Abstract
FIV establishes a latent infection in peripheral CD4+ T-cells, and the latent FIV promoter is associated with deacetylated, methylated histones, consistent with a restrictive chromatin structure. Here we explored the use of 5 histone-modifying agents - 4 histone deacetylase inhibitors (HDACi) and 1 histone methyltransferase inhibitor (HMTi) - to reactivate latent FIV ex vivo. All compounds tested were able to alter histone lysine residue modifications in feline cells, both globally and at the FIV promoter locally. When latently FIV-infected peripheral CD4+ T-cells were cultured ex vivo in the presence of these inhibitors, viral transcription was significantly activated relative to no treatment controls. Transcriptional reactivation of virus mediated by the HDACi suberoylanilide hydroxamic acid (SAHA) was dose-dependent, detected after as little as 1 hour of exposure, and resulted in virion formation as evidenced by supernatant reverse transcriptase activity. A synergistic effect was not found when SAHA was combined with HMTi under the conditions tested. At low therapeutically relevant concentrations in primary feline PBMC, SAHA was found to be minimally cytotoxic and non-immune activating. HDACi and HMTi can reactivate latent FIV ex vivo, and SAHA, also known as the anticancer drug Vorinostat, in particular is a promising candidate for in vivo use because of its efficacy, potency, and relative safety. Use of the FIV/cat model of lentiviral latency to explore in vivo treatment with SAHA and other anti-latency therapeutics will allow investigations that are either ethically or logistically not addressable in patients persistently infected with human immunodeficiency virus (HIV-1).
Keywords: FIV, latency, CD4 T-cells, histone deacetylase, SAHA, DZNep, epigenetic
Feline immunodeficiency virus (FIV) is a lentivirus of domestic cats with similar immunopathogenesis to human immunodeficiency virus (HIV) infection in humans (Burkhard and Dean, 2003; Kanzaki and Looney, 2004). FIV-infected cats are the only naturally-occurring, outbred, large animal model of lentiviral-induced immunodeficiency. Like HIV, FIV has been shown in our laboratory to establish a latent infection in peripheral CD4+ T-cells, which can be reactivated ex vivo with exposure to mitogens (Murphy et al., 2012). In peripheral CD4+ T-cells, the latent FIV promoter is associated with deacetylated, methylated histones, consistent with a restrictive chromatin structure (McDonnel et al., 2012). The ability to pharmacologically reactivate latent virus may facilitate attenuation or eradication of the lentiviral reservoir in infected individuals (Geeraert et al., 2008; Richman et al., 2009; Shen and Siliciano, 2008). Here we explore the use of 4 histone deacetylase inhibitors (HDACi) including suberoylanilide hydroxamic acid (SAHA), valproic acid (VPA), trichostatin A (TSA), and sodium butyrate (NaB), as well as a histone methyltransferase inhibitor (HMTi), 3-deazanoplanocin A (DZNep), to reactivate latent FIV ex vivo. SAHA (Vorinostat) is used as an anti-cancer agent in humans (Mann et al., 2007). Butyrate has been tested for treating a fetal globin disorder (Perrine et al., 1993), cancer (Douillard et al., 2000), and induction of latent Epstein-Barr virus (Ghosh et al., 2012). VPA (Depakote) has been used for treating certain neurological conditions and has been tested in HIV-infected individuals with either limited or no success at reducing the viral reservoir (Margolis, 2011).
We first established that the candidate HDACi and HMTi were functional in feline cells, as they had not been previously studied in a feline system. Peripheral blood mononuclear cells (PBMC) were isolated from whole blood from specific pathogen free (SPF) cats as previously reported (Murphy et al., 2012) and treated overnight (16 hr) with varying concentrations of either SAHA (100nM to 1μM), VPA (300 μM to 3mM), TSA (25nM to 250 nM), NaB (50μM to 5mM), or no treatment in cell culture medium (Murphy et al., 2012). SAHA was reconstituted in DMSO, TSA and DZNep in ethanol, and VPA and NaB in water; the final concentrations of these liquids in cell culture were negligible (<0.01% for DMSO and <0.3% for ethanol). After incubation, cells were harvested and lysates were tested by immunoblot using polyclonal rabbit IgG antibodies against either acetylated histone H3 (H3K9,14) or methylated histone H3 (H3K27me3). Density measurements for the resulting ~17 kDa bands were normalized to no treatment controls. HDACi treatment resulted in a dose-dependent increase in global histone acetylation; HMTi treatment resulted in a dose-dependent decrease in histone methylation (Fig. 1). Thus, the selected HDACi and HMTi are functional inhibitors of feline enzymes and induce the expected changes in histone modification.
Figure 1. SAHA, VPA, TSA, NaB, and DZNep cause dose-dependent alterations in chromatin modification in feline cells.
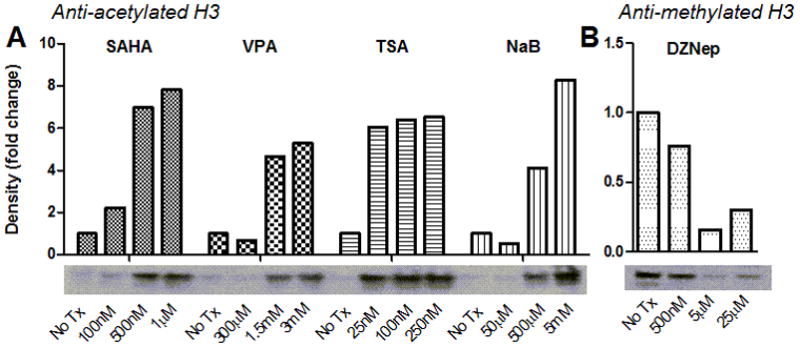
SPF feline PBMC were treated with HDACi (A), HMTi (B), or no treatment (No Tx), and Western blots were performed on cell lysates (20 μg protein per lane) using a primary rabbit anti-acetylated histone (H3K9,14ac, Millipore) antibody (A) or anti-methylated histone (H3K27me3, Millipore) antibody (B) following normalization for total protein using a commercial BCA assay (Thermo Scientific). Band densities calculated using ImageJ (U.S. National Institutes of Health) were normalized to no treatment controls.
Of particular interest is the ability of these inhibitors to alter the local chromatin environment at the FIV promoter in latently infected cells. CD4+ T-cells were isolated from the peripheral blood of cats experimentally infected with FIV-Cpgmr (Diehl et al., 1995) in the asymptomatic phase by dual magnetic column separation (Murphy et al., 2012). Cells were treated overnight as above with either 1μM SAHA, 3mM VPA, 250nM TSA, 5mM NaB, or no treatment, and chromatin immunoprecipitation (ChIP) assays were performed using antibodies specific for acetylated or methylated histone H3 (McDonnel et al., 2012). In untreated latently FIV-infected cells, the viral promoter was associated with deacetylated and methylated histones, as found previously for freshly isolated CD4+ T-cells (McDonnel et al., 2012). However, treatment with HDACi resulted in a significant increase in histone acetylation, and treatment with HMTi resulted in a small, but significant decrease in histone methylation, in association with the FIV LTR (Fig. 2). Thus, the selected HDACi and HMTi were able to at least partially reverse histone marks of restrictive chromatin physically associated with the latent FIV promoter.
Figure 2. SAHA, VPA, TSA, NaB, and DZNep alter the chromatin environment at the FIV promoter.
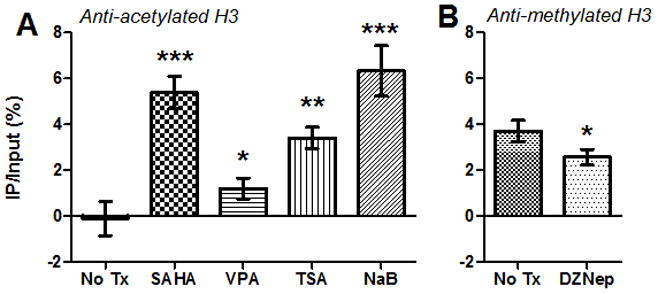
CD4+ T-cells from 2 asymptomatically FIV-infected cats were treated with HDACi (A), HMTi (B), or no treatment (No Tx), and chromatin immunoprecipitation (ChIP) assays were performed using anti-acetylated H3 (H3K9,14ac, Millipore, A) or anti-methylated H3 (H3K27me3, Millipore, B) antibodies. Following ChIP, quantitative PCR was used to detect a 180-bp fragment at the 5′ FIV LTR (forward primer GCTTCGAGGAGTCTCTCAGTTGAG at U3/R interface; reverse primer CAAATCAAGTCCCTGTTCGGGC in gag leader). Data are presented as the percentage of immunoprecipitated (IP) FIV DNA out of the total input FIV DNA with background subtraction using a normal IgG control. Error bars represent the standard deviation of triplicate qPCR measurements, and unpaired, one-tailed t-tests (GraphPad Prism, v5.04) were used to determine if there was a significant increase or decrease in histone acetylation or methylation, respectively (*p < 0.05, **p < 0.01, ***p < 0.001). Data are representative of two independent ChIP experiments for each condition.
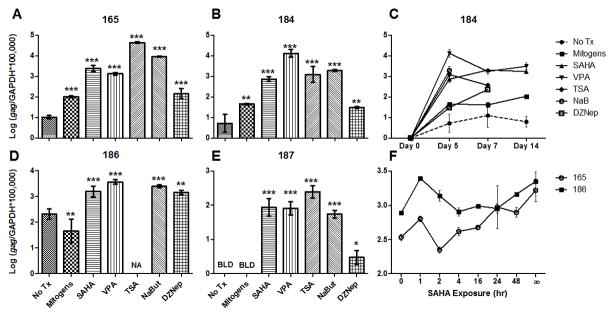
We hypothesized that treatment of latently FIV-infected cells with HDACi and HMTi would also result in transcriptional reactivation of the latent viral promoter. CD4+ T-cells were isolated as above from 4 chronically FIV-C-infected cats (Diehl et al., 1995), and shown to be latent by lack of detection of viral gag transcripts as previously reported (Murphy et al., 2012). During the asymptomatic phase of FIV-C infection, cats are naturally aviremic and were not treated with antiretroviral therapy (Murphy et al., 2012). Cells were then treated ex vivo with SAHA, VPA, TSA, or NaB. Medium alone (no treatment) or activating mitogens (Murphy et al., 2012) served as negative and positive controls, respectively. On days 0, 5, 7, and 14 post-culture, cellular RNA was isolated and measured for FIV gag and feline GAPDH by real-time PCR, as described previously (Murphy et al., 2012). Treatment with both HDACi and HMTi elicited a significant increase in FIV transcription relative to untreated controls; these results were repeatable in all 4 infected cats over a 2-week culture period (Fig. 3a–e). These chromatin-modifying agents are therefore effective in activating FIV transcription ex vivo. In cells from one of the cats (186, Fig. 3d), mitogen stimulation paradoxically resulted in a significant decrease in FIV gag RNA expression. This may have been due to activation-induced early death of infected T-cells related to the immune activation status of the animal. “A limitation of this experimental strategy is that there were likely to be multiple rounds of infection because the experiment was carried out over five days in culture. It is possible that the agents discussed above were serving to promote viral replication through mechanisms in addition to transcriptional activation. However, our data support the idea that these agents removed a transcriptional block which led to virus production and the subsequent increase in viral RNA detected in cells.”
Figure 3. SAHA, VPA, TSA, NaB, and DZNep reactivate transcription from the latent FIV promoter.
CD4+ T-cells from 4 asymptomatically FIV-infected cats were cultured ex vivo in the presence of 1μM SAHA, 3mM VPA, 250nM TSA, 5mM NaB, no treatment (No Tx), or mitogens. RNA was harvested from cells on days 0, 5, 7, and 14 post-culture, and levels of FIV gag cDNA were normalized to cellular GAPDH cDNA. Day 5 values are displayed for cats 165 (A), 184 (B) 186 (D), and 187 (E); the full 14-day time course is displayed for cat 184 (C). Values were not available (NA) for TSA, NaB, and DZNep on day 14 in (C), and TSA on day 5 in (D), due to an insufficient quantity of RNA recovered from the sample. FIV gag transcripts for no treatment and mitogen-treated cells in (E) were below the limit of detection (BLD), despite sufficient quantities of RNA. Error bars represent the standard deviation of triplicate qPCR measurements, and one-way ANOVA followed by Dunnett’s multiple comparison post-test (GraphPad Prism) was used to determine significant differences relative to the no treatment control (*p < 0.05, **p < 0.01, ***p < 0.001). In a separate experiment shown in (F), CD4+ T-cells from cats 165 and 186 were exposed to SAHA for varying amounts of time (∞ = continuous exposure), washed, and cultured for a total of 5 days. RNA was analyzed as above, and error bars represent the standard deviation of triplicate qPCR measurements. One-way ANOVA followed by post-test for linear trend revealed a positive correlation between SAHA exposure time and FIV transcriptional reactivation (165: p < 0.0001; 186: p = 0.0056).
Due to particular interest in SAHA as a candidate for anti-latency therapy in HIV-1 infected human patients (Archin et al., 2009; Contreras et al., 2009), the remaining experiments in this study were focused on this compound. Because SAHA has a relatively short half-life in vivo (T1/2≈ 1.5 hours in human serum (Kavanaugh et al., 2010)), we sought to determine whether shorter exposure times would similarly induce transcriptional reactivation. CD4+ T-cells were isolated as above and treated with 1μM SAHA for varying periods (1 to 48 hours), then washed with PBS and resuspended in cell culture medium. Media alone or continuous SAHA exposure served as negative and positive controls, respectively. Cells were harvested after 5 days in culture, and FIV gag transcriptional activation was determined as above. In general, increased viral transcription correlated with increasing SAHA exposure time (Fig. 3f). Importantly, there was a readily detectable increase in FIV transcription after only 1 hr of exposure to SAHA.
To determine if SAHA-mediated activation of FIV is dose-dependent, reactivation experiments were performed as above with a range of SAHA concentrations. After 5 or 7 days in culture, a dose-dependent reactivation was evident (Fig. 4). Importantly, treatment with as little as 100nM SAHA resulted in a readily detectable increase in FIV transcription in cells from one animal (Fig. 4c–d). We also hypothesized that the combination of HDACi and HMTi would result in synergistic transcriptional activation of latent FIV. However, the combination of SAHA and DZNep resulted in a level of activation intermediate to the two single treatments (Fig. 4), indicating a lack of synergism of these two compounds in this system.
Figure 4. SAHA-mediated FIV transcriptional activation is dose-dependent and not synergistic with DZNep.
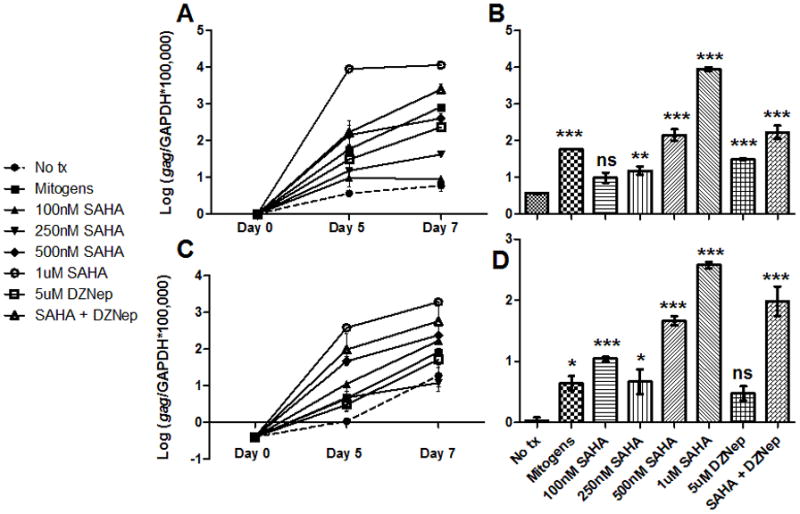
CD4+ T-cells from 2 asymptomatically FIV-infected cats (cat 184 in A and B; cat 187 in C and D) were cultured ex vivo in the presence of varying concentrations of SAHA (100nM to 1μM), 5μM DZNep, a combination of 1μM SAHA and 5μM DZNep (SAHA + DZNep), no treatment (No tx), or mitogens. RNA was harvested from cells on days 0, 5, and 7 post-culture, and levels of FIV gag cDNA were normalized to cellular GAPDH cDNA (A and C). Values for day 5 are expanded in (B) and (D). Error bars represent the standard deviation of triplicate qPCR measurements, and one-way ANOVA followed by Dunnett’s multiple comparison post-test (GraphPad Prism) was used to determine if there was a significant difference relative to the no treatment control (ns - not significant, *p < 0.05, **p < 0.01, ***p < 0.001).
Only by reactivating infectious provirus can the lentiviral reservoir be meaningfully reduced in induction eradication therapy (Geeraert et al., 2008; Richman et al., 2009). We therefore examined the ability of SAHA to induce virion formation by assaying supernatants for the presence of functional reverse transcriptase (RT) enzyme. Latently FIV-infected CD4+ T-cells from 2 cats, isolated as above, were incubated in either SAHA, DZNep, or a combination of SAHA and DZNep; media alone and mitogen treatment served as the negative and positive controls, respectively. After 7 days in culture, supernatants were harvested, clarified, and evaluated for RT activity. Commercially available RT was used to create a standard curve from 0 to 25 RT U/mL, and RT activity of each sample was calculated using the resulting linear equation. Treatment with SAHA elicited a significant increase in supernatant RT activity over the no treatment control, whereas treatment with DZNep did not (Fig. 5). The combination of SAHA and DZNep resulted in supernatant activity similar to SAHA treatment alone; this finding corroborates the lack of synergism between the two compounds under the conditions tested.
Figure 5. SAHA-mediated FIV activation results in virion formation and is not synergistic with DZNep.
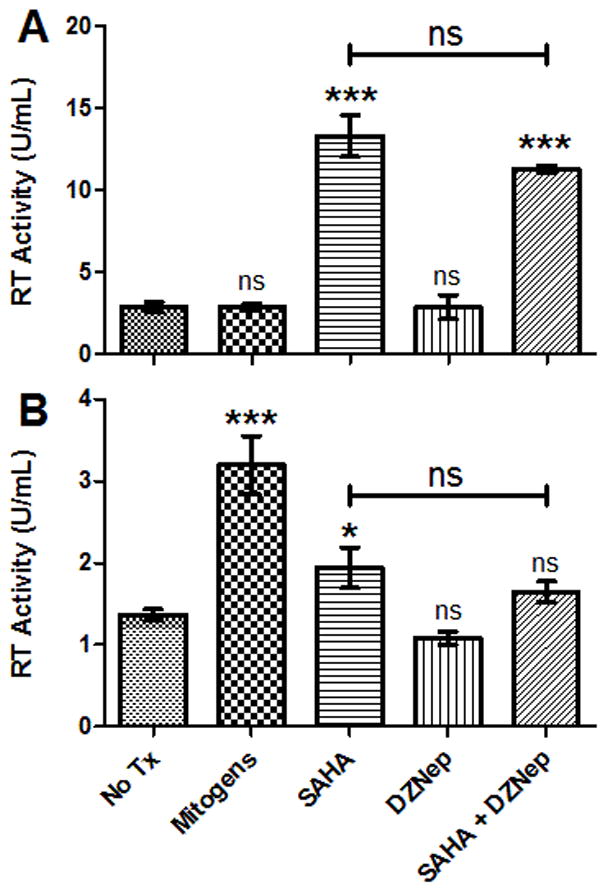
CD4+ T-cells from asymptomatically FIV-infected cats 187 (A) and 184 (B) were cultured ex vivo in the presence of 1μM SAHA, 5μM DZNep, a combination of 1μM SAHA and 5μM DZNep (SAHA + DZNep), no treatment (No Tx), or mitogens. RT activity in clarified supernatants was calculated using a standard curve generated with a commercial RT assay kit (Roche). Error bars represent the standard deviation of triplicate RT assays, and one-way ANOVA followed by Dunnett’s multiple comparison post-test (GraphPad Prism) was used to determine significant differences relative to the no treatment control (ns - not significant, *p < 0.05, ***p < 0.001).
Finally, before moving forward with in vivo SAHA studies, it is necessary to assess its potential for leukocyte activation and cytotoxicity. PBMC from asymptomatically infected cats were treated with 1μM SAHA, mitogens (positive control), or media alone (negative control) for 3 days. Cells were harvested and stained separately for the activation markers CD25 and MHC Class II, the proliferation marker Ki-67, and the co-stimulatory marker CD134, using previously described methods (Mizukoshi et al., 2009; Murphy et al., 2012; Willett et al., 2007). Flow cytometry analysis showed that treatment with SAHA did not result in a significant increase in expression of any of the leukocyte activation markers tested compared to no treatment controls (Fig. 6a). To assess drug-induced cytotoxicity, SPF feline PBMC or MCH5-4, a feline IL2-dependent T-cell lymphoid line (Lerner and Elder, 2000) were incubated for 48 hours in varying concentrations of SAHA (10nM to 5μM), mitogens, or media alone, as above. High concentrations of SAHA resulted in moderate cytotoxicity in PBMC, but at therapeutically relevant concentrations (~250nM) cells were 94% viable after 48 hours of continuous exposure (Fig. 6b). To our knowledge, the half-life of SAHA in cell culture media is not known, so the amount decay of the active compound in these studies is uncertain. A greater degree of cytotoxicity was detected in the lymphoid cell line than in primary PBMC, as might be expected for this anti-lymphoma chemotherapeutic agent. Thus, similar to what has been found in studies of human leukocytes (Archin et al., 2009; Marks et al., 2001), SAHA is minimally toxic and non-immune activating at low therapeutic levels in primary feline cells, making it suitable for use in vivo in cats. Use of the FIV/cat model may be important for determining whether dosages of SAHA with a tolerable level of toxicity can elicit a substantial effect on latent viral reservoirs, as well as in evaluating less toxic alternatives.
Figure 6. SAHA is not immune-activating or toxic in feline PBMC.
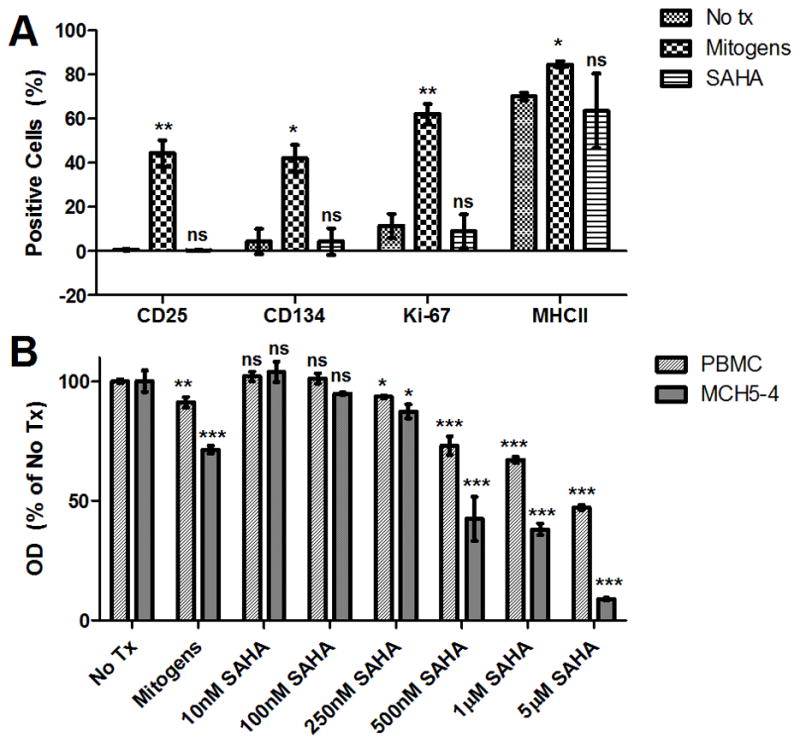
PBMC from cats 165 and 186 were cultured in the presence of 1μM SAHA, no treatment (No tx), or mitogens for 3 days, harvested, and labeled separately for CD25 (clone 9F23; gift of Koichi Ohno, University of Tokyo), MHC Class II (clone 42.3, gift of Peter Moore, UC Davis), Ki-67 (Mib-1 clone, Dako), or CD134 (AbD Serotec). Percent expression of the activation markers was determined by flow cytometry (FC500 Flow Cytometer, Beckman Coulter; FlowJo software v7.6.5, Treestar) after a broad forward- and side-scatter sizing gate (A). Mean and standard deviation expression frequencies (%) in cells from the 2 cats are displayed, and unpaired, two-tailed t-tests (GraphPad Prism) were used to determine significant differences in expression compared to no treatment controls (ns – not significant, *p < 0.05, **p < 0.01 compared to the corresponding no treatment control). SPF feline PBMC and MCH5-4 (feline T-cell lymphoma line) were incubated for 48 hours in the presence of varying concentrations of SAHA (10nM to 5μM), no treatment (No Tx), or mitogens, and a commercial MTT assay (ATCC) was performed to determine cell viability (B). Optical densities (OD) were normalized to no treatment controls after media-only background subtraction. Error bars represent the standard deviation of triplicate MTT assays, and one-way ANOVA followed by Tukey’s multiple comparison post-test (GraphPad Prism) was used to determine if there was a significant difference among treatments (ns - not significant, *p < 0.05, **p < 0.01, ***p < 0.001 compared to the corresponding no treatment control).
The FIV promoter in CD4+ T-cells of cats harboring a latent FIV infection is associated with a restrictive chromatin structure (McDonnel et al., 2012). Here, 5 different histone-modifying agents (4 HDACi: SAHA, VPA, TSA, and NaB, and 1 HMTi: DZNep) were shown to alter the FIV chromatin environment and reactivate latent virus ex vivo. The HDACi SAHA was additionally shown to be effective at low doses and short exposure times, minimally toxic, and non-immune activating. SAHA (Vorinostat) was approved by the Federal Drug Administration in 2006 for treatment of humans with cutaneous T-cell lymphoma (Mann et al., 2007), and there is much interest in its use as a potential induction eradication therapy for HIV-1 infection (Margolis, 2011). The concept is to pharmacologically activate latent virus in the presence of antiretroviral drugs so as to cause death of infected cells by direct cytopathic effect of the reactivated lentivirus and/or immune recognition of reactivated cells expressing viral proteins. Meanwhile, uninfected cells are protected from de novo infection by the antiretroviral drugs (Richman et al., 2009). In theory, this strategy could purge the latent viral reservoir and eliminate the need for life-long antiretroviral therapy (Geeraert et al., 2008). FIV is a well- established, naturally-occurring model of lentiviral infection in a large, out-bred animal, and is shown here to respond to some of the most promising pharmacologic reactivators of HIV. Therefore, use of the FIV/cat model of lentiviral latency to explore in vivo treatment with SAHA and other anti-latency therapeutics may help to address critical clinical questions which are either ethically or logistically not feasible in HIV-infected patients.
Acknowledgments
The authors are grateful for the excellent animal care provided by the staff of the University of California Davis Feline Research Laboratory and for editing assistance provided by Dr. Kirsten Murphy. Funding for this study was provided, in part, by the Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis, and NIH T32 training grants #RR021312 and #5TL1RR024145.
Abbreviations
- ChIP
chromatin immunoprecipitation
- DZNep
3-deazanoplanocin A
- FIV
feline immunodeficiency virus
- HIV-1
human immunodeficiency virus-1
- HDACi
histone deacetylase inhibitor
- HMTi
histone methyltransferase inhibitor
- LTR
long terminal repeat
- NaB
sodium butyrate
- PBMC
peripheral blood mononuclear cells
- PCR
polymerase chain reaction
- RT
reverse transcriptase
- SAHA
suberoylanilide hydroxamic acid
- SPF
specific pathogen free
- TSA
trichostatin A
- VPA
valproic acid
Footnotes
Competing Interests
UC Davis has a research agreement sponsored by Merck Research Laboratories (Whitehouse Station, NJ) to perform pharmacology studies of SAHA (Vorinostat) in rhesus macaques; P. Luciw is the principal investigator of this project. The authors declare that they have no other competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ellen E. Sparger, Email: eesparger@ucdavis.edu.
Paul A. Luciw, Email: paluciw@ucdavis.edu.
Brian G. Murphy, Email: bmurphy@ucdavise.edu.
References
- Archin NM, Espeseth A, Parker D, Cheema M, Hazuda D, Margolis DM. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses. 2009;25(2):207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhard MJ, Dean GA. Transmission and immunopathogenesis of FIV in cats as a model for HIV. Curr HIV Res. 2003;1(1):15–29. doi: 10.2174/1570162033352101. [DOI] [PubMed] [Google Scholar]
- Contreras X, Schweneker M, Chen CS, McCune JM, Deeks SG, Martin J, Peterlin BM. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem. 2009;284(11):6782–6789. doi: 10.1074/jbc.M807898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl LJ, Mathiason-Dubard CK, O’Neil LL, Obert LA, Hoover EA. Induction of accelerated feline immunodeficiency virus disease by acute-phase virus passage. J Virol. 1995;69(10):6149–6157. doi: 10.1128/jvi.69.10.6149-6157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douillard JY, Bennouna J, Vavasseur F, Deporte-Fety R, Thomare P, Giacalone F, Meflah K. Phase I trial of interleukin-2 and high-dose arginine butyrate in metastatic colorectal cancer. Cancer Immunol Immunother. 2000;49(1):56–61. doi: 10.1007/s002620050026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraert L, Kraus G, Pomerantz RJ. Hide-and-seek: the challenge of viral persistence in HIV-1 infection. Annu Rev Med. 2008;59:487–501. doi: 10.1146/annurev.med.59.062806.123001. [DOI] [PubMed] [Google Scholar]
- Ghosh SK, Perrine SP, Williams RM, Faller DV. Histone deacetylase inhibitors are potent inducers of gene expression in latent EBV and sensitize lymphoma cells to nucleoside antiviral agents. Blood. 2012;119(4):1008–1017. doi: 10.1182/blood-2011-06-362434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki LI, Looney DJ. Feline immunodeficiency virus: a concise review. Front Biosci. 2004;9:370–377. doi: 10.2741/1235. [DOI] [PubMed] [Google Scholar]
- Kavanaugh SM, White LA, Kolesar JM. Vorinostat: A novel therapy for the treatment of cutaneous T-cell lymphoma. Am J Health Syst Pharm. 2010;67(10):793–797. doi: 10.2146/ajhp090247. [DOI] [PubMed] [Google Scholar]
- Lerner DL, Elder JH. Expanded host cell tropism and cytopathic properties of feline immunodeficiency virus strain PPR subsequent to passage through interleukin-2- independent T cells. J Virol. 2000;74(4):1854–1863. doi: 10.1128/jvi.74.4.1854-1863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12(10):1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- Margolis DM. Histone deacetylase inhibitors and HIV latency. Curr Opin HIV AIDS. 2011;6(1):25–29. doi: 10.1097/COH.0b013e328341242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks PA, Richon VM, Breslow R, Rifkind RA. Histone deacetylase inhibitors as new cancer drugs. Curr Opin Oncol. 2001;13(6):477–483. doi: 10.1097/00001622-200111000-00010. [DOI] [PubMed] [Google Scholar]
- McDonnel SJ, Sparger EE, Luciw PA, Murphy BG. Transcriptional Regulation of Latent Feline Immunodeficiency Virus in Peripheral CD4+ T-lymphocytes. Viruses. 2012;4(5):878–888. doi: 10.3390/v4050878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukoshi F, Baba K, Horiuchi H, Goto-Koshino Y, Setoguchi-Mukai A, Fujino Y, Ohno K, Moore PF, Tsujimoto H. Characterization of monocyte-derived dendritic cells from cats infected with feline immunodeficiency virus. J Vet Med Sci. 2009;71(7):865–871. doi: 10.1292/jvms.71.865. [DOI] [PubMed] [Google Scholar]
- Murphy B, Vapniarsky N, Hillman C, Castillo D, McDonnel S, Moore P, Luciw PA, Sparger EE. FIV establishes a latent infection in feline peripheral blood CD4+ T lymphocytes in vivo during the asymptomatic phase of infection. Retrovirology. 2012;9(1):12. doi: 10.1186/1742-4690-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SP, Ginder GD, Faller DV, Dover GH, Ikuta T, Witkowska HE, Cai SP, Vichinsky EP, Olivieri NF. A short-term trial of butyrate to stimulate fetal-globin-gene expression in the beta-globin disorders. N Engl J Med. 1993;328(2):81–86. doi: 10.1056/NEJM199301143280202. [DOI] [PubMed] [Google Scholar]
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323(5919):1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- Shen L, Siliciano RF. Viral reservoirs, residual viremia, and the potential of highly active antiretroviral therapy to eradicate HIV infection. J Allergy Clin Immunol. 2008;122(1):22–28. doi: 10.1016/j.jaci.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett BJ, McMonagle EL, Logan N, Spiller OB, Schneider P, Hosie MJ. Probing the interaction between feline immunodeficiency virus and CD134 by using the novel monoclonal antibody 7D6 and the CD134 (Ox40) ligand. J Virol. 2007;81(18):9665–9679. doi: 10.1128/JVI.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]