Abstract
Background
Evidence suggests early events might modify adult breast cancer risk and many adolescents learn of familial and genetic risks for breast cancer. Little is known about how adolescent girls understand and respond to breast cancer risk.
Methods
Semi-structured interviews with 11-19 year-old girls at high-risk and population-risk for breast cancer evaluated knowledge and perceptions of breast cancer risk and risk modification. Framework analysis and descriptive statistics were utilized to analyze open-ended responses. Risk group and age differences were evaluated by Fisher’s exact and McNemar’s tests.
Results
54 girls (86% of invited), 35 high-risk (65%) and 19 population-risk (35%) completed interviews. The most frequently reported risk for breast cancer was family history/hereditary predisposition (66%). Only 17% of girls were aware of BRCA1/2 genes. The majority (76%) of high-risk girls perceive themselves to be at increased risk for breast cancer, compared to 22% of population-risk girls (p=0.001). Half of girls reported that women can get breast cancer before 20 years old. The majority believe there are things women (70%) and girls (67%) can do to prevent breast cancer. Mother was the most frequently reported source of information for breast cancer among both high-risk (97%) and population-risk (89%) girls.
Conclusion
In this study, many high-risk girls perceive themselves to be at increased risk for breast cancer, and many girls believe that breast cancer can occur in teens. Yet, most girls believe there are things women and girls can do to prevent breast cancer. Research evaluating the impact of awareness and perceptions of breast cancer risk on psychosocial, health and risk behaviors is needed to develop strategies to optimize responses to cancer risk.
Keywords: Adolescents, Breast cancer, Breast cancer prevention, Cancer Risk Assessment, Perceived risk of cancer
INTRODUCTION
Currently, one in eight women will be diagnosed with invasive breast cancer[1]. Breast cancer risk is increased 2-4 fold among those with a family history, and 5-7 fold among BRCA1/2 mutation carriers[2]. BRCA1/2 testing can confirm a genetic risk for many women, and ongoing research evaluating other genetic contributors is expected to be relevant to the large population of breast cancer families without a known genetic alteration in their family[3, 4]. Currently, risk reduction options for high-risk women include prophylactic surgeries, heightened surveillance and/or chemoprevention. These interventions and BRCA1/2 testing are generally not recommended before 25 years old[5, 6].
There are, however, several reasons breast cancer risk is relevant in adolescence. Adolescence is a key period of carcinogenic vulnerability[7, 8] and there is growing research evaluating how early-life events (e.g. exposures, biologic changes) might modify risks for adult breast cancer [7, 9-11]. Additionally, many health and risk behaviors begin in, or become established in adolescence [12-16]. Equally important, the majority of offspring in high-risk families learn of familial and genetic risks for breast cancer at a young age[17-20]. Additionally, breast cancer media coverage, awareness campaigns, and educational programs increasingly target youth [21-23], reaching both high-risk and population-risk girls outside the context of their families[24]. Small studies suggest that adolescent girls in the general population and girls of mothers with breast cancer are aware of, and concerned about their risk [17, 25-33]. Additionally, they are susceptible to media reports, leading to misperceptions[24, 26, 27]. What high-risk and population-risk girls know and perceive of breast cancer risks, its impact on their psychosocial well-being and adoption of preventive health and risk behaviors and how these change over time remains unknown.
While several studies have evaluated psychosocial adjustment among daughters of breast cancer patients, few studies have directly examined knowledge and perceptions of breast cancer risk and few have included girls from high-risk families without a maternal breast cancer. This study aims to address these gaps via theoretically-informed semi-structured interviews with 11-19 year-old girls at high-risk and population-risk for breast cancer. Constructs from the Self-regulation Theory of Health Behavior (SRTHB) provided a framework for the interview questions [34-37]. The goals of this study were to evaluate adolescent girls’ representations of breast cancer, health behaviors to prevent or reduce risks for breast cancer, and the impact of these health behaviors, as well as to identify sources of information among girls from both breast cancer families (i.e. high-risk) and families without breast cancer (population-risk).
MATERIALS AND METHODS
Participants
Participants included 11-19 year-old girls and their mothers. Definitions of risk were selected to reflect both objective and “common-sense” representations of breast cancer risk, which the SRTHB posits has greater impact on performance of health behaviors than objective risk[12, 15, 34, 38, 39]. Girls were classified as high-risk if they: a) had a parent with a BRCA1/2 mutation OR b) at least one first-degree or second-degree relative with breast cancer. Although the numbers are small, qualitative categories of risk were also defined (see Table 1) for exploratory analyses. Risk was determined after recruitment on the basis of mother reported family history. Institutional Review Board approval was obtained. Girls were identified through mothers in the Fox Chase Cancer Center Risk Assessment registry and clinics, a friend cohort and a local community pediatric practice between June 2009 and August 2010. Mothers provided parental permission for daughter participation. Mothers and daughters provided verbal and written consent and assent for their respective participation. We used stratified purposeful sampling to identify the range of variation between risk groups and across the age span, to inform hypotheses for future studies[40]. We sought to enroll a minimum of 10-15 girls per risk group to achieve theoretical saturation for open-ended responses in both risk groups[41]. We recruited additional girls to achieve representation across all age groups.
Table 1. Participant characteristics.
| ALL (n=54) N (%) |
HR (n=35) N (%) |
PR (n=19) N (%) |
|
|---|---|---|---|
|
| |||
| Age | |||
| 11-13 YO | 13 (24) | 9 (26) | 4 (21) |
| 14-16 YO | 26 (48) | 16 (46) | 10 (52) |
| 17-19 YO | 15 (28) | 10 (29) | 5 (26) |
|
| |||
| Mother’s history | |||
| Mother has history of breast cancer | 11 (20) | 11 (31) | 0 |
| Mother has a BRCA1/2 mutation | 6 (11) | 6 (17) | 0 |
|
| |||
| High-risk subgroups | |||
| BRCA1/2+ mother with breast cancer | 4 (11) | ||
| BRCA1/2+ mother without breast cancer | 2 ( 6) | ||
| Mother* with breast cancer AND family history of breast cancer |
4 (11) | ||
| Mother* with breast cancer only (no family history) | 2 ( 6) | ||
| 2 or more second-degree relatives** with breast cancer | 6 (17) | ||
| 1 second-degree relative** with breast cancer | 17 (49) | ||
|
| |||
| Site | |||
| Fox Chase Cancer Center | 40 (74) | 31 (86) | 9 (50) |
| Community practice | 14 (26) | 5 (14) | 9 (50) |
|
| |||
| Number unique families | 53 | 35 | 18 |
BRCA1/2 negative or unknown (i.e. not tested for BRCA1/2)
grandparent or aunt/uncle
Survey Instrument
A semi-structured telephone interview for daughters and a brief mother survey were developed to evaluate SRTHB-informed constructs. We incorporated a qualitative approach, given that few studies have explored adolescent representations of breast cancer as a disease threat. The daughter interview included 35 structured (yes/no) questions and 36 open-ended questions reflecting the key SRTHB constructs. 1. Knowledge of the disease threat, including: risk factors for breast cancer for women in general and for themselves (open-ended), familial risks (structured), inheritance of breast cancer (structured) and knowledge of BRCA1/2 genes (structured). 2. Perceived risk of Breast Cancer, including: girls’ perception of their breast cancer risk compared to other girls their age (structured), the youngest age they, women in general and women in their family could get breast cancer (structured) and why this age (open ended). 3. Perceived controllability, including things women and girls their age can do to prevent breast cancer (open-ended). 4. Sources of information, including 12 possible sources of information (structured) and additional sources (open-ended). Mothers reported daughters’ maternal and paternal family history to classify girls as high-risk and population-risk.
Statistical Analyses
Open-ended responses were analyzed using Framework analysis[42-46]. Investigators first intensively reviewed participant responses, identifying recurrent themes through methods of constant comparison[43, 47, 48]. Investigators then reviewed a subsample of responses (30%) to develop a thematic framework. Two investigators (AB, LPM) independently assigned thematic codes to the subsample (inter-rater agreement 98%)[48]. Themes were compared, definitions agreed, emerging themes added and thematic charts/indexes created. Resulting codes were then applied to the remaining sample and refined to include new themes as they emerged. Responses could be coded for more than one theme and differences in code assignments were resolved through inter-coder discussion. Descriptive statistics were utilized to characterize and describe patterns in the structured and coded responses[49, 50]. Exploratory analyses were conducted to explore differences by risk (high-risk v. population-risk and high-risk subgroups) and age (11-13, 14-16, 17-19 years old) group using Fisher’s exact tests and McNemar’s tests. As suggested by others evaluating small age ranges among adolescents from early to late adolescence can be useful to identify subtle adolescent developmental transitions in early, middle and late adolescence[51].
RESULTS
Participants
Fifty-four girls (86% of invited) completed interviews. Sixty-five percent were high-risk. High-risk subgroups and other participant characteristic are further described in Table 1. Interviews conducted by telephone lasted on average 57 minutes (range 35-90).
Knowledge of risk factors for breast cancer
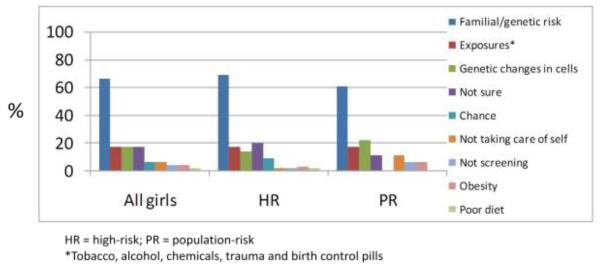
In response to an open-ended question inquiring why women, in general get breast cancer, the most frequently reported reason was family history or genetic predisposition (66%) (Fig 1a). For example, “It is mostly genetic. If you have a history you are more susceptible” (16 year-old high-risk girl). Other reasons included exposures, somatic genetic changes (i.e. in contrast to inherited genetic changes), chance, not taking care of oneself, obesity and poor diet. In addition to alcohol and tobacco, environmental exposures, such as “Pollution and radiation in our world today” (16 year-old population-risk girl), and “things in the water bottles” (16 year-old high-risk girl) were perceived as important. Of note, 17% of girls reported they did not know why women get breast cancer. Reported risk factors did not differ significantly by risk group or qualitative high-risk categories. They also did not differ by age with the exception of younger girls reporting “not sure” more frequently than older girls (31% among 11-13 year-old v. 13% among girls >13 year-old), although this was not statistically significant.
Fig 1a. Reasons women get breast cancer (n=53).
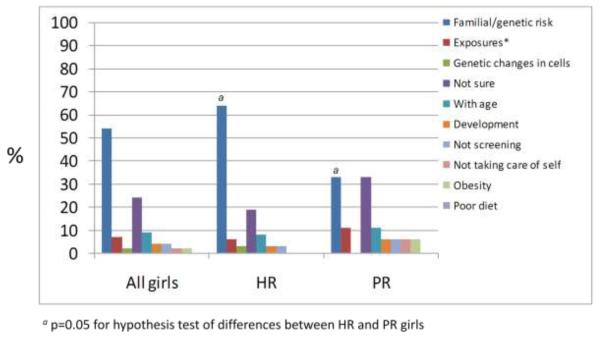
In response to a second question asking about risk factors for breast cancer for themselves, family history or genetic predisposition was again the most frequently reported theme (Fig 1b), which was reported significantly more frequently among high-risk girls (p=0.05) and did not appear to differ by high-risk subgroups. “Not sure” was the second most frequent response. Population-risk girls reporting family history or genetics as a reason they might get breast cancer frequently mentioned, in open query, other (often female) cancers in the family, or third-degree or more remote family members with a history of breast cancer. Risks factors girls reported for themselves did not differ by age.
Fig 1b. Reasons I might get breast cancer when I am an adult (n=54).
In response to close-ended questions, the majority of girls (96%) reported that a family history increases a person’s risk of developing breast cancer, which was significantly higher than the percentage of girls (83%) who reported that a family history of cancer, in general, increases a person’s risk for developing other cancers (p=0.025). Responses did not differ significantly by age or risk group. Although the majority (98%) of girls reported that one can be at increased risk if their mother or mother’s relatives have breast cancer, fewer (85%) reported an individual’s risk was increased if their father or father’s relatives have breast cancer. The majority of high-risk (83%) and population-risk girls (84%) reported that they had never heard of BRCA1/2 genes. Knowledge of BRCA1/2 genes was more frequent among girls >13 years old (8% girls 11-13 years old v. 32% girls >13 years old), although the difference was not statistically significant. The mean age of daughters of mothers who had had genetic testing (15.3 year-old, SD 2.14) did not significantly differ from that of daughters of mothers who had not had genetic testing (15.1year-old, SD 1.9), p = 0.69 by T-test. Daughters of mothers who had completed BRCA1/2 testing were more likely to report knowledge of BRCA1/2 genes than those whose mothers had never had testing (22% v. 11%), although this was not statistically significant. Among the 6 daughters of BRCA1/2 mutation carriers, 4 mothers reported sharing test results with their daughters (15-17 years old), but only one daughter (15 year-old) reported awareness of BRCA1/2 genes.
Perceived risk and timeline of breast cancer
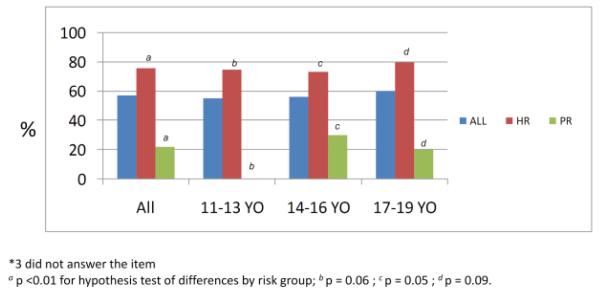
The majority (76%) of high-risk girls indicated that they perceive themselves to be at increased risk for breast cancer, compared to 22% of population-risk girls (p=0.001). These differences were significant across all age groups (Fig 2a). Girls of BRCA1/2 positive mothers and those with > 1 relative with breast cancer appeared more likely to perceive themselves to be at high-risk than daughters with only 1 relative with breast cancer (i.e. mother with breast cancer or 1 second-degree relative with breast cancer). Forty-five percent of girls who believe themselves to be at increased risk reported that they believe they can develop breast cancer before 20 year-old. The most frequent reason girls reported this age of onset was puberty or breast development (28%), e.g. “as soon as you start developing breasts” (13 year-old high-risk girl). These responses did not differ by age or risk group. Other reasons were media or experiences outside the family (9%), increasing risk with age (7%) or experience in one’s family (4%). Examples include: “I saw someone at my mom’s chemo who was 14” (16 year-old high-risk girl) and “I heard of a girl getting breast cancer at 20 in a magazine” (17 year-old high-risk girl).
Fig 2a. My risk for breast cancer is higher than other girls my age (n=51*).
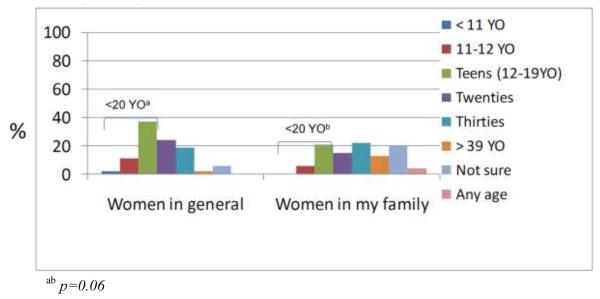
Similarly, among all girls, 50% of girls reported that women in general can get breast cancer before 20 years old (Fig 2b) and did not differ significantly by risk group or high-risk subgroups. Girls 11-13 years old were more likely to report that breast cancer can occur for women in general in their twenties (46%) compared to girls > 13 (17%, p=0.06). Again, the most frequently reported reason for earliest age of breast cancer onset among women in general was puberty or breast development (50%), media or experiences outside the family (11%), increasing risk with age (6%) and experiences in their family (6%). Additionally, 30% of girls did not provide a reason for their reported perceived earliest age of breast cancer onset in women in general. As shown in Figure 2b, fewer girls (21%) reported an age of onset for women in their family < 20 years old, as compared to the earliest age of onset for women in general (45%, p<0.01).
Fig 2b. Earliest age women in general can get breast cancer (n=54).
Perceived preventability and controllability of breast cancer
The majority (70%) of girls reported that there are things women can do to prevent breast cancer. High-risk girls were more likely than population-risk girls (74% v. 63%) to report there are things women can do to prevent breast cancer, although this difference was not statistically significant. The results did not differ significantly by age or high-risk subgroups. The methods most frequently reported were screening mammograms, self-exams, breast MRI or clinical breast exam (34%), healthy diet (24%), general health maintenance (21%), exercise (18%) and avoiding exposures (e.g. tobacco, alcohol, radiation or chemicals, 18%).
Sixty-seven percent of girls reported there are things girls their own age can do to prevent breast cancer. The data suggests that with increasing girls’ age, girls more frequently report there are things women can do to prevent breast cancer and less frequently report there are things girls can do to prevent breast cancer, although these differences were not statistically significant. While perceived controllability in adolescence did not appear to differ among high-risk and population-risk girls, girls of mothers with a BRCA1/2 mutation and mothers with breast cancer appeared less likely to perceive there are things girls can do to prevent breast cancer. The most frequently reported risk reduction methods were general health maintenance (34%), screening--most frequently self-breast exam (25%), healthy diet (23%), exercise (17%), and avoiding exposures (e.g. tobacco, alcohol, radiation or chemicals, 17%). Risk reduction methods for girls did not differ significantly by risk group or age.
The majority of girls (74%) also reported that breast cancer can be cured, with population-risk girls (79% v. 71% among high-risk) and younger girls (85% among 11-13 v. 71% among >13 years old) more frequently reporting breast cancer is curable, although these differences were not statistically significant.
Sources of information
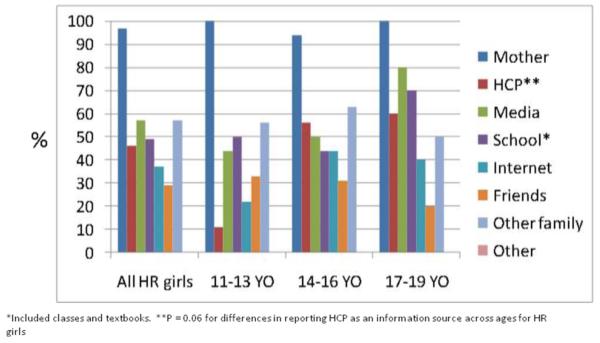
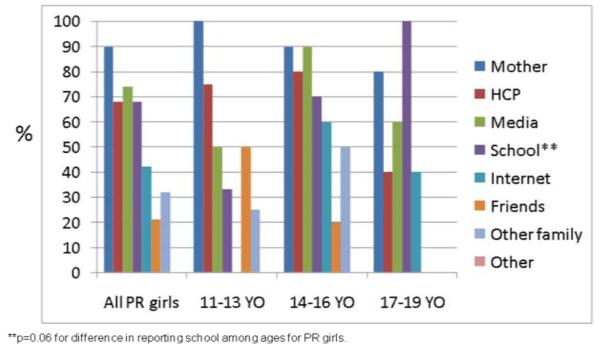
Mothers were the most frequently reported source of breast cancer information among both high-risk (97%) and population-risk (89%) girls across all age groups (Figs 3a, 3b). Other frequently reported sources included health care professionals, media (including television, radio, and print media) and school. Among high-risk girls, health care professionals were reported more frequently with increasing age (p=0.06, Fig 3a). Sources of information did not appear to differ by high-risk subgroups. School was more frequently reported with increasing age among population-risk girls (p=0.06, Fig 3b).
Fig 3a. Sources of information for breast cancer among high-risk girls by age (n=54).
Fig 3b. Sources of information for breast cancer among population-risk girls by age (n=54).
DISCUSSION
The majority of adolescents in high-risk families learn of familial and genetic risks for breast cancer at a young age[17-20]. Thus, understanding what adolescent girls know and perceive of breast cancer risks, its impact on their psychosocial well-being and adoption of preventive health and risk behaviors and how these change over time is crucial for health care providers and families, particularly as genetic and genomic testing expands[52-55]. This study examined representations of breast cancer as a disease threat, health behaviors to prevent or reduce risks for breast cancer, and the impact of these health behaviors, as well as sources of information for breast cancer. In this sample, the majority of high-risk girls perceived themselves to be at increased risk for breast cancer, while most population-risk girls did not. Other studies of adolescent girls not selected for family history and girls of mothers with breast cancer have reported similar findings[17, 25-33]. In our study, this heightened risk perception existed even among the youngest girls (11-13 years old). Consistent with the SRTHB, the majority of girls identify family history as a key risk factor for breast cancer and the data suggest that degree of family history may also impact perceived risk. Interestingly, few high-risk or population-risk girls reported knowledge of breast cancer genes, consistent with a study by Capelli et al[25]. Our data suggests that awareness of familial risk for breast cancer and breast cancer genes might be more frequent among girls >13 years old. Understanding how and when adolescent girls understand genetic risks is important given that many parents share their BRCA1/2 test results with their children[17, 18, 20], and genetic information is more frequently incorporated into medical care[52]
To our knowledge, this is the first study to report when adolescent girls believe women can get breast cancer. Fifty percent of girls reported that breast cancer can occur < 20 years oldWhile women at familial or genetic risk for breast cancer are more likely to develop premenopausal breast cancer, medical reports of breast cancer before 20 year old are rare[56]. Importantly, at the time this study was conducted, the media had recently reported breast cancer in a 10 year-old girl[57]. While this might have influenced the results, the findings from our study suggest that girls believe that women, and more importantly themselves, can develop breast cancer at young ages. In contrast, a study of 8-18 years old primarily population-risk girls in Philadelphia schools, found only 3% of girls reported that breast cancer is common in teens[27]. These data suggest that perceptions of girls from breast cancer families might differ and highlights the potential for media reports to impact health beliefs[58]. Equally important, many girls identified normal pubertal development as the rationale for perceiving themselves to be at risk in their teen years. This misperception has also been reported by Weiss et al[27], which might have greater affective, cognitive and behavioral impact among high-risk girls. Future studies of the attributes of breast cancer threat and its impact on psychosocial and behavioral outcomes among girls across the spectrum of risk have the potential to inform the development of interventions to optimize psychosocial and behavioral responses to breast cancer awareness. Education about when women can get breast cancer, and the rarity of early breast cancer reported in the media could be relevant components of future interventions. Mother, health care providers and school-based programs could be considered in the development of future interventions.
Importantly, the majority of girls believe there are things women and girls can do to prevent breast cancer, suggesting a potential “teachable moment” among adolescents that might be sustainable across the lifespan[15, 28]. Although the majority of girls who perceive breast cancer risk as modifiable might be more likely to have adaptive responses to knowledge of their breast cancer risk, girls who do not might be at risk for greater distress[34, 59]. Our data suggests that this might be particularly important for older adolescent girls, as perceptions of the efficacy of risk reductive behaviors in youth appear to decline with increasing age.
An important finding from this study was the role of mothers as a source of information about breast cancer. Studies have shown that many adult women have misperceptions about risks for breast cancer[26, 60, 61]. A better understanding of what mothers know, perceive and communicate to adolescents could be important to understanding knowledge and perceptions in adolescent girls. Educational interventions targeting mothers might be effective interventions to optimize adolescents’ responses to breast cancer information. Additionally, health care providers for women at risk for breast cancer could play an important role in addressing maternal distress and misinformation, which might optimize adaptive communication among mothers and daughters regarding breast cancer. It will also be important to better understand the impact of media and internet sources of information in adolescent girls [24].
We acknowledge several limitations to this study. This was a small and select population in a study designed to inform instrument development and hypotheses for future investigation. Participants were from highly educated families and exclusively white. Consistent with standards in pediatric and adolescent research, parental permission was required[62]. Thus, it is possible that mothers and families with more open communication about breast cancer may have been more likely to permit daughter participation. This exploratory study and was not powered to detect differences by risk group or age. Open-ended items were utilized to provide a range of responses for many constructs and confirmation with quantitative scales will be valuable. Additionally, further investigation of potential differences across the spectrum of high-risk categories is needed.
In conclusion, the majority of girls at high-risk for breast cancer perceive themselves to be at increased risk and many girls (both high-risk and population-risk) believe that breast cancer can occur in the teens. Yet, most girls believe there are things that women and girls can do to prevent breast cancer. The psychosocial and behavioral impact of growing up in a family at increased genetic or familial risk for cancer remains unknown, but will become increasingly important as genetic testing expands, creating an increasing population of families at “high-risk” for cancer. Translating these findings into effective interventions across the continuum of risk and age will require an understanding of how girls perceive and utilize breast cancer risk and risk prevention information and could provide opportunities to improve breast cancer prevention efforts across the female lifespan.
Acknowledgments
Research support:
American Cancer Society, Mentored Research Scholar Award (MRSG 07-014-01-CPPB). Support was also provided by NIH grant P30 CA006927 and the.Fox Chase Cancer Center Keystone Program in Personalized Risk and Prevention.
Footnotes
Conflict of Interest Statement: There are no conflicts of interest to disclose.
REFERENCES
- 1.American Cancer Society Cancer Facts & Figures. 2010 5/25/11]; Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-026238.pdf.
- 2.Antoniou AC, Easton DF. Models of genetic susceptibility to breast cancer. Oncogene. 2006;25(43):5898–905. doi: 10.1038/sj.onc.1209879. [DOI] [PubMed] [Google Scholar]
- 3.Nathanson KL, Weber BL. “Other” breast cancer susceptibility genes: searching for more holy grail. Hum Mol Genet. 2001;10(7):715–20. doi: 10.1093/hmg/10.7.715. [DOI] [PubMed] [Google Scholar]
- 4.Turnbull C, Rahman N. Genetic predisposition to breast cancer: past, present, and future. Annu Rev Genomics Hum Genet. 2008;9:321–45. doi: 10.1146/annurev.genom.9.081307.164339. [DOI] [PubMed] [Google Scholar]
- 5.NCCN Guidelines, version 1.2011 Breast Cancer Screening and Diagnosis. 2011 http://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- 6.Borry P, et al. Presymptomatic and predictive genetic testing in minors: a systematic review of guidelines and position papers. Clin Genet. 2006;70(5):374–81. doi: 10.1111/j.1399-0004.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 7.Wild CP. How Much of a Contribution Do Exposures Experienced between Conception and Adolescence Make to the Burden of Cancer in Adults? Cancer Epidemiol Biomarkers Prev. 2011;20(4):580–1. doi: 10.1158/1055-9965.EPI-11-0187. [DOI] [PubMed] [Google Scholar]
- 8.Okasha M, et al. Exposures in childhood, adolescence and early adulthood and breast cancer risk: a systematic review of the literature. Breast Cancer Res Treat. 2003;78(2):223–76. doi: 10.1023/a:1022988918755. [DOI] [PubMed] [Google Scholar]
- 9.Maruti SS, et al. A prospective study of age-specific physical activity and premenopausal breast cancer. J Natl Cancer Inst. 2008;100(10):728–37. doi: 10.1093/jnci/djn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colditz GA, Frazier AL. Models of breast cancer show that risk is set by events of early life: prevention efforts must shift focus. Cancer Epidemiol Biomarkers Prev. 1995;4(5):567–71. [PubMed] [Google Scholar]
- 11.Warri A, et al. The role of early life genistein exposures in modifying breast cancer risk. Br J Cancer. 2008;98(9):1485–93. doi: 10.1038/sj.bjc.6604321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmbeck GN. A developmental perspective on adolescent health and illness: an introduction to the special issues. J Pediatr Psychol. 2002;27(5):409–16. doi: 10.1093/jpepsy/27.5.409. [DOI] [PubMed] [Google Scholar]
- 13.Cohen RY, Brownell KD, Felix MR. Age and sex differences in health habits and beliefs of schoolchildren. Health Psychol. 1990;9(2):208–24. doi: 10.1037//0278-6133.9.2.208. [DOI] [PubMed] [Google Scholar]
- 14.Chassin L, et al. The natural history of cigarette smoking: predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol. 1990;9(6):701–16. doi: 10.1037//0278-6133.9.6.701. [DOI] [PubMed] [Google Scholar]
- 15.Williams PG, Holmbeck GN, Greenley RN. Adolescent health psychology. J Consult Clin Psychol. 2002;70(3):828–42. [PubMed] [Google Scholar]
- 16.Mulye TP, et al. Trends in adolescent and young adult health in the United States. J Adolesc Health. 2009;45(1):8–24. doi: 10.1016/j.jadohealth.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Tercyak KP, et al. Parental communication of BRCA1/2 genetic test results to children. Patient Educ Couns. 2001;42(3):213–24. doi: 10.1016/s0738-3991(00)00122-1. [DOI] [PubMed] [Google Scholar]
- 18.Patenaude AF, et al. Sharing BRCA1/2 test results with first-degree relatives: factors predicting who women tell. J Clin Oncol. 2006;24(4):700–6. doi: 10.1200/JCO.2005.01.7541. [DOI] [PubMed] [Google Scholar]
- 19.Bradbury AR, et al. How often do BRCA mutation carriers tell their young children of the family’s risk for cancer? A study of parental disclosure of BRCA mutations to minors and young adults. J Clin Oncol. 2007;25(24):3705–11. doi: 10.1200/JCO.2006.09.1900. [DOI] [PubMed] [Google Scholar]
- 20.Bradbury AR, et al. When parents disclose BRCA1/2 test results: Their communication and perceptions of offspring response. Cancer. 2012 doi: 10.1002/cncr.26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Early Act. http://wassermanschultz.house.gov/earlyact/index.shtml. 10/23/09]; Available from: http://wassermanschultz.house.gov/earlyact/index.shtml.
- 22.Phillips L, Susan G. Komen tour bus takes awareness on the go. http://www.collegiatetimes.com/stories/14224/susan-g.-komen-tour-bus-takes-awareness-on-the-go.
- 23.Orenstein P. The Way We Live Now; Think About Pink. The New York Times Magazine; 2010. pp. 13–14. November 12, 2010. [Google Scholar]
- 24.Driessnack M. Growing up at the intersection of the genomic era and the information age. J Pediatr Nurs. 2009;24(3):189–93. doi: 10.1016/j.pedn.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Cappelli M, et al. Psychological and genetic counseling implications for adolescent daughters of mothers with breast cancer. Clin Genet. 2005;67(6):481–91. doi: 10.1111/j.1399-0004.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- 26.Silk KJ, et al. Formative research on adolescent and adult perceptions of risk factors for breast cancer. Soc Sci Med. 2006;63(12):3124–36. doi: 10.1016/j.socscimed.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Weiss M, et al. Breast cancer fear in girls: a major “side effect” of breast cancer in loved ones and a backlash of ubiquitous media coverage; The San Antonio Breast Cancer Symposium; San Antonio, Texas. 2008. [Google Scholar]
- 28.Bradbury AR, et al. Learning of your parent’s BRCA mutation during adolescence or early adulthood: a study of offspring experiences. Psychooncology. 2009;18(2):200–8. doi: 10.1002/pon.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitch MI, Abramson T. Information needs of adolescents when a mother is diagnosed with breast cancer. Can Oncol Nurs J. 2007;17(1):16–25. doi: 10.5737/1181912x1711620. [DOI] [PubMed] [Google Scholar]
- 30.Geller G, et al. Mothers and daughters from breast cancer families: a qualitative study of their perceptions of risks and benefits associated with minor’s participation in genetic susceptibility research. J Am Med Womens Assoc. 2000;55(5):280–4. [PubMed] [Google Scholar]
- 31.Kristjanson LJ, Chalmers KI, Woodgate R. Information and support needs of adolescent children of women with breast cancer. Oncol Nurs Forum. 2004;31(1):111–9. doi: 10.1188/04.ONF.111-119. [DOI] [PubMed] [Google Scholar]
- 32.Zahlis EH. The child’s worries about the mother’s breast cancer: sources of distress in school-age children. Oncol Nurs Forum. 2001;28(6):1019–25. [PubMed] [Google Scholar]
- 33.Tercyak KP, et al. Psychological issues among children of hereditary breast cancer gene (BRCA1/2) testing participants. Psychooncology. 2001;10(4):336–46. doi: 10.1002/pon.531. [DOI] [PubMed] [Google Scholar]
- 34.Leventhal H, et al. Perceptions of Health and Illness: Current Research and Applications, in Illness representations: theoretical foundations. In: Petrie KJ, Weinman JA, editors. Amsterdam; Harwood: 1997. pp. 19–46. [Google Scholar]
- 35.Zimmerman RS, et al. The effects of health information in a worksite hypertension screening program. Health Education Quarterly. 1986;13:261–280. doi: 10.1177/109019818601300305. [DOI] [PubMed] [Google Scholar]
- 36.Petrie KJ, et al. Changing illness perceptions after myocardial infarction: an early intervention randomized controlled trial. Psychosom Med. 2002;64(4):580–6. doi: 10.1097/00006842-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 37.McAndrew LM, et al. Using the common sense model to design interventions for the prevention and management of chronic illness threats: from description to process. Br J Health Psychol. 2008;13(Pt 2):195–204. doi: 10.1348/135910708X295604. [DOI] [PubMed] [Google Scholar]
- 38.Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol. 2000;55(5):469–80. [PubMed] [Google Scholar]
- 39.Thomas AM, Peterson L, Goldstein D. Problem solving and diabetes regimen adherence by children and adolescents with IDDM in social pressure situations: a reflection of normal development. J Pediatr Psychol. 1997;22(4):541–61. doi: 10.1093/jpepsy/22.4.541. [DOI] [PubMed] [Google Scholar]
- 40.Sandelowski M. Combining qualitative and quantitative sampling, data collection, and analysis techniques in mixed-method studies. Res Nurs Health. 2000;23(3):246–55. doi: 10.1002/1098-240x(200006)23:3<246::aid-nur9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 41.Sandelowski M. Sample size in qualitative research. Res Nurs Health. 1995;18(2):179–83. doi: 10.1002/nur.4770180211. [DOI] [PubMed] [Google Scholar]
- 42.Pope C, van Royen P, Baker R. Qualitative methods in research on healthcare quality. Qual Saf Health Care. 2002;11(2):148–52. doi: 10.1136/qhc.11.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritchie J, Spencer L. Qualitative Data Analysis for Applied Policy Research. In: Bryman A, Burgess R, editors. Analysing Qualitative Data. Routledge; London: 1994. pp. 173–194. [Google Scholar]
- 44.Scott SE, et al. Patient delay in oral cancer: a qualitative study of patients’ experiences. Psychooncology. 2006;15(6):474–85. doi: 10.1002/pon.976. [DOI] [PubMed] [Google Scholar]
- 45.Velikova G, et al. The clinical value of quality of life assessment in oncology practice-a qualitative study of patient and physician views. Psychooncology. 2008;17(7):690–8. doi: 10.1002/pon.1295. [DOI] [PubMed] [Google Scholar]
- 46.Husain LS, et al. Choices in cancer treatment: a qualitative study of the older women’s (>70 years) perspective. Psychooncology. 2008;17(4):410–6. doi: 10.1002/pon.1242. [DOI] [PubMed] [Google Scholar]
- 47.Glaser BG, Strauss AL. The discovery of grounded theory. Aldine; Chicago: 1967. [Google Scholar]
- 48.Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. Bmj. 2000;320(7227):114–6. doi: 10.1136/bmj.320.7227.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334–40. doi: 10.1002/1098-240x(200008)23:4<334::aid-nur9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 50.Crabtree BF, Miller WL. Doing qualitative research. Sage; Newbury Park, CA: 1992. [Google Scholar]
- 51.Braet C, et al. Depression in Early, Middle and Late Adolescence: Differential Evidence for the Cognitive Diathesis-Stress Model. Clin Psychol Psychother. 2012 doi: 10.1002/cpp.1789. [DOI] [PubMed] [Google Scholar]
- 52.Burke W, Psaty BM. Personalized medicine in the era of genomics. Jama. 2007;298(14):1682–4. doi: 10.1001/jama.298.14.1682. [DOI] [PubMed] [Google Scholar]
- 53.Khoury MJ, et al. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet Med. 2007;9(10):665–74. doi: 10.1097/GIM.0b013e31815699d0. [DOI] [PubMed] [Google Scholar]
- 54.Scheuner MT, Sieverding P, Shekelle PG. Delivery of genomic medicine for common chronic adult diseases: a systematic review. Jama. 2008;299(11):1320–34. doi: 10.1001/jama.299.11.1320. [DOI] [PubMed] [Google Scholar]
- 55.Agurs-Collins T, et al. Public health genomics: translating obesity genomics research into population health benefits. Obesity (Silver Spring) 2008;16(Suppl 3):S85–94. doi: 10.1038/oby.2008.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kothari AS, et al. Breast carcinoma in women age 25 years or less. Cancer. 2002;94(3):606–14. doi: 10.1002/cncr.10273. [DOI] [PubMed] [Google Scholar]
- 57.Bosland K, McCarthy K. Bosland, K., McCarthy, K.11-Year-Old Breast Cancer Survivor. 2009 Oct 9; Available from: http://abcnews.go.com/GMA/11-year-youngest-breast-cancer-survivors/story?id=8782697#.Tt_fi3qt0tU.
- 58.Kasperson RE, et al. The Social Amplification of Risk: A Conceptual Framework. Risk Analysis. 1988;8(2):177–187. [Google Scholar]
- 59.Edgar KA, Skinner TC. Illness representations and coping as predictors of emotional well-being in adolescents with type 1 diabetes. J Pediatr Psychol. 2003;28(7):485–93. doi: 10.1093/jpepsy/jsg039. [DOI] [PubMed] [Google Scholar]
- 60.Covello VT, Peters RG. Women’s perceptions of the risks of age-related diseases, including breast cancer: reports from a 3-year research study. Health Commun. 2002;14(3):377–95. doi: 10.1207/S15327027HC1403_5. [DOI] [PubMed] [Google Scholar]
- 61.Lipkus IM, et al. Informing women about their breast cancer risks: truth and consequences. Health Commun. 2001;13(2):205–26. doi: 10.1207/S15327027HC1302_5. [DOI] [PubMed] [Google Scholar]
- 62.Emanuel EJ, et al. The Oxford Textbook of Clinical Research Ethics. Oxford University Press; USA: 2008. [Google Scholar]