Abstract
Vitamin D is mostly recognized for its regulation of calcium homeostasis in relation to the intestine, kidney, and bone. Although clinical studies have linked vitamin D with increased muscle function and strength, little is known of its underlying molecular mechanism. We recently demonstrated that 1,25-D3 exerts a direct pro-myogenic effect on skeletal muscle cells; this has provoked our investigation of 1,25-D’s effect on angiogenesis, a vital process for new capillary development and tissue repair. In this study, we examined the mechanism by which 1,25-D3 modulates key angiogenic growth factors and angiogenic inhibitors. C2C12 myoblasts were incubated with 100 nM 1,25-D3 or placebo for 1, 4 and 10 days. At the end of the respective incubation time, total RNA was isolated for PCR arrays and for qRT-PCR. Total proteins were isolated for western blots and proteome profiler arrays. The addition of 1,25-D3 to C2C12 myoblasts increased VEGFa and FGF-1: two pro-angiogenic growth factors that promote neo-vascularization and tissue regeneration, and decreased FGF-2 and TIMP-3: two myogenic and/or angiogenic inhibitors. Our previous study demonstrated that 1,25-D3 altered IGF-I/II expression, consistent with the observed changes in VEGFa and FGF-2 expression. These results extend our previous findings and demonstrate the modulation of angiogenesis which may be an additional mechanism by which 1,25-D3 promotes myogenesis. This study supports the mechanistic rationale for assessing the administration of vitamin D and/or vitamin D analogues to treat select muscle disorders and may also provide an alternative solution for therapies that directly manipulate VEGF and FGF’s to promote angiogenesis.
Keywords: VDR, VEGFa, FGF-1, FGF-2, TIMP-3, IGFs
1. Introduction
Vitamin D is mostly known by its role in the regulation of calcium homeostasis and bone metabolism. However, increasing evidence indicates that vitamin D plays an essential role in many other tissues including skeletal muscle, especially in relation to muscle weakness and muscle pain [1, 2]. Multiple cross-sectional studies in community-dwelling older adults have found a direct association between vitamin D status and parameters of physical performance [3, 4]. Moreover, it has been described that low vitamin D levels are associated with the frailty syndrome in men and women [5, 6]. Very notably, it has been demonstrated that a high dose of Vitamin D supplementation (>800 IU daily) leads to a significant reduction in risk fracture- with a 30% reduction in the risk of hip fracture and a 14% reduction in risk of nonvertebral fracture which was independent of age, sex, and type of dwelling amongst subjects 65 years of age and older [7]. It has been demonstrated that skeletal muscle cells are a direct target for vitamin D. Simpson et al showed the expression of 1,25-D3 receptor-like proteins by affinity binding assay in an embryonic myoblast cell line [8], although the expression of vitamin D receptor (VDR) in adult human skeletal muscle is controversial due to concerns regarding antibody specificity [9]. VDR expression in adult skeletal muscle has been shown by some authors [10, 11] and it was absent in another study [9]. However, mice lacking the VDR show a skeletal muscle phenotype with smaller and variable muscle fibers, and persistence of immature muscle gene expression during adult life suggesting a role of vitamin D in muscle development [12, 13].
We recently demonstrated that the addition of 1,25-D3 to C2C12 skeletal muscle cells decreases cell proliferation and enhances myogenic differentiation through an increased expression and nuclear translocation of the VDR and modulation of pro-and anti-myogenic factors [14]. In addition, We also have shown that 1,25-D3 exerts a direct pro-myogenic cell differentiation effect on skeletal muscle cells by increasing IGF-II and follistatin expression, while simultaneously decreasing the expression of not only IGF-I, but myostatin, a negative regulator of skeletal muscle mass [14].
Angiogenesis is a normal and vital process for new capillary development and tissue repair during the adult life span for regeneration and damaged tissues. Regenerative therapies for skeletal muscle injuries need to consider in addition to myogenic differentiation, the promotion of revascularization in order to reduce scarring and effective muscle regeneration [15].
It has been demonstrated that several angiogenic factors are also involved in myogenesis indicating a strong association between angiogenesis and skeletal muscle regeneration. Fibroblast growth factor-1 (FGF-1), a member of the heparin-binding growth factor family and a well-known pro-angiogenic factor, is induced during myogenesis and is required during myoblast differentiation [16]. Vascular endothelial growth factor alpha (VEGFa) another key inducer of angiogenesis, is regulated as part of the myogenic differentiation program and regulates myogenesis in an autocrine function [17]. Additionally, VEGFa stimulates terminal skeletal muscle cell differentiation in C2C12 myoblasts cells, as evidenced by increased myotube formation [17] and by improving skeletal muscle regeneration after acute trauma and reconstruction of the limb in a Rabbit Model [18].
There is also a growing body of evidence demonstrating that inhibitors of angiogenesis such as the tissue inhibitors of metalloproteinases (TIMPs) are key regulators of skeletal muscle function in health and disease [19]. Moreover, it has been shown that TIMP-3, the endogenous inhibitor of TNFalpha-converting enzyme (TACE), acts as an on-off switch for myogenic differentiation by regulating autocrine TNFalpha release [20]. Liu et al demonstrated that constitutively expressed TIMP-3 is transiently downregulated in the satellite cells of regenerating mouse hindlimb muscles and in differentiating C2C12 myoblasts. The same authors concluded that TIMP-3 specifically is a physiological regulator of myogenic differentiation [20].
Although it has been established that FGF-2 is an inducer of angiogenesis, proliferation and cell migration in several cells and tissue types [21], conversely, in skeletal muscle tissue, FGF-2 has been long described to be an inhibitor of skeletal muscle differentiation in myoblasts in vitro [22, 23].
Despite of the considerable body of evidence during the last recent years, the balance and contribution between angiogenic and anti-angiogenic factors in regulating skeletal muscle angiogenesis and muscle cell differentiation remains poorly understood.
Vitamin D, a fat-soluble secosteroid pro-hormone is obtained from sun exposure or from dietary sources. During exposure to sunlight 7-dehydrocholesterol (7-DHC) in the skin is converted to pre-vitamin D3 (preD3), which is immediately converted by a heat-dependent process to vitamin D3. Vitamin D2 and vitamin D3 from dietary sources are incorporated into chylomicrons, transported by the lymphatic system into the venous circulation. Vitamin D in the circulation is bound to the vitamin D-binding protein, which transports it to the liver where vitamin D is converted by the vitamin D-25 hydroxylase (25-OHase) to 25-D3 (25-hydroxivitamin D3). 25-D3 is biologically inactive and is converted primarily in the kidney by the 25-hydroxyvitamin D-1α-hydroxylase (1-OHase) to its biologically active form 1,25-dihydroxyvitamin D (1,25-D3) or calcitriol [24].
Mouse C2C12 skeletal muscle cells are an “in vitro” system that expresses the VDR [14, 25] and CYP27B1 [25], and they are widely used to study genes that regulate muscle growth and differentiation [14, 26, 27]. C2C12 myoblast cells differentiate rapidly, forming contractile myotubes and producing characteristic muscle proteins [27].
The aim of the present study was to test whether 1,25-D3, in addition to promote myogenic differentiation [14] can also modulates the expression of key angiogenic growth factors and angiogenic growth factor inhibitors that may ultimately promote muscle regeneration and repair. To accomplish this, we investigated the expression of key angiogenic growth factors and angiogenic inhibitors modulated by 1,25-D3 in a well-known and widely used skeletal muscle cell model.
2. Materials and Methods
2.1. Cell Culture
The mouse C3H myoblast cell line C2C12 (CRL-1772, ATCC, Manassas, VA) was propagated in DMEM supplemented with 10% dialyzed fetal bovine serum (FBS) at 37°C and 5% CO2 [14, 27] at 40–50% confluence in T75 flasks. FBS is dialyzed by tangential flow filtration utilizing 10,000 MW cutoff filters; this procedure eliminates many low molecular weight hormones and cytokines that could impact the cell culture. Cells were distributed on six well plates (Corning International, Lowell MA). The next day, the cells were incubated or not with 100 nM of 1,25-D3 (Sigma–Aldrich, St. Louis, MO) dissolved in less than 0.1% ethanol as vehicle in DMEM 10% dialyzed fetal bovine serum for 1 to 10 days. The 100nM concentration of 1,25-D3 employed in the experimental designed was the optimal dose established based on our prior dose-response studies and is in alignment with a commonly used dose applied in the majority of publications related to 1,25-D3 effects on different cell lines or even in primary cell cultures [14, 28–33]. Because of the 10-hour half-life of 1,25-D3, the cell culture media, incubated or not with 1,25-D3 (100 nM) was replaced daily [14, 28].
2.2. PCR Array Analysis of Angiogenesis Growth factors and Angiogenesis Inhibitors
RT2 profiler PCR pathway focused arrays (SABiosciences, Frederick, MD) were performed in triplicate to detect changes in gene expression of growth factors, receptors and cytokines that play a role in angiogenesis. Total RNA from C2C12 cells control (untreated) and treated with 1,25-D3 (100nM) for 24 hours, 4 days and 10 days were isolated with Trizol-Reagent (Invitrogen, Carlsbad, CA). Total RNA aliquots were converted by reverse transcription, and the resulting cDNA were subjected to the Mouse Angiogenesis (PAMM-024) and the Mouse Angiogenic Growth Factors & Angiogenesis Inhibitors (PAMM-072) PCR Arrays (SABiosciences, Frederick MD). The Mouse Angiogenesis RT2 Profiler™ PCR Array contains genes involved in modulating the biological processes of angiogenesis. The Mouse Angiogenic Growth Factors & Angiogenesis Inhibitors array profiles the expression of growth factors, chemokines and cytokines that promote the biogenesis of new blood vessels and the genes that encode inhibitors of this process. Real-time PCR were performed as follows: melting for 10 min. at 95 C°, 40 cycles of two-step PCR, including melting for 15 sec at 95 C°, annealing for 1 min. at 60 C°. The raw data were analyzed using ΔΔCt (cycle threshold) method following manufacturer’s instructions (SABiosciences Copr.) [14, 28, 29].
2.3. Real-time quantitative PCR
Total RNA was extracted using Trizol-Reagent (Applied Biosystems, Foster City, CA) followed by the RNeasy mini kit (QIAGEN, Valencia, CA) and equal amounts (1 μg) of RNA were reverse transcribed using the RT2 First Strand kit (CO-3) (SABiosciences, Frederick, MD). Mouse gene PCR primer sets (RT2) for VEGFa, FGF-1, FGF-2, and TIMP-3 were obtained from SABiosciences Corp. The Qiagen RT2SYBR Green/ROX qPCR MasterMix (QIAGEN, Valencia, CA) was used with the ABI Step One Plus PCR thermocycler with fluorescent detector lid (Applied Biosystems) [14, 28, 29]. The protocol included melting for 15 min. at 95 C°, 40 cycles of two-step PCR including melting for 10 min. at 95 C°, 40 cycles of two-step PCR, including melting for 15 sec. at 95 C°, annealing for 1 min at 60 C°. Samples of 25 ng of cDNA were analyzed in triplicate in parallel with glyceraldehide-3-phosphate dehydrogenase (GAPDH) and ribosomal protein, large P1, (data not shown) controls. Relative quantification of the gene expression level was carried out using the comparative Ct (ΔΔCt) method and determined as the difference between the CT for a specific mRNA gene and the CT for a reference mRNA, normalized to GAPDH and RPLP1 threshold expression.
2.4. Proteome array analysis of angiogenic growth factors
To determine the changes in angiogenesis growth factors in C2C12 cells, a proteome profiler array was employed in triplicate following the manufacturer’s instructions (R&D System Inc, Minneapolis, MN). The Proteome profiler Mouse Angiogenesis Array (ARY015) detects the relative levels of 53 angiogenesis related proteins including differentiation factors, extracellular matrix components, proteases, membrane bond receptors, and intracellular signaling molecules. Briefly, protein aliquots of 300 μg/mL were diluted and mixed with a cocktail of biotinylated detection antibodies for 1h at room temperature. The sample/antibody mixture was then incubated overnight at 4°C with the membrane array. The angiogenesis growth factor antibodies complex present were bound by its cognate immobilized capture antibodies on a nitrocellulose membrane containing 53 different anti-angiogenic antibodies in duplicate. After several washes, streptavidin-horseradish peroxidase was added and incubated for 30 min. Membranes were then exposed to western blot chemiluminescent detection reagents (GE Healthcare, Piscataway, NJ). The densitometric signal produced by each spot run in duplicate was proportional to the amount of protein bound determined by Image J (NIH, Bethesda, MD). Positive and negative controls are included in each membrane array to compensate for background and intensity differences.
2.5. Western Blot and Densitometry Analysis
Cell lysates (30–60 μg of protein) were subjected to western blot analyses after separation of proteins on 4–15% Mini-PROTEAN TGX Precast Gels (Bio-Rad, Hercules, CA) in running buffer (Tris/Glycine/SDS). Proteins were horizontally transferred for 40 minutes to nitrocellulose membranes in transfer buffer (Tris/Glycine/Methanol). The non-specific binding was blocked by immersing the membranes into 5% non-fat dried milk, 0.1% (v/v) Tween 20 in 1X PBS overnight at 4°C. After several washes with washing buffer (PBS Tween 0.1%), membranes were incubated with the primary antibodies for three hours at room temperature or overnight at 4°C, monoclonal antibodies were as follows: a) VEGFa (1: 150) (R & D Systems, Minneapolis, MN) b) glyceraldehide-3-phosphate dehydrogenase (GAPDH) (1:10,000) (Millipore, Temecula, CA). Polyclonal antibodies were used for: a) FGF-1 (1:50) b) FGF-2 (1: 3,000) and c) TIMP-3 (1:3000) (Abcam Inc., Cambridge, MA).
The washed membranes were incubated for 1 hour at room temperature with: 1:500 dilution (anti-rat horseradish peroxidase conjugated antibody, for VEGFa primary antibody) (R & D Systems, Minneapolis, MN) and 1:3,000 dilution (anti-mouse) or 1:2,000 dilution (anti-rabbit) of secondary antibody linked to horseradish peroxidase, respectively (Cell Signaling Technology, Inc., Danvers, MA). After several washes, the immunoreactive bands were visualized using the Amersham ECL western blotting detection system (GE Healthcare, Buckinghamshire, UK). The densitometry analysis of the bands was done with Image J 1.40g Image software (National Institute of Health, Bethesda, MD) [28, 29].
2.6. Statistical Analysis
All data are presented as Mean +/− S.E.M. Multiple comparisons were analyzed by a one-way analysis of variance (one-way ANOVA or T-test).
If the overall ANOVA revealed significant differences, then pair-wise comparisons between groups were performed by Tukey multiple comparison test.
All comparisons were two-tailed, and P values less than 0.05 were considered statistically significant. In vitro experiments were repeated thrice, and data from representative experiments are shown. Specifically, the RT2 Profiler PCR arrays and the proteome profiler arrays were done in triplicate and confirmed by real time quantitative PCR and western blots respectively done in triplicate.
For PCR array and real time PCR analysis we considered significant changes in gene expression values of ± 1.5 fold change respect to control.
3. Results
3.1. 1,25-D3 Modulates the expression of key angiogenic growth factors and angiogenic growth factor inhibitors in C2C12 skeletal muscle cells
The effect of 1,25-D3 on specific angiogenic growth factors and angiogenic growth factors inhibitors was evaluated at the steady state mRNA level by applying the Mouse Angiogenesis and the Mouse Angiogenic Growth Factors & Angiogenesis Inhibitors PCR Arrays. Table 1 shows the differential steady state mRNA levels between 1,25-D3 treated and untreated cells for determinations done in triplicate after 24h, 4 and 10 days of incubation with 1,25-D3. The PCR array analysis showed no changes in the expression of FGF-1 after 24h incubation with 1,25-D3, although a positive up-regulation of the expression of FGF-1 was observed at 4 and 10 days respectively.
TABLE 1.
Differential steady-state mRNA levels of angiogenic growth factors and angiogenic growth factor inhibitors between 1,25D3(100nM) treated and untreated C2C12 cells
| Fold Change | |||||
|---|---|---|---|---|---|
| Gene Symbol | Description | Reference Sequence | 24 hours | 4 days | 10 days |
| Fgf-1 | Fibroblast growth factor 1 | NM_008010 | 1.03 | +5.77b | +11.47c |
| Vegfα | Vascular endothelial growth factor A | NM_009505 | +3.28c | +6.55b | −1.09 |
| Timp-3 | Tissue inhibitor of metalloproteinase 3 | NM_011595 | −2.47c | −3.74b | −1.10 |
| Fgf-2 | Fibroblast growth factor 2 | NM_008006 | −2.13c | −2.06b | −1.87 |
| Fgf-6 | Fibroblast growth factor 6 | NM_010204 | +1.13 | +1.35 | +1.13 |
Total RNA from cells treated with or without 1,25-D3 for 24 hours, 4 days and 10 days was subjected to RT real time PCR by the Angiogenic Growth Factor and Angiogenesis Growth Factor Inhibitor arrays, and the ratios between the treated 1,25D3-treated and 1,25D3-untreated cells were corrected by GAPDH, Hprt1, Hsp90ab1, and Actb were calculated for assays performed in triplicate. Experiments were performed in triplicate.
P < 0.05
P < 0.01
P < 0.001
VEGFa was also up-regulated after incubating the cells with 1,25-D3 for 24h, and 4 days. No significant changes in the expression of VEGFa were observed at 10 days incubation with 1,25-D3 with respect to the control. In contrast, the angiogenesis growth factor inhibitor TIMP-3 was continuously down regulated upon 1,25-D3 incubation at 24h and 4 days while reverting back to control values at 10 days. Most importantly, a marked down- regulation of FGF-2, an inhibitor of skeletal muscle differentiation [22] was observed at 24h and 4 days with a marginal down-regulation observed at 10 days (−1.87). We also investigated possible changes in FGF-6 gene expression, a critical component of the muscle regeneration machinery in mammals, possibly by stimulating or activating satellite cells [34]. We found no changes in FGF-6 expression at 24h, 4 days and 10 days of continuous incubation with 1,25-D3.
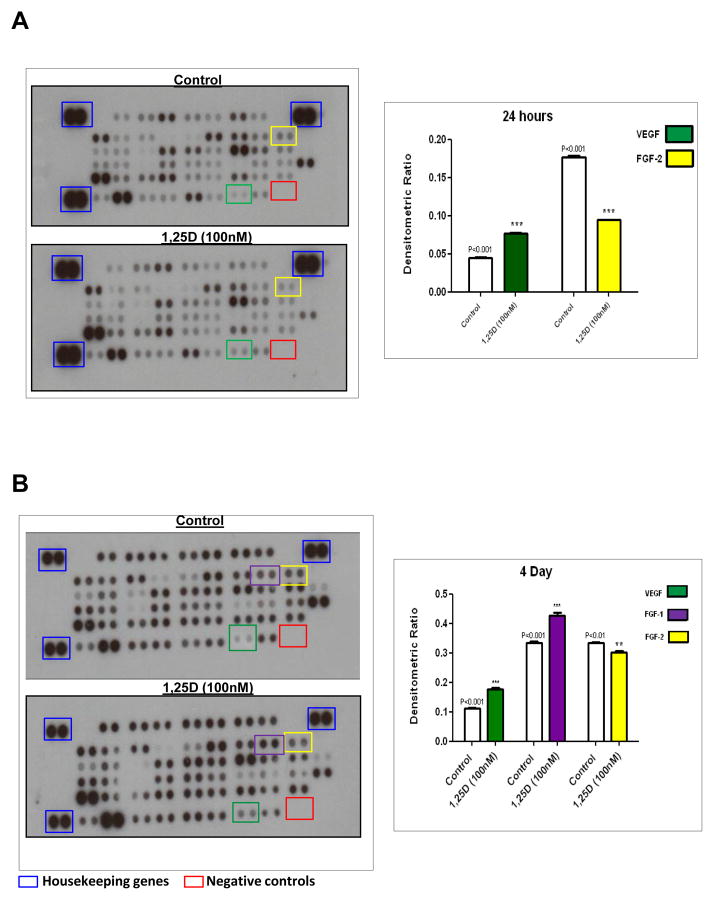
The effect of 1,25-D3 on the expression of angiogenic growth factors and inhibitors were also studied at the protein level by using the Proteome Profiler Mouse Angiogenesis Array. Figure 1, A shows the changes in protein expression profile with the corresponding densitometric analysis after incubation of 1,25-D3 for 24h. A 1.74-fold up-regulation of VEGFa protein expression with respect to the control was observed, in agreement with the findings at the mRNA levels.
Figure 1. Angiogenesis expression is regulated by 1,25-D3 treatment.
Protein extracts were incubated with a cocktail of biotinylated detection antibodies and then incubated in a membrane array containing 53 different anti-angiogenesis antibodies, in duplicates, followed by streptavin peroxidase and chemioluminescence. The densitometric signal produced by each spot run in duplicate was proportional to the amount of angiogenesis factors bound as determined by densitometry analysis. Panel A, left: Representative membranes for Control and 1,25-D3 treatment for 24hs, right: densitometric analysis. Panel B, left: Representative membranes for Control and 1,25-D3 treatment for 4 days, right: Densitometry analysis. Housekeeping genes (blue squares) and negative controls (red squares) are included in each membrane array to compensate for background and intensities differences. **p<0.01; ***p<0.001 with respect to control #p<0.05
In addition we corroborated the down regulation of the expression of FGF-2, by 1.93-fold compared with the control after 24h of incubation with 1,25-D3. Figure 1, B shows the changes in protein expression profile with the corresponding densitometric analysis after 4 days of continuous incubation with 1.25-D3, VEGFa was consistently up-regulated by 2.47-fold compared to the control, and FGF-1 was also up-regulated by 1.70-fold. At the same timepoint, FGF-2 was down-regulated by 1.50-fold compared with the control. The proteome profiler mouse angiogenic array was also done at day 10 and showed a protein expression profile comparable to the data found by PCR arrays at the same time point (not shown).
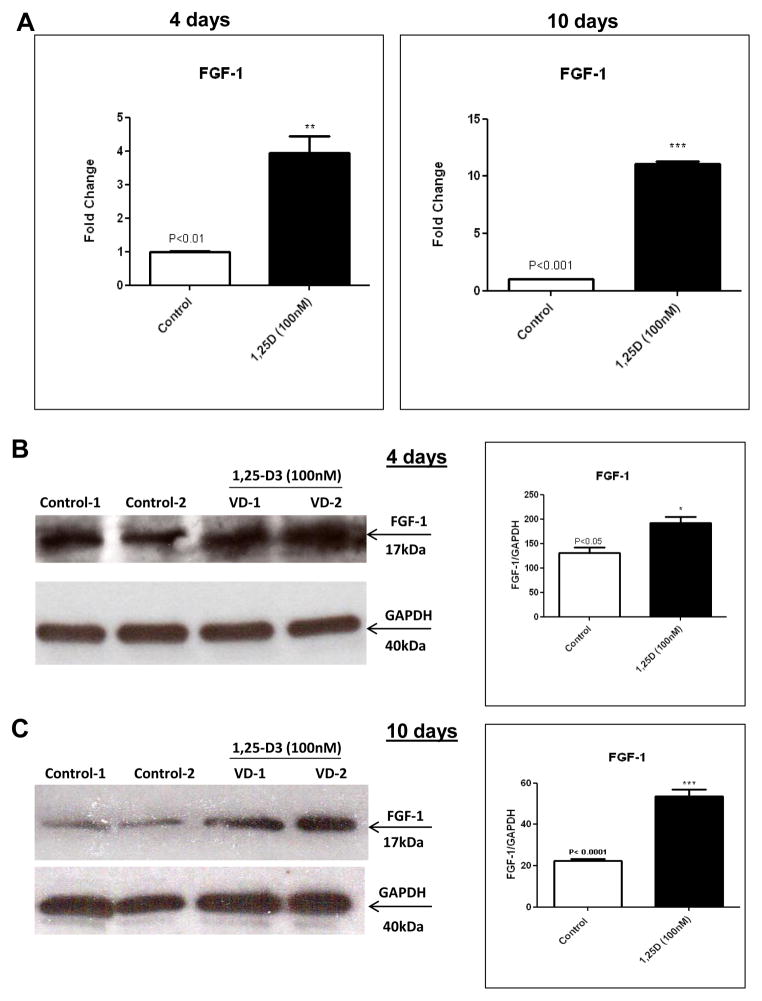
3.2. 1,25-D3 increases FGF-1 expression in C2C12 skeletal muscle cells
The data obtained by PCR arrays and proteome profiler arrays where further confirmed by individual real-time PCR and western blots respectively both done in triplicate. The real-time PCR data showed an increase in the expression of FGF-1 by 3.10-fold after 4 days and by 10.73-fold after 10 days of continuous incubation with 1,25-D3 (Figure 2, A). The changes at the level of protein expression were estimated by western blots with the corresponding densitometric analysis and showed a similar trend for FGF-1 at 4 days (Figure 2, B) and at 10 days (Figure 2, C).
Figure 2. Steady state mRNA and protein up-regulation levels of FGF-1 expression upon incubation of C2C12 cells with 1,25-D3.
Cultures of C2C12 cells were incubated with or without 1,25-D3 (100nM) for 4 and 10 days. Total RNA and whole protein extracts were isolated for qRT-PCR and western blots respectively (A) Mean ± SEM corresponds to experiments done in triplicate, **p<0.01 and ***p<0.001 and western blots (B) and (C) Mean ± SEM corresponds to experiments done in triplicate, *p<0.05 and ***p<0.001 at 4 and 10 days respectively. Control-1, Control-2, VD1 and VD2 are different pools of two samples each. In both cases real time PCRs and western blots, samples and controls were normalized with GAPDH housekeeping gene.
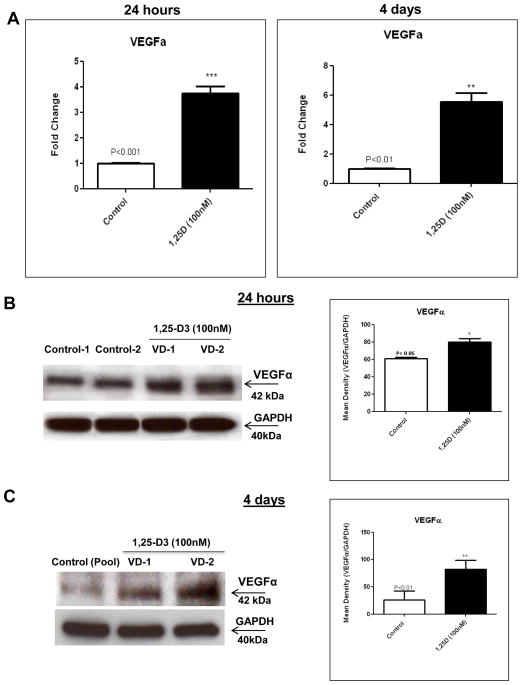
3.3. 1,25-D3 increases VEGFa expression in C2C12 skeletal muscle cells
The increased expression of VEGFa observed by PCR and proteome profiler arrays were also further confirmed by real-time PCR at 24 h (4.21-fold) and at 4 days (4.57-fold) (Figure 3, A) and at the protein level by western blots with the corresponding densitometric analysis at 24h (Figure 3, B) and at 4 days (Figure 3, C).
Figure 3. Steady state mRNA and protein up-regulation levels of VEGFa expression upon incubation of C2C12 cells with 1,25-D3.
Cultures of C2C12 cells were incubated with or without 1,25-D3 for 24hs and 4 days. Total RNA and whole protein extracts were isolated for qRT-PCR and western blots respectively (A) Mean ± SEM corresponds to experiments done in triplicate, **p<0.01 and ***p<0.001 and western blots (B) and (C) Mean ± SEM corresponds to experiments done in triplicate, *p<0.05 and ***p<0.001 at 4 and 10 days respectively. Control-1, Control-2, Control (pool), VD1 and VD2 are different pools of two samples each. In both cases real time PCRs and western blots, samples and controls were normalized with GAPDH housekeeping gene.
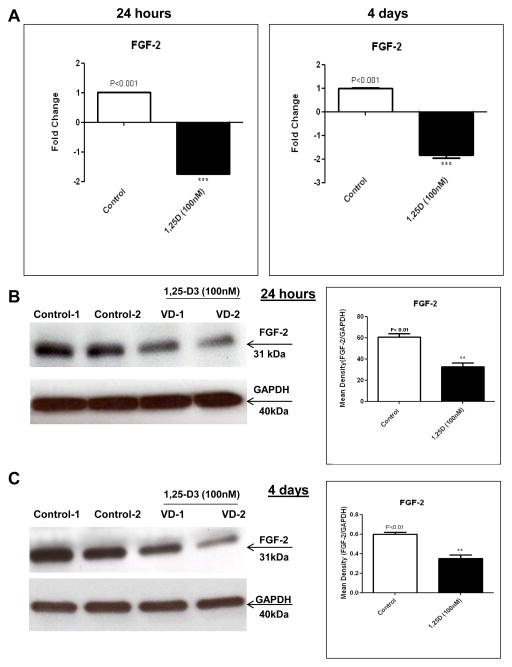
3.4. 1,25-D3 decreases FGF-2 expression in C2C12 skeletal muscle cells
In agreement with the results obtained by real time PCR and proteome profiler arrays at 24h and 4 days, a down-regulation of FGF-2 was further confirmed by real time PCR (1.74-fold, 24h) and (1.60-fold, 4 days) Figure 4, A and at the protein level by western blot with the corresponding densitometric analysis at 24hs (Figure 4, B) and at 4 days (Figure 4, C).
Figure 4. 1,25-D3 down-regulates the expression of FGF-2.
Cultures of C2C12 cells were treated as in Fig 3 for 24hs and 4 days. Total RNA and whole protein extracts were isolated for qRT-PCR and western blots respectively (A) Mean ± SEM corresponds to experiments done in triplicate, **p<0.01 and western blots (B) and (C) Mean ± SEM corresponds to experiments done in triplicate, *p<0.05 and **p<0.01 at 24hs and 4 days respectively. Control-1, Control-2, VD1 and VD2 are different pools of two samples each. In both cases real time PCRs and western blots, samples and controls were normalized with GAPDH housekeeping gene.
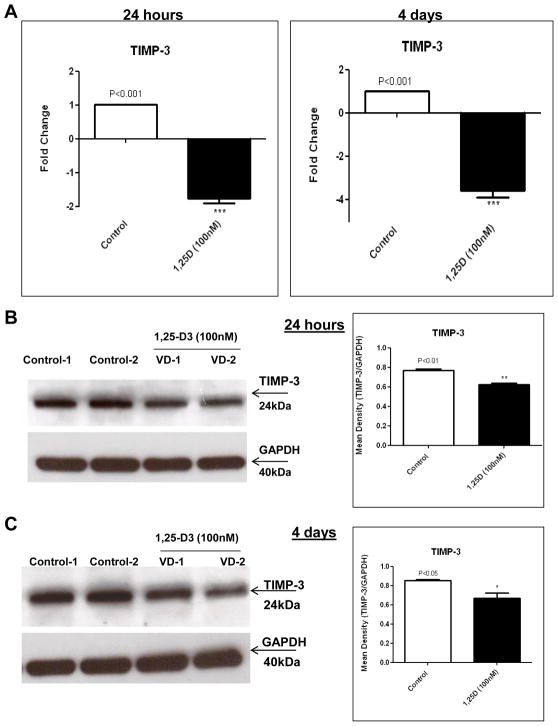
3.5. 1,25-D3 decreases TIMP-3 expression in C2C12 skeletal muscle cells
TIMP-3 is considered a potent inhibitor of angiogenesis by binding directly to VEGF-R2 and hence blocking the pro-angiogenic activity of VEGFa [17]. We demonstrated by PCR array a down-regulation of the expression of TIMP-3 upon incubation with 1,25-D3. A further confirmation of the down-regulation was observed by real-time PCR at 24h (1.76-fold) and at 4 days (3.74-fold), Figure 5, A.
Figure 5. 1,25-D3 down-regulates the expression of TIMP-3.
Cultures of C2C12 cells were treated as in Fig 3 for 24hs and 4 days. Total RNA and whole protein extracts were isolated for qRT-PCR and western blots respectively (A) Mean ± SEM corresponds to experiments done in triplicate, ***p<0.001 and western blots (B) and (C) Mean ± SEM corresponds to experiments done in triplicate, *p<0.05 and **p<0.01 at 24hs and 4 days respectively. Control-1, Control-2, VD1 and VD2 are different pools of two samples each. In both cases real time PCRs and western blots, samples and controls were normalized with GAPDH housekeeping gene.
A decreased expression of TIMP-3 at the protein level was confirmed by western blot with the corresponding densitometric analysis at 24 h (Figure 5, B) and at 4 days (Figure 5, C).
4. Discussion
The data presented in this manuscript demonstrate that the addition of 1,25-D3 to C2C12 skeletal muscle cells enhance the expression of key angiogenesis growth factors and decrease the expression of essential angiogenesis/myogenesis inhibitors, promoting the myogenic process. Specifically, we demonstrated that the angiogenic effect of 1,25-D3 on C2C12 skeletal muscle cells involves 1) an up-regulation of two pro-angiogenic growth factors: FGF-1 and VEGFa which have been shown to promote muscle differentiation as well as neo-vascularization and tissue regeneration, and 2) decreased expression of an inhibitor of skeletal muscle differentiation, FGF-2 [17]; and 3) down regulation of an angiogenic and myogenic inhibitor TIMP-3 [17, 19].
Consistent with our data, it has been proposed that the equilibrium between pro- and anti-angiogenic factors is also needed to regulate and maintain angiogenesis under physiologic conditions, such as wound healing [19]. In other words, it is unlikely that one factor solely controls the angiogenic process. Indeed, it is more likely that the delicate balance between stimulators and inhibitors, and even more importantly, the temporal expression that ultimately determines whether or not microvessel density and the differentiation process will be altered. Angiogenesis is defined not only as a vital process for new capillary development and tissue repair during the adult life span but also for tissue regeneration, differentiation and wound healing [35]. Regenerative therapies for skeletal muscle injuries and disorders such as muscle wasting require the revascularization of the scarred tissue as well as myofiber regeneration during the wound healing process, and collectively we have shown in the present manuscript and in our previous study [14] that vitamin D replenishment could be a crucial supplement to enhance the myogenic process.
The increase expression of FGF-1 induced by 1,25D-3 agrees with the concept that FGF-1 up-regulation is required for myoblast differentiation since FGF-1 knock-down by siRNA attenuates Myogenin induction originating in abnormal myotubes [16]. Moreover, it has been shown that FGF-1 is expressed in dystrophic muscle, suggesting a positive role in the regeneration of skeletal muscle fibers [36, 37]. Furthermore, previous studies have evaluated the administration of FGFs directly to the sites of wounds, similar to that of other growth factors [38]. However, when free FGF-1 solutions were injected in vivo, they rapidly lose their biological functional activity, primarily due to diffusional loss and/or enzymatic inactivation/degradation [39, 40]. Using 1,25-D3 to enhance FGF-1 endogenous expression could be beneficial rather than directly administering FGF-1 externally due its instability.
The increase in the expression of VEGFa induced after 1,25-D3 incubation reinforces the role of vitamin D as a “natural” myogenic enhancer without the difficulty of gene transfer techniques, since published data demonstrate that VEGF promotes the growth of myogenic fibers and protects the myogenic cells from apoptosis in vitro and in vivo [41]. The increase local expression of VEGFa in vivo was achieved using an AAV-VEGFa vector (adeno-associated virus-VEGF vector), which exerted a powerful effect on skeletal muscle regeneration in CD1 mice [41].
We found no change in the expression of FGF-1 at 24h but observed a constant increase from 4 to 10 days. By contrast VEGFa expression increased at 24h, peaked at 4 days, and leveled off at 10 days. Previous studies have investigated the interaction between MyoD, an early myogenic marker, with VEGFa and its receptors. They observed that MyoD is imperative for increasing the expression of VEGFa, in C2C12 differentiating cells, through its direct interaction at the VEGF promoter region [17]. These results agree and provide a possible explanation for our previous findings that showed that 1,25-D3 treated cells increased MyoD expression during the time frame that we observed [14] an increase in VEGFa expression in the present study. Notably, it has been demonstrated that the VEGFa increases IGF-II, and neither showed any changes at day 10 of incubation with 1,25-D3 in the present or previous study [42]. Myotube formation, which is a late event in myogenic differentiation, has been observed to be greatly dependent on the presence of FGF-1. Conte et al. observed that FGF-1 silenced C2C12 cells resulted in delayed and abnormal myotube formation [16]. They concluded that FGF-1 was required during muscle regeneration/differentiation and required for correct myotube formation [16]. This supports our previous and present study where we observed that the addition of 1,25-D3 resulted in an increase expression of MHC type II, a late marker of myogenesis and fiber hypertrophy [14], and at the same time an increased of FGF-1 expression. Combined with supporting literature, we interpret our results by the following: VEGFa is required at the initial stages of the myogenic differentiation while FGF-1 acts as the main driving force for mature, functional, myotube formation during muscle differentiation and repair.
Concerning the sustained decreased expression of FGF-2 upon incubation of muscle cells with 1,25-D3; FGF-2 has been described as an inhibitor of skeletal muscle differentiation, which operates by activating PDGF independent signaling pathways [43]. Furthermore, it has also been proposed that FGF-2 could possibly play a role in the genesis of muscular disorders since release of FGF-2 may be responsible for several of the abnormalities associated with muscular dystrophy, including suppression of muscular skeletal differentiation and excessive fibrosis. Certainly, the MDX mouse, which serves as a model of Duchenne’s myopathy, displays extracellular FGF-2 surrounding myofibers compared with normal mice [44]. In addition, plasma levels of FGF-2 are elevated in many muscular dystrophy patients but are undetectable in control patients [45]. These previous reports support our results that show the down-regulation of FGF-2 associated with 1,25-D3 incubation, not only would be beneficial in terms of myogenesis enhancement by 1,25-D3 but also could be a potential therapeutic option in the treatment of muscle disorders.
Moreover, muscle differentiation is characterized by a down regulation of IGF-I as well as an up-regulation of IGF-II [46]. Since FGF-2 (bFGF) is a known skeletal muscle differentiation inhibitor, Rosenthal et al. incubated BC3H-1 muscle cells with FGF-2 and found an increase in IGF-I binding as well as a decrease in IGF-II [46]. These results support our previous findings that 1,25-D3 reduced expression of FGF-2 and IGF-I while simultaneously increasing the expression of IGF-II [14].
Finally, our data demonstrates that the incubation of muscle cells with 1,25-D3 decreases the expression of TIMP-3, a factor that was previously described as a member of a family of proteins that were classified according to their ability to inhibit matrix metalloproteinases (MMP) [47, 48]. Subsequently, it was reported that TIMP-3 also functioned as a potent angiogenic inhibitor due to its ability to block the binding of VEGF to KDR (also known as VEGFR2 and FLK-1), thereby inhibiting the downstream signaling pathways necessary to stimulate cell differentiation and angiogenesis [49]. This property appeared to be independent of its MMP-inhibitory activity [49]. This is consistent with our findings that showed that TIMP-3 was down-regulated, while VEGFa is up-regulated at 24 hours and 4 days upon 1,25-D3 incubation. Moreover, since overexpression of TIMP-3 in satellite cells of regenerating mouse hindlimb muscles and in differentiating C2C12 myoblasts blocks myogenic gene expression and myotube formation [20], our data strongly indicate that the decreased expression of TIMP-3 upon incubating C2C12 myoblasts with 1,25-D3 enhanced the myogenic process in our skeletal muscle model. These results are in agreement with Rahman et all, which demonstrated that 1,25-D3 modulates the expression of MMPs and TIMPs (TIMP-1 and TIMP-3) in heart, suggesting that 1,25-D3 plays an important role in extracellular matrix remodeling [50].
In summary, the data presented in this manuscript demonstrated that supplementation of 1,25-D3 to C2C12 myoblasts increased VEGFa and FGF-1; two well described pro-angiogenic growth factors that promote neo-vascularization, tissue regeneration, and myogenesis. In addition, 1,25-D3 supplementation simultaneously decreased FGF-2 and TIMP-3 expression, two main angiogenic/myogenic inhibitors, which both have been described to promote myogenic inhibition through FGF-2’s interaction with IGFs.
These results reinforce our previous findings in skeletal muscle cells and contribute to a more comprehensive description of the mechanism by which 1,25-D3 promotes myogenesis through the orchestration of angiogenesis growth factors and inhibitors. Further “in vivo” studies need to de done, in order to demonstrate the biological significance for muscle development and also show whether 1,25-D3 can directly stimulate angiogenesis. In agreement with our results, expression of VDR has been shown in myoblasts cell lines G8 [8] and C2C12 [14, 25] and recently the expression of VDR and CYP27B1 was found in regenerating skeletal muscle in vivo [25], suggesting a potential role for vitamin D3 in skeletal muscle regeneration following injury.
Vitamin D deficiency has been linked to sarcopenia and frailty [51] and it is particularly detrimental for patients with kidney disease who are unable to produce the active form [52]. Therefore, treatment of muscle disorders by administering vitamin D and/or vitamin D analogues could potentially provide an easily administrable supplement to treat or prevent muscle wasting and can also provide an alternative solution or adjuvant for therapies that directly manipulate VEGF and FGF’s to promote angiogenesis, wound healing and muscle regeneration.
Summary.
1,25(OH)2vitamin D3 promotes myogenesis by modulating several key angiogenic growth factors and angiogenesis inhibitors; 1,25-D3 increases VEGFa, FGF-1 and decreases FGF-2 and TIMP-3. This study provides a rationale for assessing vitamin D replenishment in muscle wasting conditions.
Highlights.
1,25-D3 promotes myogenesis in C2C12 myoblast cells
1,25-D3 modulates key angiogenic growth factors and angiogenic inhibitors.
Addition of 1,25-D3 to C2C12 myoblasts increases VEGFa and FGF-1 expression.
1,25-D3 decreases FGF-2 and TIMP-3: two myogenic and/or angiogenic inhibitors.
Acknowledgments
Grant Support: RR026138, RR022762, MD000182, SC1NS064611 and MD000103
Abbreviations
- 1,25-D3
1,25-dihydroxyvitamin D3
- VDR
vitamin D receptor
- VEGFa
vascular endothelial growth factor-a
- FGF-1
fibroblast growth factor-1
- FGF-2
fibroblast growth factor-2
- TIMP-3
tissue inhibitor of metalloproteinase-3
- IGF
insulin-like growth factor
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Andersen H, Charles P, Eriksen EF. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int. 2000;66(6):419–24. doi: 10.1007/s002230010085. [DOI] [PubMed] [Google Scholar]
- 2.Bordelon P, Ghetu MV, Langan RC. Recognition and management of vitamin D deficiency. Am Fam Physician. 2009;80(8):841–6. [PubMed] [Google Scholar]
- 3.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, Dawson-Hughes B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 4.Gerdhem P, Ringsberg KA, Obrant KJ, Akesson K. 2005 Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int. 2005;16:1425–1431. doi: 10.1007/s00198-005-1860-1. [DOI] [PubMed] [Google Scholar]
- 5.Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, Knol DL, Lips P. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 6.Shardell M, Hicks GE, Miller RR, Kritchevsky S, Andersen D, Bandinelli S, Cherubini A, Ferrucci L. Association of low vitamin D levels with the frailty syndrome in men and women. J Gerontol A Biol Sci Med Sci. 2009;64:69–75. doi: 10.1093/gerona/gln007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, Meyer HE, Pfeifer M, Sanders KM, Stähelin HB, Theiler R, Dawson-Hughes B. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med 5. 2012;367(1):40–9. doi: 10.1056/NEJMoa1109617. [DOI] [PubMed] [Google Scholar]
- 8.Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem. 1985;260(15):8882–91. [PubMed] [Google Scholar]
- 9.Wang Y, DeLuca HF. Is the vitamin d receptor found in muscle? Endocrinology. 2011;152(2):354–63. doi: 10.1210/en.2010-1109. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff H, Borchers M, Gudat F, Duermueller U, Theiler R, Stahelin H, Dick W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33:19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff-Ferrari HA, Borchers M, Gudat F, Durmuller U, Stahelin HB, Dick W. 2004 Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19(2):265–9. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 12.Bouillon R, Bischoff-Ferrari H, Willett W. Vitamin D and health: perspectives from mice and man. J Bone Miner Res. 2008;23:974–9. doi: 10.1359/jbmr.080420. [DOI] [PubMed] [Google Scholar]
- 13.Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T, Kato S, Matsumoto T. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 2003;144:5138–44. doi: 10.1210/en.2003-0502. [DOI] [PubMed] [Google Scholar]
- 14.Garcia LA, King KK, Ferrini MG, Norris KC, Artaza JN. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology. 20011;152(8):2976–86. doi: 10.1210/en.2011-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunelli S, Rovere-Querini P. The immune system and the repair of skeletal muscle. Pharmacol Res. 2008;58:117–121. doi: 10.1016/j.phrs.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Conte C, Ainaoui N, Delluc-Clavières A, Khoury MP, Azar R, Pujol F, Martineau Y, Pyronnet S, Prats AC. Fibroblast growth factor 1 induced during myogenesis by a transcription-translation coupling mechanism. Nucleic Acids Res. 2009;37(16):5267–78. doi: 10.1093/nar/gkp550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryan BA, Walshe TE, Mitchell DC, Havumaki JS, Saint-Geniez M, Maharaj AS, Maldonado AE, D’Amore PA. 2008 Coordinated vascular endothelial growth factor expression and signaling during skeletal myogenic differentiation. Mol Biol Cell. 2008;19(3):994–1006. doi: 10.1091/mbc.E07-09-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frey SP, Jansen H, Raschke MJ, Meffert RH, Ochman S. VEGF Improves Skeletal Muscle Regeneration After Acute Trauma and Reconstruction of the Limb in a Rabbit. Clin Orthop Relat Res. 2012 Jul 18; doi: 10.1007/s11999-012-2456-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olfert IM, Birot O. Importance of anti-angiogenic factors in the regulation of skeletal muscle angiogenesis. Microcirculation. 2011;18(4):316–30. doi: 10.1111/j.1549-8719.2011.00092.x. Review. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Chen SE, Jin B, Carson JA, Niu A, Durham W, Lai JY, Li YP. TIMP3: a physiological regulator of adult myogenesis. J Cell Sci. 2010;1;123(Pt 17):2914–21. doi: 10.1242/jcs.057620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yun YR, Won JE, Jeon E, Lee S, Kang W, Jo H, Jang JH, Shin US, Kim HW. Fibroblast growth factors: biology, function, and application for tissue regeneration. J Tissue Eng. 2010;218142:18. doi: 10.4061/2010/218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997;18(1):26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- 23.Guthridge M, Wilson M, Cowling J, Bertolini J, Hearn MT. The role of basic fibroblast growth factor in skeletal muscle regeneration. Growth Factors. 1992;6(1):53–63. doi: 10.3109/08977199209008871. [DOI] [PubMed] [Google Scholar]
- 24.Holick MF. 2011 Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12(1):4–18. doi: 10.2174/138945011793591635. [DOI] [PubMed] [Google Scholar]
- 25.Srikuea R, Zhang X, Park-Sarge OK, Esser KA. VDR and CYP27B1 are Expressed in C2C12 Cells and Regenerating Skeletal Muscle: Potential Role in Suppression of Myoblast Proliferation. Am J Physiol Cell Physiol. 2012;303(4):C396–405. doi: 10.1152/ajpcell.00014.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willett M, Cowan JL, Vlasak M, Coldwell MJ, Morley SJ. Inhibition of mammalian target of rapamycin (mTOR) signaling in C2C12 myoblasts prevents myogenic differentiation without affecting the hyperphosphorylation of 4E-BP1. Cell Signal. 2009;21(10):1504–12. doi: 10.1016/j.cellsig.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Artaza JN, Bhasin S, Mallidis C, Taylor W, Ma K, Gonzalez-Cadavid NF. Endogenous expression and localization of myostatin and its relation to myosin heavy chain distribution in C2C12 skeletal muscle cells. J Cell Physiol. 2002;190(2):170–9. doi: 10.1002/jcp.10044. [DOI] [PubMed] [Google Scholar]
- 28.Artaza JN, Sirad F, Ferrini MG, Norris KC. 1,25(OH)2 vitamin D3 inhibits cell proliferation by promoting cell cycle arrest without inducing apoptosis and modifies cell morphology of mesenchymal multipotent cells. J Steroid Biochem Mol Biol. 2010;119(1–2):73–83. doi: 10.1016/j.jsbmb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Artaza JN, Norris KC. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J Endocrinol. 2009;200(2):207–21. doi: 10.1677/JOE-08-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardus A, Parisi E, Gallego C, Aldea M, Fernandez E, Valdivielso JM. 1,25-Dihydroxyvitamin D3 stimulates vascular smooth muscle cell proliferation through a VEGF-mediated pathway. Kidney Int. 2006;69(8):1377–84. doi: 10.1038/sj.ki.5000304. [DOI] [PubMed] [Google Scholar]
- 31.Khanna-Jain R, Vuorinen A, Sandor GK, Suuronen R, Miettinen S. 2010 Vitamin D (3) metabolites induce osteogenic differentiation in human dental pulp and human dental follicle cells. J Steroid Biochem Mol Biol. 122(4):133–41. doi: 10.1016/j.jsbmb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Barbosa EM, Nonogaki S, Katayama ML, Folgueira MA, Alves VF, Brentani MM. Vitamin D3 modulation of plasminogen activator inhibitor type-1 in human breast carcinomas under organ culture. Virchows Arch. 2004;444(2):175–82. doi: 10.1007/s00428-003-0929-5. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez AM, Wongtrakool C, Welch T, Steinmeyer A, Zugel U, Roman J. 2010 Vitamin D inhibition of pro-fibrotic effects of transforming growth factor beta1 in lung fibroblasts and epithelial cells. J Steroid Biochem Mol Biol. 2010;118(3):142–50. doi: 10.1016/j.jsbmb.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Floss T, Arnold HH, Braun T. 1997 A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 1997;15;11(16):2040–51. doi: 10.1101/gad.11.16.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 36.Oliver L, Raulais D, Vigny M. 1992 Acidic fibroblast growth factor (aFGF) in developing normal and dystrophic (mdx) mouse muscles. Distribution in degenerating and regenerating mdx myofibres. Growth Factors. 1992;7:97–106. doi: 10.3109/08977199209046399. [DOI] [PubMed] [Google Scholar]
- 37.Saito A, Higuchi I, Nakagawa M, Saito M, Uchida Y, Inose M, Kasai T, Niiyama T, Fukunaga H, Arimura K, Osame M. An overexpression of fibroblast growth factor (FGF) and FGF receptor 4 in a severe clinical phenotype of facioscapulohumeral muscular dystrophy. Muscle Nerve. 2000;23:490–497. doi: 10.1002/(sici)1097-4598(200004)23:4<490::aid-mus6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 38.Doukas J, Blease K, Craig D, Ma C, Chandler LA, Sosnowski BA, Pierce GF. Delivery of FGF genes to wound repair cells enhances arteriogenesis and myogenesis in skeletal muscle. Molecular Therapy. 2002;5(5):517–527. doi: 10.1006/mthe.2002.0579. [DOI] [PubMed] [Google Scholar]
- 39.Andreopoulos FM, Persaud I. Delivery of basic fibroblast growth factor (bFGF) from photoresponsive hydrogel scaffolds. Biomaterials. 2006;27(11):2468–2476. doi: 10.1016/j.biomaterials.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 40.Cai S, Liu Y, Xiao ZS, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26(30):6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M. 2004 Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004;10(5):844–54. doi: 10.1016/j.ymthe.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Karvinen H, Pasanen E, Rissanen TT, Korpisalo P, Vähäkangas E, Jazwa A, Giacca M, Ylä-Herttuala S. Long-term VEGF-A expression promotes aberrant angiogenesis and fibrosis in skeletal muscle. Gene Ther. 2011;18(12):166–72. doi: 10.1038/gt.2011.66. [DOI] [PubMed] [Google Scholar]
- 43.Kundla AJ, John ML, Bowen-Pope DF, Rainish B, Olwin BB. A requirement for fibroblast growth factor in regulation of skeletal muscle differentiation cannot be replaced by activation of platelet-derived growth factor signaling pathways. Mol Cell Biol. 1995;15:3238–3246. doi: 10.1128/mcb.15.6.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson JE, Kakulas BA, Jacobson PF, Johnson RD, Kornegay JN, Ground MD. Comparison of basic fibroblast growth factor in X-linked dystrophin-deficient myopathies of human, dog and mouse. Growth Factors. 1993;9:107–121. [PubMed] [Google Scholar]
- 45.D’Amore P, Brown RH, Ku RT, Hoffman E, Watanabe H, Arahala K, Ishihara T, Folkman J. Elevated basic fibroblast growth factor serum levels of patients with Duchenne’s muscular dystrophy. Ann Neurol. 1994;35:362–365. doi: 10.1002/ana.410350320. [DOI] [PubMed] [Google Scholar]
- 46.Rosenthal SM, Brown EJ, Brunetti A, Goldfine ID. Fibroblast growth factor inhibits insulin-like growth factor-II (IGF-II) gene expression and increases IGF-I receptor abundance in BC3H-1 muscle cells. Mol Endocrinol. 1991;5(5):678–84. doi: 10.1210/mend-5-5-678. [DOI] [PubMed] [Google Scholar]
- 47.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;30, 274(31):21491–4. doi: 10.1074/jbc.274.31.21491. Review. [DOI] [PubMed] [Google Scholar]
- 48.Woessner JF., Jr MMPs and TIMPs. An historical perspective. Methods Mol Biol. 2001;151:1–23. [PubMed] [Google Scholar]
- 49.Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9(4):407–15. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- 50.Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007;103(3–5):416–9. doi: 10.1016/j.jsbmb.2006.12.081. [DOI] [PubMed] [Google Scholar]
- 51.Rolland Y, Dupuy C, Abellan van Kan G, Gillette S, Vellas B. Treatment strategies for sarcopenia and frailty. Med Clin North Am. 20011;95(3):427–38. doi: 10.1016/j.mcna.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 52.Jones G. Expanding role for vitamin D in chronic kidney disease: importance of blood 25-OH-D levels and extra-renal 1alpha-hydroxylase in the classical and nonclassical actions of 1alpha, 25-dihydroxyvitamin D (3) Semin Dial. 2007;20(4):316–24. doi: 10.1111/j.1525-139X.2007.00302.x. [DOI] [PubMed] [Google Scholar]