Abstract
The addictive nature of nicotine remains a global health problem. Despite the availability of treatments for smoking cessation, relapse to smoking after quit attempts still remains very high. Here, we evaluated the effects of chronic nicotine in male C57BL/6J mice in an operant cognitive flexibility task that required the animals to progress sequentially through multiple phases including visual discrimination, strategy shifting and response reversal. As frontostriatal circuits involving discrete regions of dorsal striatum contribute directly to decision-making processes, and BDNF modulates synaptic plasticity and learning, we also assessed the effects of nicotine on striatal BDNF expression. Osmotic minipumps containing either of the two doses of nicotine (low: 6.3 mg/kg/day; high: 18 mg/kg/day) or saline (control) were implanted for chronic delivery that lasted 4 weeks. Nicotine-treated mice exhibited greater response accuracy during visual discrimination. Neither dose of nicotine affected learning of new egocentric response strategy during set-shifting. However, higher but not lower dose of nicotine impaired reversal learning by increasing perseverative responding to the previously non-reinforced stimulus. Furthermore, this effect was associated with reduced BDNF levels in the dorsal striatum. Collectively, these findings suggest that higher relapse rates often observed in high nicotine-dependent smokers may be attributed to impairments in inhibitory control processes. Moreover, striatal BDNF may play a critical role in nicotine-induced alterations in cognitive flexibility.
Keywords: Nicotine, addiction, cognitive flexibility, BDNF, mice
1. Introduction
Nicotine, a major psychoactive and addictive component of tobacco smoke, exerts diverse motivational and behavioral/cognitive effects. Smoking begins as a voluntary habit but eventually it takes the form of addiction. The addictive nature of nicotine remains a global health problem and smoking-related illness is the largest preventable cause of death in the United States [1]. Despite the availability of treatments for smoking cessation, relapse to smoking after quit attempts still remains very high [2]. Therefore, understanding the neurobiology of nicotine addiction remains an elusive goal.
Neural circuits of addiction extensively overlap with those that support cognitive functions; cognitive processes interact with chronic drug-induced neuroadaptations to promote compulsive drug use [3–5]. Although the initiation of tobacco use involves the primary reinforcing effects of nicotine, the development of nicotine dependence and relapse during withdrawal in habitual smokers is triggered by smoking-associated contextual cues that provide a stronger urge to smoke [6]. Acute administration of nicotine, like other drugs of abuse, is proposed to initiate a learning process that promotes stronger associations between the rewarding effects of drugs and environmental stimuli [5, 7]. These drug-stimulus associations following chronic drug use foster hypersensitization of neural circuits mediating incentive salience leading to drug-seeking and drug-taking [8]. Successful suppression of such maladaptive drug-stimulus associations require activation of cognitive control mechanisms that could compete with prepotent or habitual responses, and eventually refrain individuals from taking drugs. The frontostriatal circuits subserve executive functions including cognitive control and decision-making processes [9], and disruption in these circuits may contribute to compulsive drug use and loss of control in addiction [10].
Cognitive deficits in drug addicts manifest primarily as the inability to change responding to stimuli previously associated with drug stimulus or reward. The ability to switch behavioral responses adaptively between changing stimuli and environments, termed “cognitive flexibility”, is a key component of executive function, and is used to probe cognitive dysfunction in addiction [11]. Human studies indicated that several domains of executive functions, including cognitive flexibility, are compromised in drug addicts [12–14]. Likewise, substantial evidence from animal studies suggests that chronic administration of psychostimulants impair attentional set-shifting and reversal learning [15–18]. Although, the effects of nicotine on alterations in motivational states and aberrant salience attribution to drug stimulus are known to involve mesolimbic dopaminergic circuits [19], chronic nicotine use may also produce neuroadaptations in the frontostriatal circuits that disrupt top-down cognitive control and foster the switch from voluntary nicotine use to compulsive drug use [20]. However, to date only a handful of studies have evaluated the effects of nicotine dependence on measures of cognitive flexibility. More importantly, the available data from these studies are conflicting, due either to variations in the level of nicotine dependence or withdrawal duration [21–23].
The present study examined the effects of chronic nicotine on cognitive flexibility in mice using an operant-based behavioral task that required the animals to shift strategies either from one dimension to another or to reverse response strategy within the same dimension. Chronic nicotine was administered using subcutaneous osmotic minipumps and the effects of two different doses modeling low- and high-nicotine dependence states were evaluated. As brain-derived neurotrophic factor (BDNF) facilitates learning [24], promotes LTP in the corticostriatal projections [25], and is implicated in nicotine addiction [26], we also assessed the effects of chronic nicotine on BDNF levels in the dorsal striatum following completion of the behavioral testing.
2. Materials and Methods
2.1. Subjects
Male C57BL/6J mice (PND 42–56) were purchased from Jackson Laboratories (Bar Harbor, ME). The animals were housed individually in a temperature-/humidity-controlled environment with a 12-hour dark/light cycle (07:00 lights on). One week prior to the task onset, animals were progressively water-restricted to 5-min of water per day. Mice were 10 weeks old and weighed 23–28 g at the beginning of experiments. All behavioral training and testing took place 7 days/week between 9:00–16:00 h. At the completion of each operant session, mice received 5-min of water in addition to sweetened water received as a reward for each correct response (see below). Food (PMI LabDiet) was available ad libitum throughout the experiment. All experimental procedures were approved by the Institutional Care and Use Committee (IACUC) of Temple University and were in accordance with National Institute of Health guidelines.
2.2. Mouse Operant Cognitive Flexibility Task
The mouse behavioral task used in the present study to examine the effects of nicotine on flexible decision-making was adapted and modified from a rat behavioral flexibility task developed by Floresco and colleagues [27, 28]. This task uses an automated operant procedure and shares similar features as the cross-maze task to assess both complementary forms of cognitive flexibility i.e. extradimensional set-shifting and reversal learning [29–32]. The presentations of same set of stimuli remained constant throughout all the stages of the task. Moreover, animals were provided with a feedback (extinguished house light; see below) in case they made an incorrect choice. These features make it analogous to the Wisconsin Card Sorting Task used to assess cognitive flexibility in humans [33].
2.2.1. Apparatus
Behavioral training occurred in classic mouse modular operant chambers (interior dimensions: 15.9 cm L × 14.0 cm W × 12.7 cm H; MED Associates; St. Albans, VT). Each chamber was equipped with a standard grid floor, a standard house light (28 V DC, 100 mA), and a panel consisting of two large cue lights (2.5 cm; 28 V DC, 100 mA), a central reward port attached to a fluid dipper with a 10 μl cup, and two ultra-sensitive retractable levers (ENV-312-2M). Operant chambers were housed in sound-attenuating PVC cubicles equipped with an exhaust fan (28 V DC) that provided ventilation and low-level background noise. All events including visual cue presentation, lever operations and reward delivery were controlled using programs written in Medstate notation via 8 input/16 output SmrtCtrl™ interface running MED-PC IV software (Med Associates) on a PC (Dell OptiPlex™ 960).
2.2.2. Autoshaping
Mice were autoshaped on a FR-1 schedule of reinforcement to acquire the lever press response and subsequent reinforcement (10 μl of .066% saccharin solution). During this phase both levers were available for the duration of a 30 minute session. Additionally, noncontingent reinforcers were delivered every 10 minutes to facilitate the association between activation of the dipper and reward availability. To prevent the development of side bias, lever presses on the dominant lever (i.e., the lever with ≥ 5 more presses) ceased to be reinforced until the discrepancy was made up on the opposing lever. Animals were required to obtain at least 30 rewards during each 30-min session for three consecutive days. After attaining criterion, the animals were advanced to the pretraining phase.
2.2.3. Pretraining
A session began with the illumination of a house light. After an inter-trial interval (ITI) of 9 ± 3s, a lever (either left or right) was presented and remained active for 10 s or until a lever press occurred. Each lever press within the allotted time was reinforced. If no lever press occurred within 10 s, an “omission” was scored and the ITI was reinstated. Lever presentations were completely randomized with no more than 5 activations from the same side. To control for any novelty effects associated with the visual stimulus during later phases of training, trials were randomly associated with unpredictably occurring visual cues (presented only in 50% of trials) that involved illumination of the panel light above the lever. A lever press on the cued trials coterminated both the visual cue and the lever. To reach criterion, animals were required to achieve 30 rewards and <20% omissions for three consecutive days. After attaining criterion performance, mice were prepared for chronic saline/nicotine administration and progressed to subsequent stages of the task (see below). Animals that failed to complete pretraining within 15 days were removed from the study. In our pilot studies, we found minimal omissions (< 10%) over a 30-trial session as opposed to longer (40–120 trials) training sessions where omissions approached 25–45%. Unlike rats that show lower rates of omission in longer behavioral sessions, the tendency to perform operant tasks in mice decreases with time either due to different satiation or motivational mechanisms [34]. Therefore, each behavioral session was restricted to 30 trials only in subsequent phases of the task.
2.2.3. Visual Discrimination
Acquisition of visual discrimination task required animals to discriminate the lever with an activated cue light (see Figure 1A). Session began with the illumination of house light and presentation of 30 trials with an ITI of 9 ± 3 s. The house light remained illuminated throughout the entire session except for the punishment phase during this stage, as well as during the subsequent stages of the task (see below). All trials were started with the illumination of 7s visual cue (either from the left or right panel), followed by the presentation of both levers 2 s later. Levers were present for 5 s and both the stimulus light and levers were co-terminated together. Illumination of the cue lights was counterbalanced between the two panels (15 each from either side) and remained pseudorandom to avoid more than five consecutive presentations from the same side. A lever press response on the cued lever was scored as a “correct response” and was followed by reward delivery. Responses on the incorrect lever (errors) were not rewarded and resulted in a “time out” period (punishment phase) characterized by a 10s extinguishing of the houselight. Punishment on incorrect responses was introduced to discourage indiscriminate responding to levers during this phase, as well as, in the subsequent phases of the task. Following the completion of punishment phase, the houselight was turned on and the ITI was reinstated. Failure to respond to any of the levers resulted in omissions. Animals trained to criterion (≥ 80% correct responses and < 10% omissions for 3 consecutive days) were advanced to the next phase.
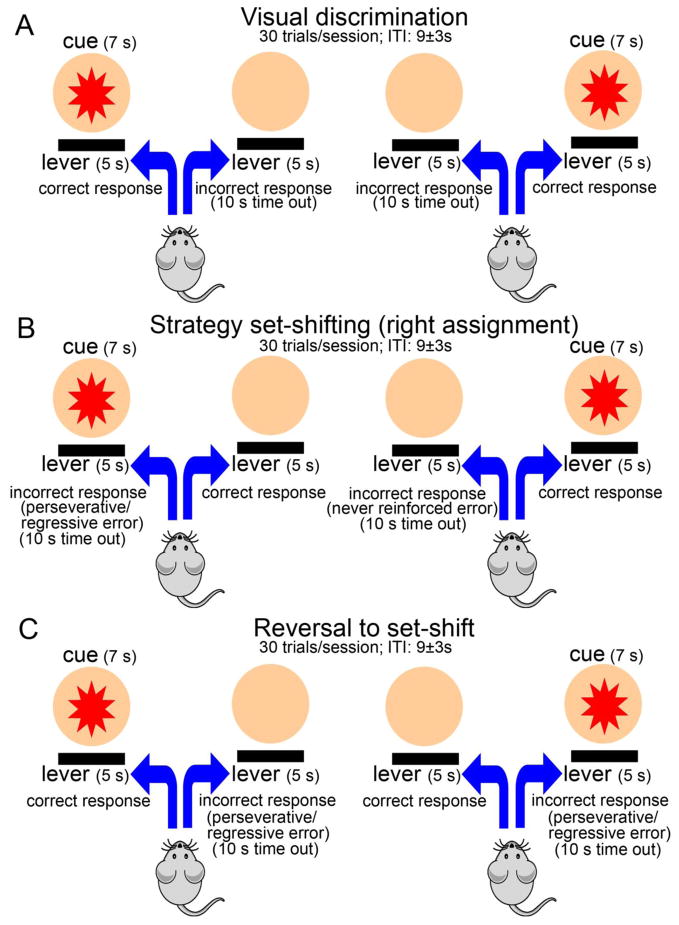
Figure 1.
Illustration of the main events constituting the mouse operant cognitive flexibility task. A session began with the illumination of house light that remained illuminated throughout the entire session except for the punishment phase (see below). Each session consisted of 30 trials separated by an ITI of 9 ± 3 s. All trials were started with the illumination of 7 s visual cue (either from the left or right panel). After 2 s of cue presentation, both levers were activated to allow the animal to make a choice for the correct lever. Levers and cue light co-terminated 5 s later. Each correct response was reinforced with sweetened water while incorrect response resulted in a time out period (punishment) characterized by a 10 s extinguishing of the houselight. (A) During visual discrimination phase, mice were required to press the lever associated with the stimulus light that illuminated above it. (B) Strategy set-shifting entailed a shift from visual cue- to egocentric spatial cue-based strategy. In this phase, the animals were required to press the lever always located at one side (e.g., right in this case) regardless of the position of the cue light. For set-shift to right, a left lever press was scored as an incorrect response (error). If the response was made while the cue light was illuminated above the incorrect lever, the errors were scored either as perseverative or regressive (see Methods for classification). An incorrect lever press in the presence of cue illuminated on the opposite side was scored as never-reinforced error. (C) For response reversal to set-shift, mice were required to always press the lever on the opposite side of the previously reinforced lever (left for set-shift to right). Incorrect responses were scored either as a perseverative or regressive error.
2.2.4. Strategy Set-shifting
Experimental parameters were identical to the previous stage except that the contingencies were altered in such a way that the animals were required to eliminate a visual cue-based strategy and adopt a new egocentric spatial response strategy to achieve rewards. This extradimensional shifting requires switching attention away from a previously reinforced dimension (visual cue) and redirecting attention to the previously irrelevant dimension (spatial location). Mice were required to press the correct lever (e.g. right only; see Figure 1B) to earn a reward irrespective of visual cue presentation which remained random. Responding on incorrect lever in this case (e.g. left if the animal set-shifts to right) resulted in an incorrect response (set-shift error) and led to punishment (10s time out). Half of the animals were trained with the reverse set of rules. Animals were moved to the reversal training after they achieved ≥ 80% correct responses for three consecutive days.
2.2.5. Response reversal learning
During this phase of the training, the proprioceptive rule of the set-shifting task was reversed. The animals were required to press the lever opposite to the one assigned to them during set-shifting, irrespective of the position of the activated cue light, to get a reinforcement. For example, if the animal was required to press the right lever during the set-shifting phase (set-shift to right) to get a reward, it should now press the left lever to make a correct response (Figure 1C). The illumination of the stimulus light above either lever remained random and served as a distracter in these sessions. Correct response reversals occurred when the animal responded on their assigned lever and resulted in a reward. Reversal errors occurred when the animal responded on the lever opposite to the assigned one and resulted in a time out phase. Daily training on 30-trial reversal sessions continued until the animals achieved criterion performance of ≥ 80% correct responses and < 10% omissions.
2.2.6. Behavioral Measures
For each behavioral session the number of correct responses, incorrect responses (errors), omissions, and latencies for both correct and incorrect responses were obtained. Additionally, response accuracy was calculated for each session using the formula: correct responses/(correct + incorrect responses) * 100. The total number of trials to criterion, errors to criterion and omissions were obtained for each training phase using the criteria described in the behavioral training section.
2.2.7. Error Analysis for Set-shifting and Reversal Phases
Given the response diversity characteristic of the set-shifting phase of training, we conducted error analysis to characterize strategy shifts by distinguishing whether an incorrect response occurred due to the perseverance of a previously learned strategy or failure to acquire/maintain a new strategy. As described earlier [27, 28], set-shift errors were classified as perseverative, regressive or never-reinforced errors. A perseverative error occurred if the animal responded to the incorrect lever when the visual cue was illuminated above it on more than 60% of trials within a session. For example, if the animal was set-shift to right and pressed the left lever in more than 9 out of 15 trials where visual stimulus was presented from the left side, the error was scored as perseverative. Depending on the training performance in the preceding session, a perseverative error was scored as a regressive (learning) error if the animal made ≤ 60% incorrect responses in subsequent sessions. At this point, the animal was making errors in 60% or less trials and was considered to be acquiring a new strategy. Never-reinforced errors occurred on trials when the animal responded on the incorrect lever but the cue was presented from the opposite (set-shift) side. Like regressive errors, never-reinforced errors were also classified as learning errors and represented an index of acquisition/execution of a new strategy [28].
Errors committed during response reversal learning were classified as perseverative and regressive, respectively. Reversal perseverative errors occurred when the animal responded on the lever opposite to the assigned one in > 60% of the performed trials (typically > 18 out of 30) in a session. If the animals made ≤ 60% incorrect lever presses, errors in all subsequent sessions were scored as regressive. Additionally, reversal errors were also analyzed based on the presence of distracter (visual cue) above the incorrect or correct lever.
2.3. Chronic Nicotine Administration and Study Design
Animals trained to criterion at the pretraining phase (above) were randomly assigned into three groups (saline, low dose nicotine, and high dose nicotine; N = 10–11/group) and prepared for chronic drug infusions. The average number of pretraining sessions required to attain criterion did not differ between the animals assigned control and nicotine-treated groups [saline: 8.2 ± 2.8; nicotine: 9.6 ± 2.0; F(1,29) = 0.39; P = 0.70]. Twenty-four hours prior to surgeries, animals were given ad libitum water to prevent dehydration. Mini-osmotic pumps (model 1004; DURECT Corporation, Cupertino, CA) designed to administer drug solutions at a rate of 0.11 uL/hr for 28 days were used. Each pump was filled with 100 uL of either sterile saline or nicotine hydrogen tartrate (Sigma Co., St Louis, MO, USA) dissolved in saline, and implanted subcutaneously in an isoflurane-anesthetized mouse under aseptic conditions. Nicotine was administered at two doses (low nic: 6.3mg/kg/day; high nic: 18mg/kg/day; doses reported as free base) to model low- and high-nicotine dependence states observed in chronic smokers [23]. These doses were selected based on earlier studies that demonstrated desensitization/upregulation of nicotinic receptors (nAChRs) following chronic use, and that produced plasma nicotine levels (10–50 ng/mL) in the range reported for smokers [35–39]. Plasma nicotine levels vary linearly as a function of the infusion dose in mice [38]. Therefore, steady-state plasma nicotine levels were predicted to be 2–3 times higher in high nic animals.
Pretraining was resumed after 24 h of recovery. Following retention of criterion performance at this phase (2–3 days), the animals progressed to subsequent stages of the task i.e. visual discrimination, response strategy set-shifting and reversal learning (see schematic for experimental design as shown in Figure 2). As the major objective of this study was to assess cognitive flexibility performance in mice while the steady-state nicotine levels are maintained, osmotic minipumps remained implanted throughout the duration of the study. Based on the individual variation in performance, the animals required 15–24 days to acquire all the three stages of the task after retaining pretraining criterion post-surgery. Therefore, a single osmotic pump was sufficient to deliver nicotine for the entire duration of the study. On average, osmotic pumps remained implanted for the duration of 21 ± 0.6 days. Mice were decapitated immediately after the last session to perform BDNF analysis (see below). Following the decapitation, the pumps were removed and examined to ensure that the contents had been released into the mouse.
Figure 2.
Schematic representation of the experimental design. Mice were initially autoshaped and then pretrained to press a lever within allotted time to receive reinforcement (refer Methods for details). Animal that reached pretraining criterion were implanted with subcutaneous osmotic minipumps (Alzet, Model #1004) to administer chronic nicotine or saline for 28 days. Following recovery, mice were trained and tested for the retention of pretraining criterion. Animals that matched criterion performance, which typically took 2–4 days including the recovery time, were progressed through subsequent stages of the task i.e. visual discrimination, strategy set-shifting, and reversal to set-shift. After completion of training at the terminal phase, animals were decapitated to isolated dorsal striatum for BDNF analysis using ELISA. Considering the variation in animals’ performance and treatment effects, the duration of training in the cognitive flexibility task varied from 15–24 days.
2.4. BDNF ELISA
Brains were removed to isolate dorsal striatum under a dissection microscope. Striatal tissues from both hemispheres were pooled together. Samples were homogenized in glass tissue grinder using 1 mL of ice-cold extraction buffer (50 mM HEPES NaOH, pH 7.4, 0.32 M sucrose, 5mM MgCl2, 0.2 mM EDTA, 0.2 mM EGTA, 1% Triton X-100 and protease inhibitor cocktail). The homogenates were kept in ice for 30 min following which they were centrifuged at 13000 × g for 15 min to obtain tissue lysates. Supernatants were stored at −80°C until analyzed. BDNF levels were quantified using a mouse BDNF ELISA kit as per recommendations from the manufacturer (IBL-America, Minneapolis, MN). All lysates were analyzed in duplicates and the data were expressed as pg/mg protein. Protein concentrations were determined by using a modified Lowry Protein Assay (Pierce, Rockford, IL).
2.5. Statistical Analyses
Statistical analyses were performed using SPSS/PC+ V19.0 (IBM SPSS software, Armonk, NY). Data for trials to criterion, errors to criterion, error types, omissions, response latencies (both correct and incorrect), and BDNF levels were analyzed by one-way ANOVAs. Analysis of response accuracy was conducted using mixed factor repeated measures ANOVAs with sessions as within-subject and treatment as between-subject variables. Because the animals required a minimum of 4 training sessions for visual discrimination, and 5 sessions for set-shifting/reversal learning to achieve criterion performance, session-based analyses for response accuracy was performed at either 4 or 5 levels for the respective phase of the task. Post hoc comparisons were made using a Fisher’s least significant difference (LSD) test.
3. Results
3.1. Nicotine improved response accuracy during visual discrimination learning
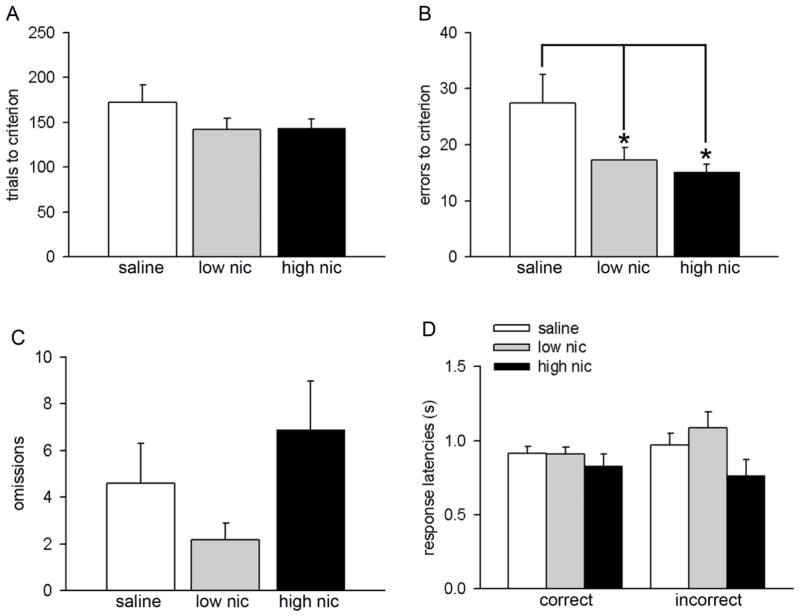
The main results on visual discrimination performance are summarized in Figure 3. Saline-treated mice acquired criterion performance in 177 ± 20 trials. Trials to criterion did not differ across the treatment groups (F(2,30) = 1.33, P = 0.28; Figure 3A). The rate of task acquisition was analyzed by comparing response accuracy for the first four discrimination sessions. Response accuracy was significantly higher in the nicotine-treated animals (means for main effect: saline 81.70% ± 0.20, low nic 86.78% ± 0.01, high nic 88.77 ± 0.01; F(2, 28) = 4.33, P = 0.02). Moreover, a significant main effect of session on this measure indicated effective learning of the visual discrimination strategy in all treatment groups (F(3, 84) = 13.55, P < 0.001). However, a lack of interaction between the two factors indicated that the rate of learning was comparable in all groups (treatment × session interaction: F(6, 84) = 0.88, P = 0.51). One-way ANOVAs revealed significant treatment effects on errors to criterion (F(2,30) = 3.83, P = 0.03; Figure 3B). In particular, animals treated with both low and high nicotine doses made fewer errors as compared to the control group (both P < 0.05; Figure 3B). These data further explain improved accuracy in both both low- and high-nic groups. The total number of omissions remained unaffected with treatment (F(2,30) = 2.30, P = 0.12; Figure 3C). Moreover, latencies for both correct (F(2,30) = 0.65, P = 0.53) and incorrect (F(2,30) = 2.68, P = 0.09) responses remained identical in all groups (Figure 3D).
Figure 3.
Effects of nicotine administration on visual discrimination performance. All data are presented as mean ± S.E.M. (A) Number of trials to reach criterion performance. (B) Both low and high dose of nicotine significantly lowered errors to criterion. Total number of omissions (C) and correct/incorrect response latencies (D) did not differ between groups. (LSD: *, P < 0.05). For this and subsequent figures: low nic and high nic groups represent mice treated with the low (6.3 mg/kg/day) and high dose (18 mg/kg/day) of nicotine, respectively; saline represent mice implanted with saline osmotic pumps and served as control group.
3.2. Acquisition of response strategy set-shifting
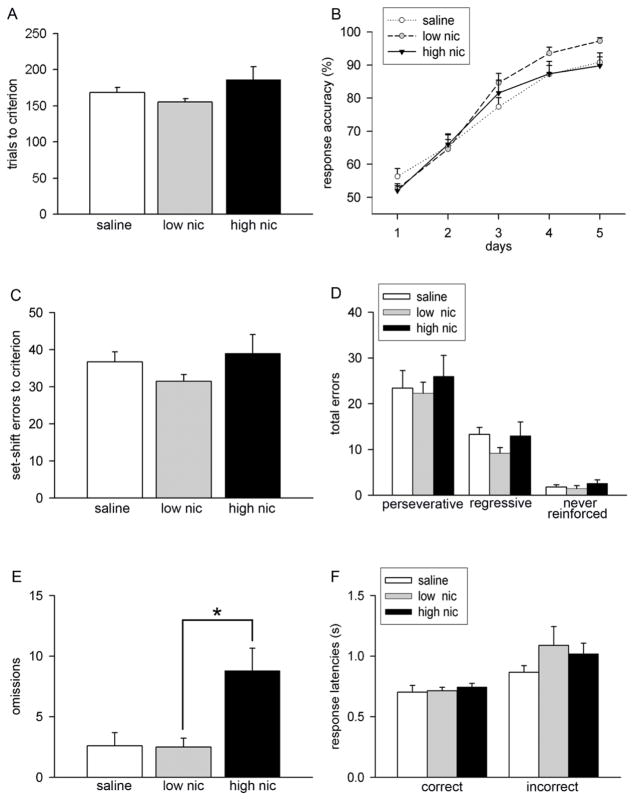
Saline- and nicotine-treated mice making > 80% correct choices for 3 consecutive days during visual discrimination phase were tested for the acquisition of an extradimensional set-shifting strategy that required the animals to shift attention from visual cue to a spatial location. Animals in all treatment conditions showed similar trials to criterion performance during the set-shifting phase (F(2,30) = 2.52, P = 0.09; Figure 4A). Learning curves for all groups are depicted in Figure 4B. Analysis of the rate of acquisition revealed a session-dependent learning effect (F(4, 112) = 148.82, P < 0.001). However, response accuracy remained unaffected by treatment (main effect: F(2, 28) = 0.83, P = 0.45) and this effect did not interact with session (session × treatment interaction: F(8, 112) = 1.54, P = 0.08). Group differences in total errors to criterion remained insignificant (F(2,30) = 1.32, P = 0.28; Figure 4C). Likewise, the analysis of types of errors made during set-shifting indicated no significant effect of treatment (perseverative: F(2,30) = 0. 82, P > 0.76; regressive, F(2,30) = 1.34, P > 0.28; non-reinforced, F(2,30) = 1.34, P > 0.28). Interestingly, the total number of omissions calculated for the entire duration of the acquisition phase differed significantly in the three groups (F(2,30) = 7.82, P < 0.01; Figure 4E). Subsequent post hoc analyses reflected higher omissions in high nic group as compared to the low nic group (P = 0.02). Nicotine treatment did not alter response latencies at criterion performance in strategy set-shifting (correct latencies: F(2,30) = 0.31, P = 0.74; incorrect latencies: F(2,30) = 1.02, P = 0.37; Figure 4F). Taken together, these data indicate that chronic nicotine treatment does not affect extradimensional shifting. As higher dose of nicotine did not alter the speed of responding for both correct and incorrect choices in mice, higher omissions in these animals may not be attributed to a decline in motor activity.
Figure 4.
Acquisition performance during the strategy set-shifting phase. Data are mean ± S.E.M. (A) Effects of chronic nicotine on trials to criterion performance. (B) Response accuracy for first 5 sessions of performance show identical session-related learning effects in all groups. (C) Chronic nicotine did not affect set-shift errors to criterion. (D) Bar charts depict the analysis of error types. Set-shift errors were classified as perseverative, regressive, and never reinforced based on whether the animal committed an error by pressing the incorrect lever in the presence or absence of cue above it. (E) Total number of omissions made during the acquisition of set-shifting strategy significantly increased in high nic group. As omissions generally remained lower (< 10%) across all sessions, set-shifting performance doesn’t seem to be confounded by this measure. (F) Correct and incorrect response latencies remained similar in all treatment groups. (LSD: *, P < 0.05)
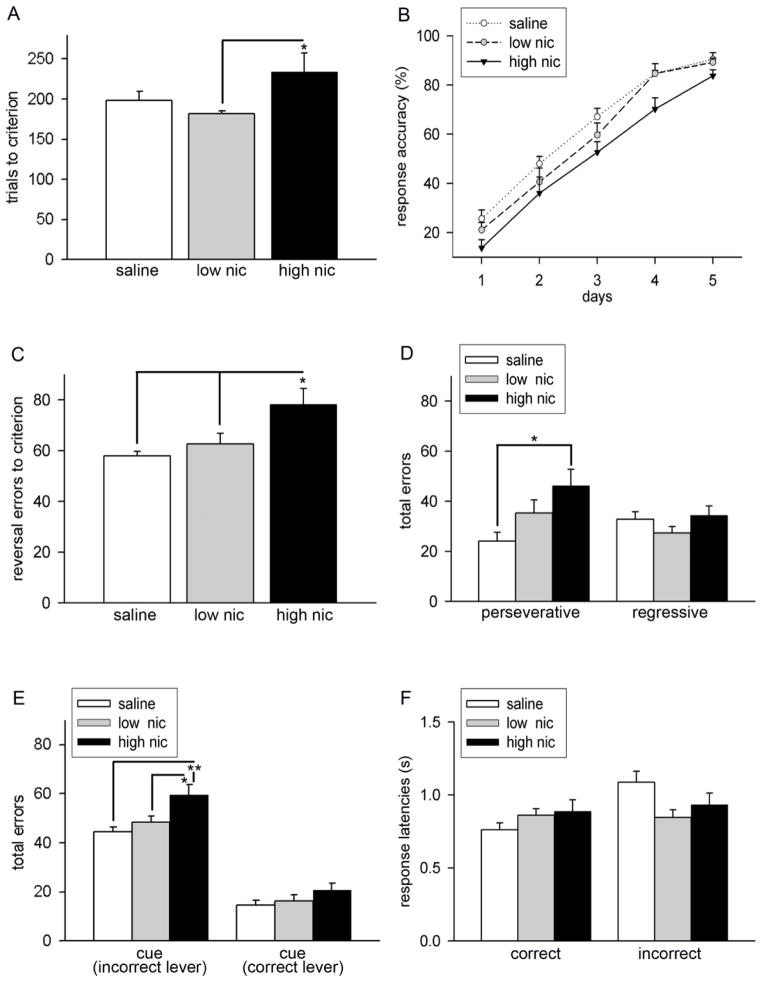
3.3. Chronic high dose nicotine impaired reversal learning
Following extradimensional shifting, a response reversal phase was implemented during which the reward contingencies were reversed. Saline- and nicotine-treated animals were continuously trained on this phase of the task until they achieve criterion performance. Statistical comparison indicated a significant group difference in the trials to criterion during reversal learning (F(2,30) = 3.30, P = 0.04). As illustrated in Figure 5A, post hoc analysis revealed that high nic animals required more trials to attain criterion as compared to saline (P = 0.09) or low nic group (P = 0.01) reflecting slower learning of the new rule in these animals. Trials to criterion did not differ between controls and animals treated with lower dose of nicotine (P = 0.97). Mixed factor ANOVAs conducted on response accuracy yielded a significant main effect of session (F(4, 112) = 211.04, P < 0.001) and treatment (F(2, 28) = 4.28, P = 0.02), but no interactions between the two factors (F(8, 112) = 0.54, P = 0.83). A general observation from the data analyzed for the learning curve was that, although the response accuracy during the initial learning phase remained lower in high nic animals, the learning trajectory remained similar in all groups (see Figure 5B). Therefore, a disrupted learning performance in high nic animals may reflect difficulty in shifting strategies rather than deficits in the rate of acquisition. Reversal error analyses further supported this interpretation (Figure 5C). As expected, errors to criterion were significantly higher in high nic group (treatment effect: F(2,30) = 4.82, P = 0.03; LSD: P < 0.05 vs. saline and low nic groups). To determine whether higher errors committed during reversals originated either from the ability to inhibit previously learned strategy or to acquire a new strategy or both, group comparisons were conducted on the types of errors. Interestingly, this analysis indicated significant group differences in perseverative errors (F(2,30) = 3.78, P = 0.04) but not regressive errors (F(2,30) = 1.41, P = 0.26). Post hoc comparisons revealed significantly higher perseverative errors in high nic group as compared to the control group (P < 0.05). Further examination of incorrect responses during the reversal phase indicated that high nic animals committed significantly more errors when the distracter (cue light) was present above the incorrect lever (F(2,30) = 5.87, P = 0.008; LSD: high nic vs. saline or low nic, both P < 0.02; Figure 5E). However, the number of errors remained insignificant in trials where the distracter was present on the correct lever (F(2,30) = 1.55, P = 0.23). Nicotine treatment did not affect total number of omissions (F(2,30) = 1.89, P = 0.17; Figure 5E) as well as latencies for correct responses (F(2,30) = 1.27, P > 0.30). Nicotine treatment marginally reduced incorrect response latencies during reversal learning and this effect approached significance (F(2,30) = 3.10, P = 0.06; Figure 5F).
Figure 5.
Characterization of the effects of chronic nicotine on response reversal learning. Data are expressed as Mean ± S.E.M. (A) High nic mice required significantly more number of trials to achieve criterion as compared to low nic mice. Although, a similar trend appeared in comparison to the saline group, this effect did not reach significance (P = 0.09). (B) Learning curves for 5 reversal sessions depict gradual increase in response accuracy with training in all groups. Response accuracy for high nic mice remained lower in comparison to other two groups. However, this effect did not interact with the session (see results for the main effect of treatment and interactions). As shown in (C), total reversal errors did not differ between saline and low nic group. In contrast, animals chronically exposed to the higher dose of nicotine committed more errors. (D) Analysis of error types exemplify that this increase resulted from increased response perseveration to the previous strategy (perseverative error) but not due to learning of the new strategy (regressive error). Furthermore, these animals made significantly more errors when the cue light was present above the incorrect lever (E). Group differences in reversal errors were not observed during the illumination of cue light above the correct lever. (F) Latencies on both the correct and incorrect responses made during the acquisition of reversal learning remained unaffected by nicotine treatment. * P < 0.05; post hoc multiple comparisons based on one-way ANOVAs.
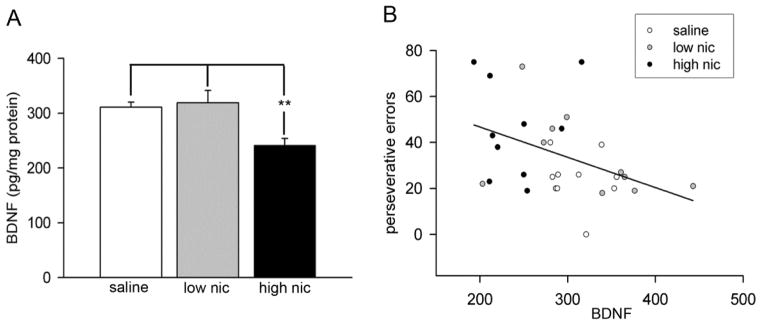
3.4. Chronic high dose nicotine reduced BDNF levels in the dorsal striatum
ELISA estimations of BDNF protein in tissues obtained from the dorsal striatum indicated significant group differences (F(2,30) = 7.30, P < 0.001). Striatal BDNF levels declined in the high nic group as compared to both the saline and low nic groups (see Figure 6A for post hoc comparisons). As high dose of nicotine reduced BDNF and also impaired reversal learning, we conducted a correlation analysis to examine any association between striatal BDNF and behavioral measures. For this analysis, data from animals across all groups were pooled together to gain statistical power. A significant negative correlation was observed between striatal BDNF and perseverative errors (Pearson’s r = −0.42; P = 0.02). As shown in Figure 6B, data points from high nic group mostly lie on the upper part of the regression line indicating higher perseverative errors and lower striatal BDNF. However, a subsequent break-up analysis by group did not reveal significant correlations between the two measures (all P > 0.1). This lack of statistical significance may be either due to a low sample size, or a low effect size as a result of limited inter-individual variation in performance within group. Furthermore, these data reflect that the strength of correlation presumably depend upon an interaction between the effects of learning and nicotine treatment on BDNF levels. Correlations with other behavioral measures including the trials to criterion, errors to criterion, regression errors and response latencies did not reach significance (all P > 0.17).
Figure 6.
Effects of chronic nicotine on striatal BDNF levels in mice. BDNF was estimated using ELISA in the homogenates prepared from dorsal striatum immediately following the completion of training and testing on the reversal phase. (A) BDNF levels significantly declined in high nic mice as compared to controls. On the other hand, chronic exposure with a lower dose of nicotine did not alter striatal BDNF. (B) Correlation analysis between BDNF levels and perseverative errors during reversal learning shows a significant negative correlation (P < 0.05). Data points from high nic animals remained scattered around the upper part of the regression line.
4. Discussion
In the present study, we assessed the effects of chronic nicotine on cognitive flexibility in mice utilizing an operant procedure that required the animals to progress through three different stages: 1) visual discrimination, 2) strategy set-shifting and 3) response reversal. Animals were exposed to nicotine using 4-week subcutaneous osmotic implants to ensure steady-state plasma nicotine levels [40] throughout the training and testing procedures. Two different doses (6.3 mg/kg/day & 18 mg/kg/day) of nicotine were used to model a low- and a high-nicotine dependence state. Nicotine doses in a similar range were earlier shown to produce plasma nicotine levels and nAChR upregulation in mice that are physiologically-relevant to human smokers [35–40]. Chronic nicotine exposure at both doses facilitated visual discrimination performance but did not improve learning of a new egocentric response strategy during set-shifting. On the other hand, higher but not lower dose of nicotine impaired response reversal learning by increasing perseverative responding to the previously reinforced lever. Furthermore, this effect was associated with reduced BDNF levels in the dorsal striatum.
Accumulating evidence from human and animal studies indicates that nicotine exerts beneficial effects on cognitive abilities by activating nicotinic receptors (nAChRs), and these effects are primarily attributed to the enhancement of fundamental attentional operations [41–43]. Attentional processes facilitate the selection of goal-relevant stimuli from the environment, and these cognitive processes are involved in learning of operant discrimination tasks [44]. In the current study, the animals performed a discrimination task where a visual cue directed attention to either the left or right lever to receive reinforcement. Chronic nicotine exposure improved visual discrimination performance by minimizing errors and increasing the response accuracy. These results substantiate the notion that nicotine facilitates attentional processes. As the response latencies remained unaltered by nicotine, our data reflect that nicotine enhanced perceptual selectivity of the discriminative stimulus but not processing speed which is mostly governed by sensorimotor processes. These findings are consistent with a previous study that demonstrated a facilitated acquisition of this form of learning using a T maze procedure [45].
The ability to flexibly adapt to a new situation with changes in environmental contingencies requires higher attentional control, and this capacity is assessed using attentional set-shifting tasks in humans [46] and animals [47]. The strategy switching paradigm used in the present study assesses shifting between different discrimination strategies also engages attentional set-shifting functions [27, 48]. Chronic administration of either the low or the high dose of nicotine did not facilitate the ability of mice to selectively shift attention from one stimulus dimension (visual cue) that previously predicted reinforcement to a new stimulus dimension (spatial position). Moreover, error analyses indicated that nicotine-treated animals were not better in suppressing previously acquired strategy or in executing new strategy as compared to the control group. These data reconcile with a previous study that demonstrated lack of performance differences during extradimensional set-shifting between low- and high-dependent smokers [23].
The beneficial cognitive effects of nicotine depend upon the duration/magnitude of nicotine exposure, task demands that explicitly tax attentional processes, and on the measures of performance. For example, nicotine-treated rats failed to show improvement in performance during the presentation of distracters or following the distracter challenge in a sustained attention task [49]. Acute nicotine administration in non-smokers or chronic nicotine in minimally-deprived smokers impaired attention selectivity and reduced perceptual speed [50, 51]. Moreover, reaction times in a divided attention task did not differ between smokers and non-smokers [52]. Collectively, the present data in conjunction with these studies imply that the cognition-enhancing effect of chronic nicotine is limited under conditions that tax attentional control. Moreover, the control of attentional bias is not influenced by the level of nicotine dependence.
However, a recent study by Allison and Shoaib [53] reported that acute/repeated nicotine administration improved attentional set-shifting in rats. The discrepancy in findings between this and our study could be attributed to multiple factors. First, the route and duration of nicotine administration were different in both studies. Nicotine is eliminated very rapidly in mice (half life: 5–10 min), and therefore, to model steady state nicotine levels following chronic use we employed subcutaneous osmotic minipumps. Acute/repeated nicotine injections mostly replicate boosts of plasma nicotine levels, and this route was chosen in the other study. Second, our operant cognitive flexibility task places a heavier emphasis on response conflict as the stimuli remain constant throughout all stages of the task making it similar to the Wisconsin Card Sorting Task. On the other hand, a perceptual set-shifting task that utilizes presentations of novel complex stimuli across multiple phases was used in the other study. Therefore, beneficial effects of nicotine observed in that study may simply reflect enhanced sensory processes and shifts in attention to novel aspects of compound stimuli. Finally, species differences in the behavioral effects of nicotine may be related to differences in nicotine binding to central nAChRs [54], and therefore may not be ruled out.
Optimization of decision-making critically depends upon inhibitory control processes. Response inhibition requires voluntary control of responses when there is a change of context. Individual variation in response inhibition including impulsive responding influences addiction vulnerability [55, 56]. Reversal learning paradigms measure the ability to suppress reward-related responding in the presence of conflicting response alternatives, and are critical in assessing impulsive and compulsive behavior in drug addiction [57]. Here we evaluated the effects of chronic nicotine in a task that required the animals to acquire the reversal of response discrimination. To reiterate, response discrimination was acquired incrementally during the penultimate (set-shifting) phase where the animals were trained to choose the correct lever (either left or right) irrespective of the position of the cue. Therefore, during reversal learning, the animals were trained to make a correct choice by pressing the lever opposite to that which was reinforced during set-shifting. Low-dose nicotine-treated animals achieved criterion performance in the reversal at the same pace as the control animals. In contrast, mice treated with the high dose of nicotine required more trials to achieve criterion indicating slower adaptation to rule reversal. These animals exhibited higher perseverative responding towards the previously reinforced lever. However, the maintenance and execution of new choice pattern remained unaffected. Interestingly, these animals committed more errors under distracting conditions i.e. when the cue was illuminated above the incorrect lever. This may suggest that animals chronically exposed to a higher dose of nicotine attempted to revert back to use the visual cue discrimination strategy. Therefore, impairments in reversal learning in these animals may be attributed to a disruption in prepotent responding to a previously rewarded stimulus. Moreover, aberrations in inhibitory control processes in high-nicotine-dependent mice could be a consequence of instrumental associations with the salient environmental stimulus. These data complement previous studies that show chronic nicotine augments the influence of reward-associated stimuli and reduce inhibitory modulation of motivational impulses [58, 59].
As noted earlier, frontostriatal circuits involving discrete regions of the frontal lobe and dorsal striatum are known to contribute directly to rule-based task switching and decision-making processes through the integration of sensorimotor, cognitive and motivational information [9]. Previous studies indicated that lesions or in activations of the medial prefrontal cortex in rodents impaired extradimensional or strategy set-shifting but left the reversal learning intact [27, 32, 47]. Learning during the reversal of stimulus-reward associations is sensitive to the orbitofrontal cortex, and inactivation of this prefrontal region does not affect learning of discrimination tasks and strategy switching [60, 61]. Intact strategy set-shifting in nicotine-treated mice in our study confirms that the function of medial prefrontal cortex is not affected with chronic nicotine. Although the pro-attentional effects of nicotine may be limited due to non-specific activation of multiple nAChR subtypes [62], facilitation of discrimination learning in the paradigm used here may indicate that chronic nicotine may improve certain aspects of attention either via activation of prefrontal α4β2 nAChRs [63–65] or α5 nAChRs [66]. A recent structural MRI study demonstrated lower thickness of the medial orbitofrontal cortex in the brain of smokers [67]. Moreover, cortical thinning in this brain region was inversely correlated with the amounts of cigarettes smoked per day. This may further support our interpretations that the level of nicotine dependence influences reversal learning as this form of learning critically depends upon the integrity of orbitofrontal cortex.
Dorsal striatum receives converging inputs from the prefrontal regions including the orbitofrontal cortex as well as other sensorimotor association areas implicated in addiction [9, 68], and this brain region appears to be recruited during compulsive drug-seeking [4]. Inactivation or lesions of the dorsomedial striatum impaired both forms of cognitive flexibility [31, 48]. Dorsolateral striatum is critical for habit formation and lesions in this region impaired generalization of previously learned actions [69, 70]. Thus, chronic nicotine-induced neuroadaptations in the dorsal striatum may underlie maladaptive decision-making associated with high-nicotine dependence.
BDNF exerts neuromodulatory effects on dopaminergic/glutamatergic transmission [71, 72], and plays a critical role in activity-dependent synaptic plasticity, learning and memory [24]. Presynaptic release of BDNF from glutamatergic synapses in dorsal striatum induced long-term potentiation [25]. BDNF heterozygous mice displayed higher tonic DA levels in the dorsal striatum [73] and reduced efficiency of presynaptic glutamate release [74]. BDNF gene polymorphism is implicated in nicotine addiction [26]. Plasma BDNF decreased during smoking while smoking cessation increased BDNF levels [75, 76]. Moreover, chronic nicotine treatment or nicotine withdrawal produced alterations in BDNF expression in multiple brain regions [77, 78]. Therefore, nicotine-induced alterations in striatal BDNF levels and consequent aberrations in dopaminergic/glutamatergic signaling in the dorsal striatum could influence learning and cognitive flexibility.
In the current study, chronic administration of a low dose of nicotine did not alter BDNF levels in the dorsal striatum. In contrast, BDNF levels declined ~ 25% in mice exposed to a higher dose of nicotine. Correlation analyses points to a negative relationship between striatal BDNF levels and perseverative errors during reversals. As indicated in Figure 6B, the data points from animals treated with high-dose nicotine are condensed closer to the upper part of the regression line. A closer inspection of these data indicates that 5% reduction in BDNF corresponded to 20% increase in perseverative errors. These findings suggest that nicotine-induced alterations in the BDNF levels depend upon the level of nicotine dependence, and reduced striatal BDNF may account for higher perseverance akin to response inhibition during reversal learning deficits. Future studies are warranted to examine whether withdrawal from chronic nicotine produces an increase in BDNF in the dorsal striatum, and whether such increases triggers a state of compulsion and loss of control for drug intake or reflect a compensatory homeostatic response to normalize withdrawal-induced cognitive deficits.
The exact mechanism by which chronic nicotine regulates BDNF levels remains unclear. Corticostriatal synapses constitute the major pool of BDNF in dorsal striatum [79], and the expression of cortical BDNF levels and subsequent anterograde transport to cortical afferents depends upon neuronal activity in striatal target areas [80]. α4β2 nAChRs regulate striatal dopaminergic transmission [19], while α7 nAChRs are mostly linked to striatal glutamate release [81, 82]. Moreover, these receptors desensitize at different rates and under different conditions, and the extent of nAChR activation/desensitization may influence relapse vulnerability [83, 84]. Thus, it may be speculated that alterations in striatal BDNF levels and cognitive flexibility during nicotine dependence is dependent upon a balance between signaling processes mediated by these two nAChR subtypes and consequent changes in glutamatergic-dopaminergic transmission.
One limitation of this study could be that stages of the task preceding the reversal learning phase, including visual discrimination and set-shifting, were completed under different states of nAChR activation and desensitization which may affect striatal BDNF levels. As these processes may influence reward sensitivity and goal-directed behavior [83, 84], we may not rule out whether absence of any effects in extradimensional shifting occurred because striatal BDNF levels in nicotine-treated animals remained identical to control or this type of behavioral flexibility remained insensitive to alterations in BDNF levels. Future studies designed to match the time course of chronic nicotine are warranted to resolve this issue.
Although the role of executive processes has been proposed to play a critical role in drug addiction [85], how these functions are altered during nicotine dependence is not known. The present evidence indicates that chronic nicotine impairs a low order decision-making process (reversal learning), while a higher order process that involves shifting of attentional sets or learning about attributes of a new stimulus/information remains intact. Moreover, reversal learning deficits that correspond to disruptions in response inhibition depend upon the level of nicotine dependence and are linked to alterations in striatal BDNF levels. There is an inverse relationship between the likelihood of quitting smoking and the level of nicotine dependence [86]. High nicotine dependence is also much more prevalent among psychiatric patients, most notably, in schizophrenic subjects [87]. Additionally, BDNF is linked to cognitive symptoms of schizophrenia [88, 89], and deficits in executive functions are prominent in schizophrenic patients [90]. Therefore, these findings have major implications not only for understanding the neurobiology of maladaptive decision-making in nicotine addicts, but also in understanding the cognitive mechanisms that link the comorbidity of nicotine addiction and schizophrenia.
Highlights.
Nicotine facilitates visual discrimination learning but not strategy shifting
Sustained nicotine (18mg/kg/day) exposure impaired reversal learning
Higher perseverative reversal errors were associated with reduced striatal BDNF
Acknowledgments
The present work was supported by a grant from the Brain and Behavior Research Foundation (V.P.) and partly by NIH grants DA # 017949 and CA 143187 (T.J.G.). We thank Dawn Guzman and Sean Naughton for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Center for Disease Control and Prevention. Cigarette smoking among adults-United States, 2006. Morbidity Mortality Weekly Report. 2007;56:1157–61. [PubMed] [Google Scholar]
- 2.Hughes JR, Peters EN, Naud S. Relapse to smoking after 1 year of abstinence: a meta-analysis. Addict Behav. 2008;33:1516–20. doi: 10.1016/j.addbeh.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 4.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–35. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould TJ. Addiction and cognition. Addict Sci Clin Pract. 2010;5:4–14. [PMC free article] [PubMed] [Google Scholar]
- 6.Thewissen R, van den Hout M, Havermans RC, Jansen A. Context-dependency of cue-elicited urge to smoke. Addiction. 2005;100:387–96. doi: 10.1111/j.1360-0443.2005.00996.x. [DOI] [PubMed] [Google Scholar]
- 7.Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008;38:101–21. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95 (Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 9.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–5. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 11.Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology. 2009;56 (Suppl 1):63–72. doi: 10.1016/j.neuropharm.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colzato LS, Huizinga M, Hommel B. Recreational cocaine polydrug use impairs cognitive flexibility but not working memory. Psychopharmacology (Berl) 2009;207:225–34. doi: 10.1007/s00213-009-1650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verdejo-Garcia AJ, Lopez-Torrecillas F, Aguilar de Arcos F, Perez-Garcia M. Differential effects of MDMA, cocaine, and cannabis use severity on distinctive components of the executive functions in polysubstance users: a multiple regression analysis. Addict Behav. 2005;30:89–101. doi: 10.1016/j.addbeh.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Ersche KD, Sahakian BJ. The neuropsychology of amphetamine and opiate dependence: implications for treatment. Neuropsychol Rev. 2007;17:317–36. doi: 10.1007/s11065-007-9033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jentsch JD, Olausson P, De La Garza R, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–90. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- 16.Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- 17.Featherstone RE, Rizos Z, Kapur S, Fletcher PJ. A sensitizing regimen of amphetamine that disrupts attentional set-shifting does not disrupt working or long-term memory. Behav Brain Res. 2008;189:170–9. doi: 10.1016/j.bbr.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 18.Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ, et al. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2012;35:505–14. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav. 2001;70:439–46. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- 20.Changeux JP. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci. 2010;11:389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- 21.Martin CA, Rayens MK, Kelly T, Hartung C, Leukefeld C, Haigler E. Card Perseveration Task performance and post-task feeling states: relationship to drug use in adolescents. Am J Drug Alcohol Abuse. 2000;26:325–33. doi: 10.1081/ada-100100608. [DOI] [PubMed] [Google Scholar]
- 22.Kalmijn S, van Boxtel MP, Verschuren MW, Jolles J, Launer LJ. Cigarette smoking and alcohol consumption in relation to cognitive performance in middle age. Am J Epidemiol. 2002;156:936–44. doi: 10.1093/aje/kwf135. [DOI] [PubMed] [Google Scholar]
- 23.Nesic J, Rusted J, Duka T, Jackson A. Degree of dependence influences the effect of smoking on cognitive flexibility. Pharmacol Biochem Behav. 2011;98:376–84. doi: 10.1016/j.pbb.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 25.Jia Y, Gall CM, Lynch G. Presynaptic BDNF promotes postsynaptic long-term potentiation in the dorsal striatum. J Neurosci. 2010;30:14440–5. doi: 10.1523/JNEUROSCI.3310-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang UE, Sander T, Lohoff FW, Hellweg R, Bajbouj M, Winterer G, et al. Association of the met66 allele of brain-derived neurotrophic factor (BDNF) with smoking. Psychopharmacology (Berl) 2007;190:433–9. doi: 10.1007/s00213-006-0647-1. [DOI] [PubMed] [Google Scholar]
- 27.Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–52. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- 29.Brown HD, Baker PM, Ragozzino ME. The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats. J Neurosci. 2010;30:14390–8. doi: 10.1523/JNEUROSCI.2167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- 31.Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116:105–15. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–94. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–41. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wrenn CC, Turchi JN, Schlosser S, Dreiling JL, Stephenson DA, Crawley JN. Performance of galanin transgenic mice in the 5-choice serial reaction time attentional task. Pharmacol Biochem Behav. 2006;83:428–40. doi: 10.1016/j.pbb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Benowitz NL, Porchet H, Jacob P. Nicotine dependence and tolerance in man: pharmacokinetic and pharmacodynamic investigations. Prog Brain Res. 1989;79:279–87. doi: 10.1016/s0079-6123(08)62487-5. [DOI] [PubMed] [Google Scholar]
- 36.Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61:743–50. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- 37.Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–13. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marks MJ, Rowell PP, Cao JZ, Grady SR, McCallum SE, Collins AC. Subsets of acetylcholine-stimulated 86Rb+ efflux and [125I]-epibatidine binding sites in C57BL/6 mouse brain are differentially affected by chronic nicotine treatment. Neuropharmacology. 2004;46:1141–57. doi: 10.1016/j.neuropharm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res. 2010;13:41–6. doi: 10.1093/ntr/ntq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–34. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- 41.Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Stolerman IP, Mirza NR, Hahn B, Shoaib M. Nicotine in an animal model of attention. Eur J Pharmacol. 2000;393:147–54. doi: 10.1016/s0014-2999(99)00886-9. [DOI] [PubMed] [Google Scholar]
- 43.Semenova S, Stolerman IP, Markou A. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacol Biochem Behav. 2007;87:360–8. doi: 10.1016/j.pbb.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muir JL. Attention and stimulus processing in the rat. Brain Res Cogn Brain Res. 1996;3:215–25. doi: 10.1016/0926-6410(96)00008-0. [DOI] [PubMed] [Google Scholar]
- 45.Besheer J, Bevins RA. Nicotine enhances acquisition of a T-maze visual discrimination: assessment of individual differences. Behav Pharmacol. 2000;11:613–20. doi: 10.1097/00008877-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Roberts AC, Robbins TW, Everitt BJ. The effects of intradimensional and extradimensional shifts on visual discrimination learning in humans and non-human primates. Q J Exp Psychol B. 1988;40:321–41. [PubMed] [Google Scholar]
- 47.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–75. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- 49.Howe WM, Ji J, Parikh V, Williams S, Mocaer E, Trocme-Thibierge C, et al. Enhancement of attentional performance by selective stimulation of alpha4beta2(*) nAChRs: underlying cholinergic mechanisms. Neuropsychopharmacology. 2010;35:1391–401. doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azizian A, Monterosso JR, Brody AL, Simon SL, London ED. Severity of nicotine dependence moderates performance on perceptual-motor tests of attention. Nicotine Tob Res. 2008;10:599–606. doi: 10.1080/14622200801979159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vangkilde S, Bundesen C, Coull JT. Prompt but inefficient: nicotine differentially modulates discrete components of attention. Psychopharmacology (Berl) 2011;218:667–80. doi: 10.1007/s00213-011-2361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hahn B, Ross TJ, Wolkenberg FA, Shakleya DM, Huestis MA, Stein EA. Performance effects of nicotine during selective attention, divided attention, and simple stimulus detection: an fMRI study. Cereb Cortex. 2009;19:1990–2000. doi: 10.1093/cercor/bhn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allison C, Shoaib M. Nicotine improves performance in an attentional set shifting task in rats. Neuropharmacology. 2012 Jul 6; doi: 10.1016/j.neuropharm.2012.06.055. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 54.Pauly JR, Stitzel JA, Marks MJ, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain. Brain Res Bull. 1989;22:453–9. doi: 10.1016/0361-9230(89)90072-5. [DOI] [PubMed] [Google Scholar]
- 55.Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–94. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 57.Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl) 2012;219:607–20. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances reward-related learning in the rat. Neuropsychopharmacology. 2003;28:1264–71. doi: 10.1038/sj.npp.1300173. [DOI] [PubMed] [Google Scholar]
- 59.Olausson P, Ericson M, Lof E, Engel JA, Soderpalm B. Nicotine-induced behavioral disinhibition and ethanol preference correlate after repeated nicotine treatment. Eur J Pharmacol. 2001;417:117–23. doi: 10.1016/s0014-2999(01)00903-7. [DOI] [PubMed] [Google Scholar]
- 60.Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–90. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- 61.McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 62.Sarter M, Parikh V, Howe WM. nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem Pharmacol. 2009;78:658–67. doi: 10.1016/j.bcp.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parikh V, Man K, Decker MW, Sarter M. Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. J Neurosci. 2008;28:3769–80. doi: 10.1523/JNEUROSCI.5251-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parikh V, Ji J, Decker MW, Sarter M. Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. J Neurosci. 2010;30:3518–30. doi: 10.1523/JNEUROSCI.5712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guillem K, Bloem B, Poorthuis RB, Loos M, Smit AB, Maskos U, et al. Nicotinic acetylcholine receptor beta2 subunits in the medial prefrontal cortex control attention. Science. 2011;333:888–91. doi: 10.1126/science.1207079. [DOI] [PubMed] [Google Scholar]
- 66.Bailey CD, De Biasi M, Fletcher PJ, Lambe EK. The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J Neurosci. 2010;30:9241–52. doi: 10.1523/JNEUROSCI.2258-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuhn S, Schubert F, Gallinat J. Reduced thickness of medial orbitofrontal cortex in smokers. Biol Psychiatry. 2010;68:1061–5. doi: 10.1016/j.biopsych.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav Brain Res. 2006;166:189–96. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 70.Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–9. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- 71.Goggi J, Pullar IA, Carney SL, Bradford HF. Modulation of neurotransmitter release induced by brain-derived neurotrophic factor in rat brain striatal slices in vitro. Brain Res. 2002;941:34–42. doi: 10.1016/s0006-8993(02)02505-2. [DOI] [PubMed] [Google Scholar]
- 72.Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol. 2008;153 (Suppl 1):S310–24. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bosse KE, Maina FK, Birbeck JA, France MM, Roberts JJ, Colombo ML, et al. Aberrant striatal dopamine transmitter dynamics in brain-derived neurotrophic factor-deficient mice. J Neurochem. 2012;120:385–95. doi: 10.1111/j.1471-4159.2011.07531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abidin I, Kohler T, Weiler E, Zoidl G, Eysel UT, Lessmann V, et al. Reduced presynaptic efficiency of excitatory synaptic transmission impairs LTP in the visual cortex of BDNF-heterozygous mice. Eur J Neurosci. 2006;24:3519–31. doi: 10.1111/j.1460-9568.2006.05242.x. [DOI] [PubMed] [Google Scholar]
- 75.Kim TS, Kim DJ, Lee H, Kim YK. Increased plasma brain-derived neurotrophic factor levels in chronic smokers following unaided smoking cessation. Neurosci Lett. 2007;423:53–7. doi: 10.1016/j.neulet.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 76.Bhang SY, Choi SW, Ahn JH. Changes in plasma brain-derived neurotrophic factor levels in smokers after smoking cessation. Neurosci Lett. 2010;468:7–11. doi: 10.1016/j.neulet.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 77.Yeom M, Shim I, Lee HJ, Hahm DH. Proteomic analysis of nicotine-associated protein expression in the striatum of repeated nicotine-treated rats. Biochem Biophys Res Commun. 2005;326:321–8. doi: 10.1016/j.bbrc.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 78.Kivinummi T, Kaste K, Rantamaki T, Castren E, Ahtee L. Alterations in BDNF and phospho-CREB levels following chronic oral nicotine treatment and its withdrawal in dopaminergic brain areas of mice. Neurosci Lett. 2011;491:108–12. doi: 10.1016/j.neulet.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 79.Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–60. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 80.Canals JM, Checa N, Marco S, Akerud P, Michels A, Perez-Navarro E, et al. Expression of brain-derived neurotrophic factor in cortical neurons is regulated by striatal target area. J Neurosci. 2001;21:117–24. doi: 10.1523/JNEUROSCI.21-01-00117.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Campos F, Alfonso M, Duran R. In vivo modulation of alpha7 nicotinic receptors on striatal glutamate release induced by anatoxin-A. Neurochem Int. 2010;56:850–5. doi: 10.1016/j.neuint.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 82.Quik M, Wonnacott S. alpha6beta2* and alpha4beta2* nicotinic acetylcholine receptors as drug targets for Parkinson’s disease. Pharmacol Rev. 2011;63:938–66. doi: 10.1124/pr.110.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–42. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hilario MR, Turner JR, Blendy JA. Reward Sensitization: Effects of Repeated Nicotine Exposure and Withdrawal in Mice. Neuropsychopharmacology. 2012 Jul 25; doi: 10.1038/npp.2012.130. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108:15037–42. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pinto RP, Abrams DB, Monti PM, Jacobus SI. Nicotine dependence and likelihood of quitting smoking. Addict Behav. 1987;12:371–4. doi: 10.1016/0306-4603(87)90052-9. [DOI] [PubMed] [Google Scholar]
- 87.Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8:1465–70. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 88.Gratacos M, Gonzalez JR, Mercader JM, de Cid R, Urretavizcaya M, Estivill X. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biol Psychiatry. 2007;61:911–22. doi: 10.1016/j.biopsych.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 89.Pillai A, Kale A, Joshi S, Naphade N, Raju MS, Nasrallah H, et al. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J Neuropsychopharmacol. 2010;13:535–9. doi: 10.1017/S1461145709991015. [DOI] [PubMed] [Google Scholar]
- 90.Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biol Psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]