health. 1997;50:113–124. doi: 10.1080/009841097160528. [DOI] [PubMed] [Google Scholar]
- 33.Jin Y, Liu J, Wang L, Chen R, Zhou C, Yang Y, Liu W, Fu Z. Permethrin exposure during puberty has the potential to enantioselectively induce reproductive toxicity in mice. Environ Int. 2012;42:144–151. doi: 10.1016/j.envint.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Roma GC, De Oliveira PR, Bechara GH, Camargo Mathias MI. Cytotoxic effects of permethrin on mouse liver and spleen cells. Microsc Res Tech. 2012;75:229–238. doi: 10.1002/jemt.21047. [DOI] [PubMed] [Google Scholar]
- 35.Vadhana MS, Carloni M, Nasuti C, Fedeli D, Gabbianelli R. Early life permethrin insecticide treatment leads to heart damage in adult rats. Exp Gerontol. 2011;46:731–738. doi: 10.1016/j.exger.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- 37.Hauser R, Sokol R. Science linking environmental contaminant exposures with fertility and reproductive health impacts in the adult male. Fertil Steril. 2008;89:e59–65. doi: 10.1016/j.fertnstert.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 38.Kumar S. Occupational exposure associated with reproductive dysfunction. J Occup Health. 2004;46:1–19. doi: 10.1539/joh.46.1. [DOI] [PubMed] [Google Scholar]
- 39.Vujovic S. Aetiology of premature ovarian failure. Menopause Int. 2009;15:72–75. doi: 10.1258/mi.2009.009020. [DOI] [PubMed] [Google Scholar]
- 40.Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004;18:671–683. doi: 10.1016/j.bpobgyn.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Xu N, Azziz R, Goodarzi MO. Epigenetics in polycystic ovary syndrome: a pilot study of global DNA methylation. Fertil Steril. 2010;94:781–783. e781. doi: 10.1016/j.fertnstert.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu N, Kwon S, Abbott DH, Geller DH, Dumesic DA, Azziz R, Guo X, Goodarzi MO. Epigenetic mechanism underlying the development of polycystic ovary syndrome (PCOS)-like phenotypes in prenatally androgenized rhesus monkeys. PLoS ONE. 2011;6:e27286. doi: 10.1371/journal.pone.0027286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DiVall, Radovick S. Endocrinology of female puberty. Curr Opin Endocrinol Diabetes Obes. 2009;16:1–4. doi: 10.1097/med.0b013e3283207937. [DOI] [PubMed] [Google Scholar]
- 44.Engelbregt MJ, Houdijk ME, Popp-Snijders C, Delemarre-van de Waal HA. The effects of intrauterine growth retardation and postnatal undernutrition on onset of puberty in male and female rats. Pediatr Res. 2000;48:803–807. doi: 10.1203/00006450-200012000-00017. [DOI] [PubMed] [Google Scholar]
- 45.Jacobson-Dickman E, Lee MM. The influence of endocrine disruptors on pubertal timing. Curr Opin Endocrinol Diabetes Obes. 2009;16:25–30. doi: 10.1097/med.0b013e328320d560. [DOI] [PubMed] [Google Scholar]
- 46.Traggiai C, Stanhope R. Disorders of pubertal development. Best Pract Res Clin Obstet Gynaecol. 2003;17:41–56. doi: 10.1053/ybeog.2003.0360. [DOI] [PubMed] [Google Scholar]
- 47.Guerrero-Bosagna CM, Sabat P, Valdovinos FS, Valladares LE, Clark SJ. Epigenetic and phenotypic changes result from a continuous pre and post natal dietary exposure to phytoestrogens in an experimental population of mice. BMC Physiol. 2008;8:17. doi: 10.1186/1472-6793-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skinner MK. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics. 2011;6:838–842. doi: 10.4161/epi.6.7.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anway MD, Rekow SS, Skinner MK. Transgenerational epigenetic programming of the embryonic testis transcriptome. Genomics. 2008;91:30–40. doi: 10.1016/j.ygeno.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guerrero-Bosagna C, Covert T, Haque MM, Settles M, Nilsson EE, Anway MD, Skinner MK. Epigenetic Transgenerational Inheritance of Vinclozolin Induced Mouse Adult Onset Disease and Associated Sperm Epigenome Biomarkers. Reproductive Toxicology. 2012 doi: 10.1016/j.reprotox.2012.09.005. Provisionally Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stouder C, Paoloni-Giacobino A. Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm. Reproduction. 2010;139:373–379. doi: 10.1530/REP-09-0340. [DOI] [PubMed] [Google Scholar]
- 52.Salian S, Doshi T, Vanage G. Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci. 2009;85:742–752. doi: 10.1016/j.lfs.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Bruner-Tran KL, Osteen KG. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol. 2011;31:344–350. doi: 10.1016/j.reprotox.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes (Lond) 2008;32:1373–1379. doi: 10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greer EL, Maures TJ, Ucar D, Hauswirth AG, Mancini E, Lim JP, Benayoun BA, Shi Y, Brunet A. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature. 2011;479:365–371. doi: 10.1038/nature10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruden DM, Lu X. Hsp90 affecting chromatin remodeling might explain transgenerational epigenetic inheritance in Drosophila. Curr Genomics. 2008;9:500–508. doi: 10.2174/138920208786241207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hauser MT, Aufsatz W, Jonak C. Luschnig C. Transgenerational epigenetic inheritance in plants. Biochim Biophys Acta. 2011;1809:459–468. doi: 10.1016/j.bbagrm.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rassoulzadegan M, Grandjean V, Gounon P, Vincent S, Gillot I, Cuzin F. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature. 2006;441:469–474. doi: 10.1038/nature04674. [DOI] [PubMed] [Google Scholar]
- 59.Wagner KD, Wagner N, Ghanbarian H, Grandjean V, Gounon P, Cuzin F, Rassoulzadegan M. RNA induction and inheritance of epigenetic cardiac hypertrophy in the mouse. Dev Cell. 2008;14:962–969. doi: 10.1016/j.devcel.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 60.Pembrey ME. Male-line transgenerational responses in humans. Hum Fertil (Camb) 2010;13:268–271. doi: 10.3109/14647273.2010.524721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


