Abstract
Rit, along with Rin and Drosophila Ric, comprises the Rit subfamily of Ras-related small GTPases. Although the cellular functions of many Ras family GTPases are well established, the physiological significance of Rit remains poorly understood. Loss of Rit sensitizes multiple mammalian cell lines and mouse embryonic fibroblasts (MEFs) derived from Rit−/− mice to oxidative stress-mediated apoptosis. However, whether Rit-mediated pro-survival signaling extends to other cell types, particularly neurons, is presently unknown. Here, to examine these issues we generated a transgenic mouse overexpressing constitutively active Rit (RitQ79L) exclusively in neurons, under control of the Synapsin I promoter. Active Rit-expressing hippocampal neurons display a dramatic increase in oxidative stress resistance. Moreover, pharmacological inhibitor studies demonstrate that p38 MAPK, rather than a MEK/ERK signaling cascade, is required for Rit-mediated protection. Together, the present studies identify a critical role for the Rit-p38 MAPK signaling cascade in promoting hippocampal neuron survival following oxidative stress.
Keywords: Ras GTPase, Hippocampal neuron, Survival, Reactive Oxygen Species (ROS), Stress
1. Introduction
Ras-related small GTPases function as guanine nucleotide-regulated molecular switches, responding to a wide variety of cellular stimuli to control a number of critical cellular processes, including proliferation and differentiation, cell survival and apoptosis, and cytoskeleton dynamics [7]. To accomplish these varied functions, Ras GTPases regulate an array of intracellular signaling pathways, with mitogen-activated protein kinase (MAPK) cascades among the most intensively studied [5]. There are three major classes of MAPKs, with ERK activation associated with cell growth and survival, and the stress-activated p38 and JNK MAPK pathways shown to play major roles in the cellular adaption to stress, including the induction of cell death [17].
Rit, along with the mammalian Rin and Drosophila Ric GTPases, comprise the Rit subfamily of Ras-related small GTP-binding proteins [12, 20, 26]. Rit is expressed in the majority of adult and embryonic tissues, including a variety of primary neurons and the developing brain [20, 26]. Despite its widespread expression within the nervous system, its cellular functions remain incompletely characterized. Expression of constitutively active Rit (RitQ79L) results in the development of highly branched neurites in pheochromocytoma cells [21, 23], in a process that relies upon the activation of both ERK and p38 MAPK cascades [19, 21, 22]. Studies in primary neurons support a role for Rit in the regulation of axonal and dendritic growth. In cultured sympathetic and hippocampal neurons, expression of RitQ79L has been found to promote axonal but inhibit dendritic growth whereas expression of a dominant-negative Rit mutant inhibits axonal but enhances dendritic growth [13]. Moreover, Rit has recently been found to contribute to IFN-γ-induced dendritic retraction [1].
We have recently demonstrated that Rit serves as a central regulator of stress-activated MAPK regulation and pro-survival signaling [4, 22]. Rit silencing renders both cultured cells and primary embryonic fibroblasts susceptible to apoptosis and results in a disruption of stress-dependent p38 and Akt signaling [4, 22]. However, while Rit loss sensitizes cells to stress-dependent cell death, it has not been established whether Rit activation promotes in vivo protective signaling.
To further explore the physiological function of Rit in the central nervous system, we generated a transgenic mouse line overexpressing constitutively active RitQ79L under the control of the neuron-specific Synapsin I promoter (termed SynCARit mice). We demonstrate that neuronal overexpression of RitQ79L does not result in any discernible morphological or anatomical abnormalities within the central nervous system. However, cultured hippocampal neurons from SynCARit mice display significantly enhanced survival compared to wild-type neurons following H2O2 exposure, supporting a pro-survival function for Rit following oxidative stress. Moreover, pharmacological inhibitor studies demonstrate that p38 MAPK, but not MEK/ERK signaling, is required for RitQ79L-mediated survival. Taken together, these data strengthen the notion that Rit-p38 signaling plays a critical role in promoting survival in neurons adapting to oxidative stress.
2. Experimental procedures
2.1 Reagents
Hydrogen peroxide (Sigma) and kinase specific inhibitors SB203580 (Tocris), and PD98059 (CalBiochem), mouse monoclonal anti-HA (12CA5) (Applied Science), mouse monoclonal anti-MAP2 (AP20) (Sigma), and fluorescein-conjugated anti-mouse IgG (H+L) (vector, Burlingame, CA) were purchased.
2.2 Generation of SynCARit transgenic mice
To generate a transgenic mouse line the rat Synapsin I promoter was fused to 3×HA-human RitQ79L followed by a Simian Virus (SV40) RNA splice donor/splice acceptor sequence and an SV40 polyadenylation sequence in the pZero-2 vector (Invitrogen). The linear fragment was released by NsiI digestion and used for microinjection at the University of Kentucky Transgenic Facility. Two lines positive for the human Rit transgene were identified and crossed back to C57BL/6 line. No differences were observed between these two lines in pilot studies, and further characterization was only carried out with Line 2.
2.3 Mouse Genomic DNA extraction and genotyping PCR
Genomic DNA was extracted from tail-snips by incubation in tail lysis buffer (100 mM Tris-HCl (pH 8.8), 5 mM EDTA, 0.2% SDS and 200 mM NaCl) containing 0.4 mg/mL proteinase K (Invitrogen) at 55°C overnight, followed by incubation with 60 μg/mL RNase (Invitrogen) at 37 °C for 1 h. DNA was precipitated, and resuspended in 10 mM Tris-HCl (pH 8.0) prior to genotyping. Primers used for PCR analysis were as follows: forward primer (Human Rit 36–51) 5′-TAGCAGCCCCGCTGGG-3′; reverse primer (Human Rit 471–453) 5′-GCTGAATTCTCGGGCCAAG-3′.
2.4 Morphological analysis of brain structure
Adult SynCARit mice and wild-type littermates were anesthetized (130 mg/kg sodium pentobarbital, intraperitoneally) and perfused intracardially with heparinized saline (1000 units/L) followed by 60 mL of 10% neutral-buffered formalin (Sigma). 24 h post-fixation, brains were removed from the skull, postfixed in formalin for 3–4 h, cryprotected in 30% sucrose, and frozen using 2-methylbutane at −40 °C. Serial 40 μm coronal sections were cut using a sliding microtome (HM400, Microm, Walldorf, Germany). The sections were subsequently stained for Nissl substance and examined with an AX80 light microscope (Olympus, Melville, NY). Images were captured using 4× and 10× objectives.
2.5 Primary cell cultures from post-natal mouse pups
Primary cultures of hippocampal neurons were prepared from C57BL/6 wild-type or SynCARit transgenic pups within 12 h after birth. Isolated hippocampi were dissociated by treatment for 30 min at 37 °C in a solution of DMEM supplemented with 4 mg/mL papain (Sigma) and 2.5 μg/mL DNase I (Sigma). Cells were then triturated in DMEM plus 10% FBS and plated at 5×105 cells per 35 mm poly-D-lysine (0.1 mg/mL in 5 mM Tris (pH 8.0)) coated tissue culture dishes or equivalent density in other dishes. After 4 h incubation at 37 °C, the medium was replaced with serum-free Neuobasal medium (Invitrogen) supplemented with 0.5 mM Glutamine (Invitrogen), B27 (Invitrogen) and 100 μg/mL streptomycin and 100 U/mL penicillin (Invitrogen) (Complete Neurobasal). Cells were incubated in a humidified incubator at 37 °C and 5% CO2. After 3 days in vitro (DIV3), half the medium was removed and replaced with fresh complete neurobasal supplemented with 2 μM AraC to prevent the proliferation of non-neuronal cells. At DIV7, the cells were re-fed with Complete Neurobasal medium. All the experiments were performed at DIV8 unless noted.
Primary cultures of mixed glial cells were prepared from C57BL/6 wild type and SynCARit transgenic pups within 24hr after birth. Cerebral hemispheres were dissociated by treatment for 30 min at 37 °C in 0.25% Trypsin-EDTA (Invitrogen) plus DNase 1 (2.5 μg/mL). Tissue was triturated in DMEM containing 10% FBS, 100 μg/mL streptomycin, 100 U/mL penicillin, and plated in 10 cm dishes at the density of one pup/dish. Cells were then incubated in a humidified incubator at 37 °C and 5% CO2. Every 2–3 days thereafter, cells were re-fed with DMEM supplemented with 10% FBS, 100 μg/mL streptomycin, and 100 U/mL penicillin.
2.6 Treatment of isolated primary hippocampal neurons
At DIV8, hippocampal neurons were left untreated or exposed to 60 μM H2O2 for 2 h at 37 °C. Cells were fixed with fresh 4% paraformaldehyde (PFA, Sigma) for 15 min. For pharmacological inhibitor studies, cultures were incubated for 30 min prior to H2O2 exposure with either 0.1% DMSO (vehicle), 10 μM PD98059 (MEK inhibitor) suspended in DMSO or 10 μM SB203580 (p38 MAPK inhibitor) suspended in distilled H2O.
2.7 Immunofluorescence of primary neuronal cultures
Cultures fixed with 4% PFA were washed three times with PBS and permeabilized for 5 min at room temperature (RT) in 0.1% Triton X-100. Cultures were blocked for 2 h at RT in 5% BSA, incubated overnight at 4°C with MAP-2 antibody (1:1000) in 5% BSA to label neuronal dendrites and cell bodies, washed three times with PBS, and incubated overnight at 4 °C with Fluorescein-conjugated anti-mouse IgG (H+L) (1:1000, Vector, Burlingame, CA) diluted in 5% BSA. The cultures were rinsed three times with PBS, incubated in 1 μg/mL Hoechst 33258 (Sigma) for 10 min to label nuclei, and washed three times with PBS. Apoptotic neurons were determined by condensed and fragmented nuclei using a Zeiss Axiovert 200M fluorescence microscope and representative images were captured with an Orca ER camera using a 32× objective.
2.8 Immunoblotting
Protein concentrations of cell lysates were determined with the Bio-Rad protein assay reagent. Equal amounts of total protein were resolved using 10% SDS-PAGE, and subjected to immunoblotting. Immunoblots were blocked in 1% casein (Sigma) in PBS plus 0.1% Tween-20 (PBST) for 1 h at RT, washed 3 times with PBST, incubated with an appropriate dilution of the primary antibody in 1% casein in PBST, washed three times with PBST, incubated with HRP-conjugated secondary antibody (1:20,000) in 1% casein in PBST, and chemiluminescence (SuperSignal West Pico system, Pierce) used to detect protein expression as described previously [4].
2.9 Statistical analysis
All data were expressed as mean ± SEM. Fisher’s PLSD post-hoc test was used to analyze the difference between the individual experiments.
3. Results
3.1 Expression of constitutively active Rit does not alter normal brain morphology
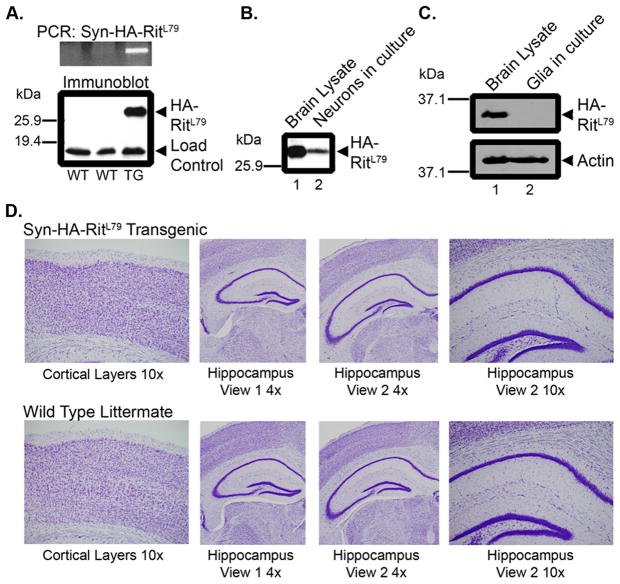
A transgenic mouse line overexpressing HA-tagged constitutively active human Rit, hRitQ79L, under the control of a neuron-specific Synapsin I (SynI) promoter was generated to examine the in vivo effect of Rit signaling on neuronal function. As shown in Figure 1A, both genotype analysis and anti-HA immunoblotting of brain lysates from transgene positive mice, but not wild-type littermates, confirmed expression of exogenous HA-Rit protein. SynCARit mice were born at the expected Mendelian ratio and grew to adulthood without displaying any discernible abnormalities.
Fig. 1. HA-RitQ79L protein under the control of the Synapsin I promoter is expressed exclusively in neurons and does not alter brain morphology.
(A) Upper panel, PCR analysis of wild-type (WT) or SynCARit (TG) animals screened with primers for the human Rit gene. Lower panel, anti-HA immunoblotting was used to detect HA-RitQ79L expression from brain lysate (100 μg) generated from the same animals as in the Upper panel. The non-specific band serves as a loading control. (B) Neurons were harvested and subjected to anti-HA immunoblotting (100 μg). Whole brain lysate (100 μg) served as positive control. (C) Mixed glial cell cultures (100 μg) were subjected to anti-HA immunoblotting. (D) Coronal sections from adult SynCARit or WT littermates were Nissl stained and images captured with an AX80 light microscope.
To verify the neural specificity of SynI-mediated HA-RitQ79L expression, primary hippocampal neuron and glial cell cultures were established from post-natal day one (P1) mouse pups. Figure 1B shows that primary neurons express HA-RitQ79L protein even after 2 weeks in culture (DIV14). In contrast, no HA-tagged Rit protein could be detected in glial cells cultured from SynCARit pups (Fig. 1C), confirming neuronal-specific HA-RitQ79L expression.
Since expression of activated Rit has been shown to modulate axonal/dendritic growth in primary hippocampal and superior cervical ganglia (SCG) neurons [13], we asked whether HA-RitQ79L expression altered normal brain structure. Examination of Nissl-stained brain sections failed to identify any overt developmental differences within the cortex or hippocampus of adult SynCARit mice when compared to wild-type littermates (Fig. 1D, see details in Materials and Methods).
3.2 Primary hippocampal neurons from SynRit mice are protected from oxidative stress
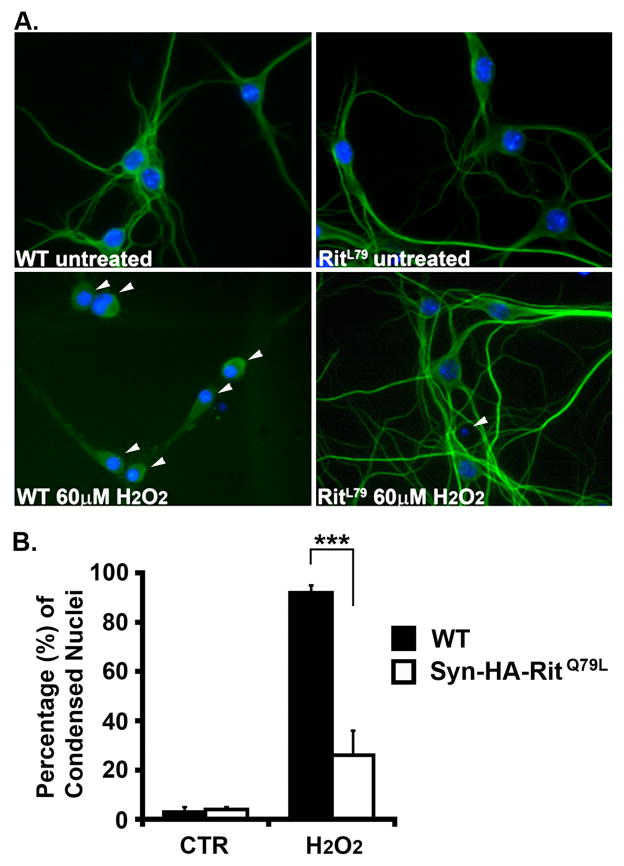
Since expression of active Rit has been shown to reduce stress-mediated apoptosis in pheochromocytoma (PC6) cells [22], and protect primary MEFs from oxidative-stress mediated cell death [4], we next asked whether neuronal Rit expression would also promote pro-survival signaling. To test this hypothesis, isolated primary hippocampal neurons from SynCARit and wild-type littermates were in vitro cultured for 8 days (DIV8), exposed to hydrogen peroxide (H2O2) (60 μM, 2 h), and cell death monitored by nuclei condensation. Figure 2A shows representative images of hippocampal neurons from SynCARit mice and wild-type littermates with or without H2O2 treatment. Approximately 3% of the transgenic and wild-type neurons showed condensed nuclei in the mock treated groups (Fig. 2B). However, following H2O2 exposure, 92.0 ± 1.9% of the wild-type neurons displayed condensed nuclei. In contrast, only 25.0 ± 10.9% of the SynCARit transgenic neurons were found to have condensed nuclei (Fig. 2B). These data indicate that RitQ79L expression protects neurons from H2O2-mediated cell death.
Fig. 2. SynRit neurons are protected from ROS-induced death.
(A) Hippocampal neurons (DIV8) isolated from WT or SynCARit P1 mouse pups were left untreated or treated with H2O2 (60 μM) for 2h and coimmunostained with MAP2 antibody (green) and Hoechst stain (blue). Arrowheads indicate neurons with condensed nuclei. (B) The percentage of MAP2+ cells with condensed nuclei are presented as mean ± SEM. Experiments were performed in triplicate. *** p<0.0001 using Fisher’s PLSD post-hoc test.
3.3 Rit-mediated protection from reactive oxygen stress is inhibited by blockade of p38 MAPK activity
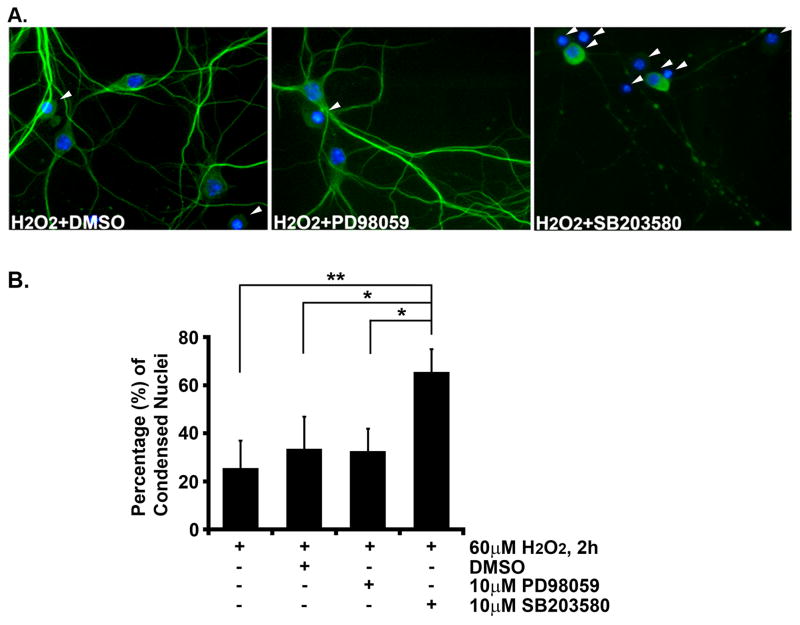
Previous studies have demonstrated a complex role for Rit in the control of MAPK signaling, including roles in activation of both ERK and p38 MAPKs [18, 21]. To determine which downstream MAPKs contribute to Rit-mediated oxidative stress protection, hippocampal neurons (DIV8) were pretreated with PD98059 (10 μM) or SB203580 (10 μM) for 30 min to block MEK/ERK or p38 MAPK signaling, respectively, or with vehicle control (DMSO). Figure 3A shows representative images of vehicle, PD98059 or SB203580 treated SynCARit hippocampal neurons following H2O2 exposure. Approximately 25 % of neurons displayed condensed nuclei following H2O2 exposure (60 μM, 2 h). DMSO or PD98059 pretreatment resulted in a slight increase of nuclear condensation (DMSO: 33.8 ± 12.8%; PD98059: 33.5 ± 8.4%, Fig. 3B). However, p38 inhibition dramatically increased nuclear condensation (64.3 ± 10.1%, Fig. 3B). Together, these data suggest that the p38 MAPK cascade plays a critical role in Rit-mediated pro-survival pathways.
Fig. 3. Inhibition of p38 MAPK but not ERK blocks RitQ79L-mediated protection from reactive oxygen species-induced death.
(A) Hippocampal neurons (DIV8) were pre-treated for 30 min with DMSO, PD98059, or SB203580, and subjected to H2O2 exposure (60 μM) for 2 h. Cells were fixed, stained (neurons, MAP2, green; nuclei, Hoechst, blue) and scored for condensed nuclei (arrowhead). Representative images are shown. (B) The percentage of MAP2+ cells with condensed nuclei are presented as mean ± SEM. Experiments were performed in triplicate. * p<0.02, ** p<0.006 using Fisher’s PLSD post-hoc test.
4. Discussion
To investigate the physiological role for the Rit GTPase in the adult nervous system, we generated a transgenic mouse model overexpressing constitutively active human Rit (HA-RitQ79L) postnatally in the brain of transgenic mice. Use of the Synapsin I promoter restricted RitQ79L expression to postnatal neurons. Neuronal specific Rit expression did not alter brain morphology (Fig. 1), but resulted in hippocampal neurons that were highly resistant to oxidative stress (Fig. 2). Moreover, in keeping with a growing literature in cultured cell lines, Rit-dependent p38 MAPK, but not ERK1/2 signaling played a central role in survival signaling [4, 22]. Taken together, these studies suggest that the Rit-p38 MAPK signaling cascade plays a key role in the survival of hippocampal neurons following oxidative stress.
The role for p38 MAPK in promoting survival, especially in neurons, is controversial, as p38 has been reported to play a prominent role in pro-apoptotic signaling [11, 25]. However, accumulating evidence supports an important role for p38 MAPK signaling in growth factor- and neuronal activity-dependent survival [15, 16, 28]. We recently identified a role for Rit in stress-mediated activation of a scaffolded p38-Akt pro-survival signaling complex [22, 27]. Indeed, this complex appears to be selectively coupled to active Rit, but not the related Ras or Rap GTPases, and p38-dependent Akt signaling is critical for Rit-mediated protection in PC6 cells and primary MEFs [4, 22]. In addition to controlling Akt, p38 has been implicated in the regulation of additional pro-survival signaling pathways. For instance, p38 can directly phosphorylate and inhibit GSK3β to promote a β-catenin-dependent pro-survival cascade [24]. p38 has also been implicated in the regulation of MSK1/2-CREB and MEF2 pro-survival transcriptional cascades, which result in transcription of a variety of anti-apoptotic genes in different cell models, including neurons [2, 15]. It remains to be determined whether Rit-p38 signaling contributes to these or additional pathways.
A major motivation for developing the SynCARit mouse model was to investigate the possible role for Rit in neuronal survival in neurodegenerative disease or in response to brain injury [6]. It has been previously shown that overexpression of activated Rit protects pheochromocytoma cells from a variety of cellular stresses including oxidative stress in a p38 MAPK-dependent manner [22]. In addition, primary MEFs derived from Rit−/− mice were much more susceptible to reactive oxygen species (ROS) exposure, which can be rescued by GFP-RitQ79L [4]. Our finding that expression of constitutively active Rit largely prevented ROS-dependent hippocampal neural death suggests that the Rit may promote protective signaling in vivo. Unfortunately, before we were able to address this important issue, our SynCARit mice lost RitQ79L protein expression. A second model, allowing regulated neuronal Rit expression, is currently under development to address this important issue.
Despite earlier studies demonstrating a role for Rit as a regulator of neuronal differentiation and axonal/dendritic growth [1, 10, 13, 18, 21, 23], SynRit mice lacked any gross morphological defects of the brain. These seemingly conflicting results may be explained by the Synapsin I-mediated expression of active Rit late in development [9, 14]. Thus, it is possible that neurons may already have progressed beyond the developmental stage in which Rit plays a critical role in controlling axonal/dendritic growth. Indeed, we have recently found that loss of Rit alters dendritic growth in the developing hippocampus, indicating that Rit contributes to the maturation or functional integration of these neurons [3]. Additional studies are therefore needed to investigate the role of Rit signaling to neuronal development and morphogenesis.
In summary, we present data to support a neuroprotective role for Rit signaling within central nervous system neurons. Expression of activated Rit stimulates a p38-dependent cascade that supports hippocampal neuronal survival in response to oxidative stress, but does not grossly alter neural development. These data also further support a unique physiological function for Rit, relative to other Ras subfamily GTPases [22]. Indeed, expression of activated H-Ras under the Synapsin I promoter has been shown to induce protective signaling in midbrain dopaminergic neurons [8]. However, postnatal H-RasG12V expression was found to induce neuronal cell body hypertrophy, resulting in an increase in total brain volume [8]. Despite similar protective effects, H-RasG12V-mediated survival relied upon upregulation of ERK MAPK signaling [8], indicating that Rit and Ras regulate distinct survival pathways in the mammalian brain. The ability of Rit to promote neuron survival without inducing neuronal hypertrophy makes enhancement of endogenous Rit signaling a possible therapeutic strategy for the treatment of neurodegenerative diseases and brain injuries, especially those in which excessive levels of reactive oxygen species result in cellular apoptosis [6].
Highlights.
Loss of the Rit GTPase sensitizes cells to oxidative-stress
A transgenic mouse expressing active Rit (RitQ79L) in neurons was generated
Expression of active Rit does not alter CNS development
Neuronal expression of RitQ79L promotes oxidative stress resistance
Inhibitor studies demonstrate a key role for p38 MAPK in Rit-mediated neuronal survival
Acknowledgments
We thank Drs. G.-X. Shi, and C. Moncman for helpful discussions. This work was supported by Public Health Service grant NS045103 from the National Institute of Neurological Disorders and Stroke (D.A.A.) and in part by NIH Grant P20GM103486 from the National Institute of General Medical Sciences, its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the NIGMS.
Abbreviations
- ROS
reactive oxygen species
- MAPK
mitogen-activated protein kinase
- MEF
mouse embryonic fibroblast
- PC6
pheochromocytoma cells
- SCG
superior cervical ganglia
- DIV
days in vitro
- SynI
synapsin I
- SynCARit
Syn-HA-RitQ79L
- RT
room temperature
- PFA
paraformaldehyde
- PBST
PBS plus 0.1% Tween-20
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andres DA, Shi GX, Bruun D, Barnhart C, Lein PJ. Rit signaling contributes to interferon-gamma-induced dendritic retraction via p38 mitogen-activated protein kinase activation. Journal of neurochemistry. 2008;107:1436–1447. doi: 10.1111/j.1471-4159.2008.05708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur JS, Fong AL, Dwyer JM, Davare M, Reese E, Obrietan K, Impey S. Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. The Journal of neuroscience. 2004;24:4324–4332. doi: 10.1523/JNEUROSCI.5227-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai W, Carlson SW, Brelsfoard JM, Mannon CE, Moncman CL, Saatman KE, Andres DA. Rit GTPase Signaling Promotes Immature Hippocampal Neuronal Survival. Journal of Neuroscience. 2012;32:9887–9897. doi: 10.1523/JNEUROSCI.0375-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai W, Rudolph JL, Harrison SM, Jin L, Frantz AL, Harrison DA, Andres DA. An evolutionarily conserved Rit GTPase-p38 MAPK signaling pathway mediates oxidative stress resistance. Molecular biology of the cell. 2011;22:3231–3241. doi: 10.1091/mbc.E11-05-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 6.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Progress in neurobiology. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Colicelli J. Human RAS superfamily proteins and related GTPases. Science’s STKE: signal transduction knowledge environment. 2004;2004:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heumann R, Goemans C, Bartsch D, Lingenhohl K, Waldmeier PC, Hengerer B, Allegrini PR, Schellander K, Wagner EF, Arendt T, Kamdem RH, Obst-Pernberg K, Narz F, Wahle P, Berns H. Transgenic activation of Ras in neurons promotes hypertrophy and protects from lesion-induced degeneration. The Journal of cell biology. 2000;151:1537–1548. doi: 10.1083/jcb.151.7.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoesche C, Sauerwald A, Veh RW, Krippl B, Kilimann MW. The 5′-flanking region of the rat synapsin I gene directs neuron-specific and developmentally regulated reporter gene expression in transgenic mice. The Journal of biological chemistry. 1993;268:26494–26502. [PubMed] [Google Scholar]
- 10.Hynds DL, Spencer ML, Andres DA, Snow DM. Rit promotes MEK-independent neurite branching in human neuroblastoma cells. Journal of cell science. 2003;116:1925–1935. doi: 10.1242/jcs.00401. [DOI] [PubMed] [Google Scholar]
- 11.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiological reviews. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 12.Lee CH, Della NG, Chew CE, Zack DJ. Rin, a neuron-specific and calmodulin-binding small G-protein, and Rit define a novel subfamily of ras proteins. The Journal of neuroscience. 1996;16:6784–6794. doi: 10.1523/JNEUROSCI.16-21-06784.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lein PJ, Guo X, Shi GX, Moholt-Siebert M, Bruun D, Andres DA. The novel GTPase Rit differentially regulates axonal and dendritic growth. The Journal of neuroscience. 2007;27:4725–4736. doi: 10.1523/JNEUROSCI.5633-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science. 1999;286:785–790. doi: 10.1126/science.286.5440.785. [DOI] [PubMed] [Google Scholar]
- 16.Ouwens DM, de Ruiter ND, van der Zon GC, Carter AP, Schouten J, van der Burgt C, Kooistra K, Bos JL, Maassen JA, van Dam H. Growth factors can activate ATF2 via a two-step mechanism: phosphorylation of Thr71 through the Ras-MEK-ERK pathway and of Thr69 through RalGDS-Src-p38. The EMBO journal. 2002;21:3782–3793. doi: 10.1093/emboj/cdf361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Current opinion in cell biology. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 18.Rudolph JL, Shi GX, Erdogan E, Fields AP, Andres DA. Rit mutants confirm role of MEK/ERK signaling in neuronal differentiation and reveal novel Par6 interaction. Biochimica et biophysica acta. 2007;1773:1793–1800. doi: 10.1016/j.bbamcr.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakabe K, Teramoto H, Zohar M, Behbahani B, Miyazaki H, Chikumi H, Gutkind JS. Potent transforming activity of the small GTP-binding protein Rit in NIH 3T3 cells: evidence for a role of a p38gamma-dependent signaling pathway. FEBS letters. 2002;511:15–20. doi: 10.1016/s0014-5793(01)03264-1. [DOI] [PubMed] [Google Scholar]
- 20.Shao H, Kadono-Okuda K, Finlin BS, Andres DA. Biochemical characterization of the Ras-related GTPases Rit and Rin. Archives of biochemistry and biophysics. 1999;371:207–219. doi: 10.1006/abbi.1999.1448. [DOI] [PubMed] [Google Scholar]
- 21.Shi GX, Andres DA. Rit contributes to nerve growth factor-induced neuronal differentiation via activation of B-Raf-extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades. Molecular and cellular biology. 2005;25:830–846. doi: 10.1128/MCB.25.2.830-846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi GX, Jin L, Andres DA. A rit GTPase-p38 mitogen-activated protein kinase survival pathway confers resistance to cellular stress. Molecular and cellular biology. 2011;31:1938–1948. doi: 10.1128/MCB.01380-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spencer ML, Shao H, Andres DA. Induction of neurite extension and survival in pheochromocytoma cells by the Rit GTPase. The Journal of biological chemistry. 2002;277:20160–20168. doi: 10.1074/jbc.M201092200. [DOI] [PubMed] [Google Scholar]
- 24.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton TM, Rincon M. Non-classical p38 map kinase functions: cell cycle checkpoints and survival. International journal of biological sciences. 2009;5:44–51. doi: 10.7150/ijbs.5.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wes PD, Yu M, Montell C. RIC, a calmodulin-binding Ras-like GTPase. The EMBO journal. 1996;15:5839–5848. [PMC free article] [PubMed] [Google Scholar]
- 27.Wu R, Kausar H, Johnson P, Montoya-Durango DE, Merchant M, Rane MJ. Hsp27 regulates Akt activation and polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. The Journal of biological chemistry. 2007;282:21598–21608. doi: 10.1074/jbc.M611316200. [DOI] [PubMed] [Google Scholar]
- 28.Xing J, Kornhauser JM, Xia Z, Thiele EA, Greenberg ME. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Molecular and cellular biology. 1998;18:1946–1955. doi: 10.1128/mcb.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]