Abstract
The mouse tibial axial compression loading model has recently been described to allow simultaneous exploration of cortical and trabecular bone adaptation within the same loaded element. However, the model frequently induces cortical woven bone formation and has produced inconsistent results with regards to trabecular bone adaptation. The aim of this study was to investigate bone adaptation to incremental load magnitudes using the mouse tibial axial compression loading model, with the ultimate goal of revealing a load that simultaneously induced lamellar cortical and trabecular bone adaptation. Adult (16 week old) female C57BL/6 mice were randomly divided into three load magnitude groups (5, 7 and 9 N), and had their right tibia axially loaded using a continuous 2-Hz haversine waveform for 360 cycles/d, 3 d/wk for 4 consecutive weeks. In vivo peripheral quantitative computed tomography was used to longitudinally assess midshaft tibia cortical bone adaptation, while ex vivo micro-computed tomography and histomorphometry were used to assess both midshaft tibia cortical and proximal tibia trabecular bone adaptation. A dose response to loading magnitude was observed within cortical bone, with increasing load magnitude inducing increasing levels of lamellar cortical bone adaptation within the upper two thirds of the tibial diaphysis. Greatest cortical bone adaptation was observed at the midshaft where there was a 42% increase in estimated mechanical properties (polar moment of inertia) in the highest (9 N) load group. A dose response to load magnitude was not clearly evident within trabecular bone, with only the highest load (9 N) being able to induce measureable adaptation (31% increase in trabecular bone volume fraction at the proximal tibia). The ultimate finding was that a load of 9 N (engendering a tensile strain of 1,833 με on medial surface of the midshaft tibia) was able to simultaneously induce measurable lamellar cortical and trabecular bone adaptation when using the mouse tibial axial compression loading model in 16 week old female C57BL/6 mice. This finding will help plan future studies aimed at exploring simultaneous lamellar cortical and trabecular bone adaptation within the same loaded element.
Keywords: bone formation, bone structure, bone mass, exercise, mechanical loading
Introduction
Animal models wherein controlled loads are introduced to the skeleton have been instrumental in advancing understanding of the response of bone to mechanical stimuli. As a result of studies utilizing animal models, we now know bone preferentially responds to dynamic rather than static stimuli, only short durations of loading are required to initiate an adaptive response, and bone cells accommodate to unique mechanical loading environments [1]. With the sequencing of the mouse genome and subsequent generation of transgenic mice, attention has shifted to exploration of molecular pathways underlying the skeletal response to loading. For instance, recent work utilizing transgenic animal models has demonstrated important mechanotransductive roles for molecules within the Wnt signaling pathway, including low-density lipoprotein receptor-related protein 5 and sclerostin [2, 3].
The rodent ulna axial compression loading model has evolved as a useful model to investigate the response of bone to mechanical loading [4, 5]. It allows controlled mechanical loads to be introduced both non-invasively and unilaterally, enabling bone responses to be explored in the absence of trauma and with the contralateral side serving as an internal control site. These features represent an advance on alternative loading models, such as jump training and treadmill running which load the skeleton bilaterally, and the rodent 4-point loading model which causes traumatic periosteal woven bone formation (see Robling et al. [6] for review). However, the ulna axial compression loading model does not readily allow investigation of trabecular bone responses to loading due to the principally cortical bone structure of the rodent ulna with virtual lack of trabeculae.
The mouse tibial axial compression loading model has recently been described to enable simultaneous exploration of cortical and trabecular bone adaptation to mechanical loading within a single bone [7, 8]. The model involves axially loading the tibia through a flexed knee and dorsiflexed ankle. Because of the natural curvature of the tibia, the compressive load applied to the bone is converted into bending with peak compressive strain engendered at the posterolateral border and peak tensile strain at the anteromedial surface of the tibial diaphysis [9–11]. Studies utilizing the model have generally demonstrated a loading benefit on trabecular bone volume fraction (bone volume [BV]/total volume [TV]) within the proximal tibia [7, 8, 10, 12–22]. However, BV/TV decreased in some studies depending on experimental conditions [7, 9, 23, 24], and a dose response to load magnitude on BV/TV within the proximal tibia has yet to be clearly demonstrated [9, 16–18]. In terms of cortical bone, previous work using the mouse tibial axial compression loading model has demonstrated a clear dose response to load magnitude at the tibial diaphysis [7, 9, 16, 18]; however, woven bone formation on the periosteal surface is often evident [9, 10, 17, 18, 19–22, 25]. As woven bone apposition is considered a pathological response to excessive load and subsequent damage accumulation [26], its frequent observation limits the ability of the mouse tibial axial compression loading model to allow simultaneous exploration of non-pathological (i.e. lamellar) cortical and trabecular bone adaptation to mechanical loading.
To further understanding of the mouse tibial axial compression loading model, the current study aimed to investigate combined cortical and trabecular bone adaptation to incremental load magnitudes. The ultimate goal was to reveal a load magnitude that simultaneously induced lamellar cortical and trabecular bone adaptation.
Methods
Animals
Forty female C57BL/6 mice were purchased (Jackson Laboratories, Bar Harbor, ME) and acclimated until 16 weeks of age. Animals were housed under standardized conditions with ad libitum access to standard mouse chow and water. All procedures were performed with prior approval from the Institutional Animal Care and Use Committee of Indiana University.
Strain gauge measurement
Four mice were randomly selected for a load-strain calibration study. The right hindlimbs were harvested after euthanasia and stored at −20°C. Limbs were allowed to warm to room temperature over several hours on the day of strain gauge measurement and the medial surface of the mid-diaphysis was minimally exposed. A single element strain gauge (EA-06-015DJ-120; Measurements Group, Inc., Raleigh, NC) was bonded with cyanoacrylate (M-Bond 200; Measurements Group, Inc., Raleigh, NC) to the middle of the medial surface of the midshaft tibia. The leg was axially loaded at a frequency of 2 Hz and peak loads of 3, 5, 7 and 9 N using the same loading system as used for experimentation (see In vivo axial tibial loading). The strain gauge voltage signal was routed through a signal conditioning amplifier (Model 2210; Measurements Group, Inc., Raleigh, NC), and the peak-to-peak voltage measured on a digital oscilloscope. Voltage was converted to strain using a calibration factor derived from measured and calculated (using beam theory) strains collected using an aluminum cantilever.
In vivo axial tibial loading
Thirty-six mice were randomly divided into three load magnitude groups—5, 7 and 9 N. The right leg was fixed between molded knee and foot cups on a computer-controlled electromagnetic mechanical actuator (Enduratec ELF 3200; Bose Corporation, Eden Prairie, MN), with the animal under isoflurane anesthesia (Abbott Laboratories, North Chicago, IL). The tibia was axially loaded across a near fully flexed knee and dorsiflexed ankle. Loading was applied with a continuous 2-Hz haversine waveform for 360 cycles/d, 3 d/wk for 4 consecutive weeks. Left legs were not loaded, with left tibiae serving as internal controls. A recent study using the tibial axial compression loading model found loading effects were isolated to loaded bones [19]. Normal cage activity was allowed between loading sessions.
In vivo pQCT
Cortical bone adaptation to mechanical loading was assessed in vivo using peripheral quantitative computed tomography (Stratec XCT Research SA+; Stratec Medizintechnik GmbH, Pforzheim, Germany). Animals were positioned on a custom scanning platform at baseline and following the 4 wk loading regime, with the animal under isoflurane anesthesia (Abbott Laboratories, North Chicago, IL). Each leg was centered in the machine gantry and a scout scan of the tibia performed for tomographic scan localization. A tomographic scan was performed at the midshaft tibia using a 0.46-mm collimation and 70 μm voxel size. This voxel size is relatively large compared to the cortical thickness of the mouse tibial midshaft increasing the potential for partial volume effects. However, good agreement has previously been shown between pQCT, micro-computerized tomography (μCT) and histological measures of cortical bone properties in mice [27]. Analyses were restricted to cortical bone using contour mode 1 with a threshold of 400 mg/cm3 within the Stratec software (version 6.20C; Stratec Medizintechnik GmbH, Pforzheim, Germany). Total bone mineral content (Tt.BMC; mg/cm), total bone area (Tt.Ar; mm2), and cortical area (Ct.Ar; mm2) were recorded for each bone, and the minimum (IMIN; mm4) and maximum second moments of area (IMAX; mm4) derived according to Gere and Timoshenko (18). Medullary area (Me.Ar; mm2) was derived as Tt.Ar minus Ct.Ar.
Ex vivo μCT
Animals were euthanized following the 4 wk loading regime, and the right and left tibias dissected free and placed in 10% neutral buffered formalin for 48 hours before being stored in 70% alcohol. A desktop micro-computerized tomography machine (μCT-20; Scanco Medical AG, Auenring, Switzerland) scanning with an isotropic voxel size of 9 μm was used to assess cortical bone geometry at 1 mm increments along the entire tibial diaphysis. Tomographic images were imported into ImageJ (National Institutes of Health, MD) wherein the polar moment of inertia (IP) was derived as the sum of the IMAX and IMIN measurements, with IMAX and IMIN being determined using standard and custom macros. The same desktop micro-computerized tomography machine was also used to assess trabecular bone properties within the proximal tibial metaphysis. 122 slices were acquired distal to the proximal tibial growth plate using a 13 μm voxel size. Images were reconstructed using a standard convolution-back projection procedure with a Shepp-Logan filter at a threshold of 275, and trabecular BV/TV (%), number (Tb.N; mm−1), thickness (Tb.Th; mm) and separation (Tb.Sp; mm) acquired.
Histomorphometry
Alizarin (15 mg/kg; Sigma Chemical Co., St. Louis, MO) was given 27 days, and calcein (10 mg/kg; Sigma Chemical Co., St. Louis, MO) was given 10 days and 6 days before euthanasia by intraperitoneal injection to permit determination of bone formation rates. The tibiae were harvested and fixed in 10% neutral buffer formalin for 48 hours before being stored in 70% ethanol. The bones were embedded undecalcified in 99% methyl-methacrylate with 3% dibutyl phthalate (Sigma-Aldrich, St. Louis, MO). Transverse thick (40–50 μm) sections were removed from the tibial midshaft using a diamond-embedded wire saw (Histo-saw; Delaware Diamond Knives), and mounted unstained to assess periosteal bone formation rate. Frontal plane thin (4 μm) sections of the proximal tibia were taken using a microtome (Reichert-Jung 2050; Reichert-Jung, Heidelberg, Germany), and mounted either unstained to enable determination of trabecular bone formation rate or stained with tartrate-resistant acid phosphatase and counterstained with hematoxylin (Sigma-Aldrich, Kit #387A-1KT, St. Louis, MO) to allow identification of trabecular osteoclasts.
Sections were montaged using Image-Pro Plus (Version 7.0; Media Cybernetics, Inc., Bethesda, MD) on a Leica DMI6000 inverted microscope (Leica Mikrosysteme Vertrieb GmbH, Wetzlar, Germany) and stored digitally. Dynamic parameters were measured from the unstained midshaft and proximal tibia sections, and included single-label perimeter (sL.Pm), double-label area (dL.Ar) and perimeter (dL.Pm), and interlabel width (Ir.L.Wi). Cortical bone dynamic parameters were measured using the alizarin and calcein labels (21 day interlabel period), whereas trabecular bone dynamic parameters were measured using the dual calcein labels (4 day interlabel period). The following were derived from the primary data: mineralizing surface (MS/BS=[1/2sL.Pm+dL.Pm]/B.Pm; %), mineral apposition rate (MAR=mean Ir.L.Wi/interlabel period; μm/d), and bone formation rate (BFR/BS=MARxMS/BSx3.65; μm3/μm2/yr). The region of interest within the proximal tibia consisted of a 1 mm2 box positioned 1.0 mm distal from the growth plate within the secondary spongiosa. Bone resorption was determined from stained sections of the proximal tibia by counting the number of bone-adherent, multinucleate, tartrate-resistant acid phosphatase positive cells (osteoclasts) within 1 mm2 of the secondary spongiosa and normalizing to bone surface (Oc.N/BS).
Statistical Analysis
Statistical analyses were performed with the Statistical Package for Social Sciences for Windows (Version 19.0; SPSS Inc., Chicago, USA), with a level of significance set at 0.05 for all tests. The effect of loading (loaded vs. nonloaded) within each load magnitude group was assessed by paired t-tests. The effect of load magnitude (5 N vs. 7 N vs. 9 N) on the loaded side was assessed using one-way analyses of covariance (ANCOVA) followed by Bonferroni pairwise comparisons, with the nonloaded side serving as the covariate.
Results
One mouse in each of the 5 N and 7 N groups experienced a tibial fracture on the first day of loading due to malfunction with the loading machine. One mouse from the 9 N group experienced a tibial fracture during the first week of loading, presumably due to mechanical overload. Each of these mice were immediately euthanized and their data excluded from the study.
Strain gauge data
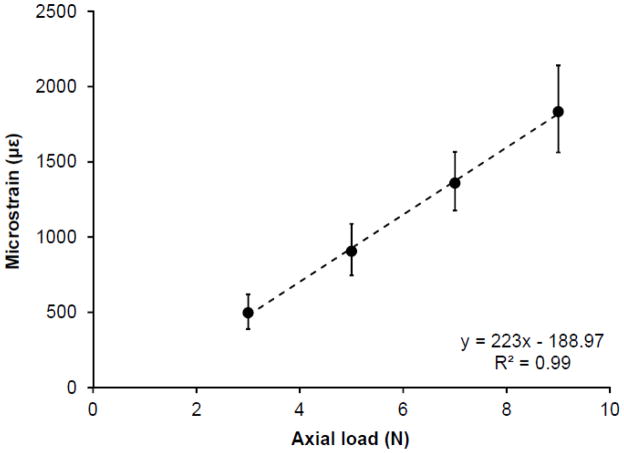
The results of the preliminary load-strain calibration are illustrated in Figure 1. Bone strain on the medial surface of the midshaft tibia demonstrated a linear increase in response to incremental externally applied loads (R2 = 0.99; P < 0.001), with 9 N inducing a tensile strain of 1,833 με (95% confidence interval [CI], 1,460 με to 2,206 με).
Fig. 1.
Strain engendered on the medial surface of the midshaft tibia in response to incremental external load magnitudes. Error bars indicate 95% confidence intervals.
Cortical bone adaptation
The effects of loading and load magnitude on in vivo adaptation of the cortical midshaft tibia are shown in Figure 2. The loaded hindlimb in all load magnitude groups had greater gains in Tt.BMC, Ct.Ar and IMIN than in the contralateral non-loaded hindlimb (all P < 0.05), with loading at 9 N inducing relative gains of 23.1% (95% CI, 17.6% to 28.7%), 19.7% (95% CI, 11.8% to 27.6%) and 27.5% (95% CI, 14.7% to 40.4%) in Tt.BMC, Ct.Ar and IMIN, respectively. The loaded hindlimb in the 5 N and 9 N groups also had greater gains in Tt.Ar and IMAX than in the non-loaded hindlimb (all P < 0.05), with loading at 9 N inducing relative gains of 13.9% (95% CI, 7.1% to 20.7%) and 42.2% (95% CI, 20.6% to 63.9%) in Tt.Ar and IMAX, respectively. There was no effect of loading on Me.Ar in any load magnitude group (all P > 0.05).
Fig. 2.
Effect of loading and load magnitude on percent change in tibial midshaft: A) total bone mineral content [Tt.BMC]; B) total bone area [Tt.Ar]; C) cortical area [Ct.Ar]; D) medullary area [Me.Ar]; E) minimum second moment of area [IMIN], and; F) maximum second moment of area [IMAX]. Bars represent mean ± SD. *indicates significant loading effect (nonloaded vs. loaded) within respective load magnitude group, as assessed by paired t-test. †indicates significant difference in loaded tibiae between respective load magnitude groups, as assessed by one-way analyses of covariance (with the nonloaded side serving as the covariate) followed by Bonferroni pairwise comparisons.
There was a dose-response to external loading magnitude at the midshaft tibia, with higher loads inducing greater adaptation. The 9 N group had greater gains in all properties of the loaded midshaft tibia compared to the 5 N group (all P < 0.05) (Fig. 2). Loading at 9 N also induced greater gains in Tt.BMC, Tt.Ar and IMAX of the loaded midshaft tibia compared to 7 N (all P < 0.05). There were no differences in midshaft tibia adaptation between loading at 5 N and 7 N (all P >0.05).
Cortical bone adaptation (as indicated by IP) was greatest at the midshaft tibia in all load magnitude groups; however, loading induced adaptation both proximal and distal to the midshaft (Fig. 3). Adaptation in the 9 N group was significantly greater in the proximal two-thirds and half of the bone than the 5 N and 7 N groups, respectively. There were no differences in cortical bone adaptation along the bone length between the 5 N and 7 N groups (all P > 0.05).
Fig. 3.
Effect of loading and load magnitude on percent difference in polar moment of inertia (IP) between loaded and nonloaded tibiae at 1 mm increments along the bone length. Loading and loading magnitude effects were predominantly in the proximal two-thirds of the bone, and greatest at the midshaft and in the 9 N load group. Error bars indicate 95% confidence intervals, with bars not crossing zero (x-axis) indicating a significant loading (loaded vs. nonloaded) effect within the respective load magnitude group. Symbols indicate that the percent difference (loaded vs. nonloaded) in IP in the 9 N differed significantly from in the 5 N (*) and 7 N (†) groups, as assessed by one-way analyses of covariance followed by Bonferroni pairwise comparisons.
Histomorphometrically, there was no evidence of woven bone formation in any loaded tibia (Fig. 4). The loaded hindlimb in all load magnitude groups had higher periosteal MS/BS at the midshaft tibia compared to the contralateral nonloaded hindlimb (all P < 0.05) (Fig. 5), with loading at 9 N inducing relative gains of 40.4% (95% CI, 31.1% to 49.7%). The loaded hindlimb in the 5 N and 9 N groups also had greater gains in periosteal MAR and BFR/BS than in the nonloaded hindlimb (all P < 0.05), with loading at 9 N increasing MAR and BFR/BS by 4- and 7-fold respectively. There was a load magnitude response at the midshaft tibia with the 9 N group having greater gains in periosteal MS/BS, MAR and BFR/BS compared to both the 5 N and 7 N groups (all P < 0.05) (Fig. 5). There were no differences in midshaft tibia adaptation between the 5 N and 7 N groups (all P >0.05).
Fig. 4.
Representative histological images of the midshaft tibia from nonloaded and loaded tibiae in each load magnitude group under: A-B) fluorescent and C) polarized light. A) Note the greater labeling in the loaded tibia, with B) uniform double labeling [lamellar bone formation] and absence of diffuse labeling [woven bone formation]. C) Lamellar bone formation in the region of alizarin and calcein labeling (white arrows) was confirmed under polarized light.
Fig. 5.
Effect of loading and load magnitude on tibial midshaft periosteal: A) mineralizing surface [MS/BS]; B) mineral apposition rate [MAR], and; C) bone formation rate [BFR/BS]. Bars represent mean ± SD. *indicates significant loading effect (nonloaded vs. loaded) within respective load magnitude group, as assessed by paired t-test. †indicates significant difference in loaded tibiae between respective load magnitudes, as assessed by one-way analyses of covariance (with the nonloaded side serving as the covariate) followed by Bonferroni pairwise comparisons.
Trabecular bone adaptation
The effects of loading and load magnitude on trabecular bone adaptation within the proximal tibia are shown in Figure 6. The loaded hindlimb in the 9 N group had 30.8% (95% CI, 12.9% to 48.7%) and 23.8% (95% CI, 16.9% to 30.8%) higher BV/TV and Tb.Th compared to the contralateral nonloaded hindlimb, respectively (all P < 0.05). There were no loading effects on any trabecular bone properties in the 5 N and 7 N groups, or on Tb.N or Tb.Sp in the 9 N group (all P > 0.05). Comparing across load magnitudes (and using the nonloaded hindlimb as a covariate), the 9 N group had 21.9% (95% CI, 3.95% to 39.8%) higher BV/TV within the proximal tibia compared to the 5 N group, and 23.0% (95% CI, 3.95% to 39.8%) and 15.3% (95% CI, 7.69 % to 23.1%) higher Tb.Th than both the 5 N and 7 N groups, respectively (all P <0.05) (Fig. 6).
Fig. 6.
Effect of loading and load magnitude on: A) representative (150 μm thick) frontal plane three-dimensional reconstructions of trabecular architecture within the proximal tibia of the highest [9N] load group; B) trabecular bone volume fraction [BV/TV]; C) trabecular thickness [Tb.Th]; D) trabecular number [Tb.N], and; E) trabecular separation [Tb.Sp]. In A), note the increased BV/TV and Tb.Th in the loaded proximal tibia. Bars in (B–E) represent mean ± SD. *indicates significant loading effect (nonloaded vs. loaded) within respective load magnitude group, as assessed by paired t-test. †indicates significant difference in loaded tibiae between respective load magnitudes, as assessed by one-way analyses of covariance (with the nonloaded side serving as the covariate) followed by Bonferroni pairwise comparisons.
Histomorphometrically, there was no effect of load on trabecular bone formation (MS/BS, MAR, BFR/BS) or resorption (Oc.N/BS) indexes within the proximal tibia of the loaded hindlimb when compared to the contralateral nonloaded hindlimb (Fig. 7). Comparing across load magnitudes (and using the nonloaded hindlimb as a covariate), the loaded hindlimb in the 9 N group had 19.3% (95% CI,.8795 % to 37.8%) higher trabecular MAR within the proximal tibia compared to the 5 N group (P = 0.038) (Fig. 7). There was no effect of load magnitude on trabecular MS/BS, BFR/BS or Oc.N/BS (all P > 0.05).
Fig. 7.
Effect of loading and load magnitude on proximal tibia trabecular: A) mineralizing surface [MS/BS]; B) mineral apposition rate [MAR]; C) bone formation rate [BFR/BS], and D) osteoclast number [N.Oc/BS]. Bars represent mean ± SD. †indicates significant difference in loaded tibiae between respective load magnitudes, as assessed by one-way analyses of covariance (with the nonloaded side serving as the covariate) followed by Bonferroni pairwise comparisons.
Discussion
The current study investigated the influence of load magnitude on cortical and trabecular bone adaptation using the mouse tibial axial compression loading model. A dose response to loading magnitude within cortical bone was observed, with increasing load magnitude inducing increasing levels of cortical bone adaptation within the upper two thirds of the tibial diaphysis. The greatest cortical adaptation was observed at the midshaft tibia where there was a 42% increase in estimated mechanical properties (IP) over the 4 week loading period in the highest (9 N) load group. The increase in estimated mechanical properties resulted from an increase in periosteal lamellar bone apposition. A dose response to load magnitude was not clearly evident within trabecular bone, with only the highest load (9 N) being able to induce measureable adaptation (31% increase in BV/TV at the proximal tibia). The ultimate finding of the study was that a load of 9 N (engendering a tensile strain of 1,833 με on medial surface of the midshaft tibia) was able to simultaneously induce lamellar cortical and trabecular bone adaptation when using the mouse tibial axial compression loading model in 16 week old female C57BL/6 mice.
The observed dose response of cortical bone to loading magnitude is consistent with the vast body of evidence provided from alternative loading models, as well as previous studies using the mouse tibial axial compression loading model [7, 9, 16, 18]. A maximum load of 9 N was chosen for the current investigation as preliminary work in our laboratory using the mouse tibial axial compression loading model demonstrated loads in excess of 10 N resulted in periosteal woven bone formation and fractures (unpublished data). Also, representative microCT and histology images in previously published studies using the mouse tibial axial compression loading model are suggestive of woven bone formation when higher load magnitudes (>10 N) are introduced [10, 17–22, 25]
When comparing skeletal responses to mechanical load across studies it is important to consider the tissue level strain engendered. Strain provides an indication of the tissue level mechanical stimulus, and varies for a given external load magnitude as a result of alterations in bone quality (which includes bone structure, geometry and composition). Cortical bone strains in the current study were on the high end of those previously reported using the mouse tibial axial compression loading model. For instance, loading in the current study engendered a tensile strain of 223 με/N on the medial surface of the midshaft tibia, which compares to strains in the range of 77 με/N to 250 με/N [7, 8, 13, 16, 18, 24, 28, 29] reported in previous studies. Possible explanations for the relatively high strains per given external load in the current study are unknown, but may include the age, sex and genotype of animals being investigated, strain gauge measurements being performed ex vivo, strain gauge position relative to the bending axis, and position of the knee joint during loading (see discussion below), to name a few. Irrespective of the reason for the difference in measured strain per unit load between studies, the fact remains that woven bone was not induced in the current study at the loads introduced and subsequent strains engendered.
Cortical bone adaptation in the current study occurred in the proximal two-thirds of the diaphysis and principally at the midshaft tibia. This pattern of adaptation differs from that reported by van der Meulen and colleagues [8, 30]. The latter investigators loaded 10 week old male C57BL/6 mice for up to 6 weeks using the mouse tibial axial compression loading model to induce adaptation in the proximal half of the tibial diaphysis (as indicated by ex vivo micro-CT measures of IMAX and IMIN) with the greatest adaptation proximally, as opposed to at the midshaft. A possible explanation for the disparity between studies in the location of maximal adaptation is the position of the knee joint during loading which may influence strain distribution within the tibia. The knee was positioned in near full flexion during loading in the current study, as opposed to 90° flexion in the studies by van der Meulen and colleagues. The greater knee flexion in the current study potentially moved the femoral condyles more caudally on the tibial plateau increasing the bending lever arm and promoting conversion of the axial compressive load into bending at the tibial midshaft.
Axial compressive loading of the tibia induced trabecular bone adaptation within the proximal tibia, with BV/TV increasing by 31% in the highest load (9 N) group over the 4 week loading period. This magnitude of change is comparable to published studies; however, a wide range has previously been reported. For instance, some studies have reported a loss of BV/TV when using the tibial axial compression loading model [7, 9, 18, 23, 24], whereas others loading for as little as 2 weeks have reported BV/TV increases of between 11% and 81% [8, 13, 16–22, 25], and changes of 15% to 68% when loading for as long as 6 weeks [8, 30]. Factors potentially contributing to these large ranges include the age, sex and genotype of animals being investigated, loading parameters (such as the loading waveform shape, load magnitude, inclusion of rest periods between cycles, duration of loading, number of loading cycles), and region analyzed within the proximal tibia.
BV/TV increased in the current study as a result of new bone being deposited on pre-existing trabeculae (as indicated by an increase in Tb.Th), as opposed to the construction of new trabeculae (as indicated by the absence of a load effect on Tb.N) or a decrease in bone resorption (as indicated by the absence of a load effect on Oc.N/BS). A concomitant increase in trabecular bone formation was not measured histomorphometrically; however, this may reflect a decline in mechanoresponsiveness with continued loading [31] and our measurement of trabecular bone formation indexes towards the end of the loading program. The absence of a significant histomorphometrical finding may also be due to the greater variance associated with histomorphometric measures of trabecular bone and consequent reduction in statistical power.
There was a lack of a clear dose response of trabecular bone to load magnitude in the current study, with trabecular bone adaptation only being detected in the highest load (9 N) group. Other investigators using a variety of loading models including the mouse tibial axial loading model have demonstrated progressive trabecular bone adaptation to increasing loads [16–18, 32, 33]. The inability to demonstrate trabecular adaptation in the lower load groups (5 N and 7 N) in the current study may relate to the inability of these lower loads to surpass the osteogenic threshold at the site assessed or the induction of lameness with the mouse tibial axial loading model. In terms of the latter, there is a possibility that the model causes some degree of lameness-induced unloading which may have obscured any trabecular bone benefits in the lower load groups. We did not assess lameness following tibial axial loading through the quantification of ground reaction forces; however, lameness may explain why some studies using this model have observed a loss of BV/TV [7, 9, 23], and needs further exploration considering axial loading of the mouse leg through a near fully flexed knee has been associated with knee joint degeneration [34].
Other factors beyond the induction of temporary lameness need to be considered when translating these data. Principally, the current data are limited to the loading parameters and animals investigated. Different levels of adaptation may be induced in mice of a different age, sex or genotype, or when loading with a different loading waveform, duration or frequency. Similarly, the current data are limited to studies loading through a near fully flexed knee. A final factor to consider when interpreting the current data is the lack of quantification of the loading induced within the proximal tibia. We assume the increasing load magnitudes resulted in incremental loading within the proximal tibia; however, this was not quantified through direct measurement or finite element modeling. This will be necessary in future studies comparing trabecular bone responsiveness to mechanical loading between mice with differing baseline skeletal phenotypes.
In summary, the current data indicate that tibial axial loading is a useful model to explore combined cortical and trabecular bone adaption in mice, and that a load of 9 N (engendering a tensile strain of 1,833 με on medial surface of the midshaft tibia) is able to simultaneously induce lamellar cortical and trabecular bone adaptation in 16 week old female C57BL/6 mice.
Highlights.
Tibial axial compression induced combined cortical and trabecular bone adaptation
Cortical bone responded to increasing load in a dose response manner
Loading induced lamellar cortical bone formation, with greatest cortical adaptation observed at the midshaft tibia
A dose response to load magnitude was not clear in trabecular bone
Only the highest load group had measurable trabecular bone adaptation
Acknowledgments
The authors thank Keith W. Condon for assistance with tissue processing. This work was supported by the National Institutes of Health (R15 AR056858 [S.J.W.] and K01 AR054408 [R.K.F.]).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Turner CH. Three rules for bone adaptation to mechanical stimuli. Bone. 1998;23:399–407. doi: 10.1016/s8756-3282(98)00118-5. [DOI] [PubMed] [Google Scholar]
- 2.Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281:23698–711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 3.Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI, Bellido T. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50:209–17. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KC, Maxwell A, Lanyon LE. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone. 2002;31:407–12. doi: 10.1016/s8756-3282(02)00842-6. [DOI] [PubMed] [Google Scholar]
- 5.Torrance AG, Mosley JR, Suswillo RF, Lanyon LE. Noninvasive loading of the rat ulna in vivo induces a strain-related modeling response uncomplicated by trauma or periostal pressure. Calcif Tissue Int. 1994;54:241–7. doi: 10.1007/BF00301686. [DOI] [PubMed] [Google Scholar]
- 6.Robling AG, Burr DB, Turner CH. Skeletal loading in animals. J Musculoskelet Neuronal Interact. 2001;1:249–62. [PubMed] [Google Scholar]
- 7.De Souza RL, Matsuura M, Eckstein F, Rawlinson SC, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37:810–8. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36:1030–8. doi: 10.1016/j.bone.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. J Bone Miner Res. 2010;25:2006–15. doi: 10.1002/jbmr.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE, Price JS. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012;23:1225–34. doi: 10.1007/s00198-011-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stadelmann VA, Hocke J, Verhelle J, Forster V, Merlini F, Terrier A, Pioletti DP. 3D strain map of axially loaded mouse tibia: a numerical analysis validated by experimental measurements. Comput Methods Biomech Biomed Engin. 2009;12:95–100. doi: 10.1080/10255840903077287. [DOI] [PubMed] [Google Scholar]
- 12.Bonnet N, Standley KN, Bianchi EN, Stadelmann V, Foti M, Conway SJ, Ferrari SL. The matricellular protein periostin is required for sost inhibition and the anabolic response to mechanical loading and physical activity. Journal of Biological Chemistry. 2009;284:35939. doi: 10.1074/jbc.M109.060335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch ME, Main RP, Xu Q, Walsh DJ, Schaffler MB, Wright TM, van der Meulen MC. Cancellous bone adaptation to tibial compression is not sex dependent in growing mice. J Appl Physiol. 2010;109:685–91. doi: 10.1152/japplphysiol.00210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marenzana M, De Souza RL, Chenu C. Blockade of beta-adrenergic signaling does not influence the bone mechano-adaptive response in mice. Bone. 2007;41:206–15. doi: 10.1016/j.bone.2007.04.184. [DOI] [PubMed] [Google Scholar]
- 15.Pierroz DD, Bonnet N, Bianchi EN, Bouxsein ML, Baldock PA, Rizzoli R, Ferrari SL. Deletion of beta-adrenergic receptor 1, 2, or both leads to different bone phenotypes and response to mechanical stimulation. J Bone Miner Res. 2012;27:1252–62. doi: 10.1002/jbmr.1594. [DOI] [PubMed] [Google Scholar]
- 16.Saxon LK, Jackson BF, Sugiyama T, Lanyon LE, Price JS. Analysis of multiple bone responses to graded strains above functional levels, and to disuse, in mice in vivo show that the human Lrp5 G171V High Bone Mass mutation increases the osteogenic response to loading but that lack of Lrp5 activity reduces it. Bone. 2011;49:184–93. doi: 10.1016/j.bone.2011.03.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxon LK, Galea G, Meakin L, Price J, Lanyon LE. Estrogen receptors alpha and beta have different gender-dependent effects on the adaptive responses to load bearing in cancellous and cortical bone. Endocrinology. 2012;153:2254–66. doi: 10.1210/en.2011-1977. [DOI] [PubMed] [Google Scholar]
- 18.Sugiyama T, Meakin LB, Browne WJ, Galea GL, Price JS, Lanyon LE. Bones’ adaptive response to mechanical loading is essentially linear between the low strains associated with disuse and the high strains associated with the lamellar/woven bone transition. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiyama T, Price JS, Lanyon LE. Functional adaptation to mechanical loading in both cortical and cancellous bone is controlled locally and is confined to the loaded bones. Bone. 2010;46:314–21. doi: 10.1016/j.bone.2009.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugiyama T, Meakin LB, Galea GL, Jackson BF, Lanyon LE, Ebetino FH, Russell RG, Price JS. Risedronate does not reduce mechanical loading-related increases in cortical and trabecular bone mass in mice. Bone. 2011;49:133–9. doi: 10.1016/j.bone.2011.03.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugiyama T, Saxon LK, Zaman G, Moustafa A, Sunters A, Price JS, Lanyon LE. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1–34) on trabecular and cortical bone in mice. Bone. 2008;43:238–48. doi: 10.1016/j.bone.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Sugiyama T, Meakin LB, Galea GL, Lanyon LE, Price JS. The cyclooxygenase-2 selective inhibitor NS-398 does not influence trabecular or cortical bone gain resulting from repeated mechanical loading in female mice. Osteoporos Int. 2012 doi: 10.1007/s00198-012-1922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva MJ, Brodt MD, Lynch MA, Stephens AL, Wood DJ, Civitelli R. Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice. PLoS One. 2012;7:e34980. doi: 10.1371/journal.pone.0034980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimston SK, Watkins MP, Brodt MD, Silva MJ, Civitelli R. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin43 deficient mice. PLoS One. 2012;7:e44222. doi: 10.1371/journal.pone.0044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch ME, Main RP, Xu Q, Schmicker TL, Schaffler MB, Wright TM, van der Meulen MC. Tibial compression is anabolic in the adult mouse skeleton despite reduced responsiveness with aging. Bone. 2011;49:439–46. doi: 10.1016/j.bone.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch JA, Silva MJ. In vivo static creep loading of the rat forelimb reduces ulnar structural properties at time-zero and induces damage-dependent woven bone formation. Bone. 2008;42:942–949. doi: 10.1016/j.bone.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt C, Priemel M, Kohler T, Weusten A, Muller R, Amling M, Eckstein F. Precision and accuracy of peripheral quantitative computed tomography (pQCT) in the mouse skeleton compared with histology and microcomputed tomography (microCT) J Bone Miner Res. 2003;18:1486–96. doi: 10.1359/jbmr.2003.18.8.1486. [DOI] [PubMed] [Google Scholar]
- 28.Stadelmann VA, Bonnet N, Pioletti DP. Combined effects of zoledronate and mechanical stimulation on bone adaptation in an axially loaded mouse tibia. Clin Biomech (Bristol, Avon) 2011;26:101–5. doi: 10.1016/j.clinbiomech.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Niziolek PJ, Warman ML, Robling AG. Mechanotransduction in bone tissue: The A214V and G171V mutations in Lrp5 enhance load-induced osteogenesis in a surface-selective manner. Bone. 2012;51:459–65. doi: 10.1016/j.bone.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Bone mass is preserved and cancellous architecture altered due to cyclic loading of the mouse tibia after orchidectomy. J Bone Miner Res. 2008;23:663–71. doi: 10.1359/JBMR.080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saxon LK, Robling AG, Alam I, Turner CH. Mechanosensitivity of the rat skeleton decreases after a long period of loading, but is improved with time off. Bone. 2005;36:454–64. doi: 10.1016/j.bone.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Webster D, Wasserman E, Ehrbar M, Weber F, Bab I, Muller R. Mechanical loading of mouse caudal vertebrae increases trabecular and cortical bone mass-dependence on dose and genotype. Biomech Model Mechanobiol. 2010;9:737–47. doi: 10.1007/s10237-010-0210-1. [DOI] [PubMed] [Google Scholar]
- 33.Chow JW, Jagger CJ, Chambers TJ. Characterization of osteogenic response to mechanical stimulation in cancellous bone of rat caudal vertebrae. Am J Physiol. 1993;265:E340–7. doi: 10.1152/ajpendo.1993.265.2.E340. [DOI] [PubMed] [Google Scholar]
- 34.Poulet B, Hamilton RW, Shefelbine S, Pitsillides AA. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 2011;63:137–47. doi: 10.1002/art.27765. [DOI] [PubMed] [Google Scholar]