Abstract
Lanthionines are novel neurotrophic and neuroprotective small molecules that show promise for the treatment of neurodegenerative diseases. In particular, a recently developed, cell permeable lanthionine derivative known as LKE (lanthionine ketimine 5-ethyl ester) promotes neurite growth at low nanomolar concentrations. LKE also has neuroprotective, anti-apoptotic, and anti-inflammatory properties. Its therapeutic potential in cerebral ischemia and its mechanisms of neurotrophic action remain to be fully elucidated. Here, we hypothesize that the neuroprotective actions of LKE could result from induction or modulation of CRMP2. We found that treating primary cultured mouse neurons with LKE provided significant protection against t-butyl hydroperoxide-induced neuronal death possibly through CRMP2 upregulation. Similarly, in-vivo studies showed that LKE pre and/or post-treatment protects mice against permanent distal middle cerebral artery occlusion (p-MCAO) as evidenced by lower stroke lesions and improved functional outcomes in terms of rotarod, grip strength and neurologic deficit scores in treated groups. Protein expression levels of CRMP2 were higher in brain cortices of LKE pretreated mice, suggesting that LKE’s neuroprotective activity may be CRMP2 dependent. Lower activity of cleaved PARP and higher activity of SERT1 was also observed in LKE treated group suggesting its anti-apoptotic properties. Our results suggest that LKE has potential as a therapeutic intervention in cerebral ischemia and that part of its protective mechanism may be attributed in- part to CRMP2 mediated action and PARP-1/SIRT-1 modulation.
Keywords: lanthionines, stroke, CRMP2, PARP-1, Ischemia, SERT-1
1. Introduction
Lanthionine is a nonproteogenic amino acid formed in the central nervous system (CNS) through the transsulfuration pathway and possibly through other glutathione-dependent biochemical pathways (Hensley et al., 2010b). In the mammalian brain, Lan undergoes aminotransferase conversion to yield an unusual cyclic thioether, lanthionine ketimine (LK; 2H-1,4-thiazine-5,6-dihydro-3,5-dicarboxylic acid). LK has been observed to demonstrate neuroprotective, neurotrophic and anti-inflammatory activities(Hensley et al., 2010b). Recently, a novel cell-permeable derivative, lanthionine ketimine-ethyl ester (LKE), was shown to protect NSC-34 motor neuron-like cells from oxidative toxicity mediated by hydrogen peroxide (H2O2) and also to promote the growth factor-stimulated outgrowth of neurites in these cells (Hensley et al., 2010a; Hensley et al., 2011).
One of the most significant events triggered by the loss of blood supply to the brain during ischemia is axonal injury due to the imbalance of Na2+ and/or Ca2+ influx into axons, which leads to the breakdown of the cytoskeleton, the final outcome of which is neuronal degeneration and death. Collapsin response mediator proteins (CRMPs) are cytoskeleton-regulating proteins expressed in dendrites, axons and growth cones. CRMPs have important roles, both developmentally and following axonal damage, as exemplified by the observation that expression of semaphoring-3A and its downstream effector CRMP2 was associated with neuronal injury in the brains of patients with epilepsy, Alzheimer’s disease (AD), and cerebral ischemia (Gu et al., 2000; Kee et al., 2001).
Poly(ADP-ribose) polymerase-1 (PARP-1) activation, among several other mechanisms, has been implicated in the oxidative stress-mediated cell death during ischemic injury. Release of apoptotic inducing factor (AIF) mediated by poly (ADP-ribose) polymerase-1 (PARP-1) also plays a critical role in the cascade of neuronal loss and death (Alano et al., 2010). The histone deacetylase sir2 (silent information regulator 2) orthologue sirtuin-1 (SIRT-1), another NAD+ dependent enzyme, is involved in regulating energy homeostasis by controlling the acetylation status and the activities of a number of enzymes and transcriptional regulators. Since both PARP-1 and SIRT-1 use NAD+ for their activity, it has been thought that the increased activity of one molecule might interfere with the activity of the other.
In the present study, we were interested in evaluating the therapeutic potential of LKE in stroke and its implications for CRMP2 modulation as well as its ability to modulate the expression of other proteins involved in the apoptotic process following ischemia, specifically the PARP-1/SIRT-1 system. This is the first study to evaluate the role of LKE in delayed ischemic response induced by permanently occluding the distal part of middle cerebral artery (p-MCAO).
2. Materials and Methods
2.1. Animals
All animal studies were conducted in accordance to the protocol approved by The University of Toledo Health Science Campus Institutional Animal Care and Use Committee and the guidelines prescribed by the National Institutes of Health. C57BL/6 male and female (timed 17 days pregnant) mice (5–10 weeks old; 25–30 g) were procured from Charles River Laboratories, Wilmington, MA and were housed at 22 ± 1 °C with a 12 h:12 h light/dark cycle with water and food available ad libitum.
2.2. Permanent Middle cerebral artery occlusion (p-MCAO)
We used our previously optimized method to occlude the distal portion of the MCA (Shah et al., 2011). Briefly, mice were anesthetized with isoflurane (Baxter Healthcare, Deerfield, IL), initially with 3–5% in the induction chamber and then maintained at 1% throughout the surgical procedure via the nasal cone. With the aid of a surgical microscope, a 10mm vertical skin incision was made between the right eye and ear, and the underlying temporal bone was exposed by moving the temporal muscle aside. Accordingly, a 2.0-mm burr hole was drilled and the distal part of MCA was occluded directly using a bipolar coagulator, and complete cessation of blood flow at the occlusion site was confirmed by laser Doppler flowmetry. Mice having complete cessation of the blood flow were selected for the study. Rectal temperature was monitored continuously with a rectal probe and maintained at 37.0 ± 0.5 °C during the surgery with a heating blanket. After the surgery, animals were left to recover in a temperature-regulated recovery chamber before shifting to home cages. No mortality was observed in this model and all the mice survived.
2.3. Drug treatment
Mice were randomized into different treatment groups, and personnel working on the study were blinded from the experimental design. LKE was synthesized as described previously (Hensley et al., 2010a) and solubilized to 25mg/mL in physiological saline by careful titration to neutrality with 2N NaOH. In the pre-treatment paradigm, LKE (100mg/kg p.o.) or vehicle (physiological saline) was administered for 7 days prior to p-MCAO. In the post-treatment paradigm, LKE (100 mg/kg) was administered via an intraperitoneal (i.p.) injection 4 h after the p-MCAO and then daily for 7 days. This dose was chosen based on the limits of LKE solubility in saline and reported dose efficacy in a mouse model of ALS (Hensley et al., 2010b).
2.4. Locomotor activity
Locomotor activity was evaluated by means of a rotarod task by a person blinded to the treatment groups. Mice were placed on a horizontal rod (Columbus Instruments, OH) that was made to rotate at 1 r.p.m. with an acceleration rate of 1 r.p.m. every 10 seconds until the animal fell from the rod. Each animal was tested three times per trial. All the animals were trained on the rotarod assembly prior to surgery. The duration for which each animal was able to stay on the accelerating rod was recorded as the latency to fall and registered manually. Locomotor activity was monitored 4 h before and 2, 5 and 7 days after p-MCAO surgery.
2.5. Grip Strength
Grip strength was evaluated by holding the mice by tails and placing their forelimbs on a specially designed pull bar assemblies (Grip strength meter, Columbus Instruments, OH). Peak amount of force animals exert was displaced on the digital display and noted. Each animal was tested three times per trial at 4 h before, 2 and 7 days after p-MCAO surgery.
2.6. Neurological deficit Scores (NDS)
NDS were evaluated by a 28-point score pattern optimized by our group (Shah et al., 2011). A person blinded to the treatment evaluated NDS 7 days after p-MCAO; the evaluation included both sensory and motor deficits, such as body symmetry, gait, climbing, circling behavior, front limb symmetry, compulsory circling, and whisker response. Each of the seven tests included in the 28-point NDS was graded from 0 to 4, with higher scores indicating severe deficits.
2.7. Infarct volume analyses
Animals from all the groups were euthanized 7 days after p-MCAO. Brains were dissected out and sliced into five 2-mm-thick coronal sections before incubating in 1% triphenyltetrazolium chloride (TTC) (Sigma Co, MI). The infarct area was estimated from five slices of each brain, measuring rostral and caudal sides of each individual slice in conjunction with the thickness and expressed as a percentage of the volume of the contralateral hemisphere. A person blinded to the treatment groups measured the infarct volume with the help of ImageJ software provided by NIH.
2.8. Western blots
Brain cortices of ischemic and non-ischemic mice were dissected out, weighed, and homogenized as described previously (Shah et al., 2011). Protein samples were analyzed by loading equivalent amounts of protein (25 μg) onto 10% SDS-polyacrylamide gels and incubated with different primary antibodies; rabbit anti-actin, 1:3000 (Sigma Co, MI); rabbit anti-CRMP2, 1:50,000 (Millipore, Billerica, MA), rabbit anti PARP-1, 1:1000 (cell signaling, Danvers, MA); rabbit anti SIRT-1, 1:1000 (Cell Signaling, Danvers, MA) and /or rabbit anti histone H3, 1:3000 (ThermoScientific, Waltham, MA). Images were analyzed using ImageJ software provided by the NIH. The densitometric values were normalized with respect to β-actin/histone H3.
2.9. Cell cultures and viability assay
Isolation of embryonic cortical neuronal cells was performed according to Shah et al. (Shah et al., 2007) with minor modifications. Timed 17-day pregnant mice were terminated by CO2 overdose, and fetuses were decapitated and cortices collected thoroughly homogenized, triturated and centrifuged at 1000g at 18 °C for 3 min. Cells were resuspended in fresh 5 mL of DMEM medium (Fisher scientific) containing 0.25% of trypsin followed by incubation at 37 °C/5% CO2 for 15 min. Cells were then centrifuged and re-suspended in 5 mL of neurobasal medium (Invitrogen, Carlsbad, CA) containing penicillin and streptomycin (50U/mL of medium), glutamine (2 mmol/L), and B27 serum free (Invitrogen). This step was repeated twice, after which the cells were counted using trypan blue and plated onto poly-L-lysine coated plates. Cultures were maintained at 37 °C in a 5% CO2 atmosphere and all the in vitro experiments were performed after 14 days in culture. Primary cortical neurons were seeded at a population 0.5 × 106 in pre-coated 24-well dishes. Two hours prior to drug treatment, the neurobasal medium was replaced with the medium containing B-27 minus antioxidant (Invitrogen). Cells were pre-treated with LKE with increasing concentrations of 100–500 μmol/L for 6 h and then exposed to 100 μmol/L hydrogen peroxide (H2O2) and/or 60 μmol/L tert-butyl hydroperoxide (t-BuOOH) for an additional 18 h. Cell viability assay was performed using the 3-(4,5-dmethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay kit (Promega, San Luis Obispo, CA). Optical density was measured at 570 nm. All the experiments were conducted in triplicate with three separate batches of cultures.
2.10. CRMP2 and phalloidin staining for immunocytochemistry
As per the our previously published method (Nada and Shah, 2012), primary cortical neurons were seeded at a 0.75 × 106 on a sterilized coverslip pre-coated with poly-L-lysine in 6-well dishes and pre-treated with/or without LKE (200 μmol/L) for 6 hours followed by t-BuOOH treatment (60 μmol/L) for an additional 6 h. Cells were then washed, fixed with freshly prepared 4% para-formaldehyde, and permeabilized using 0.3% Triton X-100 in 1X PBS. The fixed cells were blocked and incubated with CRMP-2 antibody (1:8000). Slides were washed and incubated in secondary antibody, 1:800 (Jackson Immuno Research, West Grove, PA) followed by incubation with phalloidin (Invitrogen) and later on mounted with DAPI.
2.11. Statistical analyses
Infarct volumes for vehicle and ischemia/treatment groups were analyzed by one-way ANOVA with Newman–Keuls post hoc test. Neurological deficits and rotarod outcomes were analyzed by the non-parametric Kruskal–Wallis test. Data are presented as mean ± SEM. A value of p < 0.05 was considered to be statistically significant.
3. Results
3.1. Oral pre-treatment of LKE reduces infract volume and improves functional outcomes in mice subjected to p-MCAO
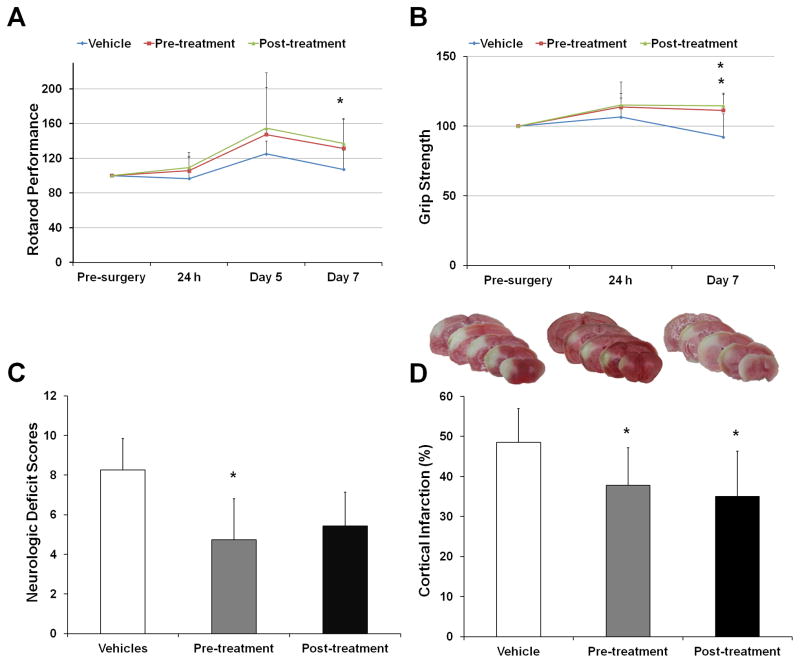
Initial proof of concept studies with LKE in the p-MCAO model used a pre-treatment protocol to maximize the likelihood of achieving the drug effects. Mice were given LKE (100mg/kg) daily for seven days by oral gavage and subjected to p-MCAO, after which mice were allowed to survive for 7 days. Although generally better recovery of locomotor activity was observed at 2, 5 and 7 days of p-MCAO in the LKE treated mice as compared to the ones treated with vehicle, these were not statistically significant (Figure 1A). Grip strength was significantly improved in LKE treated mice at day 7 (p < 0.04) (Figure 1B). Likewise, LKE also significantly reduced neurological deficits in mice as compared to the vehicle group (NDS: 4.7 ± 0.4 vs. 8.2 ± 1.5; p < 0.04) (Figure 1C). LKE administration significantly reduced infarct volume in mice when compared to those treated with vehicle (37.7 ± 9.4% vs. 48.5 ± 8.3%; p < 0.03) (Figure 1D).
Figure 1.
Effect of LKE pre-and post-treatment on the cortical infarct volume and functional outcomes in mice subjected to p-MCAO. (A, B C and D) The grip strength test results and NDS were significantly reduced in case of LKE pre-treatment (100mg/kg, orally) while as rotarod test results and grip strength were significantly reduced in case of LKE post-treatment (100mg/kg, i.p.). (D) Representative coronal brain sections showing cortical infarct volume (white part) in different treatment groups. The corrected cortical infarct volumes were significantly reduced in both pre- and post-treatment groups (graph with infarct volume analysis). Data are expressed as mean ± SEM; *p<0.05, vs vehicle-treated control; number of mice, n = 13 (control); n = 15 (pre-treatment); n = 15 (post-treatment)
3.2. Post-treatment of LKE reduces infarct volume in mice subject to p-MCAO
To address the potential clinical relevance of LKE, we administered LKE (100mg/kg, i.p.) to a separate cohort of mice 4 hours after p-MCAO and then daily for 7 days. Compared to vehicle, LKE treated mice showed better recovery in their locomotor activity measured after 2, 5 and 7 days of p-MCAO (Figure 1A) with significant results observed at day 7 (p < 0.04). Grip strength was significantly improved in LKE treated mice at day 7 (p < 0.04) (Figure 1B). Reduced trend in neurological deficits were observed in mice compared to the vehicle group (Figure C). However, LKE significantly lowered infarct volumes in mice when compared to the vehicle group in the post-treatment regimen (34.9 ± 11.3% vs. 48.5 ± 8.3%; p < 0.01) (Figure 1D).
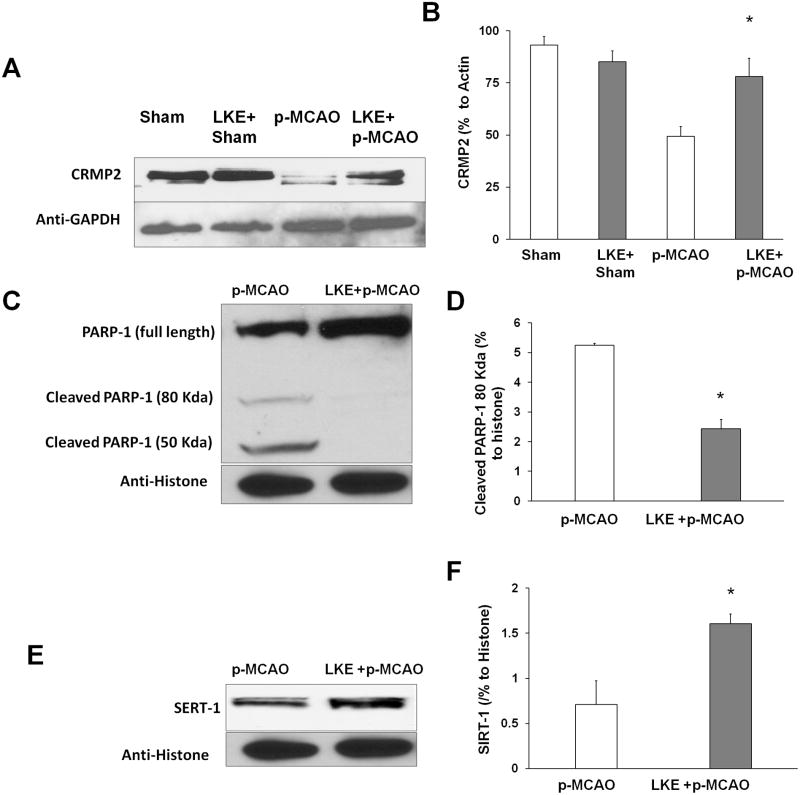
3.3. LKE upregulates the expression of CRMP2 and SIRT-1 and attenuates the expression of caspase-cleaved PARP-1 in the mouse brain cortex
A separate randomized cohort of mice pre-treated with LKE (100mg/kg p.o.) daily for seven days was subjected to p-MCAO and sacrificed seven days later for the removal of their brain cortices and subsequent western blot analyses. There were no differences observed in sham (no p-MCAO) and LKE pre-treated sham group (No p-MCAO). LKE significantly upregulated the expression of CRMP2 and the anti-apoptotic SIRT-1, whereas it elicited lower levels of the caspase cleaved fragment of PARP-1 (Figure 2A, B and C).
Figure 2.
Effect of LKE on the expression of CRMP2, PARP-1 and SIRT-1. (A, B and C) No differences were observed between sham and LKE pre-treated sham. LKE pre-treatment significantly upregulated the expression of total CRMP2 and SIRT-1 and decreased the expression of the caspase cleaved fragment of PARP-1. Corresponding graphs show the densitometric analysis normalized to actin and histone proteins, respectively.
3.4. LKE protects primary neuronal cells from oxidative stress in a cell viability assay
In primary neuron cultures, H2O2 decreased cell viability as measured by the MTT assay, whereas LKE dose-dependently protected neurons against H2O2 mediated oxidative stress (Figure 3A). Pre-treatment with LKE (100 μmol/L) for 6 h followed by t-BuOOH (60 μmol/L) exposure for another 18 h significantly protected neurons against t-BuOOH-induced oxidative stress (Figure 3B). These findings correlate with prior report of LKE neuroprotection against ROS (Hensley et al., 2010b).
Figure 3.
Effect of LKE on neuronal survival in vitro. (A) LKE showed significant reversal of neuronal death induced by H2O2 in a dose-dependent manner (B) In case of t-BuOOH mediated cell death, the lowest protective concentration of LKE (200 μmol/L) showed significant reversal of cell death. Each experiment was conducted in triplicate and repeated four times with different primary culture batches. Data are expressed as mean ± SEM; *p < 0.05 vs. control, #p<0.05 vs. stressor.
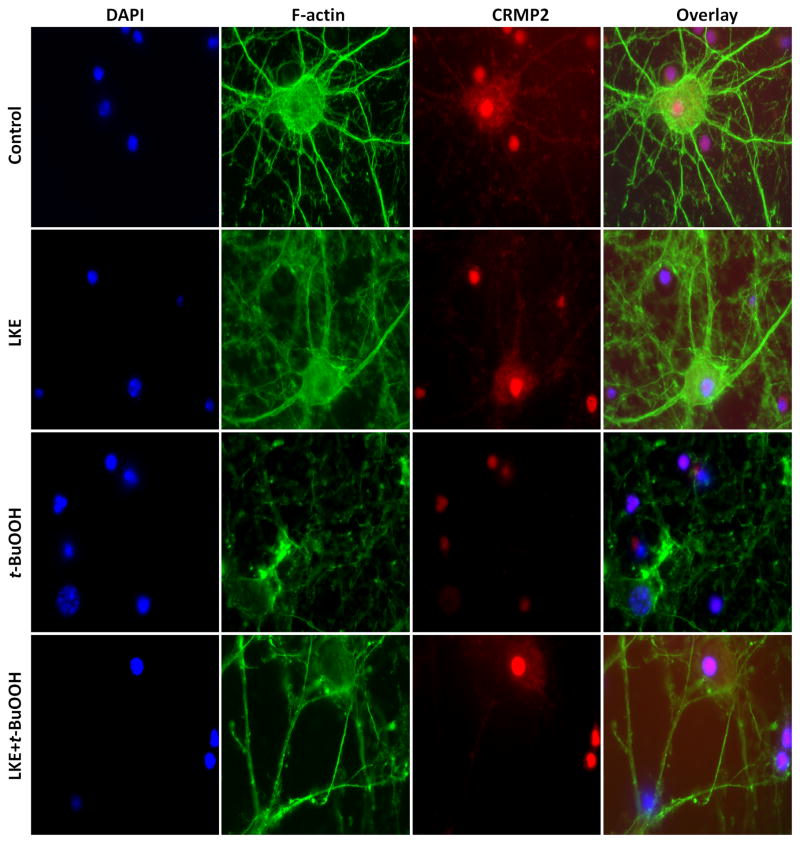
Immunofluorescence microscopy revealed intact F-actin and CRMP2 expression in axons and dendrites, but a drastic breakdown of the F-actin filaments and somatic relocation of CRMP2 was observed in neurons when exposed to 6 h t-BuOOH, with actin no longer extending into the axonal or dendritic structures (Figure 4). The CRMP2 protein appeared to be collapsed and localized mainly in the nuclei. Pre-incubation with LKE (200 μmol/L) 6 h prior to t-BuOOH exposure restored F-actin filament network and extended the expression of CRMP2 into both the axons and dendrites (Figure 4).
Figure 4.
LKE elicits neuritogenesis in mice cortical neurons. Top panel: control neurons without any treatment. The nucleus is stained with DAPI (blue stain), F-actin (phalloidin green stain), and CRMP2 (red stain). The far right image shows the merged overlay of all the pictures. t-BuOOH treatment induced CRMP2 localization into the nucleus and collapse of F-actin filaments. Pre-treatment with LKE (200 μmol/L) induced the expression of CRMP2 and F-actin in axons and dendrites. Each experiment was conducted in triplicate and repeated three times with different primary culture batches.
4. Discussion
In this study, we demonstrated that LKE is neuroprotective in a p-MCAO model of cerebral ischemia as well as in in vitro models of oxidative stress. The effect of LKE in cerebral ischemia is a hitherto unexplored area and, to the best of our knowledge, we are the first to report that both LKE pre- and post-treatment offers significant reversal of cortical tissue damage in an animal model of delayed ischemia. Moreover, these protective effects were resonant with improved functional outcomes in terms of locomotor activity (significant in post-treatment), grip strength (significant in both preand post-treatment) as well as neurological deficits (significant in pre-treatment). In addition, we have successfully attributed LKE’s putative neuroprotective mechanisms, at least in part to its CRMP2 mediated neurotrophic and anti-apoptotic effects. The ability of LKE to salvage neurons subjected to H2O2 and t-BuOOH-mediated oxidative stress in vitro and its veracity of the anti-apoptotic hypothesis was confirmed by its ability to modulate the expression of key proteins involved in the apoptotic cascade-PARP1 and SIRT1.
Our results with animal studies suggest that the daily dose of LKE (100mg/kg, orally) in pre-treatment paradigm produces prophylactic effects against subsequent p-MCAO-induced ischemic brain injury. This led us to hypothesize that LKE may have clinical relevance, which was supported by the fact that LKE showed promise in the post-treatment paradigms, therefore highlighting its clinical relevance. These results are in agreement with previous studies showing that LKE (100mg/kg/d, i.p.) slows down progression of paralytic disease in the SOD1G93A mouse model of familial amyotrophic lateral sclerosis (ALS), as was seen in improved performance on rotarod, delayed onset-of-paralysis, and increased lifespan (Hensley et al., 2010b). Since LKE is a bioavailable pro-drug (data not shown), we speculate that it may be relatively fast-acting and potent, which could be crucial in stroke therapy. Successful results with SOD1G93A mice and pMCAO-induced stroke justify the idea to test LKE in other neurodegenerative diseases marked by oxidative stress, glutamate excitotoxicity, neuron degeneration and apoptotic cell death.
Based on the prior work (Hensley et al., 2010a) indicating that LK binds CRMP2 and provides neurotrophic action, we initially focused on CRMP2-related pathways. CRMP2 is a multifunctional adaptor protein that binds cytoskeletal partners to affect microtubule dynamics, neurite outgrowth and retraction, neural differentiation, neurotransmitter release, and other important neurophysiological functions (Hensley et al., 2011). CRMP2 phosphorylation by negative regulator lysophosphatidic acid results in growth cone collapse and neurite retraction in dorsal root ganglia, but LKE acts as a positive regulator in neurite stability (Hensley et al., 2010a; Hensley et al., 2010b; Hensley et al., 2011). Our CRMP2 upregulation was found to promote resistance to glutamate excitotoxicity, a hallmark of ischemic damage (Hou et al., 2009) and protection in hypoxic ischemia (Zhou et al., 2008) and traumatic brain injury (Zhang et al., 2007). These findings led us to believe that CRMP2 upregulation could be an effective means of inducing neurite regeneration and subsequent neuronal survival under ischemic conditions. We observed that LKE pre-treatment induced significantly higher expression levels of CRMP2 in the brain cortices of mice subjected to p-MCAO. Our immunocytochemical studies also showed that LKE induced CRMP2 levels in primary cortical neurons in culture, which correlated with correspondingly increased levels of F-actin in axons and dendrites. These findings are in contrast to previous studies where no LKE was observed to affect CRMP2 protein expression but instead affected LKE’s affinity for CRMP2 binding to its various partners.
Next, we were interested in investigating the anti-apoptotic potential of LKE in more detail to help elucidate its neuroprotective mechanism. It is well known that proteins partaking in energy metabolism are closely involved in the apoptotic machinery under ischemic conditions. PARP-1 and SIRT-1 are two such enzymes, with both their activities dependent on NAD+. PARP-1 is activated in response to DNA damage such as single-strand breaks, which could develop as a response to various pathological conditions such as inflammatory diseases, diabetes, reperfusion injury, or oxidative stress. Overactivation of PARP-1 consumes NAD+ and results in cell death due to depletion of intracellular NAD+ stores (Ha and Snyder, 1999). This NAD+ deficit could consequently affect the activity of NAD+ dependent SIRT-1, which is considered to be a nuclear sensor of the redox state of the cell (Saunders and Verdin, 2007). SIRT-1 has been implicated in transcriptional silencing, genetic control of aging, cell metabolism, and calorie restriction-mediated longevity of the organism (Haigis and Guarente, 2006; Pallas et al., 2008). SIRT-1 also regulates neuronal differentiation (Guo et al., 2011; Hisahara et al., 2008; Prozorovski et al., 2008) and participates in the ischemic preconditioning mediated neuronal protection in the hippocampus (Raval et al., 2008), and prevents neurodegeneration in mouse models of neuronal diseases, including amyotrophic lateral sclerosis (ALS) (Kim et al., 2007) and AD (Chen et al., 2005). We report that LKE pre-treatment inhibited the activation and thus cleavage of PARP1 into its pro-apoptotic fragment, as well as upregulated the expression of SIRT1 under ischemic conditions. It is well established that activation of PARP-1 in ischemia results in neuronal death (Virag and Szabo, 2002) and that its inhibition could result in increased ischemic protection (Eliasson et al., 1997). On the other hand, expression of SIRT-1 promotes cell survival (Guo et al., 2011; Rajamohan et al., 2009) Thus, our results suggest that interplay between PARP1 and SIRT1 could have partially contributed to the observed protective effects of LKE and inhibition of PARP-1 could be responsible for activating SIRT-1 by replenishing the reserves of NAD+.
Conclusion
We demonstrated the neuroprotective properties of the experimental therapeutic LKE in an animal model of permanent middle cerebral occlusion in both pre-treatment and post-treatment regimens as well as in neuronal cell viability experiments. Furthermore, we uncovered evidence that LKE mediated its beneficial effects, at least in part, through CRMP2 and SIRT1 upregulation and PARP1 inhibition. The neuroprotective and morphogenic potential inherent to LK metabolites in ischemic conditions may begin to suggest that LK or its derivatives may be useful for therapeutic benefit against multiple neurodegenerative diseases such as stroke and ischemia. Research is underway in our laboratory to explore such possibilities.
We have demonstrated that LKE is neuroprotective in a p-MCAO model of stroke.
LKE’s putative mechanism is in-part due to its CRMP2 mediated neurotrophic effects.
LKE modulates the expression of apoptotic cascade- proteins, PARP1 and SIRT1.
LKE salvaged neurons against H2O2 and t-BuOOH-mediated oxidative stress in vitro.
LKE may be useful for therapeutic benefits in diseases such as stroke and ischemia.
Acknowledgments
Sources of Funding—This work was supported partly by grants from the National Institutes of Health- R00-AT004197 to ZAS and R01-AE031553, R21-NS066279 to KH.
Abbreviations
- CRMP2
collapsin response mediator protein 2
- H2O2
hydrogen peroxide
- LKE
lanthionine ketimine-ethyl ester
- MCAO
middle cerebral artery occlusion
- NDS
neurologic deficit score
- t-BuOOH
tertiary butylhydroperoxide
- TFM
terpene free material
- p-MCAO
permanent distal middle cerebral artery occlusion
- PARP-1
Poly(ADP-ribose) polymerase-1
- TTC
triphenyltetrazolium chloride
- SERT-1
situin-1
Footnotes
Disclosures
KH is inventor of an issued U.S. patent 7,683,055 concerning LK derivatives.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alano CC, et al. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30:2967–78. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, et al. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–74. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- Eliasson MJ, et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–95. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- Gu Y, et al. Neurofibrillary tangle-associated collapsin response mediator protein-2 (CRMP-2) is highly phosphorylated on Thr-509, Ser-518, and Ser-522. Biochemistry. 2000;39:4267–75. doi: 10.1021/bi992323h. [DOI] [PubMed] [Google Scholar]
- Guo W, et al. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J Neurosci Res. 2011;89:1723–36. doi: 10.1002/jnr.22725. [DOI] [PubMed] [Google Scholar]
- Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A. 1999;96:13978–82. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–21. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Hensley K, et al. Proteomic identification of binding partners for the brain metabolite lanthionine ketimine (LK) and documentation of LK effects on microglia and motoneuron cell cultures. J Neurosci. 2010a;30:2979–88. doi: 10.1523/JNEUROSCI.5247-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K, et al. Emerging biological importance of central nervous system lanthionines. Molecules. 2010b;15:5581–94. doi: 10.3390/molecules15085581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K, et al. Collapsin Response Mediator Protein-2: An Emerging Pathologic Feature and Therapeutic Target for Neurodisease Indications. Mol Neurobiol. 2011 doi: 10.1007/s12035-011-8166-4. [DOI] [PubMed] [Google Scholar]
- Hisahara S, et al. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci U S A. 2008;105:15599–604. doi: 10.1073/pnas.0800612105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou ST, et al. CaMKII phosphorylates collapsin response mediator protein 2 and modulates axonal damage during glutamate excitotoxicity. J Neurochem. 2009;111:870–81. doi: 10.1111/j.1471-4159.2009.06375.x. [DOI] [PubMed] [Google Scholar]
- Kee NJ, et al. Enhanced neurogenesis after transient global ischemia in the dentate gyrus of the rat. Exp Brain Res. 2001;136:313–20. doi: 10.1007/s002210000591. [DOI] [PubMed] [Google Scholar]
- Kim D, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–79. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada SE, Shah ZA. Preconditioning with Ginkgo biloba (EGb 761(R)) provides neuroprotection through HO1 and CRMP2. Neurobiol Dis. 2012;46:180–9. doi: 10.1016/j.nbd.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas M, et al. Modulation of sirtuins: new targets for antiageing. Recent Pat CNS Drug Discov. 2008;3:61–9. doi: 10.2174/157488908783421492. [DOI] [PubMed] [Google Scholar]
- Prozorovski T, et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10:385–94. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- Rajamohan SB, et al. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol Cell Biol. 2009;29:4116–29. doi: 10.1128/MCB.00121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval AP, et al. Resveratrol and ischemic preconditioning in the brain. Curr Med Chem. 2008;15:1545–51. doi: 10.2174/092986708784638861. [DOI] [PubMed] [Google Scholar]
- Saunders LR, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26:5489–504. doi: 10.1038/sj.onc.1210616. [DOI] [PubMed] [Google Scholar]
- Shah ZA, et al. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience. 2007;147:53–9. doi: 10.1016/j.neuroscience.2007.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZA, et al. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience. 2011;180:248–55. doi: 10.1016/j.neuroscience.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- Zhang Z, et al. Calpain-mediated collapsin response mediator protein-1, -2, and -4 proteolysis after neurotoxic and traumatic brain injury. J Neurotrauma. 2007;24:460–72. doi: 10.1089/neu.2006.0078. [DOI] [PubMed] [Google Scholar]
- Zhou Y, et al. Proteomic analysis of neonatal mouse brain: evidence for hypoxia- and ischemia-induced dephosphorylation of collapsin response mediator proteins. J Proteome Res. 2008;7:2507–15. doi: 10.1021/pr800108k. [DOI] [PubMed] [Google Scholar]