SUMMARY
Age related macular degeneration (AMD) is the leading cause of irreversible blindness in the elderly population worldwide. While recent studies have demonstrated strong genetic associations of single nucleotide polymorphisms within a number of genes and AMD, other modes of regulation are also likely to play a role in its etiology. We identified a significantly decreased level of methylation on the IL17RC promoter in AMD patients. Further, we showed that hypomethylation of the IL17RC promoter in AMD patients led to an elevated expression of its protein and mRNA in peripheral blood as well as in the affected retina and choroid, suggesting that the DNA methylation pattern and expression of IL17RC may potentially serve as a biomarker for the diagnosis of AMD and likely plays a role in disease pathogenesis.
INTRODUCTION
Age-related macular degeneration (AMD) is the most common cause of irreversible central blindness in the older population worldwide. It currently affects more than 1.75 million individuals in the United States alone and it has been estimated that this number will increase to almost 3 million in 10 years (Friedman et al., 2004). AMD leads to progressive loss of central vision because of macular atrophy or choroidal neovascularization. Currently, no medical or surgical treatment is available for central geographic atrophy (GA), the “dry” form of advanced AMD, while anti-vascular endothelial growth factor (anti-VEGF) drugs including ranibizumab (Lucentis) and bevacizumab (Avastin) have been used to treat choroidal neovascular AMD (CNV), the “wet” form of advanced AMD (Campa and Harding, 2010).
In the past two decades, genetic susceptibility factors for AMD have been extensively studied and documented. DNA variants in a growing list of genes have been identified as strong genetic contributors to the etiology of AMD (Swaroop et al., 2009), including complement factor H (CFH) gene, CFB, complement 2, complement 3 (C3), and complement factor I (CFI). In addition to genes within the complement pathway, the ARMS2/HTRA1 region, apolipoprotein E (APOE), tissue inhibitor of metalloproteinase 3 (TIMP3), cholesteryl ester transfer protein (CETP), ATP-binding cassette sub-family A member 1 (ABCA1), and hepatic lipase gene (LIPC) have also been associated with AMD (Tuo et al., 2012). Despite considerable success in deciphering the genetic risk factors associated with the etiology of AMD, the lack of direct functional links between genes and pathogenesis of disease, as well as an inconsistency between genetic studies are generally recognized limitations of these studies (Peter and Seddon, 2010). In addition, several major non-genetic risk factors for AMD are reported, including aging, smoking, diet, and inflammation (De Jong, 2006, Swaroop et al., 2009, Baird et al., 2006). However, the mechanism by which these environmental factors affect the retina during pathogenesis of AMD is largely unknown.
Epigenetic regulation, including DNA methylation and histone modifications, is the main mechanism by which gene expression patterns can be altered depending on the environmental stimulus, without a change in DNA sequence. In vertebrates, DNA can be methylated by DNA methyltransferase and this modification occurs mostly in the context of CpG dinucleotides. DNA methylation has a substantial influence on chromatin structure and mediates silencing of gene expression (Bernstein et al., 2007). Recent studies have indicated that dynamic regulation of DNA methylation plays an important role in pluripotent stem cell differentiation and tumorigenesis (Feinberg, 2007). A number of therapeutic agents modulating DNA methylation patterns have been tested in clinics for cancer treatment (Cedar and Bergman, 2009).
We were interested in assessing whether methylation changes could be identified through the analysis of twin pairs as these provide a means of dissecting apart genetic and environmental components of disease. While the majority of identical twins are concordant for end stage AMD, in a small minority they present with a discordant phenotype. We recently identified twins and siblings who display such a discordant disease phenotype for AMD. This argues that non-genetic factors also play a potentially crucial role in the pathogenesis of AMD. Recent studies of genome-wide DNA methylation and histone modifications in identical twins with discordant disease phenotypes support the notion that environmentally driven epigenetic changes contribute to the etiology of diseases such as systemic lupus erythematosus and multiple sclerosis (Javierre et al., 2009, Baranzini et al., 2010, Kaminsky et al., 2009, Fraga et al., 2005). In this study, we initially investigated genome-wide differences in DNA methylation patterns between twins (both monozygotic and dizygotic) with discordant AMD and further validated methylation changes identified at the IL17RC promoter in discordant siblings for AMD as well as in an AMD case control cohort where cases presented with either the dry or the wet form of AMD. Furthermore, we evaluated IL17RC expression in the eyes and blood of AMD patients.
RESULTS
Difference in DNA methylation patterns between twins and siblings with discordant AMD
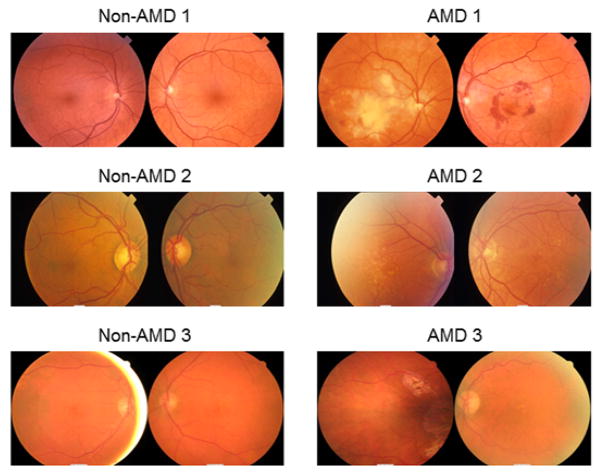
Three pairs of twins (one monozygotic and two dizygotic) with phenotypic discordance of AMD were identified from our twin patient cohort at The Centre for Eye Research Australia (CERA). Their age and gender information is listed in Table S1. As shown in Figure 1, the fundus photographs of the patients “non-AMD 1”, “non-AMD 2”, and “non-AMD 3” have a normal appearing retina and no evidence of drusen in any of the eyes. In contrast, the photographs of their monozygotic (AMD 1) and dizygotic (AMD 2 and AMD 3) twins showed evidence of macular haemorrhage that was secondary to choroidal neovascularization (AMD 1), multiple large drusen (AMD 2), and geographic atrophy (AMD 3). Monozygotic and dizygotic twins have either an identical or very similar genetic background. Therefore, we hypothesized that the phenotypic difference of the AMD twins could be due to epigenetic diversity, in addition to the difference in genetic information between twin pairs.
Figure 1.
Fundus photographs of twins with discordant AMD
Although AMD has been traditionally considered as a neurodegenerative disease, the recent association between AMD and single nucleotide polymorphisms (SNPs) in genes involving the immune response, such as CFH, CFB, CFI, C3, ARMS2/HTRA1, CX3CR1, and TIMP3, argues that a disregulated immune system may play an essential role during the onset and duration of AMD (Swaroop et al., 2009). In addition, we have recently reported in a small randomized study that immunotherapy positively alters the clinical course of late stage neovascular AMD (Nussenblatt et al., 2010). Therefore, we next obtained genomic DNA from peripheral blood mononuclear cells (PBMCs), which could be reflective of the status of the immune system, to investigate the DNA methylome difference between AMD twins. DNA was subjected to DNA methylation microarray (MeDIP-chip) analysis using NimbleGen Human DNA Methylation 2.1M Deluxe Promoter Array. A total of 231 genes showed consistent differential methylation patterns on their promoters between AMD twins and their non-AMD co-twins (Table S2). Consistent with the speculation that AMD might be an immunological disease, our gene ontology enrichment analysis using Ingenuity Systems (http://www.ingenuity.com/) identified “Immunological Disease” as one of the 5 most significantly enriched gene categories among the list of genes with differential DNA methylation patterns between twins (Table S3). However, we did not find consistent DNA methylation changes in AMD patients on the promoters of CFH, HTRA1, ARMS2 (Figure S1A), CFB, CFI, C3, TIMP3, TLR3, and TLR4 (data not shown).
Recently we have demonstrated elevated serum levels of Th17 cytokines, IL-17A and IL-22, in AMD patients (Liu et al., 2011). Previous studies have also indicated the induction of IL-17A by complement C5a (Hashimoto et al., 2010, Lajoie et al., 2010). Importantly, our microarray analysis identified IL22, IL17A, and IL17F as the top 3 most differentially induced genes by C5A in CD4+ T cells between non-AMD controls and AMD patients (Figure S1B). Therefore, among our list of 231 genes with differential methylation patterns between twin pairs, we first focused on molecules contributing to Th17 immunity. Intriguingly, as shown in Figure 2A, MeDIP-chip data suggested that methylated CpG sites were only found in the twins without AMD but not in their AMD co-twins in the promoter regions of IL17RC, which encodes the receptor for IL-17A and IL-17F dimers, as well as CCL22, which is the chemokine mediating trafficking of activated T lymphocytes to inflammatory sites. In contrast, we did not find consistent DNA methylation pattern differences on the promoters of Th17 or Th1 cytokines themselves, including IL17A, IL17F, IL22, IL26, and IFNG (Figure S1C).
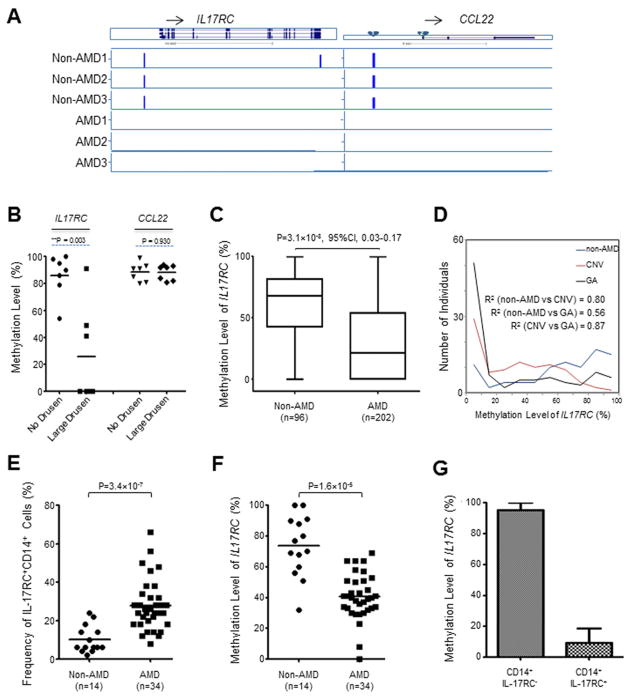
Figure 2. Hypomethylated IL17RC Promoters in AMD Patients.
(A) The genome browser view of DNA methylation peaks, identified by MeDIP-chip analysis, on the loci of IL17RC and CCL22. Twins with AMD were named AMD 1, AMD 2, and AMD3; twins without AMD were named Non-AMD 1, Non-AMD2, and Non-AMD 3, respectively.
(B) The promoter methylation levels of IL17RC and CCL22 in 7 pairs of siblings with discordant AMD phenotype.
(C) The promoter methylation level of IL17RC in 96 non-AMD controls and 202 AMD patients.
(D) Methylation levels of IL17RC promoters and their distribution in CNV and GA patients.
(E) Summary of the frequency of IL-17RC+ monocytes in the peripheral blood of NEI patient cohort of 14 non-AMD controls and 34 AMD patients.
(F) The promoter methylation level of IL17RC in the NEI patient cohort of 14 non-AMD controls and 34 AMD patients.
(G) The promoter methylation level of IL17RC in CD14+IL-17RC− and CD14+IL-17RC+ monocytes isolated from peripheral blood of AMD patients. Data are expressed as the means ± S.E from three AMD patients. See also Figure S1 and S2.
To test whether a similar DNA methylation pattern could be found in other AMD patients with more diverse genetic backgrounds, we identified 7 pairs of siblings, from our patient cohort at the Casey Eye Institute (OR), who had discordant AMD phenotypes (for all sibling pairs, one of the two had large drusen greater than 125 micron in diameter in at least one eye, and the other had no drusen). We next carried out Methyl-Profiler assays to detect the methylation status on the promoters of IL17RC and CCL22 (Figure 2B). Intriguingly, a significant differential methylation status between siblings with discordant AMD was found on the promoter of IL17RC (P=0.003, Mann-Witney test). A hypomethylated IL17RC promoter was only found in the DNA from the siblings with AMD similar to that observed in the discordant twins. We also noted that no difference in methylation status was found on the promoters of CCL22 in the sibling pairs from Oregon, USA, which was different to the promoter methylation patterns found in the twin pairs from Australia. To rule out that the methylation pattern difference of CCL22 promoters between these two patient cohorts was CpG site dependent, we designed two more primer pairs targeting specifically two other CpG sites as indicated in the Figure 2A (#1 and #2 sites). Using PBMC DNA samples from 34 AMD patients and 14 non-AMD controls collected in our NEI clinics, we performed Methyl-Profiler assays on the CCL22 promoter targeting CpG site 1 and 2. As shown in Figure S2A, no difference in methylation status was found on these two CpG sites. In addition to the IL17RC and CCL22 promoters, we performed Methyl-Profiler assays on the promoters of IL17F, IFNG, IL26, CFH, and HTRA1. Consistent with our MeDIP results, we did not find a consistent difference of methylation status on these promoters in our NEI collected patient cohorts (34 AMD patients and 14 non-AMD controls) (Figure S2B). Taken together, these data suggest that a hypomethylated IL17RC promoter is associated with AMD disease, not only in twins, but also in siblings.
Hypomethylated IL17RC promoter in 202 AMD patients but not in 96 non-AMD controls
It is well known that microarray based methylation detection technologies, such as MeDIP-chip are prone to low specificity (Fazzari and Greally, 2010). In addition, NimbleGen MeDIP-chip analysis only provides binary and fragment-level measurement of DNA methylation on gene promoters. The genetic similarity within a twin pair and within sibling pairs could also lead to bias. Therefore, to investigate the methylation status of IL17RC promoters in patients and healthy controls who do not share known genetic similarities, we collected genomic DNA from the PBMCs of 202 AMD patients (95 CNV and 107 GA) as well as 96 healthy individuals without AMD (non-AMD) from our clinics at the National Eye Institute, NIH and the Casey Eye Institute, OHSU. The average age of AMD patients and non-AMD controls were 80.5 and 70.2 years old, respectively (Table S1, Case-Control Patient Cohort). Similar to the findings with both twins and siblings, a hypomethylated IL17RC promoter was also found in AMD patients, as compared to a hypermethylated IL17RC promoter in non-AMD controls (95% CI, 0.03–0.17, P=3.1×10−8, multivariable logistic regression adjusted for age and gender) (Figure 2C and Table S4). Interestingly, the methylation status of the IL17RC promoters was not significantly different between wet and dry AMD patients (P=0.41, multivariable logistic regression adjusted for age and gender) (Figure S2C). However, their distribution was not identical between CNV and GA patients (Figure 2D). Collectively, we show an association of a hypomethylated IL17RC promoter with AMD.
Elevated expression of IL-17RC in AMD patients’ peripheral blood
DNA methylation generally associates with gene silencing. To test whether DNA hypomethylation found on the promoter of IL17RC resulted in gene de-silencing and hence increased expression in only AMD patients, we performed a FACS analysis of whole blood samples, together with other cell surface markers identifying peripheral hematopoietic cell lineages as well as the isotype control for IL-17RC staining. Intriguingly, in the NEI patient cohort with 14 non-AMD controls and 34 AMD patients, we found a significantly elevated frequency of IL-17RC+CD14+ monocytes in the peripheral blood of AMD patients (Figure S3A and 3E). Consistently, we found hypomethylated IL17RC promoters in these AMD patients (Figure 3F). Importantly, we found that the methylation level of IL17RC promoter in the CD14+IL-17RC+ cells were significantly lower than its level in the CD14+IL-17RC− cells (Figure 3G), indicating that the elevated IL-17RC expression was the result of demethylation of its promoter. In addition, an increased frequency of IL-17RC+ cells were also found among other types of cells from AMD patients as compared to age matched non-AMD controls, including CD3+CD4−CD8− and CD8+ T cells, but not on CD4+ T cells (Figure S3B). These data suggest that the hypomethylation of IL17RC promoter results in the elevated expression of the IL-17RC protein on selected cells in peripheral blood of AMD patients.
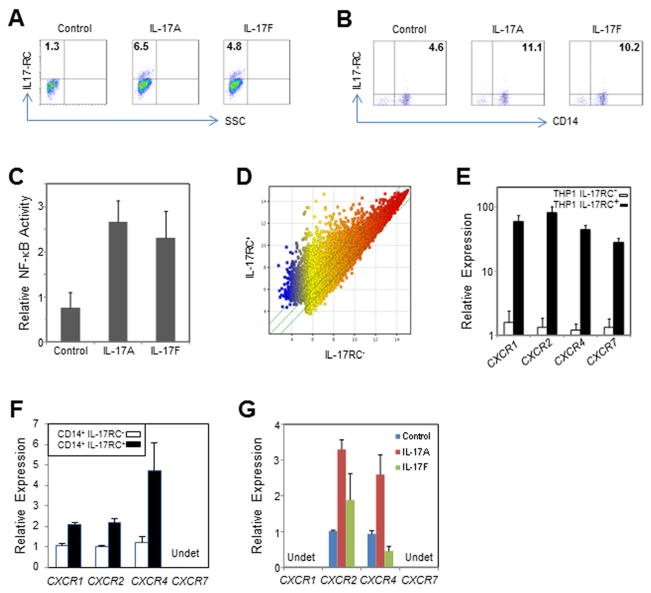
Figure 3. Induction of IL-17RC by IL-17A and IL-17F in monocytes and characteristics of IL-17RC+ monocytes. (Data are expressed as the means ± S.E.).
(A) FACS staining of IL-17RC induced by IL-17A and IL-17F in THP1 cells.
(B) FACS staining of IL-17RC induced by IL-17A and IL-17F in primary CD14+ monocytes. See also Figure S3.
(C) Relative activity of NF-κB measured by TransAM NF-κB p65 Transcription Factor ELISA Kit.
(D) Scatter plot showing the genome-wide expression difference between sorted IL-17RC+ and IL-17RC− THP1 cells.
(E) Relative expression of CXCR1, CXCR2, CXCR4, and CXCR7 mRNA in sorted IL17RC+ and IL17RC− THP1 cells.
(F) Relative expression of CXCR1, CXCR2, CXCR4, and CXCR7 mRNA in sorted IL17RC+CD14+ and IL17RC−CD14+ monocytes from two AMD patients. Undet, undetectable.
(G) Relative expression of CXCR1, CXCR2, CXCR4, and CXCR7 mRNA in primary CD14+ monocytes stimulated by IL-17A or IL-17F overnight. Undet, undetectable.
Induction of IL-17RC expression by IL-17A and IL-17F
Previous studies have demonstrated that IL-17RC mediates both IL-17A and IL-17F signalling (Ho and Gaffen, 2009). However, it is not clear which factors regulate the expression of IL17RC. As shown in Figure 4A and 4B, we found that both IL-17A and IL-17F, through activation of NF-κB (Figure 4C), induced the expression of IL-17RC in THP1 cells (a human acute monocytic leukemia cell line) as well as in primary CD14+ monocytes. Our microarray analysis also indicated a great difference in genome-wide gene expression profiles between IL-17RC+ and IL-17RC− THP1 cells (Figure 4D). Using semi-quantitative real-time PCR, we confirmed that IL-17RC+ THP1 cells expressed higher levels of chemokine receptors, CXCR1, CXCR2, CXCR4, and CXCR7 (Figure 4E). Similarly, we also found elevated expression of CXCR1, CXCR2, CXCR4, but not CXCR7 in CD14+IL-17RC+ cells from AMD patients as compared to their expression in CD14+IL-17RC− cells (Figure 4F). Moreover, we found that IL-17A induced the expression of CXCR2 and CXCR4 (Figure 4G). Therefore, our results suggest that IL-17RC can be induced by both IL-17A and IL-17F, and IL-17RC+ monocytes highly express chemokine receptors CXCR2 and CXCR4.
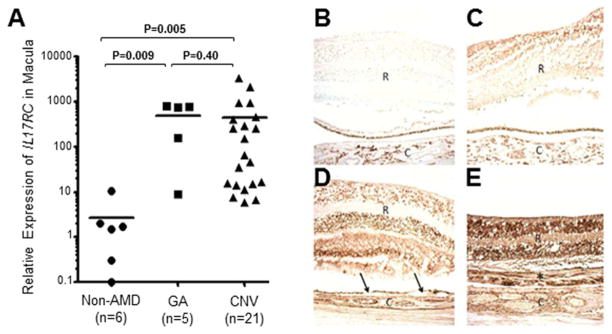
Figure 4. Expression of IL17RC in the Macular Tissues of AMD Patients.
(A) Relative expression of IL17RC mRNA in archived microdissected macular tissues from 5 eyes with GA, 21 eyes with CNV and 6 eyes from non-AMD controls.
(B–E) Immunohistochemistry staining using non-specific serum in AMD eyes. Immunohistochemistry staining of IL-17RC protein in non-AMD control (C), GA (D), as well as CNV eyes (E). Arrows, drusen; asterisk, chorioreintal neovascular fibrous tissue; R, retina; C, choroid. See also Figure S4A and S4B.
Elevated expression of IL17RC in AMD patients’ eyes
Previous studies have suggested the involvement of monocytes/macrophages in AMD pathogenesis (Nussenblatt et al., 2009, Cao et al., 2011). In order for monocytes to exit the vasculature through endothelial cells in the choroid or retina, the chemokine receptors expressed on their cell surface play critical roles. Since we found that IL-17RC+ monocytes highly expressed chemokine receptors CXCR2 and CXCR4, we hypothesized that they might be able to migrate to the macular regions of the eye. To test whether IL17RC expression could be found in the macular tissues of AMD patients, we obtained formalin-fixed, paraffin-embedded (FFPE) archived slides from 26 eyes with either GA (5 eyes) or CNV AMD (21 eyes), as well as 6 eyes from age-matched non-AMD controls. The macular tissue was microdissected into RNA lysis buffer (Ambion, TX) and total RNA was extracted using a RNA extraction kit (Ambion, TX). Quantitative real-time PCR was performed to detect the expression of IL17RC mRNA. Interestingly, IL17RC was only highly expressed in the macular tissues of AMD patients but not the non-AMD controls (Figure 4A). Moreover, immunohistochemistry assays were carried out to detect the expression of IL-17RC protein in the macular regions of both AMD and control eyes. As shown in Figure 4B–E, the immunoreactivity against IL-17RC was stronger in the macular tissues from patients with GA AMD (Figure 4D and S4A) and CNV AMD (Figure 4E and S4B) than in the macular tissues from non-AMD controls (Figure 4C) or in the macular tissues stained with non-specific antibody (Figure 4B). Thus elevated IL17RC expression was found not only in the peripheral blood but also in the chorioretinal tissues with AMD lesions.
DISCUSSION
Twin and sibling designs are broadly used in studies dissecting the genetic versus environmental contributions to various diseases. Earlier studies using small patient cohorts indicated a significantly higher concordance rate of AMD in monozygotic than in dizygotic twins or families, strongly suggesting a genetic predisposition to AMD (Meyers, 1994, Gottfredsdottir et al., 1999, Klein et al., 1994, Heiba et al., 1994). In several twin studies, Hammond et al. (Hammond et al., 2002) showed that the concordance for AMD in monozygotic twins was 0.37 compared with 0.19 in dizygotic twins, while Seddon et al. (Seddon et al., 2005) suggests that genetic factors can explain 46% to 71% of the variation in the overall severity of AMD. However, the later study also elucidated that for specific macular drusen and retinal pigment epithelial characteristics, both significant genetic (0.26–0.71) and unique environmental (0.28–0.64) proportions of variance were detected (Seddon et al., 2005). Similarly, Seddon et al. (Seddon et al., 2011) suggests that smoking and diet associated with epigenetic mechanisms are involved in the etiology of AMD, in addition to genetic susceptibility. We were able to identify three twin pairs (one monozygotic and two dizygotic) in our patient collection that displayed significant discordance for end stage disease, providing a unique opportunity to study the potential epigenetic differences between them. Our MeDIP-chip analysis identified differences in ~1.5% of the total CpG sites within 231 gene promoters between AMD twins. To our knowledge, this is the first attempt to address genome-wide epigenetic regulation in AMD patients. Our current study has only confirmed the association of hypomethylated IL17RC promoter with the onset of AMD. However, we are in the process of investigating whether epigenetic regulation of the other 230 genes associates with AMD as well. Our results also suggest that epigenetic modifications of DNA may be crucial for understanding the molecular basis of AMD.
IL-17RC is found highly expressed on the endothelium and epithelium of lung, intestine, kidney, prostate, and joint (Ge and You, 2008). However, it was not clear what types of cells normally express IL-17RC in the human peripheral blood or eyes. IL-17RC serves as an essential subunit of the IL-17 receptor complex that mediates the signal transduction and proinflammatory activities of IL-17A and IL-17F. Its binding affinity and ligand preference, as well as tissue specific distribution are slightly different between the murine and human systems. Recent studies have suggested that high levels of IL-17RC expression are found in prostate cancer cells, as well as in synoviocytes and PBMCs from patients with rheumatoid arthritis (Gaffen, 2009). In our study, we found hypomethylated IL17RC promoter in patients with both GA and CNV as compared to non-AMD controls, which resulted in the increased expression of its protein in CD14+ monocytes. Moreover, we demonstrated that IL17A, which was found elevated only in AMD patients’ serum and macular tissues, induced IL-17RC expression in primary CD14+ monocyte and human RPE cells, as well as THP1 and ARPE19 cell lines, suggesting a potentially crucial role for IL-17A and IL-17RC in the pathogenesis of AMD. Intriguingly, our results suggested that CD14+IL-17RC+ monocytes expressed elevated CXCR4 and CXCR2, which serve as the receptors for chemokines such as IL8, CXCL1, CXCL5, and CXCL6. These chemokines were also highly induced in RPE cells by both IL-17A and IL-17F. Therefore, Th17 cytokine induced intraocular inflammation may promote monocyte trafficking into the inflammatory sites within macular tissues of AMD patients and eventually causing the pathology of AMD. Therefore, our results suggest a potential mechanism by which proinflammatory monocytes could promote AMD pathology.
IL-17 producing Th17 cells are essential in clearing pathogens during host defence and in inducing inflammation in autoimmune disease (Korn et al., 2009). Genetic mutations in genes involved in IL-17 signalling have been associated with multiple diseases (Puel et al., 2011, Milner et al., 2008). Our study demonstrated that epigenetic regulation of IL17RC may also play an important role in the pathogenesis of AMD, one of the best genetically characterized complex disorders. More importantly, promoter DNA methylation patterns and expression of IL17RC may serve as potential biomarkers for AMD diagnosis and targets for AMD therapy.
EXPERIMENTAL PROCEDURES
Patients
All protocols were approved by institutional review boards, and written informed consent for epigenetic and genetic testing and participation was provided by the patients to the National Institutes of Health (NIH), Casey Eye Institute (CEI) (Oregon Health and Science University), or Centre for Eye Research Australia (CERA) through the Royal Victorian Eye and Ear Hospital (RVEEH) Human Research and Ethics Committee and the Australian Twin Registry (ATR) approved the project. Twin volunteers, both identical and nonidentical with or without known eye disease, of either gender, aged 18 years or older were invited to participate through the ATR. The non-AMD controls were from the NIH eye clinics and were screened for AMD. All patients and non-AMD controls are Caucasian. The age and gender information was listed in Table S1.
DNA Methylation Microarray (MeDIP-chip) and Data Analysis
DNA sample preparation and hybridization to DNA methylation microarray were performed according to the manufacture’s protocols provided by Roche-NimbleGen, WI. Standard data analysis service was provided by NimbleGen. All raw data and processed bed files were deposited in GEO with accession number GSE28033.
Detection of DNA Methylation on Selected Gene Promoters
Genomic DNA was extracted from PBMCs of AMD patients or healthy controls, as well as from THP1 cells using DNeasy Blood & Tissue Kit (Qiagen, CA) according to the manufacture’s protocol. One μg total genomic DNA from PBMCs was digested for 6 hours at 37 °C us ing the Methyl-Profiler DNA Methylation Enzyme Kit (SABiosciences, MD) according to the manufacture’s instruction. Quantitative Real-time PCR was performed with an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, CA). The percentage of methylated DNA was calculated by the difference of CT cycle numbers between samples with total undigested DNA and methylation-sensitive restriction enzyme digested DNA.
Cell Culture
PBMCs from healthy controls or AMD patients were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, and 1X penicillin-streptomycin antibiotics (Invitrogen, CA). They were treated with or without C5A (50 ng/ml), IL-17A (20 ng/ml), or IL-17F (20 ng/ml) overnight, followed by FACS sorting for CD3+CD4+ T cells or CD14+ monocytes using FACSAria II (BD). THP1 cells (ATCC, VA) were cultured in RPMI-1640 medium supplemented with 10% FBS, 2 mM L-glutamine, and 1X penicillin-streptomycin antibiotics (Invitrogen, CA). ARPE-19 cells and primary human RPE cells (ATCC, VA) were cultured in complete Dulbecco’s Modified Eagle’s Medium supplemented with 10% FBS, 2 mM L-glutamine, and 1X penicillin-streptomycin antibiotics (Invitrogen, CA) with or without IL-17A (20 ng/ml), or IL-17F (20 ng/ml) overnight.
Detection of RNA expression in THP1 cells, RPE cells, and CD14+ monocytes
Total RNA was isolated using mirVana miRNA isolation kit (Ambion, TX). Reverse transcription of RNA and quantitative real-time PCR were performed according to manufacturer’s protocols, using the primers and probes for 18s rRNA (Hs03928990_g1), CXCR1 (Hs00174146_m1), CXCR2 (Hs01011557_m1), CXCR4 (Hs00607978_s1), CXCR7 (Hs00604567_m1), IL6 (Hs00174131), IL8 (Hs00174103), CXCL1 (Hs00236937), CXCL5 (Hs00607029), and CXCL6 (Hs00605742) from Applied Biosystems. The comparative threshold cycle method and 18s rRNA was used to normalize the expression.
Detection of IL17A and IL17RC Expression in Human Retinal and Choroidal Tissues
Archived formalin-fixed paraffin-embedded (FFPE) ocular sections were first deparaffinized in 100% Xylene 10 times, followed by sequential washes in 100% ethanol, 95% ethanol, 75% ethanol, and water (10 times in each solution). Total RNA was isolated from the microdissected macular regions of 5 eyes with GA, 21 eyes with CNV, as well as 6 age-matched eyes without AMD pathology, using mirVana miRNA kit (Ambion, TX). Reverse transcription of RNA was performed for 12 hours at 37 °C, followed by quantitative real-time PCR, using the primers and probes for 18s rRNA (Hs03928990_g1), IL17A (Hs00936345), and IL17RC (Hs00994305_m1) from Applied Biosystems. The comparative threshold cycle method and 18s rRNA was used to normalize the expression of IL17A and IL17RC.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad, CA). The Mann-Whitney test was used to compare differences between two groups and the significance level was set at P<0.05. Multivariable logistic regression analysis was used to detect the difference of the IL17RC promoter methylation status, adjusted by age, gender, genotypes of CFH, ARMS2, or HTRA1, using SAS 9.2. The significance level was set at P<0.05.
Supplementary Material
1.5% of CpG sites are differentially methylated between twins with discordant AMD
Hypomethylated IL17RC promoter and elevated expression of IL17RC are associated with AMD
IL-17 mediated inflammatory responses contribute to AMD pathogenesis
Acknowledgments
The authors thank the Australian Twin Registry for access to this national resource, the twins who volunteered and Andrea Richardson for DNA extraction and genotyping of twin samples. We also thank Dr. Neal Young for critically reading of the manuscript. This study is supported by the intramural research program of NEI (to Drs. Nussenblatt, Chan, Ferris, and Chew), NCCAM (to Drs. Wei and Nussenblatt), and NHLBI (to Dr. McCoy); by grants from the Foundation Fighting Blindness, Owing Mills, MD (to Dr. Francis), the Macular Degeneration Center Research Fund, Casey Eye Institute, Oregon Health & Science University, Portland (to Drs. Klein and Francis), Research to Prevent Blindness, New York, NY (unrestricted grant to Casey Eye Institute, Career Development Award to Dr. Francis); and by the National Health and Medical Research Council Centre for Clinical Research Excellence #529923 - Translational Clinical Research in Major Eye Diseases and through Practitioner Fellowship to Dr. Guymer. The Centre for Eye Research Australia (CERA) receives operational infrastructure support from the Victorian Government.
Footnotes
ACCESSION NUMBERS
Microarray data are available at the NCBI Gene Expression Omnibus database under the series accession number GSE28033.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baird PN, Richardson AJ, Robman LD, Dimitrov PN, Tikellis G, Mccarty CA, Guymer RH. Apolipoprotein (APOE) gene is associated with progression of age-related macular degeneration (AMD) Hum Mutat. 2006;27:337–42. doi: 10.1002/humu.20288. [DOI] [PubMed] [Google Scholar]
- Baranzini SE, Mudge J, Van Velkinburgh JC, Khankhanian P, Khrebtukova I, Miller NA, Zhang L, Farmer AD, Bell CJ, Kim RW, May GD, Woodward JE, Caillier SJ, Mcelroy JP, Gomez R, Pando MJ, Clendenen LE, Ganusova EE, Schilkey FD, Ramaraj T, Khan OA, Huntley JJ, Luo S, Kwok PY, Wu TD, Schroth GP, Oksenberg JR, Hauser SL, Kingsmore SF. Genome, epigenome and RNA sequences of monozygotic twins discordant for multiple sclerosis. Nature. 2010;464:1351–6. doi: 10.1038/nature08990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–81. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Campa C, Harding SP. Anti-VEGF Compounds in the Treatment of Neovascular Age Related Macular Degeneration. Curr Drug Targets. 2010;2010:1. doi: 10.2174/138945011794182674. [DOI] [PubMed] [Google Scholar]
- Cao X, Shen D, Patel MM, Tuo J, Johnson TM, Olsen TW, Chan CC. Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathol Int. 2011;61:528–35. doi: 10.1111/j.1440-1827.2011.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- De Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355:1474–85. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- Fazzari MJ, Greally JM. Introduction to epigenomics and epigenome-wide analysis. Methods Mol Biol. 2010;620:243–65. doi: 10.1007/978-1-60761-580-4_7. [DOI] [PubMed] [Google Scholar]
- Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–40. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604–9. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DS, O’colmain BJ, Munoz B, Tomany SC, Mccarty C, De Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–67. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge D, You Z. Expression of interleukin-17RC protein in normal human tissues. Int Arch Med. 2008;1:19. doi: 10.1186/1755-7682-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfredsdottir MS, Sverrisson T, Musch DC, Stefansson E. Age related macular degeneration in monozygotic twins and their spouses in Iceland. Acta Ophthalmol Scand. 1999;77:422–5. doi: 10.1034/j.1600-0420.1999.770413.x. [DOI] [PubMed] [Google Scholar]
- Hammond CJ, Webster AR, Snieder H, Bird AC, Gilbert CE, Spector TD. Genetic influence on early age-related maculopathy: a twin study. Ophthalmology. 2002;109:730–6. doi: 10.1016/s0161-6420(01)01049-1. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Hirota K, Yoshitomi H, Maeda S, Teradaira S, Akizuki S, Prieto-Martin P, Nomura T, Sakaguchi N, Kohl J, Heyman B, Takahashi M, Fujita T, Mimori T, Sakaguchi S. Complement drives Th17 cell differentiation and triggers autoimmune arthritis. J Exp Med. 2010;207:1135–43. doi: 10.1084/jem.20092301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiba IM, Elston RC, Klein BE, Klein R. Sibling correlations and segregation analysis of age-related maculopathy: the Beaver Dam Eye Study. Genet Epidemiol. 1994;11:51–67. doi: 10.1002/gepi.1370110106. [DOI] [PubMed] [Google Scholar]
- Ho AW, Gaffen SL. IL-17RC: a partner in IL-17 signaling and beyond. Semin Immunopathol. 2009;32:33–42. doi: 10.1007/s00281-009-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javierre BM, Fernandez AF, Richter J, Al-Shahrour F, Martin-Subero JI, Rodriguez-Ubreva J, Berdasco M, Fraga MF, O’hanlon TP, Rider LG, Jacinto FV, Lopez-Longo FJ, Dopazo J, Forn M, Peinado MA, Carreno L, Sawalha AH, Harley JB, Siebert R, Esteller M, Miller FW, Ballestar E. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res. 2009;20:170–9. doi: 10.1101/gr.100289.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky ZA, Tang T, Wang SC, Ptak C, Oh GH, Wong AH, Feldcamp LA, Virtanen C, Halfvarson J, Tysk C, Mcrae AF, Visscher PM, Montgomery GW, Gottesman Ii, Martin NG, Petronis A. DNA methylation profiles in monozygotic and dizygotic twins. Nat Genet. 2009;41:240–5. doi: 10.1038/ng.286. [DOI] [PubMed] [Google Scholar]
- Klein ML, Mauldin WM, Stoumbos VD. Heredity and age-related macular degeneration. Observations in monozygotic twins. Arch Ophthalmol. 1994;112:932–7. doi: 10.1001/archopht.1994.01090190080025. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–35. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Wei L, Meyerle C, Tuo J, Sen HN, Li Z, Chakrabarty S, Agron E, Chan CC, Klein ML, Chew E, Ferris F, Nussenblatt RB. Complement component C5a Promotes Expression of IL-22 and IL-17 from Human T cells and its Implication in Age-related Macular Degeneration. J Transl Med. 2011;9:111. doi: 10.1186/1479-5876-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers SM. A twin study on age-related macular degeneration. Trans Am Ophthalmol Soc. 1994;92:775–843. [PMC free article] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O’shea J, Holland SM, Paul WE, Douek DC. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–6. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenblatt RB, Byrnes G, Sen HN, Yeh S, Faia L, Meyerle C, Wroblewski K, Li Z, Liu B, Chew E, Sherry PR, Friedman P, Gill F, Ferris F., 3rd A randomized pilot study of systemic immunosuppression in the treatment of age-related macular degeneration with choroidal neovascularization. Retina. 2010;30:1579–87. doi: 10.1097/IAE.0b013e3181e7978e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenblatt RB, Liu B, Li Z. Age-related macular degeneration: an immunologically driven disease. Curr Opin Investig Drugs. 2009;10:434–42. [PubMed] [Google Scholar]
- Peter I, Seddon JM. Genetic epidemiology: successes and challenges of genome-wide association studies using the example of age-related macular degeneration. Am J Ophthalmol. 2010;150:450–452. e2. doi: 10.1016/j.ajo.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, Gumbleton M, Toulon A, Bodemer C, El-Baghdadi J, Whitters M, Paradis T, Brooks J, Collins M, Wolfman NM, Al-Muhsen S, Galicchio M, Abel L, Picard C, Casanova JL. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–8. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, Cote J, Page WF, Aggen SH, Neale MC. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol. 2005;123:321–7. doi: 10.1001/archopht.123.3.321. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Reynolds R, Shah HR, Rosner B. Smoking, dietary betaine, methionine, and vitamin D in monozygotic twins with discordant macular degeneration: epigenetic implications. Ophthalmology. 2011;118:1386–94. doi: 10.1016/j.ophtha.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A, Chew EY, Rickman CB, Abecasis GR. Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu Rev Genomics Hum Genet. 2009;10:19–43. doi: 10.1146/annurev.genom.9.081307.164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Grob S, Zhang K, Chan CC. Genetics of immunological and inflammatory components in age-related macular degeneration. Ocul Immunol Inflamm. 2012;20:27–36. doi: 10.3109/09273948.2011.628432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.