Abstract
Peptide substrates of well-defined protein kinases were microinjected into aleurone protoplasts of barley (Hordeum vulgare L. cv Himalaya) to inhibit, and therefore identify, protein kinase-regulated events in the transduction of the gibberellin (GA) and abscisic acid signals. Syntide-2, a substrate designed for Ca2+- and calmodulin (CaM)-dependent kinases, selectively inhibited the GA response, leaving constitutive and abscisic acid-regulated events unaffected. Microinjection of syntide did not affect the GA-induced increase in cytosolic [Ca2+], suggesting that it inhibited GA action downstream of the Ca2+ signal. When photoaffinity-labeled syntide-2 was electroporated into protoplasts and cross-linked to interacting proteins in situ, it selectively labeled proteins of approximately 30 and 55 kD. A 54-kD, soluble syntide-2 phosphorylating protein kinase was detected in aleurone cells. This kinase was activated by Ca2+ and was CaM independent, but was inhibited by the CaM antagonist N-(6-aminohexyl)-5-chloro-1-naphthalene-sulfonamide (250 μm), suggesting that it was a CaM-domain protein kinase-like activity. These results suggest that syntide-2 inhibits the GA response of the aleurone via an interaction with this kinase, implicating the 54-kD kinase as a Ca2+-dependent regulator of the GA response in these cells.
The aleurone of the barley (Hordeum vulgare L.) grain secretes hydrolases that mobilize endosperm reserves during germination (for review, see Fincher, 1989; Jones and Jacobsen, 1991). The synthesis and secretion of these hydrolases (principally α-amylases) is under hormonal regulation. GA stimulates the synthesis and secretion of α-amylase and ABA reverses this GA effect (Fincher, 1989; Jones and Jacobsen, 1991). In addition, ABA induces a series of proteins such as RaB (van der Veen et al., 1992) and the amylase subtilisin inhibitor (Mundy and Rogers, 1986). Hence, the barley aleurone has been used extensively as a model system for the study of signal transduction in response to GA and ABA. However, the molecular basis of GA and ABA signal transduction remains poorly understood.
Changes in the levels of cytoplasmic Ca2+, often mediated through the regulatory protein CaM, are recognized as ubiquitous signal transduction elements in animal and plant cells (for review, see Poovaiah and Reddy, 1993; Bush, 1995). The cytoplasmic [Ca2+] of barley and wheat aleurone cells is elevated from 100 to around 500 nm by GA, whereas ABA reduces Ca2+ levels (Gilroy and Jones, 1992; Bush, 1996; Gilroy, 1996). CaM expression is also increased in response to GA (Gilroy and Jones, 1993; Schuurink et al., 1996), implicating Ca2+ and CaM in the GA response of these cells. Alternative regulatory events, such as changes in membrane potential and cytoplasmic pH, also seem to be critical to aleurone function (van der Veen et al., 1992; Heimovaara-Dijkstra et al., 1994a, 1994b), and thus it has become clear that distinct Ca2+-dependent and -independent regulatory pathways exist in this cell (Bush, 1996; Gilroy, 1996). However, the Ca2+- and CaM-dependent systems have proved to be central elements in the events that maintain secretory activity in response to GA and ABA in aleurone cells in vivo (Gilroy, 1996). The molecular identity of the activities regulated by such changes in Ca2+ and CaM has remained elusive.
The regulation of protein phosphorylation by kinases and phosphatases is accepted as a universal mechanism of cellular control (Cohen, 1992), and Ca2+ and CaM signals are frequently transduced via Ca2+- and CaM-dependent kinases and phosphatases (Roberts and Harmon, 1992; Roberts, 1993). Okadaic acid, a protein phosphatase inhibitor, has been found to affect both GA and ABA pathways, but not hypoxic responses in the wheat aleurone (Kuo et al., 1996). It inhibited the expression of high-pI amylase, the increase in cytoplasmic [Ca2+], and the cell death normally induced by GA, and antagonized the induction of the PHVA1 gene by ABA (Kuo et al., 1996). A variety of Tyr and Ser/Thr phosphatase inhibitors have also been shown to antagonize the regulation of RaB gene expression by ABA in barley aleurone (Heimovaara-Dijkstra et al., 1996). The unknown targets of these inhibitors in situ in the aleurone have made interpretation of these data difficult. However, further support for the involvement of kinases in the regulation of the hormone responses of the aleurone arises from reports that indicate that the slow vacuolar channel is regulated by phosphatase action (Bethke and Jones, 1997) and that ABA activates an aleurone mitogen-activated protein kinase-like activity (Knetsch et al., 1996), whereas GA down-regulates expression of ASPK9. ASPK9 has homology to the regulatory mitogen-activated protein kinase ERK1 of mammalian cells (Huttly and Phillips, 1995). In addition, cDNAs corresponding to putative Ca2+-dependent CDPKs (Roberts and Harmon, 1992; Roberts, 1993) have been detected in oat aleurone (Huttly and Phillips, 1995).
Findings from other systems also strongly suggest that protein kinase and phosphatase activities may be critical elements in the regulation of plant responses to ABA. For example, the Abi-1 and Abi-2 genes encode protein phosphatase 2C homologs, which, when defective, confer ABA insensitivity to Arabidopsis (Leung et al., 1994, 1997; Meyer et al., 1994). These phosphatase activities may regulate guard cell responses to ABA (Armstrong et al., 1995; Pei et al., 1997). ABA has also been shown to lead to rapid induction of a Ca2+-independent kinase (AAPK) in guard cells (Li and Assmann, 1996; Mori and Muto, 1997). Additionally, Ca2+-independent mutants of Arabidopsis CDPKs can induce expression driven by the ABA-regulated HVA-1 promoter in maize (Sheen, 1996). ABA has also been found to up-regulate the PKABA1 kinase in wheat embryos (Anderberg and Walker-Simmons, 1992), which has homology to kinases of oat aleurone, Aspk4 and Aspk5 (Huttly and Phillips, 1995).
We therefore initiated a screen to determine where protein kinases are involved in the regulation of the aleurone GA or ABA response, and whether Ca2+ is involved in these events. A range of kinase substrate peptides was introduced into the cytoplasm of aleurone protoplasts using electroporation or microinjection. These peptides could compete with the endogenous kinase substrate(s), hence blocking downstream events. This strategy has proved highly successful in, for example, defining the timing of kinase activities during mitotic progression in Tradescantia virginiana stamen hairs (Wolniak and Larsen, 1995), or highlighting the involvement of a kinase that specifically phosphorylates myosin light-chain kinase in flagellar activity (Ashizawa et al., 1995). Data are presented showing that the peptide syntide-2, a substrate designed for selectivity toward CaM kinase II (Hashimoto and Soderling, 1987), selectively inhibits the GA response in aleurone cells while leaving constitutive and ABA-regulated events unaffected. This peptide is phosphorylated by a soluble, 54-kD Ca2+-activated protein kinase with which it appears to interact in vivo.
MATERIALS AND METHODS
Protoplast Isolation
Barley (Hordeum vulgare L. cv Himalaya; Department of Agronomy, Washington State University, Pullman) grains were de-embryonated, cut into quarters, and prepared for protoplast isolation as described by Hillmer et al. (1992), except that all cellulase solutions were supplemented with 0.1% (w/v) BSA. Freshly isolated protoplasts were incubated in Gamborg's B5 medium (Sigma) supplemented with 0.6 m mannitol and 10 mm CaCl2, in the presence or absence of either 5 μm GA3 or 10 μm ABA. GA3 was used in all experiments. After incubation, protoplasts were purified on a Nycodenz (Sigma) density gradient and amylase secretion was assayed as described by Bush and Jones (1988).
Embedding Protoplasts for Microinjection and Monitoring α-Amylase Secretion from Individual Protoplasts
Single aleurone protoplasts were embedded in a gel matrix according to the work of Gilroy and Jones (1994). Protoplasts were monitored on a microscope (Diaphot 300, Nikon) using a 40×, 0.7 numerical aperture, dry objective and differential interference contrast optics. Images were captured using a CH250A cooled, charge-coupled device camera (Photometrics, Tucson, AZ) and digitized using a computer (Quadra 800, Apple Computer Inc., Cuppertino, CA) running IPLabs spectrum image-acquisition software (Signal Analytics, Vienna, VA). The gel matrix contained 3% (w/v) ultralow-melting-point agarose (Sigma) and 3% (w/v) soluble potato starch (Baker Chemical Co., Philadelphia, PA) in Gamborg's B5 medium supplemented with 0.6 m mannitol. This preparation was used for microinjection, single-cell secretion assay, and reporter gene assays as previously described (Hillmer et al., 1992; Gilroy and Jones, 1994; Gilroy, 1996).
Microinjection of Protoplasts
Protoplasts were embedded in agarose thin films, impaled with micropipettes (10–20 MΩ resistance) pulled from 1.5-mm-external-diameter filament electrode glass (World Precision Instruments, New Haven, CT), and microinjected as described by Gilroy (1996). For fluorescent indicator injections, the micropipettes were loaded with 1 mm indo-1 conjugated to a 10-kD dextran, with or without 0.5 mm peptide, as appropriate. Fluorescent dye was then pressure injected using a pneumatic picopump (PV830, World Precision Instruments, Sarasota, FL) and a series of 0.14-MPa pressure pulses. Cytoplasmic concentrations of injected compounds were assessed from the fluorescence intensity of co-injected lucifer yellow, according to the method of Gilroy (1996).
Protoplasts were allowed to recover from the microinjection for 20 min before additional imaging was performed. Protoplasts that failed to maintain a turgid appearance, that exhibited disruption of normal cytoplasmic structure (typically a rapid condensation of the cytoplasm), or that failed to exhibit more than 500 counts per second LUC activity in the transient-expression experiments were not studied further. Using these criteria, the efficiency of microinjection was approximately 20%.
Confocal Microscopy and Measurement of Cytoplasmic [Ca2+]
Indo-1-dextran-microinjected protoplasts were placed on the stage of an inverted microscope (Axiovert, Zeiss) attached to a LSM-410 laser-scanning confocal microscope and imaged using a Zeiss 40×, 0.75 numerical aperture, dry objective. Ca2+ levels were then determined by confocal ratio imaging as described by Gilroy (1996). Autofluorescence and dark current represented <5% of the indo-1 fluorescence signal at each detector and were subtracted before ratio analysis.
FITC-dextran was visualized with the LSM-410 confocal microscope using 488-nm excitation, 488 dichroic, and 510- to 540-nm emission, selected using Zeiss interference filters. 4′,6′-Diamidino-2-phenylindole staining (Schuurink et al., 1996) and N-phenyl-1-naphthylamine staining (Saunders and Hepler, 1981) were visualized using 354-nm excitation, 80/20 beam splitter, and >460-nm emission. 2′,7′-bis-(2-Carboxyethyl)-5-(and 6)carboxyfluorescein, acetoxymethyl ester staining was performed as described by Swanson and Jones (1996) and visualized using 488-nm excitation, 488 dichroic, and 510- to 540-nm emission.
Fluorescent and Photoaffinity Labeling of Peptides
Ten milligrams of syntide-2 or malantide was labeled with 5:1 molar excess of fluorescein succinimidyl ester for 2 h at 22°C in PBS, pH 8.0 (Banks and Paquette, 1995). The reaction products were separated by TLC on silica-coated plates (Fisher Scientific) using 2:2:8 (v/v) water:butanol:glacial acetic acid as a solvent (Aromatorio et al., 1983), and the fluorescent products were visualized on a transilluminator (FBTIV-88, Fisher Scientific). The fluorescent products were each scraped from the plate and eluted in water. The eluted, labeled peptide was identified as the only fluorescently labeled product that could be phosphorylated by aleurone kinase extracts (using the assay outlined below) and that reacted with the Coomassie brilliant blue protein stain (Bio-Rad).
To generate photoaffinity forms of syntide-2 and malantide, the fluorescently labeled peptides were stirred for 4 h in the dark with benzophenone-isothiocyanate (Molecular Probes, Eugene, OR) at a 10:1 molar excess in PBS, pH 8.0 (Miller, 1991; Banks and Paquette, 1995). Labeled peptide was purified by preparative TLC as described above.
Protoplast Electroporation
Protoplasts were sedimented at 1g and resuspended to 1 × 106 mL−1 in 150 mm Suc, 250 mm sorbitol, 500 mm mannitol, 1 mm EDTA-K2, 5 mm Hepes-KOH, pH 7.1, in 0.8-mL electroporation cuvettes (Bio-Rad). The compound to be loaded into the protoplasts (peptide, BSA, or 10-kD FITC-dextran) was then added to the cuvette. Protoplasts were then permeabilized with a single 3 kV cm−1 pulse delivered from a 1-μF capacitor (Gene Pulser, Bio-Rad) and after 5 min the electroporation buffer was diluted 1:1 with 380 mm mannitol, 6 mm KNO3, 12 mm l-Arg, 10 mm Mes, pH 5.1, and sufficient Gamborg's B5 micronutrients (Sigma) to restore the medium to the composition of full-strength Gamborg's B5 medium. Protoplasts were then incubated as normal. The efficiency of permeabilization was 55 ± 5% (n = 500) as determined by ethidium bromide staining (Gilroy et al., 1986). Viability after electroporation and 24 h of incubation was >80% as assessed by fluorescein diacetate staining (Huang et al., 1986). Cytoplasmic concentrations of compounds were assessed from the fluorescence intensity of co-electroporated FITC-dextran (0.05%, w/v) as described above for microinjection experiments. Electroporation in the presence of 100 μm peptide or BSA led to an internal concentration of approximately 25 μm.
Kinase Extraction
Aleurone layers from sterile half-grains were prepared as described by Jones and Jacobsen (1983), treated with or without 5 μm GA or 5 μm ABA, frozen in liquid N2, and stored at −80°C for subsequent extraction. All of the following steps were carried out at 4°C or on ice unless otherwise stated. All buffers were supplemented with 1 mm DTT, 0.5 mm PMSF, and 10 μg mL−1 each of leupeptin, aprotinin, and pepstatin. Layers were ground with liquid N2 in a pestle and mortar and homogenized in 10× volume extraction buffer, 250 mm Suc, 0.1 mm MgCl2, 1 mm EDTA, 50 mm Tris-acetate, pH 8.0. The homogenate was filtered through two layers of Miracloth (Calbiochem) and centrifuged at 25,000g for 30 min. The supernatant (crude fraction) was used in subsequent chromatographic steps. When soluble and microsomal fractions were required, the homogenate was first centrifuged at 10,000g for 10 min, and the supernatant was centrifuged at 100,000g for 45 min. The resulting pellet (microsomal fraction) was washed with and then resuspended in extraction buffer. The supernatant was referred to as the soluble fraction.
Kinase Assays
Protein kinase activity was determined by measuring the labeling of protein by [γ-32P]ATP. Substrates used were histone (type III-S, Sigma H5505) or the peptides LRRASLG (kemptide, a cAMP-dependent protein kinase substrate), VRKRTLRRL (a protein kinase C substrate), PLARTLSVAGLPGKK (syntide-2, a Ca2+/calmodulin-dependent protein kinase-II substrate), or RTKRSGSVYEPLKI (malantide, a cAMP-dependent protein kinase substrate). The assay mixture (200 μL) contained a 50-μL sample, 50 mm Hepes, pH 7.0, 10 mm MgCl2, 200 μm EGTA, or 50 μm CaCl2 (free Ca2+ determined using a Ca2+-selective electrode; model 93.20, Orion Research Inc., Boston, MA), and either 25 μm histone or substrate peptide. The reaction was initiated with 20 μm [γ-32P]ATP (1000 cpm pmol−1; Amersham) incubated at room temperature for 2 min and stopped by pipetting 40 μL of the reaction mixture onto a square of 2- × 2-cm phosphocellulose paper (type P81, Whatman) and immediately immersing the paper into 78 mm phosphoric acid. The paper squares were washed five times for 5 min in phosphoric acid (5 mL per filter), allowed to dry for 30 min, and counted by liquid scintillation. All filter assays were performed in duplicate. The relationship between activity and time of reaction was linear for up to 6 min when syntide or histone was used as the substrate (data not shown); therefore, assays were conducted for 2 min.
Assays of autophosphorylation activities of aleurone extracts were performed as described above, except that the final volume was 50 μL, the [γ-32P]ATP added was 100 nm (10,000 cpm pmol−1), and no exogenous substrate (histone or peptide) was added. Samples were incubated at room temperature for 2 min and then TCA was added to give a final concentration of 10% (w/v). After incubation on ice for 2 h the samples were centrifuged at 1,000g in a bench-top centrifuge (Tomy Kogyo Co. Ltd., Fukushima, Japan) for 10 min and the supernatants removed. The tubes were dried and 2 μL of 1 n NaOH was added. SDS-gel electrophoresis was performed using 12% polyacrylamide gels with either a Protean II or a mini-Protean II gel system (Bio-Rad) according to the manufacturer's instructions. After electrophoresis, gels were stained with Coomassie blue, sealed in polyethylene bags, and exposed to XAR-5 autoradiographic film (Kodak) overnight before being developed. In-gel kinase assays were performed as described by Kameshita and Fujisawa (1989), except that we used 12% SDS-PAGE gels, urea as the denaturing agent, and no protein was supplemented in the gel matrix.
Protein concentration was determined by the method of Bradford (1976) using BSA as a standard and the Bio-Rad protein assay kit.
RESULTS
Microinjection of Syntide-2 Selectively Inhibits the GA Response of Aleurone Protoplasts
Aleurone cells were microinjected with a range of protein kinase substrate peptides to screen for peptides that inhibited the responses to GA or ABA. The peptides were chosen to have selectivity as substrates for a range of well-defined protein kinase activities: cAMP-dependent protein kinase (malantide, kemptide), protein kinase C, and Ca2+/calmodulin-dependent protein kinase-II (syntide-2). These peptides, plus BSA as a control, were microinjected into aleurone protoplasts at up to 50 μm cytoplasmic concentration. The protoplasts were then treated with or without 5 μm GA and the development of their GA response was assessed.
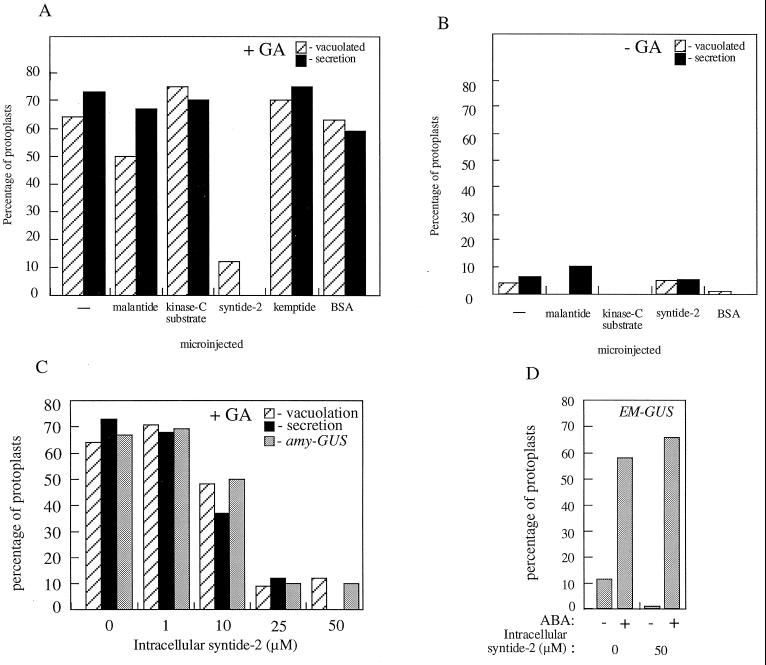
Cereal aleurone cells underwent a series of well-characterized responses to GA. Expression of α-amylase genes was strongly up-regulated by GA, and this can be monitored at the single-cell level using an AMY-GUS transient expression assay (Gilroy, 1996). Protoplasts were simultaneously microinjected with a plasmid containing the promoter of α-amylase gene fused to a GUS gene, together with a LUC gene that has a ubiquitin promoter (and that is constitutively expressed) (Gilroy, 1996). The ratio of GUS:LUC reflects the level to which the amylase promoter was being induced by GA. The second assay we used to assess the GA response monitored the α-amylase secreted by GA-responding protoplasts. α-Amylase secretion can also be assayed on a single-cell basis using a starch gel assay (Hillmer et al., 1993). The third parameter monitored in the aleurone protoplasts was the development of large vacuoles characteristic of the GA response (Bush et al., 1986). Figure 1, A and B, shows that none of the microinjected peptides induced GA-like responses in protoplasts. Only syntide-2 inhibited the response of aleurone protoplasts to GA at concentrations > 25 μm (Fig. 1C). The continued GA response in protoplasts injected with peptides other than syntide, or injected with BSA, indicates that neither the microinjection procedure nor the introduction of exogenous protein into the cytoplasm inhibited the GA response. Protoplasts injected with syntide-2 remained turgid and did not exhibit the condensation of cytoplasm associated with cytotoxicity for as long as we observed them (48 h).
Figure 1.
The effect of microinjection of protein kinase peptide substrates on GA-induced amylase secretion, vacuolation, and amylase-GUS and EM-GUS expression. A and B, Freshly isolated protoplasts were microinjected with 50 μm of the indicated protein kinase substrate peptides or BSA and then treated for 24 h with (+GA) or without (−GA) 5 μm GA. C, Freshly isolated protoplasts were microinjected with a range of syntide-2 concentrations and then treated for 24 h with 5 μm GA. Secretion of amylase, development of GA-induced vacuolated morphology, and amylase-GUS expression were then assessed on a single-cell basis. D, Freshly isolated protoplasts were co-microinjected with 50 μm syntide-2 or BSA and the EM-GUS construct and treated with or without 10 μm ABA. Induction of EM-GUS expression was then assessed on a single-cell basis. Protoplasts were scored as showing induction of amylase-GUS or EM-GUS expression if they exhibited a GUS:LUC of more than 4000 (Gilroy, 1996). Protoplasts were scored as secreting amylase if they showed a cleared “halo” of >25 μm in the starch thin-film assay (Hillmer et al., 1992). Vacuolated protoplasts were those showing development to stage 3 or 4 as defined by Bush et al. (1986). The responses of at least nine microinjected protoplasts per treatment are shown.
Microinjection of syntide-2 did not inhibit ABA-regulated or constitutive gene expression. Protoplasts injected with syntide-2 showed expression of the constitutive ubiquitin-LUC construct in transient-expression assays to levels similar (>500 counts per second) to what was seen in nonsyntide-2-injected controls (data not shown). ABA-regulated gene expression was monitored using a construct of the promoter of the ABA-responsive EM gene fused to a GUS gene (Jacobsen and Close, 1991; Gilroy, 1996). Figure 1D shows that in response to ABA, syntide-2-injected protoplasts exhibited levels of induction of the Em promoter to levels equivalent to those seen in noninjected or BSA-injected controls.
Syntide-2 Does Not Prevent Changes in Cytosolic Ca2+
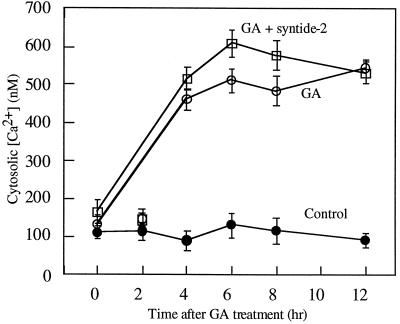
Transduction of the GA signal is known to be accompanied by an increase in cytosolic [Ca2+], and to be Ca2+ dependent (Gilroy, 1996). Therefore, we tested whether syntide-2 affected the aleurone response to GA before or after this change in [Ca2+]. Aleurone protoplasts were co-microinjected with the Ca2+-sensitive dye indo-1-dextran with or without 50 μm syntide-2, treated with GA, and the cytoplasmic [Ca2+] was determined by confocal ratio imaging (Gilroy, 1996). Figure 2 shows that protoplasts incubated without GA showed a stable resting [Ca2+] of approximately 100 nm, whereas protoplasts treated with GA showed the expected GA-induced increase in [Ca2+]. GA also induced this increase in cytoplasmic [Ca2+] in protoplasts microinjected with 50 μm syntide-2 (Fig. 2).
Figure 2.
The effect of microinjection of syntide-2 on GA-induced cytoplasmic Ca2+ elevation. Freshly isolated protoplasts were microinjected with dextran-conjugated indo-1, with or without 50 μm syntide-2. These protoplasts were then treated without hormone (control) or with 5 μm GA and their cytoplasmic [Ca2+] was monitored at the indicated times using confocal ratio imaging. The results represent the mean ± se of at least nine microinjected protoplasts per treatment.
Microinjection Loads Syntide-2 into the Cytosol of Aleurone Protoplasts
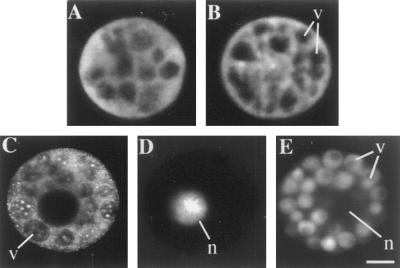
To assess the site of action of the microinjected peptide, syntide-2 was fluorescently labeled with fluorescein succinimidyl ester and microinjected into aleurone protoplasts. For comparison, protoplasts were also treated with fluorescent probes to visualize defined cytoplasmic compartments. Figure 3A shows a protoplast microinjected with fluorescein conjugated to a 10-kD dextran to visualize the cytosolic compartment. As shown in Figure 3B, fluorescently tagged syntide-2, introduced at a final concentration of approximately 1 μm and imaged at up to 24 h after microinjection, showed a distribution like that of the FITC-dextran. This indicates that the syntide-2 remains in the cytoplasm and does not accumulate in the vacuoles or nucleus, and does not associate with the endomembrane system (Fig. 3, C–E). A low concentration of fluorescently labeled syntide-2, approximately 1 μm cytoplasmic concentration, that did not inhibit the GA response was used in these experiments for two reasons. First, the visualization of different cellular compartments is more easily performed in cells that are exhibiting the GA response because of the development of large vacuoles. Second, it proved difficult to synthesize the fluorescently labeled peptide at concentrations high enough to inject an amount sufficient to completely inhibit the GA response.
Figure 3.
Cytoplasmic distribution of fluorescently labeled syntide-2 loaded into protoplasts by microinjection. A, Fluorescence from a protoplast microinjected with FITC-dextran. B, Fluorescence from a protoplast microinjected with 1 μm cytoplasmic concentration of fluorescently labeled syntide-2. C, Membranous components of protoplasts visualized by incubation with 5 μm N-phenyl-1-naphthylamine. D, Nuclear fluorescence from a protoplast stained with 4′,6′-diamidino-2-phenylindole. E, Fluorescence from vacuoles labeled with 2′,7′-bis-(2-carboxyethyl)-5-(and 6)carboxyfluorescein, acetoxymethyl ester. Freshly isolated protoplasts were microinjected with FITC-labeled 10-kD dextran fluorescein (A) or fluorescein labeled syntide-2 (B), treated for 24 h with 5 μm GA, and then fluorescence visualized using the Zeiss LSM-410 confocal microscope. C to E, Protoplasts were treated for 24 h with 5 μm GA and then stained for the appropriate compartment. Results are representative of more than 10 replicates. v, Vacuole; n, nucleus. The scale bar represents 10 μm.
Interactions between Syntide-2 and Aleurone Proteins in Vivo
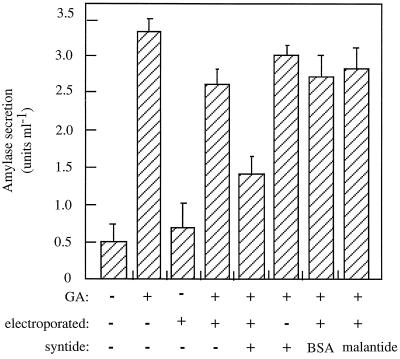
Having established that syntide-2 selectively inhibited the GA response, we sought to identify candidates for the cytosolic factor(s) with which the peptide was interacting. We first developed an electroporation protocol to load sufficient aleurone protoplasts with syntide-2 to allow biochemical analysis of the sample. We first ensured that electroporation did not alter the GA responsiveness of aleurone protoplasts (Fig. 4) and that the introduction of syntide-2 into aleurone protoplasts by electroporation inhibited the GA response (Fig. 4), as predicted from the microinjection experiments described above (Fig. 1A). The apparent degree of inhibition of the GA response by electroporation of syntide-2 (55%; Fig. 4) was not as great as that when the peptide was microinjected (approximately 90%; Fig. 1A). This result occurred because if the peptide was not successfully injected into the protoplast during the microinjection experiments, the result was excluded from the data. Thus, 100% of cells analyzed for the microinjection data were loaded with syntide-2. In contrast, the percentage of cells permeabilized by the electroporation protocol was 55 to 60%, leaving a background of 40 to 45% of protoplasts not loaded with syntide-2. Because these two pools of protoplasts could not be separated after electroporation, the amylase activity data contain a background of these unelectroporated (nonsyntide-2-loaded) protoplasts.
Figure 4.
The effect of electroporation of protein kinase substrate peptides on GA-induced amylase secretion. Freshly isolated protoplasts were loaded with 25 μm syntide-2, malantide, or BSA by electroporation and treated for 48 h with or without 5 μm GA. Secreted amylase activity was then assessed. A set of nonelectroporated control protoplasts was treated identically. Results represent the mean ± se of three separate experiments.
Having established that protoplasts loaded with syntide-2 by electroporation showed the expected inhibition of the GA response (Fig. 4), we then used this as a tool in combination with a photoaffinity technique to label the proteins targeted by syntide-2. Syntide-2 or malantide were fluorescently tagged and then covalently labeled with the UV-activated cross-linking agent benzophenone-4-isothiocyanate. When irradiated with UV, this agent cross-links the peptide and whatever molecule it is interacting with in vivo. Protoplasts were loaded with this photoaffinity, fluorescent syntide-2 or malantide by electroporation.
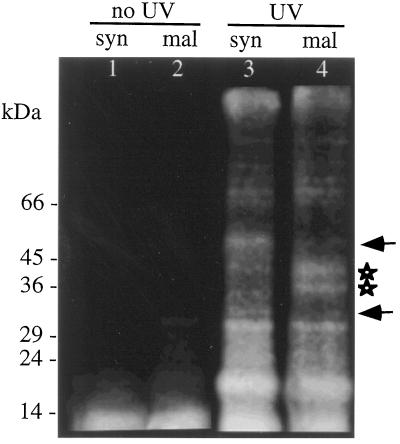
Figure 5 (lanes 1 and 2) shows that when the above procedure was performed without subsequent UV illumination, no cross-linking was evident and little protein labeling could be observed after SDS-PAGE of the protoplast extracts. However, with UV activation, both photoaffinity-labeled malantide and syntide-2 became cross-linked to a range of proteins. Most of these proteins were labeled identically by both syntide-2 and malantide. Because malantide is not inhibitory to the GA response (Figs. 1A and 4), the proteins labeled in common between syntide and malantide probably reflect nonspecific interactions with aleurone cell components. However, 33- and 41-kD proteins were selectively labeled by malantide, whereas syntide-2 showed enhanced cross-linking to 30- and 55-kD proteins. The proteins showing selective interaction (labeling) with syntide-2 represent tentative in vivo candidates for the site of action of syntide-2. UV irradiation of unelectroporated protoplasts incubated in the photoaffinity syntide-2 or malantide showed no detectable labeling of protoplast proteins (data not shown). This indicates that the labeled proteins seen in Figure 5 (lanes 3 and 4) are most likely intracellular in origin.
Figure 5.
Fluorograph of proteins labeled in vivo by photoaffinity syntide-2 or photoaffinity malantide. Protoplasts were electroporated with fluorescent, photoaffinity-labeled syntide-2 or malantide and allowed to recover for 1 h. The protoplasts were then frozen in liquid N2 to freeze molecular interactions, and total proteins were extracted in the dark (lanes 1 and 2). In parallel experiments the peptide-loaded protoplasts were UV irradiated to cross-link the photoaffinity peptides to closely associated proteins and then extracted (lanes 3 and 4). Proteins were then separated by SDS-PAGE. Peptide-labeled proteins were visualized using the fluorescein attached to the photoaffinity peptides. Note the 30- and 55-kD proteins selectively labeled by syntide-2 (arrows) and the 33- and 41-kD specific to malantide (stars). The positions of molecular mass markers are indicated in kilodaltons on the left.
Biochemical Identification of the Target of Syntide-2
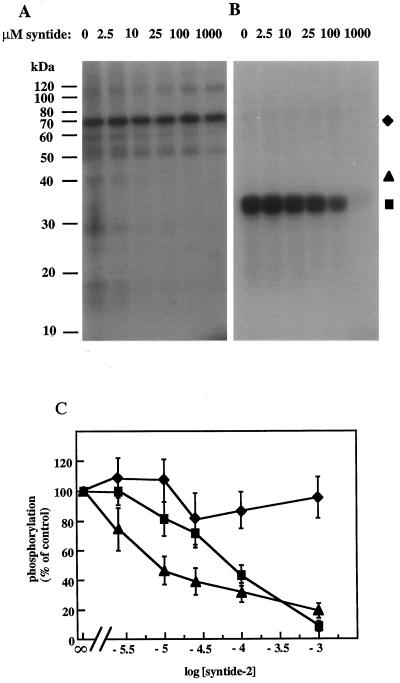
Because syntide-2 was designed as a protein kinase substrate, we hypothesized that the introduction of syntide-2 into aleurone protoplasts and the resulting inhibition of the GA response was attributable to syntide competing with the endogenous substrate(s) for a protein kinase. To determine if this competition could occur in vitro, a range of syntide-2 concentrations was added to kinase assays and the effects on the phosphorylation of endogenous proteins and histone were assessed. Figure 6 shows that the phosphorylation of histone and some but not all endogenous proteins was inhibited by increasing concentrations of syntide-2, indicating selectivity of the syntide-2 inhibitory effect. Quantification of phosphorylation levels showed that phosphorylation of a 40-kD endogenous protein and exogenous histone were inhibited with an IC50 of approximately 8 and 80 μm syntide-2, respectively, whereas phosphorylation of a 70- kD endogenous protein was unaffected by syntide (Fig. 6C).
Figure 6.
The inhibition of histone and endogenous protein phosphorylation by syntide-2. Syntide-2 was added to in vitro substrate phosphorylation assays carried out without (A) or with (B) 25 μm histone. The samples were precipitated with 10% TCA and processed for SDS-PAGE and autoradiography. C, Phosphorylation levels from the 70- and 40-kD endogenous substrate bands and histone was assessed by cutting sections of the gel that corresponded to bands on the autoradiograph, and counting duplicate samples in a scintillation counter. Results are typical of two independent experiments. ▪, Histone; ♦, 70 kD; ▴, 40 kD.
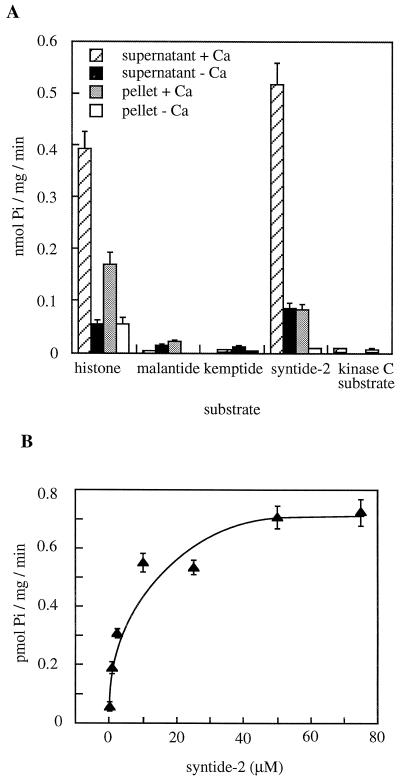
We next tested whether syntide-2 was a substrate of aleurone kinase(s). The same range of kinase substrate peptides that was microinjected into protoplasts (Fig. 1A), as well as histone, was assayed for phosphorylation in vitro for protein kinase assays by crude aleurone extracts. Syntide-2 and histone were phosphorylated in a Ca2+-activated manner, the activity being predominantly in the soluble fraction (Fig. 7A). The other peptides were phosphorylated to much lesser extents even when assayed for much longer times (up to 20 min; data not shown). None of the other peptides showed Ca2+-activated phosphorylation. The Michaelis-Menten kinetics of syntide-2 phosphorylation (Fig. 7B) suggest a kinase activity with an apparent Km of 8 μm for syntide-2.
Figure 7.
Peptide phosphorylation activity in barley aleurone extract. A, Microsomal (pellet) and soluble (supernatant) fractions were prepared and their kinase activity assessed using 25 μm substrate peptides or histone. Assays were carried out in the presence of 50 μm CaCl2 (+Ca) or 1 mm EGTA (−Ca). B, A range of syntide-2 concentrations was used on the soluble fraction in assays containing 50 μm CaCl2, and the level of 32P incorporation into the peptide during the initial 2 min of reaction was calculated. The reaction rate was linear over this time period. The results are the mean ± se of two experiments, with two replicates per experiment.
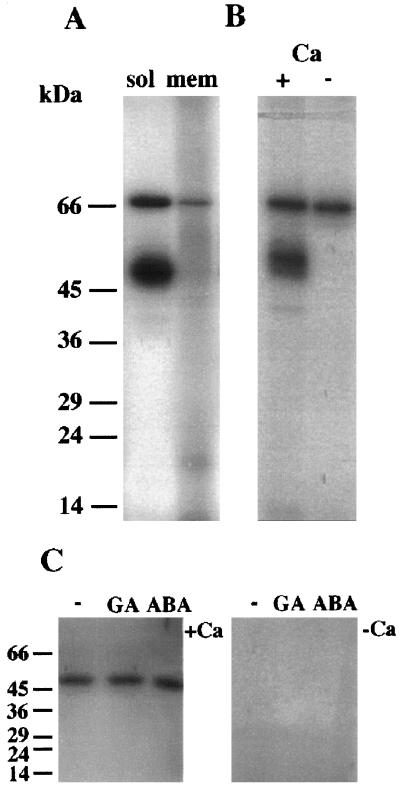
Many protein kinases show a highly active autophosphorylating activity. Therefore, we used this property as an initial screen for candidate protein kinases for the syntide-2 phosphorylating activity in extracts of aleurone layers. The same autophosphorylation profile was seen when proteins were extracted from protoplasts (data not shown) or intact tissue (aleurone layers). Layers were therefore routinely used for biochemical experiments. Figure 8A shows that two major autophosphorylating bands of 54 and 68 kD were visible on autoradiographs. Fainter signals at around 60 and 50 kD were sometimes visible, but they varied in relative intensity between experiments. The degree of syntide-2 phosphorylation did not change between experiments, regardless of whether or not these intermediate bands were visible on autoradiographs. Autophosphorylation of the 54-kD band was Ca2+ activated, whereas that of the 68-kD band was not (Fig. 8B). The 54-kD activity was found predominantly in the soluble fraction, whereas the 68-kD activity was found in both soluble and membrane-associated fractions. When autophosphorylation was assayed “in gel,” the only kinase activity detected was that of a Ca2+-dependent 54-kD protein (Fig. 8C). The pattern of autophosphorylation was identical in samples extracted from aleurone layers that had been treated with CaCl2, GA, or ABA for up to 24 h.
Figure 8.
Autophosphorylation characteristics of barley aleurone kinase activities. A, Autophosphorylating activities of kinases in soluble or microsomal fractions extracted from aleurone layers. Assays were carried out in the presence of 50 μm CaCl2 using 50 μg of protein per fraction. B, Autophosphorylation activities of kinases in a crude fraction were carried out in the presence of 50 μm CaCl2 (+) or 500 μm EGTA (−). C, Aleurone layers were treated for 24 h with no hormones (−), 5 μm GA, or 5 μm ABA. Crude extracts of proteins were then prepared, run on 12% SDS-PAGE, renatured, and assayed for autophosphorylating activity in gel. For these assays incubation with [32P]ATP at a concentration of 1 μm (50,000 dpm pmol−1) was carried out in the presence of 50 μm CaCl2 (+Ca) or 1 mm EGTA (−Ca). Note that only the 54-kD kinase is renatured under these conditions. The positions of molecular mass markers are indicated in kilodaltons on the left.
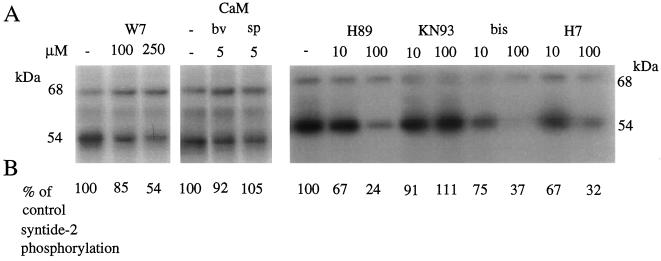
The CaM antagonist W7 (250 μm) inhibited autophosphorylation of the 54-kD kinase and syntide-2 phosphorylation (Fig. 9), whereas the much-less-active analog N-(6-aminohexyl)-1-naphthalene-sulfonamide (W5) had no effect on either parameter (data not shown). W7 did not affect autophosphorylation of the 68-kD kinase. Exogenous spinach or bovine CaM had no effect on autophosphorylation or syntide-2 phosphorylation.
Figure 9.
The effect of protein kinase inhibitors, CaM antagonist W7, and exogenous CaM on autophosphorylation and syntide-2 phosphorylation activities in the barley aleurone. Four protein kinase inhibitors, W7, or CaM (bv, bovine; sp, spinach [Sigma]) were added to autophosphorylation and syntide-2 phosphorylation assays at the indicated concentrations. Aleurone layers were extracted and assays were carried out in the presence of 50 μm CaCl2 as described in Methods. A, Autophosphorylation of the 68- and 54-kD proteins was visualized by autoradiography; B, the effect of protein kinase inhibitors on syntide-2 phosphorylation expressed as a percentage of control (no inhibitor) is shown below the appropriate inhibitor lane on the autoradiograph. The inhibitors used were obtained from Calbiochem: H89, specific for cAMP-dependent protein kinase; KN93, specific for Ca2+/CaM-dependent protein kinase II; bisindolylmaleimide (bis), specific for protein kinase C; and 1-(5-isoquinolinesulfonyl)-2-methylpiperazine (H7), a broad-range Ser/Thr kinase inhibitor. The experiments were repeated twice with two replicates each. The largest se for syntide-2 phosphorylation was 12%.
A range of protein kinase inhibitors was also added to syntide-2 phosphorylation and autophosphorylation assays. Figure 9 shows that all of the inhibitors used (H89, bisindolylmaleimide, and 1-(5-isoquinolinesulfonyl)-2-methylpiperazine) (H7), except KN93, inhibited syntide-2 phosphorylation at high concentrations (100 μm). KN93 did not affect syntide-2 phosphorylation or autophosphorylation of the 54-kD band but it partially affected autophosphorylation of the 68-kD band. Conversely, H89 had no affect on autophosphorylation of the 68-kD band, whereas it did inhibit autophosphorylation of the 54-kD band. H89 also inhibited syntide-2 phosphorylation with an IC50 of 25 μm.
DISCUSSION
The high selectivity of protein kinases for their substrate peptides allowed us to use these peptides as inhibitors of kinase action in vivo. We reasoned that the phosphorylation of the synthetic peptide by a kinase would competitively inhibit the phosphorylation of the “normal” in vivo target, and hence block the effect of the in vivo substrate phosphorylation. In aleurone we found that microinjection or electroporation of syntide-2, but not the other kinase substrate peptides tested, led to a reduction in the amylase secretion, the vacuolation, and the activation of amylase gene transcription normally associated with the GA response. Constitutive gene expression (ubiquitin-LUC) and the ABA response (induction of EM-GUS) were unaffected by concentrations of syntide-2 that inhibited the GA response. These results suggest that this peptide is highly selective for affecting transduction of the GA signal, possibly through competitive inhibition of an aleurone protein kinase.
GA causes an elevation of cytosolic [Ca2+] in aleurone (Bush and Jones, 1987, 1988; Gilroy and Jones, 1992; Bush, 1996; Gilroy, 1996), which is essential for maintaining secretory activity in these cells (Gilroy, 1996). This increase was not detectably altered by the microinjected syntide-2, suggesting that an event downstream of the GA-regulated Ca2+ signal was affected by the peptide. Although we cannot determine from this result whether syntide-2 is inhibiting a Ca2+-dependent step in the GA response, this is a strong possibility. In barley aleurone we have found that syntide-2 phosphorylation was enhanced approximately 6-fold in the presence of CaCl2. Syntide-2 was designed as a selective substrate for mammalian CaM kinase II (Hashimoto and Soderling, 1987). Very few CaM-modulated protein phosphorylation activities have been found in plants (Roberts and Harmon, 1992), and a plant CaM kinase II homolog has not been reported. However, a family of plant Ca2+-regulated protein kinases (CDPKs) containing an endogenous CaM-like domain has been characterized from a range of plant sources (e.g. Harper et al., 1991; Roberts and Harmon, 1992; DasGupta, 1994; Saha and Singh, 1995; Furumoto et al., 1996) and two putative CDPKs were found in a PCR-based screen of kinases in mRNA from oat aleurone (Huttly and Phillips, 1995). Proposed sites of Ca2+-regulated events in the aleurone cell include fluxes into the ER to sustain amylase production (Bush et al., 1993; Gilroy and Jones, 1993), regulation of vacuolar ion channel activity (Bethke and Jones, 1994), and targeting and fusion of secretory vesicles with the plasma membrane (Zorec and Tester, 1992). Exocytosis in plant cells has been proposed to require elevated Ca2+ localized at the zone of vesicle fusion, where Ca2+-dependent lipid-binding proteins, such as annexins, may play a critical role in the exocytotic event (Battey and Blackbourn, 1993). It is possible the 54-kD kinase may act to regulate one of these activities.
The kinase inhibitors KN93 and H89 revealed that inhibition of autophosphorylation of the 54-kD band was closely correlated with inhibition of syntide-2 phosphorylation, suggesting that the 54-kD kinase may be the Ca2+-dependent syntide-2 phosphorylating activity in vivo. Thus, KN93 failed to inhibit both autophosphorylation of the 54-kD protein and syntide-2 phosphorylation but had a partial effect on the autophosphorylation of the 68-kD kinase. Conversely, H89 left the 68-kD band unaffected but inhibited both syntide-2 phosphorylation and autophosphorylation of the 54-kD band. The in vivo photoaffinity labeling of a 55-kD protein by syntide-2 (Fig. 5) is noteworthy in this context, because this labeled protein has a similar molecular mass to that predicted for the 54-kD syntide-2 kinase coupled to syntide-2. Peptide-based photoaffinity probes have previously been used to successfully photoaffinity label protein kinases for which they are a specific substrate (Miller, 1991). These data led us to the hypothesis that the 54-kD kinase activity was responsible for the phosphorylation of syntide-2 in vitro and, hence, was a possible target for the effect of syntide-2 on the GA response in vivo. This idea is reinforced by the observation that in vitro, syntide-2 competed with endogenous substrates for phosphorylation by the Ca2+-activated kinase at a concentration range (>10 μm) similar to that which affected the GA response when microinjected into protoplasts in vivo.
The precise identity of the 54-kD kinase and the molecular basis of its Ca2+ activation remain to be determined. Although the Ca2+ dependence of many animal kinases is mediated by CaM (Hanson and Schulman, 1992), these CaM-activated processes are generally inhibited by the CaM antagonist W7 in the range of 0.1 to 10 μm. Proteins containing a CaM-like domain, e.g. CDPK, often require 100 to 200 μm W7 for inhibition (Roberts and Harmon, 1992). W7 inhibited the 54-kD kinase activity with an IC50 of 250 μm, suggesting that the enzyme contains a CaM-like domain (Roberts and Harmon, 1992) consistent with its in-gel Ca2+ activation (Fig. 8C). The Ca2+ activation of this kinase probably does not represent tightly bound co-purifying CaM because this CaM would have had to remain attached to the kinase despite extraction in 1 mm EDTA and SDS-PAGE. Spinach or bovine brain CaM had no effect on either autophosphorylation or histone phosphorylation activity of the 54-kD kinase, further suggesting that the kinase is of the CDPK class. Both the spinach and bovine brain CaM used in these experiments activated cyclic nucleotide phosphodiesterase (assayed according to the method of Cheung [1971]; data not shown). Additionally, bovine CaM enhances the activity of an aleurone Ca2+-activated phosphatase (data not shown) and the Ca2+-ATPase of the aleurone ER (Gilroy and Jones, 1993), and spinach CaM has been shown to alter the ABA response of aleurone protoplasts (Gilroy, 1996) and to regulate ion channels at the aleurone tonoplast (Bethke and Jones, 1994). Thus, it is unlikely that the failure of these CaMs to activate the 54-kD kinase can be attributed to a general inactivity toward CaM-regulated events or a lack of potential to influence aleurone enzymes.
Despite this circumstantial evidence that the 54-kD kinase is of the CDPK class, no proteins in crude, soluble, or microsomal fractions cross-reacted with antibody against soybean CDPK (A.C. Harmon and S. Gilroy, unpublished data). Furthermore, the kinase does not exhibit the Ca2+-dependent mobility shift on SDS-PAGE characteristic of CDPK (data not shown). Since the discovery and characterization of CDPK from soybean (Putnam-Evans et al., 1990; Harper et al., 1991), several kinases have been identified, forming a family of CDPK-like activities (Hrabak et al., 1996, and refs. therein). However, not all CDPKs share the same characteristics. For example, in Arabidopsis CDPKs with varying numbers of EF hand Ca2+-binding sites have been characterized (Hrabak et al., 1996). Similarly, a carrot kinase that contains the CaM-like domain of CDPK did not require Ca2+ for maximum activity when expressed in Escherichia coli (Furumoto et al., 1996), and CDPK-like proteins from potato tubers (MacIntosh et al., 1996) and groundnut (DasGupta, 1994) were both recognized by an anti-soybean CDPK monoclonal antibody but did not show Ca2+-dependent mobility shift on SDS-PAGE. Thus, the failure of the 54-kD kinase to exhibit all of the characteristics of classic CDPKs does not preclude it from being a CaM-like domain kinase. Current research is aimed at defining the precise molecular identity of this kinase.
ACKNOWLEDGMENT
The authors thank Elison Blancaflor for critical reading of the manuscript.
Abbreviations:
- CaM
calmodulin
- CDPK
calmodulin-like domain protein kinase
- FITC
fluorescein isothiocyanate
- H89
N-[2-((p-bromocinnamyl)amino)ethyl]-5-isoquinolinesulfonamide
- IC50
inhibitor concentration for 50% inhibition
- KN93
N-[2-(N(4-chlorocinnamyl)-N-methylaminomethyl)phenyl]-N-[2-hydroxyethyl]-4-methoxybenzenesulfonamide)
- LUC
luciferase
- W7
N-(6-aminohexyl)-5-chloro-1-naphthalene-sulfonamide
Footnotes
This work was supported by U.S. Department of Agriculture grant no. 94-37304-0955. The confocal microscope was supported by U.S. Department of Energy equipment grant no. DE-FG05-93ER79239.
LITERATURE CITED
- Anderberg RJ, Walker-Simmons MK. Isolation of a wheat cDNA clone for an abscisic acid-inducible transcript with homology to protein kinases. Proc Natl Acad Sci USA. 1992;89:10183–10187. doi: 10.1073/pnas.89.21.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong F, Leung J, Grabov A, Brearley J, Blatt MR. Sensitivity to abscisic acid of guard cell K+ channels is suppressed by ABI1-1, a mutant Arabidopsis gene encoding a putative protein phosphatase. Proc Natl Acad Sci USA. 1995;92:9520–9524. doi: 10.1073/pnas.92.21.9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aromatorio DK, Parker J, Brown WE. High-resolution analytical and preparative peptide mapping by a combination of ion-exchange chromatography and thin-layer chromatography. Methods Enzymol. 1983;91:384–391. doi: 10.1016/s0076-6879(83)91036-4. [DOI] [PubMed] [Google Scholar]
- Ashizawa K, Wishart GJ, Hashimoto K, Tsuzuki Y. Dephosphorylation of a 30 kD protein kinase of fowl spermatozoa by the addition of myosin light chain substrate peptide inhibits the flagellar motility. Biochem Biophys Res Commun. 1995;215:706–712. doi: 10.1006/bbrc.1995.2521. [DOI] [PubMed] [Google Scholar]
- Banks PR, Paquette DM. Comparison of three common reactive fluorescent probes used for conjugation to biomolecules by capillary zone electrophoresis. Bioconjugate Chem. 1995;6:447–458. doi: 10.1021/bc00034a015. [DOI] [PubMed] [Google Scholar]
- Battey NH, Blackbourn HD. The control of exocytosis in plant cells. New Phytol. 1993;125:307–338. doi: 10.1111/j.1469-8137.1993.tb03883.x. [DOI] [PubMed] [Google Scholar]
- Bethke PC, Jones RL. Ca2+-calmodulin modulates ion channel activity in storage protein vacuoles of barley aleurone cells. Plant Cell. 1994;6:277–285. doi: 10.1105/tpc.6.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke PC, Jones RL. Reversible protein phosphorylation regulates the activity of the slow-vacuolar ion channel. Plant J. 1997;11:1227–1235. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of micrograms of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bush DS. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- Bush DS. Effects of gibberellic acid and environmental factors on cytosolic calcium in wheat aleurone cells. Planta. 1996;199:89–99. [Google Scholar]
- Bush DS, Biswas AK, Jones RL. Hormonal regulation of Ca2+ transport in the endomembrane system of the aleurone. Planta. 1993;189:507–515. [Google Scholar]
- Bush DS, Cornejo MJ, Huang CM, Jones RL. Ca2+-stimulated secretion of α-amylase during development in barley aleurone protoplasts. Plant Physiol. 1986;82:566–574. doi: 10.1104/pp.82.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DS, Jones RL. Measurement of cytoplasmic calcium in aleurone protoplasts using indo-1 and fura-2. Cell Calcium. 1987;8:455–472. doi: 10.1016/0143-4160(87)90029-7. [DOI] [PubMed] [Google Scholar]
- Bush DS, Jones RL. Cytoplasmic calcium and α-amylase secretion from barley aleurone protoplasts. Eur J Cell Biol. 1988;46:466–469. [Google Scholar]
- Cheung WY. Cyclic 3′,5′-nucleotide phosphodiesterase: evidence for and properties of a protein activator. J Biol Chem. 1971;246:2859–2869. [PubMed] [Google Scholar]
- Cohen P. Signal integration at the level of protein kinases, protein phosphatases and their substrates. Trends Biochem Sci. 1992;17:408–413. doi: 10.1016/0968-0004(92)90010-7. [DOI] [PubMed] [Google Scholar]
- DasGupta M. Characterization of a calcium-dependent protein kinase from Arachis hypogea (groundnut) seeds. Plant Physiol. 1994;104:961–969. doi: 10.1104/pp.104.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincher GB. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:305–346. [Google Scholar]
- Furumoto T, Ogawa N, Hata S, Izui K. Plant calcium-dependent protein kinase-related kinases (CRKs) do not require calcium for their activities. FEBS Lett. 1996;396:147–151. doi: 10.1016/0014-5793(96)01090-3. [DOI] [PubMed] [Google Scholar]
- Gilroy S. Calcium-dependent and -independent signal transduction in the barley aleurone. Plant Cell. 1996;8:2193–2209. doi: 10.1105/tpc.8.12.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Jones RL. Gibberellic acid and abscisic acid coordinately regulate cytoplasmic calcium and secretory activity in barley aleurone protoplasts. Proc Natl Acad Sci USA. 1992;89:3591–3595. doi: 10.1073/pnas.89.8.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Jones RL. Calmodulin stimulation of unidirectional calcium uptake by the endoplasmic reticulum of barley aleurone. Planta. 1993;190:289–296. [Google Scholar]
- Gilroy S, Jones RL. Perception of gibberellin and abscisic acid at the external face of the plasma membrane of barley (Hordeum vulgare L.) aleurone protoplasts. Plant Physiol. 1994;104:1185–1192. doi: 10.1104/pp.104.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu Rev Biochem. 1992;61:559–601. doi: 10.1146/annurev.bi.61.070192.003015. [DOI] [PubMed] [Google Scholar]
- Harper JF, Sussman MR, Schaller GE, Putnam-Evans C, Charbonneau H, Harmon AC. A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science. 1991;252:951–954. doi: 10.1126/science.1852075. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Soderling TR. Calcium/calmodulin dependent protein kinase II and calcium/phospholipid-dependent protein kinase activities in rat tissue assayed with a synthetic peptide. Arch Biochem Biophys. 1987;252:418–425. doi: 10.1016/0003-9861(87)90048-8. [DOI] [PubMed] [Google Scholar]
- Heimovaara-Dijkstra S, Heistek JC, Wang M. Counteractive effects of ABA and GA3 on extracellular and intracellular pH and malate in barley aleurone. Plant Physiol. 1994a;106:359–365. doi: 10.1104/pp.106.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovaara-Dijkstra S, Nieland TJF, van der Meulen RM, Wang M. Abscisic acid-induced gene expression requires the activity of protein(s) sensitive to the protein-tyrosine phosphatase inhibitor phenylarsine oxide. Plant Growth Regul. 1996;18:115–123. [Google Scholar]
- Heimovaara-Dijkstra S, Vanduijn B, Libbenga KR, Heidekamp F, Wang M. Abscisic acid-induced membrane potential changes in barley aleurone protoplasts: a possible relevance for the regulation of Rab gene expression. Plant Cell Physiol. 1994b;35:743–750. [Google Scholar]
- Hillmer S, Gilroy S, Jones RL. Visualization of secretion from single barley aleurone protoplasts. Plant Physiol. 1992;102:279–286. doi: 10.1104/pp.102.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabak EM, Dickmann LJ, Satterlee JS, Sussman MR. Characterization of eight new members of the calmodulin-like domain protein kinase gene family from Arabidopsis thaliana. Plant Mol Biol. 1996;31:405–412. doi: 10.1007/BF00021802. [DOI] [PubMed] [Google Scholar]
- Huang C-N, Cornejo MJ, Bush DS, Jones RL. Improved methods for the determination of viability of plant protoplasts. Protoplasma. 1986;135:80–87. [Google Scholar]
- Huttly AK, Phillips AL. Gibberellin-regulated expression in oat aleurone cells of two kinases that show homology to MAP kinase and a ribosomal protein kinase. Plant Mol Biol. 1995;27:1043–1052. doi: 10.1007/BF00037031. [DOI] [PubMed] [Google Scholar]
- Jacobsen J, Close TJ. Control of transient expression of chimaeric genes by gibberellic acid and abscisic acid in protoplasts prepared from mature barley aleurone layers. Plant Mol Biol. 1991;16:713–724. doi: 10.1007/BF00023435. [DOI] [PubMed] [Google Scholar]
- Jones RL, Jacobsen JV. Calcium regulation of the secretion of α-amylase isoenzymes and other proteins from barley aleurone. Planta. 1983;158:1–9. doi: 10.1007/BF00395396. [DOI] [PubMed] [Google Scholar]
- Jones RL, Jacobsen JV. Regulation of the synthesis and transport of secreted proteins in cereal aleurone. Int Rev Cytol. 1991;126:49–88. doi: 10.1016/s0074-7696(08)60682-8. [DOI] [PubMed] [Google Scholar]
- Kameshita I, Fujisawa H. A sensitive method for detection of calmodulin-dependent protein kinase II activity in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1989;183:139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Knetsch MLW, Wang M, Snaar-Jagalska BE, Heimovaara-Dijkstra S. Abscisic acid induces mitogen-activated protein kinase activation in barley aleurone protoplasts. Plant Cell. 1996;8:1061–1067. doi: 10.1105/tpc.8.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo A, Cappelluti S, Cervantes-Cervantes M, Rodriguez M, Bush DS. Okadaic acid, a protein phosphatase inhibitor, blocks calcium changes, gene expression, and cell death induced by gibberellin in wheat aleurone cells. Plant Cell. 1996;8:259–269. doi: 10.1105/tpc.8.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Bouvier-Durand M, Morris P-C, Guerrier D, Chefdor F, Giraudat J. Arabidopsis ABA-response gene ABI1: features of a calcium-modulated protein phosphatase. Science. 1994;264:1448–1452. doi: 10.1126/science.7910981. [DOI] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J. The Arabidopsis abscisic acid-insensitive2 (ABI-2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell. 1997;9:759–771. doi: 10.1105/tpc.9.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Assmann SM. An abscisic acid-activated and calcium-independent protein kinase from guard cells of fava bean. Plant Cell. 1996;8:2359–2368. doi: 10.1105/tpc.8.12.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh GC, Ulloa RM, Raices M, Tellez-Inon MT. Changes in calcium-dependent protein kinase activity during in vitro tuberization in potato. Plant Physiol. 1996;112:1541–1550. doi: 10.1104/pp.112.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Leube MP, Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994;264:1452–1455. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- Mori IC, Muto S. Abscisic acid activates a 48-kilodalton protein kinase in guard cell protoplasts. Plant Physiol. 1997;113:833–839. doi: 10.1104/pp.113.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WT. Peptide-based affinity labeling of adenosine cyclic monophosphate-dependent protein kinase. Methods Enzymol. 1991;200:500–508. doi: 10.1016/0076-6879(91)00166-t. [DOI] [PubMed] [Google Scholar]
- Mundy J, Rogers JC. Selective expression of an amylase/protease inhibitor in barley aleurone cells: comparison to the barley amylase/subtilisin inhibitor. Planta. 1986;169:51–63. doi: 10.1007/BF01369775. [DOI] [PubMed] [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell. 1997;9:409–423. doi: 10.1105/tpc.9.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah BW, Reddy ASN. Calcium and signal transduction in plants. Crit Rev Plant Sci. 1993;12:185–211. doi: 10.1080/07352689309701901. [DOI] [PubMed] [Google Scholar]
- Putnam-Evans CL, Harmon AC, Cormier MJ. Purification and characterization of a novel calcium-dependent protein kinase from soybean. Biochemistry. 1990;29:2488–2495. doi: 10.1021/bi00462a008. [DOI] [PubMed] [Google Scholar]
- Roberts DM. Protein kinases with calmodulin-like domains: novel targets of calcium signals in plants. Curr Opin Cell Biol. 1993;5:242–246. doi: 10.1016/0955-0674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- Roberts DM, Harmon AC. Calcium-modulated proteins: targets of intracellular signals in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:375–414. [Google Scholar]
- Saha P, Singh M. Characterization of a winged bean (Psophocarpus tetragonolobus) protein kinase with calmodulin-like domain: regulation by autophosphorylation. Biochem J. 1995;305:205–210. doi: 10.1042/bj3050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders MJ, Hepler PK. Localization of membrane-associated calcium following cytokinin treatment in Funaria using chlorotetracycline. Planta. 1981;152:272–281. doi: 10.1007/BF00385156. [DOI] [PubMed] [Google Scholar]
- Schuurink RC, Chan PV, Jones RL. Modulation of calmodulin mRNA and protein levels in barley aleurone. Plant Physiol. 1996;111:371–380. doi: 10.1104/pp.111.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Ca2+-dependent protein kinases and stress signal transduction in plants. Science. 1996;274:1900–1902. doi: 10.1126/science.274.5294.1900. [DOI] [PubMed] [Google Scholar]
- Swanson SJ, Jones RL. Gibberellic acid induces vacuolar acidification in barley aleurone. Plant Cell. 1996;8:2211–2221. doi: 10.1105/tpc.8.12.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen R, Heimovaara-Dijkstra S, Wang M. Cytosolic alkalinization mediated by abscisic acid is necessary, but not sufficient, for abscisic acid-induced gene expression in barley aleurone protoplasts. Plant Physiol. 1992;100:699–705. doi: 10.1104/pp.100.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolniak SM, Larsen PM. The timing of protein kinase activation events in the cascade that regulates mitotic progression in Tradescantia stamen hair cells. Plant Cell. 1995;7:431–445. doi: 10.1105/tpc.7.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorec R, Tester M. Cytoplasmic calcium stimulates exocytosis in a plant secretory cell. Biophys J. 1992;63:864–867. doi: 10.1016/S0006-3495(92)81662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]