Abstract
Objective
To describe the relationship between mental health diagnosis and treatment with antipsychotics among U.S. Medicaid-enrolled children over time.
Data Sources/Study Setting
Medicaid Analytic Extract (MAX) files for 50 states and the District of Columbia from 2002 to 2007.
Study Design
Repeated cross-sectional design. Using logistic regression, outcomes of mental health diagnosis and filled prescriptions for antipsychotics were standardized across demographic and service use characteristics and reported as probabilities across age groups over time.
Data Collection
Center for Medicaid Services data extracted by means of age, ICD-9 codes, service use intensity, and National Drug Classification codes.
Principal Findings
Antipsychotic use increased by 62 percent, reaching 354,000 youth by 2007 (2.4 percent). Although youth with bipolar disorder, schizophrenia, and autism proportionally were more likely to receive antipsychotics, youth with attention deficit hyperactivity disorder (ADHD) and those with three or more mental health diagnoses were the largest consumers of antipsychotics over time; by 2007, youth with ADHD accounted for 50 percent of total antipsychotic use; 1 in 7 antipsychotic users were youth with ADHD as their only diagnosis.
Conclusions
In the context of safety concerns, disproportionate antipsychotic use among youth with nonapproved indications illustrates the need for more generalized efficacy data in pediatric populations.
Keywords: Antipsychotics, mental health, pediatrics, Medicaid
Background
The last two decades have been marked by a steady growth in the use of psychotropic medications among the nation's youth (Zito et al. 2000; Olfson et al. 2002, 2006; Mojtabai and Olfson 2010). Although use is growing for all classes of psychotropic medications, the increase in use of second-generation antipsychotics (SGAs) has exceeded all other classes (Patel et al. 2005). Between 1987 and 1996, SGA use among children increased twofold to fivefold (Zito et al. 2003), and between 1993 and 2002, office visits involving the prescription of SGAs to children also increased fivefold (Cooper et al. 2006; Olfson et al. 2006). By 2010, annual sales of SGAs reached $16.1 billion, representing growth of $1.4 billion for the year (IMS Institute for Healthcare Informatics 2011); they had also become the most costly drug class within the Medicaid program, far exceeding the costs of any other drug class (Crystal et al. 2009).
Increasing SGA use has been described for a variety of mental health conditions in children, including those with Food and Drug Administration (FDA) approval, as well as off-label indications. Some approved indications of SGAs, adjunctive treatment for major depressive disorder as an example, carry approval for adult populations and not for pediatric populations. As the number of children diagnosed with bipolar disorder has increased in recent years (Moreno et al. 2007), so too has SGA use, given its routine and approved indication for youth with this condition. At the same time, there has been growth in prescribing for nonapproved indications; children with aggressive or disruptive behaviors, including children with attention deficit hyperactivity disorder (ADHD) or conduct disorder, are receiving SGAs as an adjunctive therapy (Findling 2003; Zito et al. 2005; Crystal et al. 2009). Evidence of concomitant SGA use among youth with disruptive behavior disorders has raised concern about the safety and efficacy in the absence of an empirical evidence base to support such practice (dosReis et al. 2011).
A comprehensive picture of the clinical and practice conduits for SGA growth remains unclear. To date, many studies that have examined diagnosis and treatment have relied on measures of association to make inferences about the growth of SGA use among children (Staller, Wade, and Baker 2005; Cooper et al. 2006; Olfson et al. 2006; Sivaprasad, Hassan, and Handy 2006; Mojtabai and Olfson 2010; Findling et al. 2011). These studies have provided valuable insight into the clinical characteristics of children and youth receiving SGAs. Moreover, this research has highlighted the complexity of the diagnosis/treatment relationship, illustrating how comorbidity might overstate the relationship of diagnoses like ADHD with SGA treatment. Reliance on chart review or surveys to capture diagnostic and treatment information in such studies has limited scope to smaller sample sizes or study samples with a narrow diagnostic profile.
A number of adverse effects have been documented in pediatric SGA use, with major safety concerns centering on metabolic complications (Correll 2008a,b; Correll et al. 2009; Panagiotopoulos, Ronsley, and Davidson 2009; De Hert et al. 2011). Recent research has shown a threefold higher incidence of diabetes among youth receiving SGAs compared with youth who did not receive psychotropic medication (Andrade et al. 2011). In response to concerns around SGA growth grounded in uncertain efficacy across diagnostic profiles and in safety, federal and state agencies have sought to implement monitoring and oversight regulations for SGA use in pediatric populations (Sheldon, Hyde, and Berwick 2000; Leslie et al. 2010; Kutz 2011). These efforts have proceeded while lacking the empiric population data to guide them. Needed are representative data that examine how changing diagnostic and treatment practices of providers may have influenced SGA growth over time. Such data can be complementary to clinically grounded research and can inform policy and practice, as they are reflective of diagnostic labeling and prescribing practice across a more generalizable group of children.
This study therefore employed a population-based approach that aimed to better describe the relationship between changing diagnostic patterns in children and SGA prescriptions over time. Given the particularly high use of SGA that has been described previously for publicly versus privately insured children (Crystal et al. 2009), the study population was U.S. Medicaid-enrolled children between 2002 and 2007, comprising more than a third of the pediatric population during this time period.
Methods
Design and Sample Selection
The data source was the Centers for Medicare and Medicaid Services Medicaid Analytic Extract (MAX) files for 50 states and the District of Columbia for years 2002–2007. Child-level demographic, eligibility, encounter, and pharmacy data were extracted from the personal summary, outpatient, inpatient, and pharmacy MAX files. The sample was restricted to children aged 3–18 years with continuous Medicaid eligibility, defined as at least 10 of 12 months in a given year. Separate sensitivity analyses of the noncontinuously eligible population revealed similar levels of psychotropic medication use and trajectories of prescribing over the period (data available in Appendix). Because of uncertainty around coverage and service receipt during periods of disenrollment, trends are reported only for continuously enrolled children.
The main outcome measure was use of a second-generation antipsychotic (SGA). SGA use was identified from the pharmacy claims file containing an 11-digit national drug code (NDC), specific to each drug. The file was merged with the First Data Bank® in order to extract the medication name, dose, and unit of use. Secondary outcome measures of use of other psychotropic medications were similarly identified using the NDC, and then grouped by therapeutic class for comparison to observed trends in SGA use. Psychotropic classes included stimulants, antidepressants, sedative/hypnotics, anxiolytics, mood stabilizers, and alpha agonists. Antidepressants included selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), and other antidepressants. Mood stabilizing agents included carbamazepine, valproic acid, gabapentin, lamotrigine, and oxcarbazepine anticonvulsants and lithium. As alpha agonists, such as clonidine and guanfacine, can also be prescribed for medical conditions, these agents were only included in the analysis if a youth also had a claim for a psychotropic medication in one of the above-mentioned classes. Sedatives/hypnotics excluded antihistamines, which in pediatric practice most often have a nonpsychiatric indication for use.
Independent variables included demographic information (age, race/ethnicity, sex, Medicaid eligibility category, state of residence), mental health diagnoses, and a count of outpatient and inpatient mental health encounters. Age was categorized within calendar years as 3–5, 6–11, and 12–18 years. Race/ethnicity was coded as white, black or African American, Latino (including black Hispanic and white Hispanic), or other (including American Indian, Asian, Hawaiian, and more than on race). Children with race classified as unknown were excluded from the analysis (n = 4,152,651; 4.4 percent). Medicaid eligibility category was defined as foster care, SSI (including all state categories for blind/disabled coverage), or TANF (including all state categories for poverty and “other” categories). Mental health diagnoses were classified using the Diagnostic and Statistical Manual of Mental Disorders IV-TR and coded using the International Classification of Diseases, Ninth Revision (ICD-9) classification. Ten diagnostic categories were included: schizophrenia (295), bipolar disorder (296.00–296.10, 296.36–296.89), depression (296.20–296.35, and 311), anxiety disorder (300.00–300.29 and 301.4), conduct disorder (312.00–313.89), autism (299), attention deficit disorder (314), intellectual disability (formerly known as mental retardation) (317–319), developmental delay (315), and a composite variable of miscellaneous mental health diagnoses inclusive of the following: mental disorders due to conditions classified elsewhere (293, 294); delusional disorders (297); other nonorganic psychoses (298); dissociative and somatoform disorders (300.10–300.19, 300.30–300.99); personality disorders (301.10–301.30, 301.50–301.99); special symptoms or syndromes, not elsewhere classified (307); acute reaction to stress (308); adjustment reaction (309); and disturbance of emotions specific to childhood and adolescence (313.90–313.99). A separate covariate was identified for children who received a diagnosis of seizure disorder (345) to control for the overlapping use of anticonvulsant agents for mood stabilization in this population. Diagnostic profile variables were derived from these 10 diagnostic categories to encode for single, two-diagnosis, and three or more diagnosis combinations for a given child. Single diagnosis was coded for any child that had only 1 of the 10 diagnostic categories labeled in a given year. Two-diagnosis combinations were coded for children with two mental health diagnoses in a given year. An “other combinations” category was created as a composite variable of two-diagnosis combinations in which there were <5,000 children over the 6-year period. Outpatient and inpatient mental health encounters were enumerated from outpatient and inpatient claims in which a mental health diagnosis was present. Mental health diagnoses were identified by ICD-9 codes and included codes for the 10 diagnostic categories assessed in this study. Multiple claims with the same ICD-9 code occurring on the same date for any given child were counted as one.
To ensure a level of comparability across states, state-level data quality reviews were conducted to identify states in which data files were incomplete or contained extreme outliers in the proportion of children with mental health diagnoses over time. Following review, two states were deemed ineligible for use in this study: Connecticut and Maine. Outpatient mental health claims were unavailable in CT MAX files, and the ME outpatient files were unavailable for 2005–2007.
Statistical Analyses
Demographic, clinical, and medication use characteristics were summarized as frequencies across year and categories of age (3–5, 6–11, 12–18). Included in these descriptive analyses were the proportions of other major psychotropic medication classes. Next, aggregate data on SGA use among diagnostic profiles (including single and multiple diagnosis combinations) were described for years 2002 and 2007 in three ways: first, by calculating the total number (and proportion) of children with a given diagnostic profile; second, by calculating the total number (and proportion) of children with a given diagnostic profile who had at least one SGA claim; and third, by calculating the overall number (and proportion) of SGA use accounted from children with a given diagnostic profile. This approach allowed for an unadjusted accounting over time in the growth of diagnostic profiles and associated SGA treatment. To complement this approach and highlight the leading diagnostic profiles responsible for SGA use in unadjusted analysis, the single, double, and multiple diagnostic profiles were ranked based on SGA prevalence in 2002 and 2007; from these rankings, the leading 10 diagnostic profile variables were used to create bar graphs showing proportional SGA use by various diagnostic combinations over time.
Finally, due to the high level of comorbidity and potential for confounding across diagnosis, generalized linear models (logit link) were used to estimate the probability of diagnosis for each the primary 10 diagnostic categories over time within age strata, controlling for demographics clustered at the state level. Similar generalized linear models, restricted to cohorts of children with the primary 10 diagnoses, were then used to estimate the probability of SGA treatment. Results for all models were standardized by child-level characteristics, including age group, gender, race, mental health diagnoses, diagnosis of seizure disorder, and frequency of outpatient and inpatient mental health visits. Results were transformed into probabilities using predictive margins and robust variance estimates (Graubard and Korn 1999).
Analyses were conducted using Stata version 12.0 (College Station, TX, USA; 2011) and SAS v9.2 (SAS Institute, Cary, NC, USA; 2011). The Institutional Review Board at the Children's Hospital of Philadelphia approved this study.
Results
An average of 15.2 million children were identified as continuously eligible within any given year from 2002 to 2007. Of these children, 51 percent were male. Twenty-three percent were aged 3–5 years, 38 percent 6–11 years, and 38 percent 12–18 years. Forty percent were identified as white, 30 percent black, 25 percent Hispanic, and 5 percent other. The most significant demographic changes over the time period were within race and ethnicity classifications, where the proportion of Hispanic children grew over the 6-year period across all age groups; all other demographic characteristics were fairly stable over time (Table 1).
Table 1.
Demographic and Clinical Characteristics of U.S. Medicaid-Enrolled Children
| Age 3–5 (Annual average = 3,545,735) | Age 6–11 (Annual average = 5,832,631) | Age 12–18 (Annual average = 5,777,763) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Average Percentage of Population | 2002, 2007 (Percentage) | Percentage Point Change 2002–2007 | Average Percentage of Population | 2002, 2007 (Percentage) | Percentage Point Change 2002–2007 | Average Percentage of Population | 2002, 2007 (Percentage) | Percentage Point Change 2002–2007 | |
| Sex | |||||||||
| Male | 51.1 | 51.1, 51.1 | 0.0 | 51.1 | 51.0, 51.2 | +0.2 | 49.7 | 49.5, 49.8 | +0.3 |
| Female | 49.0 | 48.9, 48.9 | 0.0 | 48.9 | 49.0, 48.8 | −0.2 | 50.3 | 50.5, 50.2 | −0.3 |
| Race | |||||||||
| White | 39.0 | 39.7, 37.9 | −1.8 | 39.6 | 39.4, 39.3 | −0.1 | 40.6 | 41.4, 39.7 | −1.7 |
| Black | 27.8 | 29.7, 26.1 | −3.6 | 30.3 | 32.2, 28.7 | −3.5 | 32.3 | 33.1, 31.3 | −1.8 |
| Hispanic* | 28.6 | 26.2, 31.2 | +5.0 | 25.3 | 23.6, 27.1 | +3.5 | 21.6 | 20.0, 23.5 | +3.5 |
| Other† | 4.6 | 4.3, 4.8 | +0.5 | 4.8 | 4.8, 4.9 | +0.1 | 5.4 | 5.6, 5.4 | −0.2 |
| Eligibility group | |||||||||
| Foster care | 2.8 | 2.9, 3.0 | +0.1 | 4.1 | 4.2, 4.2 | 0.0 | 6.0 | 6.1, 6.3 | +0.2 |
| TANF | 94.5 | 94.5, 94.2 | −0.3 | 91.0 | 90.9, 90.6 | −0.3 | 86.1 | 85.6, 85.7 | +0.1 |
| SSI | 2.7 | 2.6, 2.8 | +0.2 | 4.9 | 4.9, 5.3 | +0.4 | 7.9 | 8.2, 8.1 | −0.1 |
| Chronic conditions | |||||||||
| Seizure disorder | 0.4 | 0.4, 0.5 | +0.1 | 0.5 | 0.5, 0.6 | +0.1 | 0.6 | 0.6, 0.7 | +0.1 |
| Psychotropic medication | |||||||||
| SGA | 0.3 | 0.3, 0.4 | +0.1 | 1.9 | 1.5, 2.1 | +0.6 | 3.2 | 2.6, 3.7 | +1.1 |
| Alpha agonist | 0.3 | 0.3, 0.3 | 0.0 | 1.5 | 1.3, 1.8 | +0.5 | 1.2 | 1.0, 1.3 | +0.3 |
| Antidepressant | 0.2 | 0.3, 0.2 | −0.1 | 2.0 | 2.5, 1.5 | −1.0 | 5.3 | 6.0, 4.5 | −1.5 |
| Anxiolytic | 0.3 | 0.3, 0.3 | 0.0 | 0.5 | 0.4, 0.5 | +0.1 | 0.9 | 0.9, 1.0 | +0.1 |
| Hypnotic/sedative | 0.2 | 0.2, 0.1 | −0.1 | 0.1 | 0.1, 0.1 | 0.0 | 0.3 | 0.3, 0.2 | −0.1 |
| Mood stabilizer | 0.4 | 0.5, 0.4 | −0.1 | 1.2 | 1.3, 1.1 | −0.2 | 2.6 | 2.5, 2.5 | 0.0 |
| Stimulant | 1.1 | 1.0, 0.9 | −0.1 | 7.5 | 6.5, 7.4 | +0.9 | 6.4 | 5.2, 6.6 | +1.4 |
| Any psychotropic‡ | 2.1 | 2.1, 1.8 | −0.3 | 9.5 | 8.6, 9.3 | +0.6 | 13.2 | 11.3, 11.9 | +0.6 |
Hispanic category includes black Hispanic and white Hispanic classifications.
Other category includes American Indian, Asian, Hawaiian, and more than one race classifications.
Any psychotropic category includes second-generation antipsychotics, alpha agonists, antidepressants, anxiolytics, hypnotics/sedatives (excluding antihistamines), mood stabilizers (including lithium), and stimulants.
Between 2002 and 2007, SGA use increased across all age groups, with relative increases that surpassed other medication classes. This was particularly evident among school-aged children and adolescents, whose rates climbed between 1.5–2.1 and 2.6–3.7 percent, respectively (Table 1). At the same time, the prevalence of any psychotropic modestly increased (<10 percent) and only among school-aged children and adolescents. This blunted increase was reflective of an early high peak in use by 2005 that began to decline over the last 3 years of the study period (data not shown).
Among 3- to 5-year olds, the prevalences of other individual medication classes were similar and remained stable and over time. The only exception was prevalence of stimulant use (1.1 percent), which although stable over time, exceeded all other medication classes. Stimulants remained the most prevalent medication class among 6- to 11-year olds and 12- to 18-year olds as well (7.5 and 6.4 percent, respectively). Of import, prevalence of antidepressants dropped from 2.5 to 1.5 percent over the period among this age group and continued a pattern of decline among 12- to 18-year olds, where prevalence dropped 1.5 percentage points from 2002 to 2007.
Over the study period, there were some notable shifts in diagnostic trends. Among 3- to 5-year olds, rates of diagnosis remained stable over time for many single diagnostic categories, excepting developmental delay or learning disability (from 3.0 to 3.6 percent, or 30 to 36 per 1,000); miscellaneous mental health diagnosis (from 13 to 15 per 1,000); conduct disorder (from 5 to 7 per 1,000); and autism (from 1 to 2 per 1,000) (Table 2A). Apart from single diagnosis profiles was growth among children carrying dual diagnoses and those with three or more diagnoses. The ADHD/conduct disorder profile, for example, grew by 0.7 percentage points and was also the most prevalent among youth with two diagnoses (from 1.6 to 2.3 per 1,000). The profile of children with three or more diagnoses reached a prevalence of 4 per 1,000 in 2007, representing a growth of 5,900 children over the 6-year period.
Table 2.
Frequencies and Rates of Diagnosis and Second-Generation Antipsychotic (SGA) Treatment among U.S. Medicaid-Enrolled Children
| 2002 | 2007 | |||||
|---|---|---|---|---|---|---|
| A: 3- to 5-Year Olds Diagnostic Cohort | Diagnoses* (%) N = 3,162,731 | SGA*(%) N = 7,838 0.25% | % of Total SGA* | Diagnoses* (%) N = 3,623,195 | SGA*(%) N = 13,059 0.36% | % of Total SGA* |
| No diagnoses | 92.87 | 0.04 | 14.19 | 91.35 | 0.04 | 11.28 |
| Single diagnosis | ||||||
| ADHD | 0.73 | 5.61 | 16.46 | 0.78 | 6.36 | 13.79 |
| Conduct disorder | 0.52 | 2.84 | 5.97 | 0.65 | 3.06 | 5.53 |
| Autism | 0.10 | 7.72 | 2.97 | 0.18 | 8.11 | 3.98 |
| Miscellaneous | 1.27 | 0.77 | 3.98 | 1.45 | 0.82 | 3.30 |
| Devel. delay or learning | 2.99 | 0.22 | 2.68 | 3.58 | 0.25 | 2.47 |
| Bipolar | 0.01 | 33.92 | 0.98 | 0.01 | 29.95 | 0.93 |
| Intellectual disability | 0.10 | 0.84 | 0.33 | 0.10 | 0.85 | 0.24 |
| Anxiety | 0.15 | 0.54 | 0.33 | 0.19 | 0.44 | 0.23 |
| Depression | 0.04 | 2.60 | 0.41 | 0.03 | 1.26 | 0.11 |
| Schizophrenia | 0.00 | 7.69 | 0.08 | 0.00 | 0.00 | 0.00 |
| Two diagnoses† | ||||||
| ADHD/conduct disorder | 0.16 | 12.78 | 8.39 | 0.23 | 15.89 | 10.12 |
| ADHD/miscellaneous | 0.10 | 10.62 | 4.34 | 0.12 | 12.74 | 4.09 |
| Autism/devel. delay or learning | 0.08 | 5.90 | 1.80 | 0.14 | 7.51 | 3.02 |
| ADHD/devel. delay or learning | 0.12 | 5.66 | 2.67 | 0.13 | 8.06 | 2.99 |
| Conduct/miscellaneous | 0.10 | 5.40 | 2.21 | 0.13 | 6.38 | 2.24 |
| ADHD/autism | 0.01 | 28.74 | 1.25 | 0.02 | 30.05 | 1.75 |
| ADHD/bipolar | 0.01 | 49.48 | 1.22 | 0.01 | 56.68 | 1.72 |
| Conduct/devel. delay or learning | 0.08 | 3.24 | 1.00 | 0.11 | 4.52 | 1.34 |
| ADHD/anxiety | 0.01 | 9.56 | 0.31 | 0.01 | 13.29 | 0.42 |
| Bipolar/conduct disorder | 0.00 | 58.82 | 0.51 | 0.00 | 52.58 | 0.39 |
| Other two diagnoses combinations | 0.26 | 2.86 | 2.96 | 0.30 | 3.68 | 3.11 |
| ≥ 3 diagnoses | 0.25 | 22.58 | 23.02 | 0.38 | 23.98 | 25.58 |
| 2002 | 2007 | |||||
|---|---|---|---|---|---|---|
| B: 6- to 11-Year Olds Diagnostic Cohort | Diagnoses (%) N = 5,462,434 | SGA (%) N = 80,006 1.5% | SGA(%) N = 80,006 1.5% | Diagnoses (%) N = 5,909,130 | SGA (%) N = 124,984 2.1% | % of Total SGA |
| No diagnoses | 86.31 | 0.20 | 11.63 | 84.26 | 0.25 | 10.11 |
| Single diagnosis | ||||||
| ADHD | 3.67 | 7.16 | 17.93 | 4.48 | 9.27 | 19.61 |
| Conduct disorder | 0.91 | 6.89 | 4.29 | 0.97 | 8.10 | 3.70 |
| Miscellaneous | 2.28 | 3.09 | 4.81 | 2.38 | 2.92 | 3.28 |
| Autism | 0.15 | 19.19 | 2.02 | 0.25 | 18.51 | 2.19 |
| Bipolar | 0.04 | 50.81 | 1.22 | 0.07 | 57.18 | 1.78 |
| Devel. delay or learning | 2.21 | 0.76 | 1.15 | 2.16 | 0.97 | 0.99 |
| Depression | 0.26 | 7.96 | 1.44 | 0.24 | 6.49 | 0.72 |
| Intellectual disability | 0.25 | 3.42 | 0.58 | 0.26 | 4.49 | 0.55 |
| Anxiety | 0.23 | 2.58 | 0.40 | 0.31 | 3.00 | 0.44 |
| Schizophrenia | 0.01 | 29.50 | 0.10 | 0.01 | 21.22 | 0.05 |
| Two diagnoses† | ||||||
| ADHD/conduct disorder | 0.55 | 18.95 | 7.09 | 0.79 | 22.90 | 8.59 |
| ADHD/miscellaneous | 0.50 | 14.59 | 5.01 | 0.61 | 17.02 | 4.90 |
| ADHD/bipolar | 0.04 | 63.05 | 1.81 | 0.10 | 69.98 | 3.26 |
| ADHD/devel. delay or learning | 0.36 | 7.66 | 1.90 | 0.43 | 10.45 | 2.12 |
| Conduct/miscellaneous | 0.22 | 13.04 | 1.92 | 0.23 | 14.26 | 1.56 |
| ADHD/autism | 0.03 | 35.63 | 0.82 | 0.07 | 39.27 | 1.34 |
| ADHD/depression | 0.11 | 20.03 | 1.52 | 0.11 | 21.48 | 1.09 |
| ADHD/anxiety | 0.05 | 12.87 | 0.48 | 0.10 | 15.97 | 0.74 |
| Autism/devel. delay or learning | 0.07 | 15.80 | 0.76 | 0.10 | 15.57 | 0.71 |
| Bipolar/conduct disorder | 0.01 | 59.72 | 0.47 | 0.02 | 68.32 | 0.64 |
| Other two diagnoses combinations | 0.36 | 11.24 | 2.75 | 0.38 | 15.14 | 2.73 |
| ≥ 3 Diagnoses | 1.01 | 38.26 | 26.38 | 1.32 | 42.34 | 26.33 |
| 2002 | 2007 | |||||
|---|---|---|---|---|---|---|
| C: 12-18 Year Olds Diagnostic Cohort | Diagnoses(%)N= 5,071,583 | SGA(%)N= 130,9192.6% | % of TotalSGA | Diagnoses(%)N= 5,929,130 | SGA(%)N= 216,3823.6% | % of TotalSGA |
| No diagnoses | 84.21 | 0.43 | 14.08 | 82.71 | 0.58 | 13.03 |
| Single diagnosis | ||||||
| ADHD | 2.42 | 9.43 | 8.86 | 3.19 | 12.01 | 10.50 |
| Conduct disorder | 1.62 | 8.29 | 5.21 | 1.62 | 9.88 | 4.37 |
| Bipolar | 0.15 | 44.33 | 2.65 | 0.30 | 49.44 | 4.04 |
| Miscellaneous | 2.79 | 5.22 | 5.65 | 2.52 | 5.48 | 3.78 |
| Depression | 1.29 | 8.48 | 4.24 | 1.31 | 8.88 | 3.18 |
| Autism | 0.11 | 31.18 | 1.36 | 0.19 | 28.68 | 1.53 |
| Intellectual disability | 0.45 | 5.14 | 0.89 | 0.44 | 7.59 | 0.92 |
| Anxiety | 0.41 | 3.28 | 0.52 | 0.54 | 4.01 | 0.59 |
| Devel. delay or learning | 1.30 | 1.44 | 0.72 | 0.96 | 2.20 | 0.58 |
| Schizophrenia | 0.03 | 61.43 | 0.74 | 0.03 | 56.67 | 0.40 |
| Two diagnoses† | ||||||
| ADHD/conduct disorder | 0.45 | 22.84 | 4.01 | 0.65 | 27.79 | 4.96 |
| ADHD/bipolar | 0.06 | 57.23 | 1.39 | 0.16 | 63.55 | 2.87 |
| ADHD/miscellaneous | 0.35 | 18.50 | 2.49 | 0.42 | 21.61 | 2.51 |
| Depression/miscellaneous | 0.56 | 14.87 | 3.24 | 0.49 | 14.94 | 2.00 |
| Conduct/miscellaneous | 0.41 | 14.58 | 2.29 | 0.37 | 16.93 | 1.73 |
| Bipolar/conduct disorder | 0.05 | 54.21 | 1.07 | 0.09 | 60.49 | 1.46 |
| ADHD/depression | 0.18 | 20.19 | 1.42 | 0.24 | 21.26 | 1.38 |
| Conduct/depression | 0.25 | 20.00 | 1.92 | 0.24 | 20.76 | 1.35 |
| Bipolar/depression | 0.06 | 43.06 | 0.97 | 0.09 | 49.61 | 1.17 |
| ADHD/devel. delay or learning | 0.16 | 10.41 | 0.65 | 0.16 | 14.18 | 0.62 |
| Other two diagnoses combinations | 0.59 | 22.23 | 5.09 | 0.63 | 27.67 | 4.79 |
| ≥ 3 Diagnoses | 1.70 | 42.79 | 28.12 | 2.15 | 49.93 | 29.36 |
Estimated from unadjusted conditional probabilities. From left to right within each year, the columns display the proportion of children receiving a diagnostic profile, the proportion of children receiving SGAs among those with the diagnostic profile, and finally, the proportion of all SGA use attributable to children with the diagnostic profile. For example, in 2002, 0.7% of children aged 3–5 had ADHD only. Of these 0.7%, 5.6% received SGA. Finally, children with ADHD only accounted for 16.5% of the total SGA users aged 3–5 in 2002.
Shown are the 10 two-diagnoses profiles with the greatest proportion of attributable SGA use in 2007. Unabbreviated two-diagnoses profile information is included in the online Appendix.
Among school-aged children and adolescents, ADHD, the most common single diagnosis, grew from 37 to 45 per 1,000 among 6- to 11-year olds and from 24 to 32 per 1,000 among 12- to 18-year olds (Table 2B and C). Anxiety, autism, and bipolar diagnoses also experienced notable growth from 2002 to 2007. ADHD/conduct disorder was the most common two-diagnosis profile, and rose over the period in both age groups, from 6 to 8 per 1,000 and from 5 to 7 per 1,000, respectively. Youth with three or more diagnoses increased from 10 to 13 per 1,000 among 6- to 11-year olds and from 19 to 22 per 1,000 among 12- to 18-year olds.
Aggregating across diagnostic profiles, the number of children receiving SGAs increased by 62 percent over the period, with 354,000 children aged 3–18 receiving SGAs in 2007 (13,000 3- to 5-year olds, 125,000 6- to 11-year olds, 216,000 12- to 18-year olds, Table 2A–C). Figure 1 displays the 10 diagnostic profiles in 2002 and 2007 that were most responsible for this growth. The leading 10 diagnostic profiles were responsible for approximately 80 percent of the SGA use in 2002 and 2007. Some diagnoses (e.g., ADHD and conduct disorder) were identifiable among the leading diagnostic profiles in both years. Other diagnoses shifted among the leading diagnostic profiles between the 2 years. Bipolar disorder, for example, both singularly and in combination with ADHD, was evident by 2007, seemingly replacing depression diagnoses that were represented within several diagnostic profiles in 2002. SGA use in all of the leading diagnostic profiles grew over time (median increase: 63 percent; mean: 81 percent), with the largest proportional increase for ADHD/bipolar combination (213 percent from 3,300 to 10,500 children). Youth with ADHD (single and in combination) and those with three or more diagnoses accounted for the majority of children; therefore, their proportional increase represented the largest numeric growth of children receiving SGA over time (increase of 88,000 children). Of significance, ADHD, conduct disorder, and miscellaneous mental health diagnoses were disproportionately represented among youth with three or more diagnoses, the largest category numerically; among these youth, 68 percent had an ADHD diagnosis, 63 percent conduct disorder diagnosis, and 66 percent other miscellaneous mental health diagnoses. Autism (10 percent), intellectual disability (10 percent), and schizophrenia (4 percent) were less frequently represented.
Figure 1.
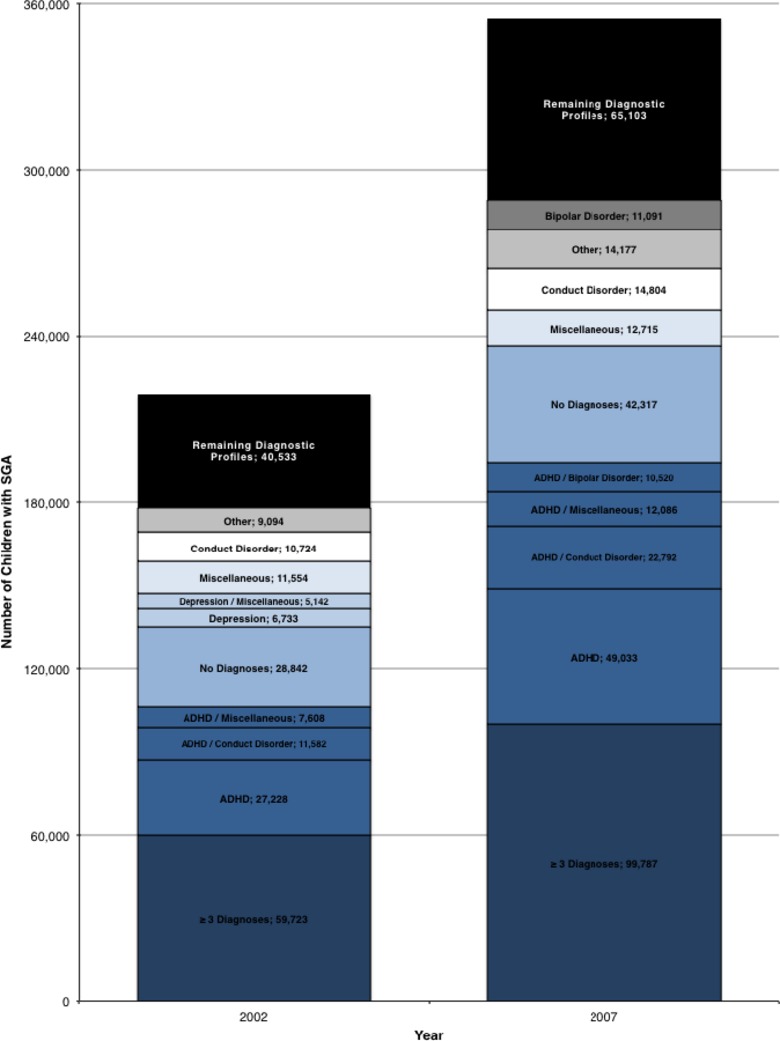
Leading Diagnostic Profiles Ranked by SGA Use, 2002 and 2007 Notes. Diagnostic profile variables were derived from primary diagnostic categories (ADHD; autism; anxiety; bipolar disorder; conduct disorder; depression; intellectual disability; schizophrenia; developmental delay or learning disability) to encode for single, two-diagnosis, and three or more diagnosis combinations for a given child. An “other” category was created as a composite variable of two-diagnosis combinations in which there were <5,000 children over the 6-year period. The diagnostic profiles were ranked by proportion of total SGA use in each year; the leading 10 diagnoses in 2002 and 2007 are shown. The remaining diagnostic profiles were aggregated.
Finally, multivariable models that examined the relationship of diagnosis with SGA use, standardized by changes in comorbidities over time, revealed several trends by age category. Figures 2–4 graphically display the standardized probabilities of diagnosis and treatment with SGA within the population; the figure also depicts the trend over time in the proportion of children with a given diagnosis who were treated with SGA. Among 3- to 5-year olds, proportionally, the highest users of SGA were found among children with bipolar disorder (57 percent). However, the overall probability of treatment and therefore highest number of children treated were among children with ADHD and miscellaneous mental health diagnoses. The strongest growth in SGA use within diagnosis was among younger children receiving the diagnosis of bipolar disorder or ADHD, in whom the likelihood of SGA use increased by 27 and 25 percent, respectively, over the period.
Figure 2.
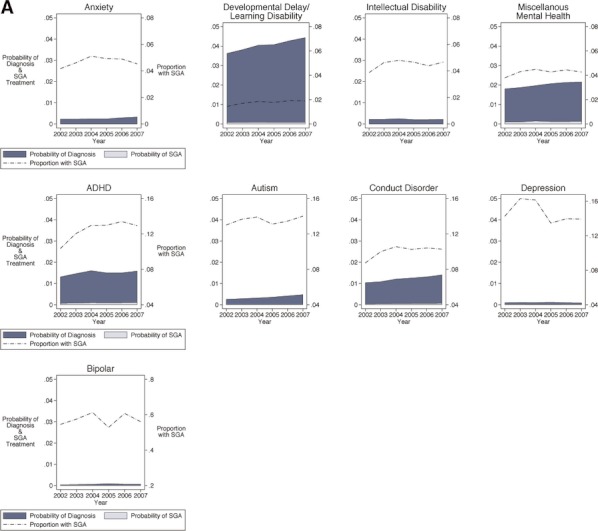
Adjusted Trends in Mental Health Diagnosis and SGA Treatment among U.S. Medicaid-Enrolled Children: 3- to 5-Year Olds Note. Schizophrenia not shown due to low prevalence in this age group. Plots are ordered in rows by proportion of SGA use by diagnosis, with the top row having the lower proportions (y scale, right axis, 0–0.08) and the bottom row having the higher proportions (y scale, right axis, 0–0.8). Each graph plots three values from 2002 to 2007 (x axis). For example, for the 3- to 5-year olds with a diagnosis of ADHD (middle row), the probability of diagnosis increased from 0.01 to 0.02 over these 6 years (y axis, left scale), the probability of treatment increased from 0 to 0.001 (y axis, left scale), and the proportion of SGA use among those with a diagnosis of ADHD increased from 0.10 to 0.13 (y axis, right scale).
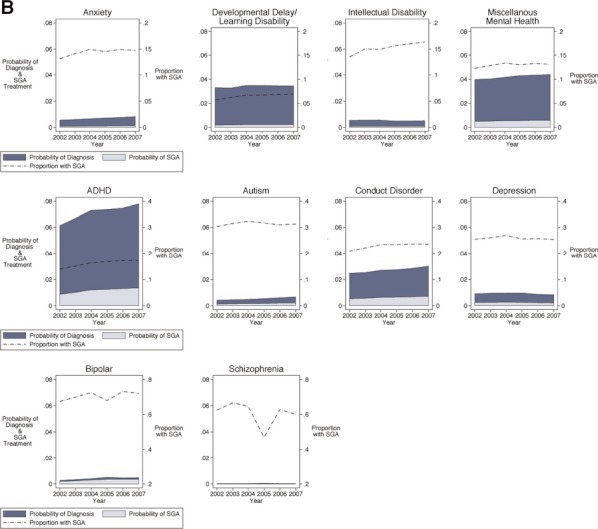
Figure 4.
Adjusted Trends in Mental Health Diagnosis and SGA Treatment among U.S. Medicaid-Enrolled Children: Twelve- to Eighteen-Year Olds Note. Plots are ordered in rows by proportion of SGA use by diagnosis, with the top row having the lower proportions (y scale, right axis, 0–0.2) and the bottom row having the higher proportions (y scale, right axis, 0–0.8). Each graph plots three values from 2002 to 2007 (x axis). For example, for the 12- to 18-year olds with a diagnosis of ADHD (middle row), the probability of diagnosis increased from 0.05 to 0.07 over these 6 years (y axis, left scale), the probability of treatment increased from 0.010 to 0.016 (y axis, left scale), and the proportion of SGA use among those with a diagnosis of ADHD increased from 0.20 to 0.25 (y axis, right scale).
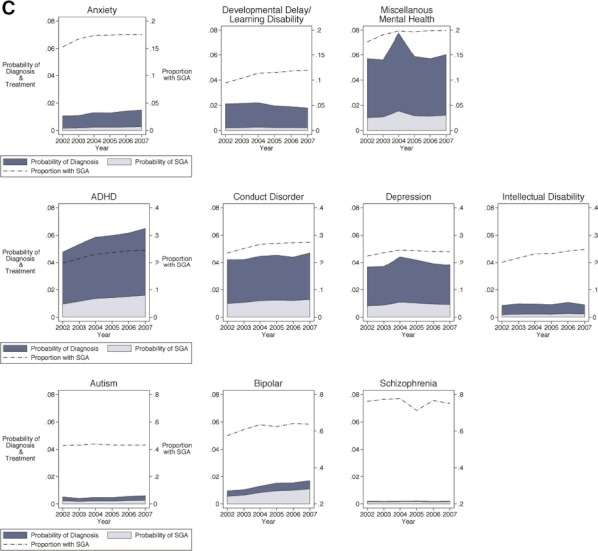
Figure 3.
Adjusted Trends in Mental Health Diagnosis and SGA Treatment among U.S. Medicaid-Enrolled Children: Six- to Eleven-Year Olds Note. Plots are ordered in rows by proportion of SGA use by diagnosis, with the top row having the lower proportions (y scale, right axis, 0–0.2) and the bottom row having the higher proportions (y scale, right axis, 0–0.8). Each graph plots three values from 2002 to 2007 (x axis). For example, for the 6- to 11-year olds with a diagnosis of ADHD (middle row), the probability of diagnosis increased from 0.06 to 0.08 over these 6 years (y axis, left scale), the probability of treatment increased from 0.010 to 0.014 (y axis, left scale), and the proportion of SGA use among those with a diagnosis of ADHD increased from 0.14 to 0.17 (y axis, right scale).
Among 6- to 11-year olds, proportional use of SGA was highest among children with the diagnoses of bipolar disorder and schizophrenia (70 and 61 percent, respectively). Yet the overall probability of treatment and, therefore, highest number of children treated were among children with ADHD, conduct disorder, and miscellaneous mental health diagnoses. Within a given diagnosis, the largest increase in SGA use over time was among children with an ADHD diagnosis, in whom the likelihood of treatment increased by 24 percent over the period. Other significant increases were seen among children with a diagnosis of intellectual disability (22 percent) and developmental delay or learning disability (21 percent).
In adolescents aged 12–18 years, those with a diagnosis of schizophrenia, bipolar disorder, and autism were proportionally the highest SGA users (76, 62, 43 percent, respectively). Again, as in younger children, the overall probability of treatment and, therefore, highest number of children treated were among adolescents with ADHD, conduct disorder, and miscellaneous mental health diagnoses, although they were also joined by adolescents with depression and bipolar disorder. Within a given diagnosis, the largest increases in SGA use over time were among adolescents with developmental delay or learning disability (26 percent), followed by ADHD (23 percent) and intellectual disability (23 percent).
Discussion
As the number of Medicaid-enrolled children receiving SGA treatment climbed by 62 percent between 2002 and 2007, ADHD and conduct disorder among all children, and bipolar disorder and depression among adolescents, were disproportionately represented among those children receiving treatment. To some degree, the increased rate of diagnosis over time matched the rate of increase in SGA use and partially explained this trend. However, there were notable exceptions; namely, there was strong growth in the proportion of children treated with SGAs given a diagnosis, with the highest rates of growth among school-aged children and adolescents with ADHD, intellectual disabilities, and developmental delay and/or learning disabilities. ADHD, not only the most prevalent diagnosis, but the diagnosis with the highest increase in rates of treatment across all ages, was increasingly represented among children and adolescents receiving treatment over time. By 2007, half of all children receiving SGAs had ADHD listed as one of their diagnoses; 1 in 7 (14 percent) had ADHD as their only mental health diagnosis. This unabated growth was alarming given an insufficient evidence base for their safety and effectiveness in this population and growing concerns that the metabolic effects of these agents may be worse among children than in adults (Correll 2008a,b; Correll et al. 2009; De Hert et al. 2011). Neither the American Academy of Pediatrics nor the American Academy of Child and Adolescent Psychiatry recommends SGA treatment for the management of ADHD.
A strength of this study was in its population approach to examining the growth in SGA use over time. This study prioritized the estimation of SGA use that was attributable to different pediatric diagnostic profiles from Medicaid claims. Such an approach is important and congruous to research examining the association of clinical indications with treatment, as it focuses on population prevalence in addition to association. For example, prior studies have demonstrated the high degree of association of bipolar disorder diagnosis with SGA treatment (Findling et al. 2011), as was also noted in our study. However, excepting the adolescent population, such a diagnosis was infrequent among most children. Therefore, the absolute numbers of children receiving SGA with a bipolar diagnosis paled in comparison with the numbers of children with the more frequent diagnoses of ADHD or conduct disorder receiving treatment, even if the probability of treatment given those diagnoses was lower.
Another strength of this study was the ability to longitudinally examine treatment patterns within diagnosis. Such an analysis revealed that ADHD was not the only diagnosis for which SGA treatment increased over time; school-aged children and adolescents with intellectual disability and developmental delay and/or learning disabilities exhibited strong growth in their receipt of SGA over time, even if their contribution to overall prevalence still remained low. It is likely comorbidity was a contributor to SGA treatment among this group, as 87 percent of children with intellectual disability and 90 percent of those with developmental delay/learning disabilities receiving SGAs had comorbid mental health conditions. Although most prior research has focused solely on risperidone, the finding of utilization among children with intellectual disability is consistent with prior research and not surprising, given several clinical trials demonstrating positive effects on behavioral problems in this population (Unwin and Deb 2011). However, the longitudinal growth in SGA treatment among children with intellectual disability and comorbid conditions may require additional safety and efficacy trials reflective of the complex diagnostic profiles of children receiving SGA over time. It is unclear the motivation for SGA use among children with no mental health diagnosis present, which represented 12 percent of children receiving SGA. An analysis of a single-state Medicaid population from 2001 to 2003 also showed a large proportion (31.8 percent) of SGA receivers without a mental health diagnosis (Penfold et al. 2010). Off-label use of SGA for sleep disturbance in youth has been cited as a common practice (Coe and Hong 2012); however, among children in the study cohort receiving SGA in the absence of a mental health diagnosis, only 0.4 percent (2007) carried a diagnosis for sleep disturbance. Further research is needed to identify the symptomatology, indications, and safety of SGA use among the population of children with no mental health diagnoses.
While some diagnoses were more prominently represented than others among children receiving SGAs over time, it is not possible to conclude that these diagnoses were responsible for the growth in SGA use. In fact, the number of children receiving SGAs numerically increased for almost every diagnostic category; children with three or more diagnoses, a rapidly growing category of diagnosis, consistently represented a quarter of children using SGA over the time period. Such a finding raises the concern of confounding by indication given the complexity of a diagnosis/treatment relationship that may be bi-directional. It is certainly possible, for example, that such diagnoses reflect the accuracy of the clinical assessment, but they could also be used as a vehicle to underwrite treatment once a concern about a “not-so-easily” encoded behavior is raised. The breadth of growth in SGA use across so many diagnoses might suggest a strong market pressure to treat symptoms that do not fit a diagnostic category. In addition, state and local jurisdiction prior authorization and utilization review policies for SGAs may further complicate the diagnosis/treatment relationship, as diagnostic coding may be reflective of prescriber labeling of preferential diagnoses to circumvent such policies and not clinical symptomatology.
This study is not without limitations. Foremost is the concern about misclassification of diagnosis, a frequent challenge when using administrative data to characterize the basis for a clinical decision. Because clinical assessments are richer than diagnoses listed on claims, Medicaid data cannot describe at a case level whether the treatment decisions were appropriate or inappropriate. Claims diagnoses might also be incomplete because of the high rates of comorbidity in children as well as the challenge in classifying concerning behaviors in children within DSM diagnostic categories. For example, aggressive and disruptive behaviors are often a target for SGA treatment (Biederman et al. 2006; Crystal et al. 2009; De Deyn and Buitelaar 2006; Findling 2008; Unwin and Deb 2011), but they are not easily encoded into DSM diagnostic categories. Therefore, administratively encoded diagnoses might not adequately capture this prescribing trend. In addition, co-morbid mental health conditions in children often confound the relationship between diagnosis and treatment, and it may be difficult to determine which medication is used for which condition. Finally, medication use in this study was assessed with filled prescription claims. Filled prescription data are limited by an inability to draw conclusions about medication consumption at a child level.
Concerns about diagnostic accuracy on claims, however, should not undermine the value of this study to inform a public health response to this issue. Although misclassification and inaccuracy of diagnosis is a relevant concern, the consistency of diagnostic coding over time, particularly among youth with ADHD and conduct disorder—both in magnitude and growth—suggests a clear practice pattern. In addition, concerns around accuracy of diagnosis should not detract from the valid concerns around appropriate treatment assuming diagnoses are accurate. The growing intensity of SGA use across so many diverse diagnostic categories suggests a malleable environment in which almost any diagnosis might illicit treatment. Consequently, any single-dimension policy reaction to limit the use of SGAs, for instance, among children with ADHD, is likely to be flawed. A likely consequence is that prescribers would substitute other diagnoses to meet the demand for treatment. Our data would therefore suggest that practice and policy efforts must be sensitive to the complex and dynamic diagnosis/treatment relationship to minimize unintended consequences, such as diagnosis drift, or alternatively, restricted access to needed medical treatment. Given the disproportionate representation of children with an ADHD diagnosis among SGA users, provider education around the safe and limited use of SGAs in pediatric populations might prove an appropriate component of a multidimensional public health response to this issue.
In summary, this national analysis revealed persistent growth in SGA use among U.S. Medicaid-enrolled children during the past decade. Across all age groups, youth with ADHD and conduct disorder, as well as those with multiple diagnoses, were significant contributors to this growth and represented a large proportion of children using SGAs over time. At the same time, SGA growth was evident across a range of diagnoses, illustrating practice patterns beyond approved indications for treatment in pediatric populations. In the context of a complex relationship between childhood behavior, diagnosis, and treatment, future research will need to more broadly examine the efficacy and safety of SGA use in pediatric populations and the potential consequences of efforts to restrict their use.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This study was funded by grant 5R01HS018550 from the Agency for Healthcare Research and Quality and a fellowship to Dr. David Rubin from the Stoneleigh Foundation. The findings have been presented at the June 2012 Academy Health Annual Research Meeting, Orlando, FL.
Disclosures: None.
Disclaimers: None.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article:
Appendix AS1: Author Matrix.
Appendix AS2: Demographic and Clinical Characteristics of U.S. Noncontinuously (<10 Months/Year) Medicaid-Enrolled Children.
Appendix AS3: Predictive Margins of Second-Generation Antipsychotic use among Continuously (≥10 Months/Year), Noncontinuously (<10 Months/Year), and All (1–12 Months/Year) Medicaid-Enrolled U.S. Children.
Appendix AS4: Frequencies and Rates of Diagnosis and Second-Generation Antipsychotic (SGA) Treatment among U.S. Medicaid-Enrolled Children: Three- to Five-Year Olds.
Appendix AS5: Frequencies and Rates of Diagnosis and Second-Generation Antipsychotic (SGA) Treatment among U.S. Medicaid-Enrolled Children: Six- to Eleven-Year Olds.
Appendix AS6: Frequencies and Rates of Diagnosis and Second-Generation Antipsychotic (SGA) Treatment among U.S. Medicaid-Enrolled Children: Twelve- to Eighteen-Year Olds.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missingmaterial) shouldbedirected tothe corresponding author for the article.
References
- Andrade S, Lo J, Roblin D, Fouayzi H, Connor D, Penfuld R, Chandra M, Reed G, Gurwitz J. “Antipsychotic Medication Use among Children and Risk of Diabetes Mellitus”. Pediatrics. 2011;128(6):1135–41. doi: 10.1542/peds.2011-0855. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV, Wozniak J, Spencer T, Pandina G. “Risperidone for the Treatment of Affective Symptoms in Children with Disruptive Behavior Disorder: A Post Hoc Analysis of Data from a 6-Week, Multicenter, Randomized, Double-Blind, Parallel-Arm Study”. Clinical Therapeutics. 2006;28(5):794–800. doi: 10.1016/s0149-2918(06)00132-9. [DOI] [PubMed] [Google Scholar]
- Coe H, Hong I. “Safety of Low Doses of Quetiapine When Used for Insomnia”. Pharmacotherapy. 2012;46(5):718–22. doi: 10.1345/aph.1Q697. [DOI] [PubMed] [Google Scholar]
- Cooper WO, Arbogast PG, Ding H, Hickson GB, Fuchs DC, Ray WA. “Trends in Prescribing of Antipsychotic Medications for US Children”. Ambulatory Pediatrics. 2006;6(2):79–83. doi: 10.1016/j.ambp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Correll CU. “Assessing and Maximizing the Safety and Tolerability of Antipsychotics Used in the Treatment of Children and Adolescents”. Journal of Clinical Psychiatry. 2008a;69(suppl 4):26–36. [PubMed] [Google Scholar]
- Correll CU. “Monitoring and Management of Antipsychotic-Related Metabolic and Endocrine Adverse Events in Pediatric Patients”. International Review of Psychiatry. 2008b;20(2):195–201. doi: 10.1080/09540260801889179. [DOI] [PubMed] [Google Scholar]
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. “Cardiometabolic Risk of Second-Generation Antipsychotic Medications during First-Time Use in Children and Adolescents”. Journal of the American Medical Association. 2009;302(16):1765–73. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal S, Olfson M, Huang C, Pincus H, Gerhard T. “Broadened Use of Atypical Antipsychotics: Safety, Effectiveness, and Policy Challenges”. Health Affairs. 2009;28(5):w770–81. doi: 10.1377/hlthaff.28.5.w770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M, Dobbelaere M, Sheridan EM, Cohen D, Correll CU. “Metabolic and Endocrine Adverse Effects of Second-Generation Antipsychotics in Children and Adolescents: A Systematic Review of Randomized, Placebo Controlled Trials and Guidelines for Clinical Practice”. European Psychiatry. 2011;26(3):144–58. doi: 10.1016/j.eurpsy.2010.09.011. [DOI] [PubMed] [Google Scholar]
- De Deyn PP, Buitelaar J. “Risperidone in the Management of Agitation and Aggression Associated with Psychiatric Disorders”. European Psychiatry. 2006;21:21–8. doi: 10.1016/j.eurpsy.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Findling RL. “Dosing of Atypical Antipsychotics in Children and Adolescents”. Primary Care Companion Journal of Clinical Psychiatry. 2003;161:677–84. [Google Scholar]
- Findling RL. “Atypical Antipsychotic Treatment of Disruptive Behavior Disorders in Children and Adolescents”. Journal of Clinical Psychiatry. 2008;69(suppl 4):9–14. [PubMed] [Google Scholar]
- Findling RL, Horwitz SC, Birmaher B, Kowatch RA, Fristad MA, Youngstrom EA, Frazier TW, Axelson D, Ryan N, Demeter CA, Depew J, Fields B, Gill M, Deyling EA, Rowles BM, Arnold LE. “Clinical Characteristics of Children Receiving Antipsychotic Medication”. Journal of Child and Adolescent Psychopharmacology. 2011;21(4):311–9. doi: 10.1089/cap.2010.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graubard BI, Korn EL. “Predictive Margins with Survey Data”. Biometrics. 1999;55:652–9. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- IMS Institute for Healthcare Informatics. The Use of Medicines in the United States: Review of 2010. Parsippany, NJ: IMS Institute for Healthcare Informatics; 2011. pp. 1–36. [Google Scholar]
- Kutz GD. Foster Children: HHS Guidance Could Help States Improve Oversight of Psychotropic Prescriptions. Washington, DC: General Accounting Office Report; 2011. GAO-12-270T. [Google Scholar]
- Leslie LK, Mackie T, Dawson EH, Bellonci C, Schoonover DR, Rodday AM, Hayek M, Hyde J. Multi-State Study on Psychotropic Medication Oversight in Foster Care. Boston: Tufts Clinical and Translational Science Institute; 2010. [Google Scholar]
- Mojtabai R, Olfson M. “National Trends in Psychotropic Medication Polypharmacy in Office-Based Psychiatry”. Archives of General Psychiatry. 2010;67(1):26–36. doi: 10.1001/archgenpsychiatry.2009.175. [DOI] [PubMed] [Google Scholar]
- Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M. “National Trends in the Outpatient Diagnosis and Treatment of Bipolar Disorder in Youth”. Archives of General Psychiatry. 2007;64(9):1032–9. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Druss B, Pincus HA. “National Trends in the Use of Outpatient Psychotherapy”. American Journal of Psychiatry. 2002;159(11):1914–20. doi: 10.1176/appi.ajp.159.11.1914. [DOI] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Liu L, Moreno C, Laje G. “National Trends in the Outpatient Treatment of Children and Adolescents with Antipsychotic Drugs”. Archives of General Psychiatry. 2006;63(6):679–85. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- Panagiotopoulos C, Ronsley R, Davidson J. “Increased Prevalence of Obesity and Glucose Intolerance in Youth Treated with Second-Generation Antipsychotics Medications”. Canadian Journal of Psychiatry. 2009;54(11):734–9. doi: 10.1177/070674370905401104. [DOI] [PubMed] [Google Scholar]
- Patel NC, Crismon ML, Hoagwood K, Johnsrud MT, Rascati KL, Wilson JP, Jensen PS. “Trends in the Use of Typical and Atypical Antipsychotics in Children and Adolescents”. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44(6):548–56. doi: 10.1097/01.chi.0000157543.74509.c8. [DOI] [PubMed] [Google Scholar]
- Penfold R, Kelleher K, Wang W, Strange B, Pajer K. “Pediatric Uptake of a Newly Available Antipsychotic Medication”. Pediatrics. 2010;125(3):475–82. doi: 10.1542/peds.2009-1288. [DOI] [PubMed] [Google Scholar]
- dosReis S, Yoon Y, Rubin DM, Riddle MA, Noll E, Rothbard A. “Antipsychotic Treatment among Youth in Foster Care”. Pediatrics. 2011;128(6):e1459–66. doi: 10.1542/peds.2010-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon GH, Hyde PS, Berwick D. DHHS Joint Letter to State Directors. Washington, DC: Department of Health and Human Services; 2000. pp. 1–7. [Google Scholar]
- Sivaprasad L, Hassan T, Handy S. “Survey of Atypical Antipsychotic Medication Use by Child and Adolescent Psychiatrists”. Child and Adolescent Mental Health. 2006;11(3):164–7. doi: 10.1111/j.1475-3588.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- Staller JA, Wade MJ, Baker M. “Current Prescribing Patterns in Outpatient Child and Adolescent Psychiatric Practice in Central New York”. Journal of Child & Adolescent Psychopharmacology. 2005;15(1):57–61. doi: 10.1089/cap.2005.15.57. [DOI] [PubMed] [Google Scholar]
- Unwin GL, Deb S. “Efficacy of Atypical Antipsychotic Medication in the Management of Behaviour Problems in Children with Intellectual Disabilities and Borderline Intelligence: A Systematic Review”. Research in Developmental Disabilities. 2011;32:2121–33. doi: 10.1016/j.ridd.2011.07.031. [DOI] [PubMed] [Google Scholar]
- Zito J, Safer D, dosReis S, Gardner J, Boles M, Lynch F. “Trends in the Prescribing of Psychotropic Medications to Preschoolers”. Journal of the American Medical Association. 2000;283(8):1025–30. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, DosReis S, Gardner JF, Magder L, Soeken K, Boles M, Lynch F, Riddle MA. “Psychotropic Practice Patterns for Youth: A 10-Year Perspective”. Archives of Pediatric and Adolescent Medicine. 2003;157(1):17–25. doi: 10.1001/archpedi.157.1.17. [DOI] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, Zuckerman IH, Gardner JF, Soeken K. “Effect of Medicaid Eligibility Category on Racial Disparities in the Use of Psychotropic Medications among Youths”. Psychiatric Services. 2005;56(2):157–63. doi: 10.1176/appi.ps.56.2.157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


