Abstract
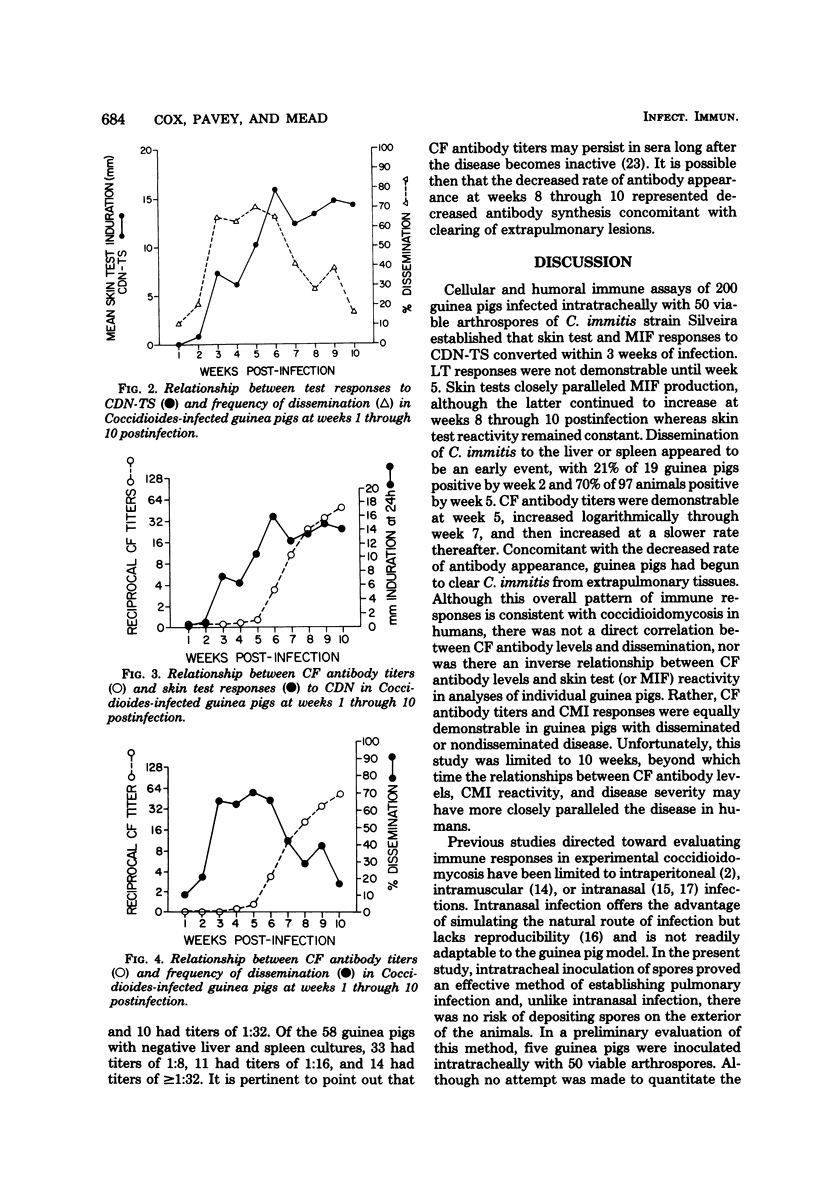
Two hundred Hartley-inbred guinea pigs were infected intratracheally with 50 viable arthrospores of Coccidioides immitis. At weeks 1 through 10 postinfection, groups of 20 guinea pigs were assayed for skin test, macrophage migration inhibitory factor (MIF), and lymphocyte transformation (LT) responses to coccidioidin. Forty-eight hours after skin testing and just before MIF and LT assays, blood was obtained for complement-fixing (CF) antibody titers and the animals were autopsied to assess the extent of fungal dissemination. Immunological assays established that skin tests and MIF responses converted within 3 weeks of infection. LT responses were not demonstrable until week 5. Dissemination of C. immitis to the liver or spleen was an early event, with 21% of guinea pigs positive by week 2 and 70% positive by week 5. CF antibody titers were demonstrable at week 5, increased logarithmically through week 7, then increased at a slower rate thereafter. Concomitant with the decreased rate of antibody production, guinea pigs began to clear C. immitis from their extrapulmonary tissues. Skin test responses peaked at 6 weeks postinfection when CF antibody titers were less than or equal to 1:16 and then plateaued with increased CF titers. Although this overall immunological profile is consistent with the disease in humans, there was not a direct correlation between CF antibody titer and dissemination to the liver or spleen, nor was there an inverse correlation between CF antibody titers and skin test or MIF responses. Rather, CF antibody titers and cell-mediated immune responses were equally demonstrable in guinea pigs with disseminated or nondisseminated disease.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artz R. P., Bullock W. E. Immunoregulatory responses in experimental disseminated histoplasmosis: depression of T-cell-dependent and T-effectory responses by activation of splenic suppressor cells. Infect Immun. 1979 Mar;23(3):893–902. doi: 10.1128/iai.23.3.893-902.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L. V., Pappagianis D., Benjamini E. Mechanisms of resistance to infection with Coccidioides immitis in mice. Infect Immun. 1979 Mar;23(3):681–685. doi: 10.1128/iai.23.3.681-685.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Pappagianis D., Benjamini E. Significance of T cells in resistance to experimental murine coccidioidomycosis. Infect Immun. 1977 Sep;17(3):580–585. doi: 10.1128/iai.17.3.580-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro A., Spitler L. E., Moser K. M. Cellular immune response in coccidioidomycosis. Cell Immunol. 1975 Feb;15(2):360–371. doi: 10.1016/0008-8749(75)90014-3. [DOI] [PubMed] [Google Scholar]
- Cox R. A., Arnold D. R. Immunoglobulin E in coccidioidomycosis. J Immunol. 1979 Jul;123(1):194–200. [PubMed] [Google Scholar]
- Cox R. A., Mead C. G., Pavey E. F. Comparisons of mycelia- and spherule-derived antigens in cellular immune assays of Coccidioides immitis-infected guinea pigs. Infect Immun. 1981 Feb;31(2):687–692. doi: 10.1128/iai.31.2.687-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Vivas J. R., Gross A., Lecara G., Miller E., Brummer E. In vivo and in vitro cell-mediated responses in coccidioidomycosis. I. Immumologic responses of persons with primary, asymptomatic infections. Am Rev Respir Dis. 1976 Nov;114(5):937–943. doi: 10.1164/arrd.1976.114.5.937. [DOI] [PubMed] [Google Scholar]
- Cox R. A., Vivas J. R. Spectrum of in vivo and in vitro cell-mediated immune responses in coccidioidomycosis. Cell Immunol. 1977 Jun 1;31(1):130–141. doi: 10.1016/0008-8749(77)90012-0. [DOI] [PubMed] [Google Scholar]
- Harrington J. T., Jr, Stastny P. Macrophage migration from an agarose droplet: development of a micromethod for assay of delayed hypersensitivity. J Immunol. 1973 Mar;110(3):752–759. [PubMed] [Google Scholar]
- Huppert M., Sun S. H., Gross A. J. Evaluation of an experimental animal model for testing antifungal substances. Antimicrob Agents Chemother. 1972 May;1(5):367–372. doi: 10.1128/aac.1.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A. B., Pappagianis D. Experimental induction of anergy to coccidioidin by antigens of Coccidioides immitis. Infect Immun. 1973 May;7(5):786–794. doi: 10.1128/iai.7.5.786-794.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashkin K. P., Likholetov S. M., Lipnitsky A. V. Studies on mediators of cellular immunity in experimental coccidioidomycosis. Sabouraudia. 1977 Mar;15(1):59–68. doi: 10.1080/00362177785190111. [DOI] [PubMed] [Google Scholar]
- Kong Y. M., Savage D. C., Levine H. B. Enhancement of immune responses in mice by a booster injection of Coccidioides spherules. J Immunol. 1965 Dec;95(6):1048–1056. [PubMed] [Google Scholar]
- Lawrence R. M., Huston A. C., Hoeprich P. D. Reproducible method for induction of pulmonary coccidioidomycosis in mice. J Infect Dis. 1977 Jan;135(1):117–119. doi: 10.1093/infdis/135.1.117. [DOI] [PubMed] [Google Scholar]
- PAPPAGIANIS D., LEVINE H. B., SMITH C. E., BERMAN R. J., KOBAYASHI G. S. Immunization of mice with viable Cocidioides immitis. J Immunol. 1961 Jan;86:28–34. [PubMed] [Google Scholar]
- PAPPAGIANIS D., LINDSEY N. J., SMITH C. E., SAITO M. T. ANTIBODIES IN HUMAN COCCIDIOIDOMYCOSIS: IMMUNOELECTROPHORETIC PROPERTIES. Proc Soc Exp Biol Med. 1965 Jan;118:118–122. doi: 10.3181/00379727-118-29773. [DOI] [PubMed] [Google Scholar]
- Pappagianis D., Saito M., Van Hoosear K. H. Antibody in cerebrospinal fluid in non-meningitic coccidioidomycosis. Sabouraudia. 1972 Jul;10(2):173–179. doi: 10.1080/00362177285190341. [DOI] [PubMed] [Google Scholar]
- Rea T. H., Einstein H., Levan N. E. Dinitrochlorobenzene responsivity in disseminated coccidioidomycosis: an inverse correlation with complement-fixing antibody titers. J Invest Dermatol. 1976 Jan;66(1):34–37. doi: 10.1111/1523-1747.ep12478071. [DOI] [PubMed] [Google Scholar]
- SMITH C. E., SAITO M. T., SIMONS S. A. Pattern of 39,500 serologic tests in coccidioidomycosis. J Am Med Assoc. 1956 Feb 18;160(7):546–552. doi: 10.1001/jama.1956.02960420026008. [DOI] [PubMed] [Google Scholar]
- Sawaki Y., Huppert M., Bailey J. W., Yagi Y. Patterns of human antibody reactions in coccidioidomycosis. J Bacteriol. 1966 Jan;91(1):422–427. doi: 10.1128/jb.91.1.422-427.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Broder S. Suppressor cells in the regulation of the immune response. Prog Clin Immunol. 1977;3:155–199. [PubMed] [Google Scholar]
- Waldron J. A., Jr, Horn R. G., Rosenthal A. S. Antigen-induced proliferation of guinea pig lymphocytes in vitro: obligatory role of macrophages in the recognition of antigen by immune T-lymphocytes. J Immunol. 1973 Jul;111(1):58–64. [PubMed] [Google Scholar]
- Ward E. R., Jr, Cox R. A., Schmitt J. A., Jr, Huppert M., Sun S. H. Delayed-type hypersensitivity responses to a cell wall fraction of the mycelial phase of Coccidioides immitis. Infect Immun. 1975 Nov;12(5):1093–1097. doi: 10.1128/iai.12.5.1093-1097.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinoya S., Cox R. A., Pope R. M. Circulating immune complexes in coccidioidomycosis. Detection and characterization. J Clin Invest. 1980 Oct;66(4):655–663. doi: 10.1172/JCI109901. [DOI] [PMC free article] [PubMed] [Google Scholar]


