Abstract
The number of older adults undergoing kidney transplantation has increased, yet little is known about calcineurin inhibitor (CNI) metabolism in this group. We studied CNI troughs and doses to determine if there were age related differences in metabolism and dose requirements. We studied 348 young (18–34 years), 1831 middle (35–64 years) and 374 older (65–84 years) adult kidney transplant recipients enrolled in a 7-center prospective study. Troughs were obtained from each patient 2x/week in weeks 1–8 and 2x/month in months 3–6. A multivariable linear mixed model examined the effect of age on log dose and weight normalized troughs. Older recipients had higher normalized tacrolimus troughs than middle or young age adults despite receiving doses a median of 1–2 mg/day lower. Age and CYP3A5*1 genotype had the largest effect on tacrolimus troughs. Older recipients also had higher normalized cyclosporine troughs than middle or young adults despite receiving median doses 100 mg/day lower. After normalization for dose and weight, CNI troughs were more than 50% higher in older adults than young adults. These data support age related changes in CNI metabolism. Further studies are needed to determine optimal dosing of CNIs in the elderly.
Keywords: tacrolimus, cyclosporine, pharmacokinetics, calcineurin inhibitor, kidney transplant, trough, elderly
Introduction
The number of older adults undergoing kidney transplantation has increased dramatically over the past decade, as demonstrated by the most recent data (OPTN and SRTR Annual Data Report 2010 at http://www.srtr.org/annual_reports/2010/). In the past 10 years, the number of kidney transplants has increased by 25% in those 50–64 years old and almost doubled in those ≥ 65 years old, whereas transplants in those < 50 years have declined. Transplantation of older recipients introduces a number of clinical problems including additional comorbidities, reduced immunologic response, poorer cognitive abilities and memory, and a greater number of concomitant medications. In addition, increasing age is associated with changes in drug disposition and susceptibility to adverse drug reactions.(1–3) However, little is known about immunosuppressive drug characteristics in the older transplant recipient despite their potential toxicity. Importantly, older recipients have been underrepresented in pivotal immunosuppression trials but clinically are usually treated with those same regimens.(4) Therefore we studied CNI disposition in a large population of kidney transplant recipients in three adult age groups; young (18–34 years), middle (35–64 years) and older (65–84 years) to define age related drug disposition changes adjusting for important demographic, clinical, and genetic factors.
Materials and Methods
Patient Recruitment and Data Collection
Subjects were enrolled from seven centers participating in the Long-term Deterioration of Kidney Allograft Function (DeKAF) and DeKAF Genomics studies. The DeKAF studies were designed to characterize clinical and genetic causes of late allograft failure and to define pharmacogenomic effects on immune suppressants pharmacokinetics and toxicity.(5–11) This study is registered at www.clinicaltrials.gov (NCT00270712). Subjects were eligible for this study if they underwent a living or deceased donor kidney or simultaneous kidney-pancreas transplantation, were ≥ 18 years of age, and received tacrolimus or cyclosporine at any time during the first 6 months posttransplant. Subjects in this analysis received a CNI-based immunosuppressive regimen; over 95% received mycophenolate either with standard steroids or a rapid discontinuation protocol. Antibody induction was used in most individuals per transplant center preference. The total number of subjects was 2,553. Tacrolimus was received by 1,809; 586 received cyclosporine, and 158 were switched from one CNI to the other within the first 6 months posttransplant and were studied on both drugs. Subjects were enrolled at time of transplant and signed informed consents approved by each institution’s Institutional Review Boards (UMN IRB 0407M62262 and 0603M83926).
CNI Trough Concentrations and Doses, and Clinical Characteristics
Tacrolimus and cyclosporine trough concentrations, measured just prior to an oral dose, were obtained as part of routine clinical care for the first 6 months posttransplant. Two measurements, if available, were obtained in each of weeks 1–8 and in each of months 3, 4, 5 and 6 posttransplant, for a maximum of 24 measurements per patient. CNI dosing, dose adjustments and trough targets were determined by the center. Prior to analysis, troughs were normalized for dose and recipient weight by taking the ratio of the trough concentration (ng/ml) by the total daily CNI dose (mg/kg). Tacrolimus concentrations were measured from whole blood by each institution’s clinical laboratory. Ninety-three percent of tacrolimus concentrations were measured using high pressure liquid chromatography mass spectroscopy. Similarly, cyclosporine concentrations were determined by each institution’s clinical laboratory; 71.8% were measured with liquid chromatography mass-mass spectroscopy.
Donor and recipient clinical characteristics were obtained from the medical record and included cause of kidney failure, age, race, diabetes at time of transplant, gender, transplant type, CMV status, immunosuppressive regimen, induction therapy, new onset diabetes after transplant (NODAT), delayed graft function in deceased donor recipients and acute CNI related nephrotoxicity. Concomitant drugs (calcium channel blockers, angiotensin converting enzyme inhibitors, steroid use, antivirals) and serum creatinine (SCr) at time of each trough concentration were obtained from the medical record. Creatinine clearance (CrCl) was estimated using the Cockcroft and Gault formula.(12)
Genotyping for CYP3A5*3 in the Tacrolimus Recipients
Tacrolimus recipients were genotyped for the CYP3A5 genotype (rs776746). The CYP3A5*3 allele is associated with reduced CYP3A5 enzyme activity.(13) CYP3A5*3 has not been shown to be an clinically important in the metabolism of cyclosporine so it was not genotyped in the cyclosporine recipients.(14–16) Pretransplant recipient DNA was isolated from lymphocytes obtained from peripheral blood after RBC lysis, with DNA purity and concentration determined by ultraviolet spectroscopy (Thermo Scientific, Wilmington, DE). Genotyping in the first 689 subjects was conducted using a customized Affymetrix GeneChip (Affymetrix, Santa Clara, CA). Details of GeneChip genotyping have been previously described.(5) The remaining 1864 subjects were genotyped using Illumina VeraCode platform using primers designed by Illumina (Illumina, San Diego, CA). Genotypes were visualized using the BeadXpress reader and data analyzed using Illumina Genome BeadStudio. Genotyping of SNP rs776746 resulted in a 100% call rate. Tests for Hardy Weinberg equilibrium showed no evidence for disequilibrium in either African American or non-African American subgroups (p > 0.05 in both subgroups). Two hundred twelve SNPs were duplicated on both platforms with a mean concordance rate of 97.5% per individual. For the CYP3A5*3 which was used in this analysis there was 100% concordance.
Statistical Analysis
Linear mixed effects regression models were used to test the association between recipient age group (18–34, 35–64, or 65–84 years) and the log of dose and weight normalized CNI trough concentrations. The correlation structure consisted of random slopes and intercept per individual and a model correlation between troughs within each individual. Each trough was considered a discrete value. Separate models were created for tacrolimus and cyclosporine troughs. Visual inspection showed that dose and weight normalized troughs initially started low, rose quickly, and then plateaued in the early weeks posttransplant. Therefore we used a simple spline to model the effect of time on CNI trough concentrations, with the change in slope occurring at day 9 for tacrolimus troughs and day 17 for cyclosporine troughs.
Confounding clinical factors were identified for tacrolimus and cyclosporine troughs separately by backwards selection with a retention p-value of 0.10. Tested clinical factors for tacrolimus troughs included as fixed covariates: transplant center, CYP3A5*3 (rs776746), CYP3A5*3 by recipient age group interaction, donor age (0–34, 35–64 or 65–84 years) and gender, recipient gender and race, diabetes at baseline, donor type (living or deceased), antibody induction, and simultaneous kidney-pancreas transplantation. Steroid use, closest CrCl to the trough (linear and quadratic), calcium channel blocker use, ACE inhibitor, and antiviral drug use were defined at each observation. Tested clinical factors for cyclosporine troughs included as fixed covariates were transplant center, donor age (0–34, 35–64 or 65–84 years) and gender, recipient gender and race, diabetes at baseline, donor type (living or deceased), and antibody induction. Steroids use, closest CrCl to the trough (linear and quadratic), calcium channel blocker use, ACE-inhibitor use and antiviral use were defined at each observation. CrCl was mean centered at 70 ml/min.
Cochran Mantel Hanszel general association tests controlling for unit were used to test the association between recipient age group (18–34, 35–64, or 65–84 years) and outcomes such as delayed graft function in recipients of deceased donors, new onset diabetes after transplant (NODAT) in those without diabetes pretransplant, and acute CNI related nephrotoxicity. Once a significant association was identified between NODAT and age group, logistic regression was used to estimate odds ratios. Delayed graft function was defined as need for dialysis within 7 days posttransplant for recipients with deceased donors. NODAT was defined as elevated glucose requiring glucose lowering drug(s) within the first 6 months posttransplant in subjects without a diabetes diagnosis at time of transplant. CNI-related acute nephrotoxicity was any rise in SCr that resulted in a lowering of the CNI dose, discontinuation of CNI, or switching to an alternate CNI within 14 days after the rise, followed by any reduction in the SCr within 14 days after the last of these changes. Additionally, if a biopsy was obtained in conjunction with the rise in SCr, the primary diagnosis on biopsy must not rule out CNI nephrotoxicity. All statistical analyses were conducted using SASv9.2 (The SAS Institute, Cary, NC, USA, http://www.sas.com).
Results
We studied 348 young (18–34 years), 1,831 middle aged (35–64 years) and 374 older (65–84 years) adult kidney transplant recipients. Subject characteristics by CNI type are shown in Tables 1 and 2. A median of 18.0 (interquartile range [IQR] 15.0–22.0) tacrolimus trough values and 14.0 (10.0–22.0) cyclosporine trough values per subject were available. Of the 34,479 tacrolimus troughs, 411 were obtained during once daily dosing, 33,995 during twice daily dosing and 72 during three times daily administration. Of the 11,133 cyclosporine toughs, 28 troughs were obtained during once daily administrations, 11,092 during twice daily dosing and 12 during three times daily. The dosing interval was missing for one cyclosporine trough measurement.
Table 1.
Kidney Recipient and Donor Demographics and Clinical Factors in Recipients Receiving Tacrolimus
| Young Age (18–34 yrs, n=266) | Middle Age (35–64 yrs, n=1425) | Old Age (65–84 yrs, n=276) | |
|---|---|---|---|
| Recipient age, median (range) | 29.3(18.4–35.0) | 51.1(35.0–64.9) | 68.5 (65.1–83.8) |
| Recipient gender, male | 153 (57.5%) | 906 (63.6%) | 178 (64.5%) |
| Recipient African American | 72 (27.1%) | 284 (19.9%) | 18 (6.5%) |
| Recipient weight (kg), median (range) | 71.8 (38.6–152.0) | 81.6 (37.3–164.2) | 81.0 (44.2–130.2) |
| Prior kidney transplant | 56 (21.1%) | 217 (15.2%) | 16 (5.8%) |
| Primary cause of kidney failure | |||
| diabetes mellitus | 48 (18.1%) | 462 (32.4%) | 84 (30.4%) |
| glomerulonephritis | 85 (32.0%) | 301 (21.1%) | 45 (16.3%) |
| polycystic kidney disease | 9 (3.4%) | 215 (15.1%) | 30 (10.9%) |
| hypertension | 27 (10.2%) | 195 (13.7%) | 49 (17.8%) |
| others | 82 (30.8%) | 218 (15.3%) | 54 (19.6%) |
| unknown | 15 (5.6%) | 34 (2.4%) | 14 (5.1%) |
| Diabetes at time of transplant | 52 (19.6%) | 592 (41.6%) | 119 (43.1%) |
| SPK1 | 24 (9.0%) | 128 (9.0%) | 1 (0.4%) |
| Donor age, median (range) | 36.9 (7.5–63.9) | 42.3 (1.7–70.9) | 48.3 (10.4–76.7) |
| Donor gender, male | 126 (47.4%) | 690 (48.6%) | 120 (43.8%) |
| Living donor | 175 (65.8%) | 791 (55.5%) | 168 (61.1%) |
| Dialysis after transplantation2 | 19 (7.1%) | 134 (9.4%) | 23 (8.3%) |
| Calcium channel blocker use3 | 1764 (40.3%) | 10585 (42.0%) | 2259 (46.0%) |
| Prophylactic antiviral use 3 | 2261 (51.7%) | 13652 (54.5%) | 2520 (51.4%) |
| ACE Inhibitor use3 | 623 (14.2) | 3565 (14.2%) | 665 (13.5%) |
| Steroids use3 | 2859 (65.4%) | 14623 (58.5%) | 3464 (71.0%) |
simultaneous pancreas kidney transplant
one or more dialysis sessions within first 30 days posttransplant in living and deceased subjects
drug from this class use at the time of trough measurement
calcium channel blocker was present at time of trough measurement; primarily amlodipine and diltiazem.
Table 2.
Kidney Recipient and Donor Demographics and Clinical Factors in Recipients Receiving Cyclosporine (n=744)
| Young Age (18–34 years, n=107) | Middle Age (35–64 years, n=520) | Old Age (65–84 years, n=117) | |
|---|---|---|---|
| Recipient age, median (range) | 26.9 (18–34.7) | 51.7 (35–65) | 69.0 (65.3–81.3) |
| Recipient gender, male | 71 (66.4%) | 321 (61.7) | 67 (57.3%) |
| Recipient African American | 17 (15.9%) | 64 (12.3%) | 5 (4.3%) |
| Recipient weight (kg), median (range) | 81.8 (37.7–158.0) | 82.0 (39.0–150.3) | 78.2(44.2–136.4) |
| Prior kidney transplant | 15 (14.0%) | 77 (14.8%) | 6 (5.1%) |
| Primary cause of kidney failure | |||
| diabetes mellitus | 8 (7.5%) | 159 (30.6%) | 39 (33.3%) |
| glomerulonephritis | 35 (32.7%) | 125 (24.0%) | 12 (10.3%) |
| polycystic kidney disease | 1 (.93%) | 67 (12.9%) | 14 (12.0%) |
| hypertension | 4 (4.0%) | 43 (8.3%) | 25 (21.4%) |
| others | 50 (46.8%) | 105 (20.2%) | 21 (18.0%) |
| unknown | 9 (8.4%) | 21 (4.0%) | 6 (5.1%) |
| Diabetes at time of transplant | 13 (12.2%) | 195 (37.5%) | 52 (44.4%) |
| SPK1 | 0 (0.0%) | 9 (1.7%) | 0 (0.0%) |
| Donor age, median (range) | 37.6 (6.1–65.3) | 41.9 (2.9–66) | 46.4 (0.8–69.4) |
| Donor gender, male | 44 (41.1%) | 241 (46.4%) | 53 (45.3%) |
| Living donor | 83 (77.6%) | 326 (62.9%) | 66 (56.4%) |
| Dialysis after transplantation2 | 4 (3.7%) | 69 (13.3%) | 17 (14.5%) |
| Calcium channel blocker use3,4 | 857 (51.4%) | 4150 (54.0%) | 1089 (61.2%) |
| Prophylactic antiviral use 3 | 911 (55.2%) | 3945 (51.3%) | 894 (50.3%) |
| ACE inhibitor use3 | 291 (17.5%) | 1251(16.3%) | 253 (14.2%) |
| Steroid use3 | 766 (46.0%) | 4046 (53.0%) | 922 (52.5%) |
simultaneous pancreas kidney transplant
one or more dialysis sessions within first 30 days posttransplant in living and deceased subjects
drug from this class use at the time of trough measurement
calcium channel blocker was present at time of trough measurement; primarily amlodipine and diltiazem.
Effect of Age on CNI Troughs and Dose
Tacrolimus and cyclosporine troughs, both unnormalized and dose and weight normalized, increased with age, whereas daily dose decreased with age (Table 3, Figures 1 and 2). There was a weak but significant association between CNI dose and troughs in all age groups (r=0.14–0.41, all p-values < 0.0001).
Table 3.
Effect of Age on Tacrolimus and Cyclosporine Trough Concentrations (median, IQR)
| Age group | Young (18–34 years) | Middle (35–64 years) | Old (65–84 years) |
|---|---|---|---|
|
Tacrolimus
| |||
| No. subjects | 266 | 1425 | 276 |
| No. troughs | 4374 | 25194 | 4911 |
| TAC trough (ng/mL) | 7.8(5.8–10.0) | 8.1(6.1–10.1) | 8.2(6.4–10.3) |
| TAC daily dose (mg) | 7.0(4.0–10.0) | 6.0(4.0–8.0) | 5.0(3.0–8.0) |
| Weight (kg) | 71.8(60.0–89.2) | 81.6(70.2–95.3) | 81.0(69.7–93.5) |
| TAC dose-and weight-normalized trough | 77.1(48.5–127.0) | 111.4(68.9–180.0) | 129.8(84.5–201.2) |
|
| |||
|
Cyclosporine
| |||
| No. subjects | 107 | 520 | 117 |
| No. troughs | 1666 | 7687 | 1779 |
| CSA trough (ng/mL) | 169.0(133.0–222.0) | 179.0(139.0–228.0) | 181.0(139.0–233.0) |
| CSA daily dose (mg) | 350(300–450) | 350(250–400) | 250(200–350) |
| Weight (kg) | 69.3(60.9–87.5) | 83.1(70.3–98.2) | 78.2(65.9–89.9) |
| CSA dose and weight normalized trough | 34.6(24.1–51.4) | 46.7(33.0–65.2) | 54.6(39.9–74.1) |
TAC, tacrolimus; CSA, cyclosporine
Data are median and interquartile range (IQR)
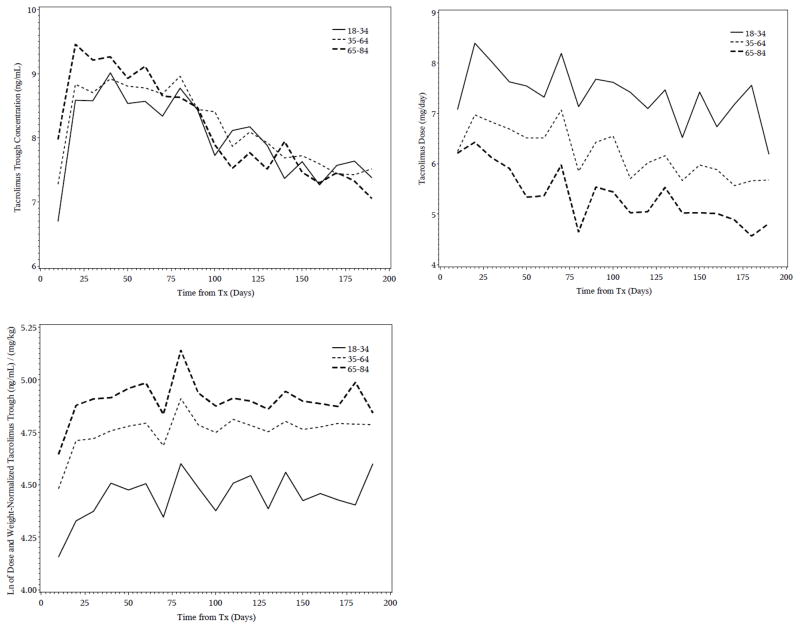
Figure 1.
Mean tacrolimus troughs, daily doses and dose and weight normalized troughs by age group (18–34, 35–64, and 65–84 years old). Observations are grouped into 10-day periods.
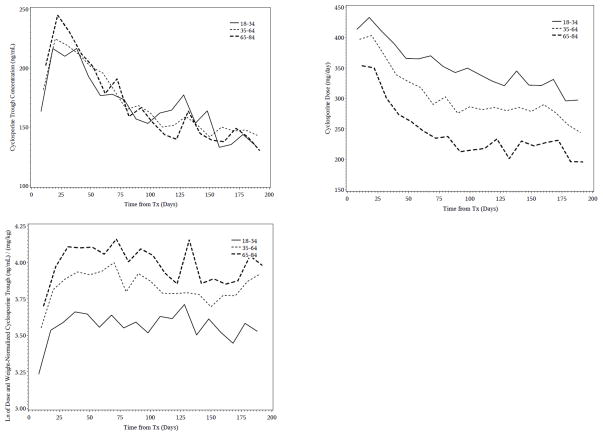
Figure 2.
Mean cyclosporine troughs, daily doses and dose and weight normalized troughs by age group (18–34, 35–64, and 65–84 years old). Observations are grouped into 10-day periods.
Over the entire first 6 months posttransplant older adults had higher median unnormalized tacrolimus troughs than either middle aged or young adults (older: 8.2 ng/mL; middle: 8.1 ng/mL; younger: 7.8 ng/mL) despite receiving 1 mg/day lower median dose (5 vs 6 mg/day) than middle age adults and 2 mg/day lower median dose (5 vs 7 mg/day) than younger adults. Unnormalized tacrolimus troughs were the highest in the older age adults compared to the other age groups for approximately the first 70 days posttransplant (Figure 1) after which time troughs were similar across the age groups. The effect of age was even more striking after normalizing the trough for dose and weight. The tacrolimus troughs were 17% (129.8 vs 111.4 ng/mL per mg/kg) higher in older adults than middle aged adults and 68% higher in older adults than young adults (129.8 vs 77.1 ng/mL per mg/kg) and remained substantially higher throughout follow-up (Figure 1).
The findings were similar for cyclosporine. Over the entire first 6 months posttransplant older adults had higher median unnormalized cyclosporine troughs than either middle aged or young adults (older: 181 ng/mL; middle aged: 179 mg/mL; younger: 169 ng/mL) but received 100 mg/day lower median doses than either younger or middle aged adults (older: 250 mg/day, both middle aged and younger: 350 mg/day). Cyclosporine troughs were higher in the older age adults compared to the other age groups for approximately the first 40 days (Figure 2) after which time troughs were similar across the age groups. Cyclosporine troughs, normalized for dose and weight, was 58% higher in older adults compared to the young adults (54.6 vs 34.6 ng/mL per mg/kg) and 17% (54.6 vs 46.7 ng/mL per mg/kg) higher than middle aged adults and remained substantially higher throughout follow-up (Figure 2).
Effect of CYP3A5 Genotype on Tacrolimus Dose and Troughs by Age
The CYP3A5 genotype (rs776746) is well-known to result in a significant reduction of tacrolimus metabolism and we considered this genotype in our analysis. Both age and genotype independently impacted tacrolimus dose and weight normalized trough levels (Table 4). Subjects in all three age groups with the CYP3A5*3/*3 genotype had a median dose and weight normalized trough twice as high as that of subjects with one or more *1 alleles. Older subjects with the CYP3A5*3/*3 genotype had a median dose and weight normalized trough that was three times as high as troughs of young individuals with one or more CYP3A5*1 alleles (145.9 vs 48.1 ng/mL per mg/kg). In addition, the median trough was higher (8.4 vs 6.7 ng/mL) and dose substantially lower (4 vs 10 mg/day) in the older subjects with the CYP3A5*3/*3 genotype compared to young subjects with one or more CYP3A5*1 alleles. The effect of age within a given genotype was substantial. In individuals with the CYP3A5*3/*3 genotype, dose and weight normalized troughs were 45% higher in the older subject, and 35% higher in the middle aged individuals relative to the younger adults. There was a similar increase in trough with increasing age in individuals with the CYP3A5*1/*1 or *1/*3 genotype.
Table 4.
Tacrolimus Troughs by CYP3A5 Genotype (rs776746) and Age (median, IQR)
| Age 18–34 years | CYP3A5*1/*1 and *1/*3 | CYP3A5*3/*3 |
|---|---|---|
| No. subjects | 103 | 163 |
| No. troughs | 1677 | 2697 |
| Trough ng/mL | 6.7(4.6–8.8) | 8.5(6.5–10.5) |
| Daily dose (mg) | 10.0(6.0–14.0) | 6.0(4.0–8.0) |
| Weight (kg) | 75.0(59.5–93.1) | 70.8(60.4–86.5) |
| Dose and weight normalized trough | 48.1(34.9–68.4) | 101.1(69.6–161.9) |
|
| ||
|
Age 35–64 years
| ||
| No. subjects | 421 | 1004 |
| No. troughs | 7396 | 17798 |
| Trough ng/mL | 6.9(5.0–9.1) | 8.4(6.7–10.5) |
| Daily dose (mg) | 8.0(6.0–11.0) | 5.0(3.5–7.0) |
| Weight (kg) | 83.0(70.8–96.0) | 81.0(70.0–95.0) |
| Dose and weight normalized trough | 65.8(45.4–100.8) | 134.9(89.4–207.3) |
|
| ||
|
Age 65–84 years
| ||
| No. subjects | 59 | 217 |
| No. troughs | 1070 | 3841 |
| Trough ng/mL | 7.3(5.4–9.4) | 8.4(6.7–10.5) |
| Daily dose (mg) | 7.0(5.0–10.0) | 4.0(3.0–6.0) |
| Weight (kg) | 79.2(67.8–88.6) | 81.8(71.0–94.9) |
| Dose and weight normalized trough | 76.4(51.3–108.9) | 145.9(102.0–227.3) |
IQR=interquartile range
Multivariate Models for Log Dose and Weight Normalized CNI Trough Concentrations Tacrolimus
A multivariate model for log dose and weight normalized tacrolimus trough considering multiple clinical factors was developed (Table 5). Age and CYP3A5 genotype had the largest effects on tacrolimus troughs. Log dose and weight normalized troughs were 0.34 higher in the older vs younger age group, and 0.11 higher in older vs middle age group. Each CYP3A5*1 allele was associated with a 0.46 reduction in troughs. There was no interaction between age and genotype (p=0.33). In the final model older recipient age, younger donor age, male recipient, African American recipient, diabetes at time of transplant, living donor, lack of induction therapy or monoclonal antibody use vs. polyclonal induction, and concomitant calcium channel blocker treatment and antiviral use at time of trough were each independently associated with higher troughs. Because the frequency of the CYP3A5*1 allele is much higher in African Americans than Caucasians we explored a model without genotype. In the model without CYP3A5*1, African American race was significantly associated with lower troughs; however, the model fit was better when both race and genotype were included. Therefore all models included both covariates. Increasing days posttransplant from day 1–9 were also associated with higher troughs. After day 9, there was little change in log dose and weight normalized troughs.
Table 5.
Final Model for Tacrolimus Log Dose and Weight Normalized Trough Concentrations in the First 6 Months Posttransplanta
| Variable | Effect on Troughb (95% confidence interval) | p-valueb |
|---|---|---|
| Time posttransplant days 1–180 | 0.062(0.057 to 0.068)c | 3.2 ×10−92 |
| Additional effect of time posttransplant from day 10–180 | −0.062(−0.0767 to−0.056)c | 3.9 ×10−89 |
| Recipient age | ||
| 65–84 vs 18–34 years | 0.34(0.26 to 0.41)d | 3.5×10−18 |
| 65–84 vs 35–64 years | 0.11(0.05 to 0.17)e | |
| Donor age | ||
| 0–34 vs 6–584 years | 0.15(0.03 to 0.27)f | 3.9 × 10−2 |
| 35–64 vs 65–84 years | 0.15(0.04 to 0.27)f | |
| CYP3A5*1 allele (rs776746) | −0.46(−0.50 to −0.42)g | 1.2×10−105 |
| Male recipient | 0.15(0.11 to 0.19) | 1.3×10−13 |
| African American recipient | 0.12(0.04 to 0.20) | 2.8×10−3 |
| Diabetes at time of transplant | 0.12(0.08 to 0.16) | 5.4×10−9 |
| Living donor | 0.038(−0.006 to 0.08) | 9.1 × 10−2 |
| Steroid use | −0.03(−0.06 to −0.002)h | 3.4×10−2 |
| Calcium channel blocker use | 0.040(0.027 to 0.06)h | 5.4×10−8 |
| Antiviral use | 0.04(0.03 to 0.05) h | 3.0×10−14 |
| Antibody induction | ||
| Monoclonal vs polyclonal | 0.03(−0.015 to 0.082) i | 3.6×10−5 |
| Monoclonal and polyclonal combination vs polyclonal | −0.17(−0.052 to −0.30) i | |
| None vs polyclonal | 0.22(0.12 to 0.34) i | |
Multiple linear regression model on the log transformed dose and weight normalized tacrolimus trough concentration using stepwise variable selection. Model is adjusted for enrolling center.
Effect on log transformed, dose and weight normalized troughs, 95% CI and p-values are for the final model.
0.062 increase in log dose and weight normalized tacrolimus trough for every one day posttransplant, there is also an additional reduction in trough by 0.062 for each day after day 10 up to 180.
Log dose and weight-normalized tacrolimus trough is higher in 65–84 yrs relative to the 18–34 yr group.
Log dose and weight-normalized tacrolimus trough is higher in 65–84 yrs relative to the 35–64 yr group.
Log dose and weight-normalized tacrolimus trough is higher in younger age group relative to the 65–84 yr group.
Effect of one *1 allele on log dose and weight normalized tacrolimus trough. The reduction is −0.92 for two *1 alleles.
Effect on log dose and weight normalized tacrolimus trough when given with this concomitant therapy.
Effect on log dose and weight normalized tacrolimus trough when given with this concomitant therapy relative to a polyclonal induction agent (the reference).
Cyclosporine
A multivariate model for log dose and weight normalized cyclosporine trough considering multiple clinical factors as developed (Table 6). Recipient age had the largest effect on cyclosporine troughs. Log dose and weight normalized troughs were 0.35 higher in the older vs younger age group, and 0.12 higher in older vs middle age group. In the final model, older recipient age, male recipient, increasing CrCl and antiviral use were each associated with higher troughs. Time was associated with an increase in troughs through day 17; thereafter the effect of time on log dose and weight normalized troughs was small and not clinically relevant. African American recipient was retained in the final model; however, its effect on the model was minimal (estimated trough only 0.061 lower in African-Americans, p=0.12) and removal from the model had only a negligible effect on the estimates of the other variables.
Table 6.
Final Model for Cyclosporine Log Dose and Weight Normalized Trough Concentrations in the First 6 Months Posttransplanta
| Variable | Effect on Troughb (95% confidence interval) | p-value |
|---|---|---|
| Time posttransplant days 1–180 | 0.029(0.026 to 0.032)c | 2.8×10−70 |
| Additional effect of time posttransplant from day 18–180 | −0.029(−0.032 to −0.026)c | 7.2×10−69 |
| Recipient age | 1.8×10−15 | |
| 65–84 vs 18–34 yrs | 0.35(0.27 to 0.44)d | |
| 65–84 vs 35–64 yrs | 0.12(0.052 to 0.18)e | |
| African American recipient | −0.061(−0.14 to 0.016) | 1.2×10−1 |
| Male recipient | 0.08(0.032 to 0.13) | 1.1×10−3 |
| Creatinine clearance rate (centered) | 0.011(0.0042 to 0.018)f | 1.4×10−3 |
| Prophylactic antiviral use | 0.029(0.015 to 0.044)g | 1.0×10−4 |
Multiple linear regression models on the log transformed dose-normalized tacrolimus trough concentration using stepwise variable selection. Model is adjusted for enrolling center.
Estimated effect on log transformed, dose and weight normalized troughs, 95% CI and p-values are for the final model. Effects are adjusted for transplant center.
0.029 increase in log dose and weight-normalized cyclosporine trough for every one day posttransplant, there is also an additional reduction in trough by 0.029 for each day from 18–180.
Log dose and weigh normalized cyclosporine trough were higher in the 65–84 yr group relative to the 18–34 yr group.
Log dose and weight normalized cyclosporine trough were higher in the 65–84 yr group relative to the 35–64 yr group.
Effect on log dose and weight normalized for each increase of 10 ml/min in CrCl, calculated by the Cockcroft and Gault equation.
Effect on log dose and weight normalized cyclosporine trough when concomitant prophylactic antiviral therapy was present.
Age and CNI Related Toxicities
There was no association between delayed graft function or CNI related nephrotoxicity and increasing age for either tacrolimus or cyclosporine (data not shown). However, older recipients had a higher incidence of NODAT than the middle and younger age groups. Overall NODAT by 6 months occurred in 99 (8.3%) of the 1194 tacrolimus recipients and in 31 (7.1%) of the 440 cyclosporine recipients who did not have diabetes before transplant and had evaluable data. For tacrolimus, NODAT in the 18–34, 35–65 and 65–84 year age groups the incidence was 1.4%, 8.5% and 16.7%, respectively. The odds ratio (95% CI) for developing NODAT in the older age group was 16.7 (4.85, 58.8) (p<0.0001) relative to the young age group and 2.16 (1.30, 3.60) (p=0.0032) relative to the middle age group. For cyclosporine, NODAT in the 18–34, 35–65 and 65–84 year age groups the incidence was 2.4%, 7.1% and 12.7%, respectively. The odds ratio (95% CI) for developing NODAT in the older age group was 5.49 (1.09, 27.8) (p=0.039) relative to the young age group and 2.01 (0.82, 4.93) (p=0.12) relative to the middle age group.
Discussion
To our knowledge this is the largest published analysis of CNI troughs in adult kidney transplant recipients and is the first to demonstrate significant age related changes in CNI disposition in older adults. It is well known that older individuals have changes in drug disposition with increasing age. The extent and relevance of these changes and identifying which drugs are most affected by age related changes have been the matter of investigation in the field of geriatrics.(17) Drug disposition in the aging transplant population has been poorly studied despite that the number of kidney transplants in individuals age >65 years has increased dramatically in the last 10 years. Therefore, it has become critical to understand metabolic and pharmacologic changes in the older transplant recipient and to define the best immunosuppressive regimens for this age group. A prerequisite to informing and designing better immunosuppressive regimens is a comprehensive understanding of the clinical pharmacology and adverse effect potential in this population.
Previous studies have shown a modest or no effect of increasing adult age on immune suppressant pharmacokinetics; however, these studies suffer from a limited number of older individuals.(18–20) This present study includes CNI pharmacokinetics in 2553 adults (393 between 65–84 years of age), and we have found that increasing age resulted in a progressive decline in CNI clearance, even after adjustment for clinical factors. Older subjects received a median of 1 and 2 mg per day lower tacrolimus dose than middle and young aged adults, respectively. Despite the lower doses, older adults achieved higher median tacrolimus troughs for at least the first 70 days posttransplant relative to the younger adults. This was also true for cyclosporine where older subjects received a median of 100 mg per day lower dose but yet achieved higher troughs for the first 40 days posttransplant than the younger adults. Especially when CNI troughs were normalized for dose and weight they were significantly higher in the older adults. These data strongly support an age related decline in CNI apparent clearance. The mechanism of decreased metabolism and higher troughs in the older recipient is not known. However, aging is associated with multiple physiologic changes of which may affect drug disposition. Older individuals have a decreased ability to metabolize certain substrates including those requiring phase I enzymes (e.g. cytochrome P450’s) possibly due to a reduction in liver size, lower hepatic blood flow, reduced drug transport across the hepatic endothelium, changes in drug protein binding and alterations in gastrointestinal motility and pH.(21–24) Older individuals also have increased body fat, decreased hemostatic reserve, more comorbid conditions and drug therapies which increase the risk of drug-drug interactions. Specifically, reductions in CNI clearance may be due to reduced hepatic or gut CYP3A enzyme, they may also have a decrease in p-glycoprotein activity, slowed gastric emptying and/or intestinal transit time or more drug-drug interactions that inhibit CNI metabolism.(25, 26)
Despite all patients undergoing regular trough monitoring, the older recipients had tacrolimus troughs that were higher than the younger aged groups for about the first 70 days posttransplant. The findings were similar for cyclosporine, where troughs were higher in the older age group for at least the first 40 days. Should older patients have been initiated on lower doses of CNIs they may have had troughs similar to the middle and younger age groups much sooner. However, future randomized trials will be needed to define whether reducing CNI troughs in the elderly increases acute rejection and/or reduces toxicity. This data provides a basis for determining a rationale starting doses in future trials in the elderly.
Our finding of higher early median CNI troughs in older adults is interesting from the immunologic perspective since it is widely thought that older adults have lower immunological responsiveness allowing for lower levels of immunosuppression and/or fewer immunosuppressive drugs.(27, 28) Although clinicians may acknowledge that lower immunosuppression is reasonable, it is not apparent within our consortium that CNI reduction in the elderly is occurring. Older adults have a lower risk of acute rejection which is thought to be due to lower immune responsiveness, but yet they are at a higher risk of immunosuppression related adverse effects such as infections, metabolic disturbances, cardiovascular complications and new onset diabetes after transplant.(29–32) In this analysis we found a higher incidence of new onset diabetes after transplant by 6 months in the older recipients receiving a CNI. It may be possible that CNI related adverse effects would be reduced and efficacy retained if lower doses of calcineurin inhibitors were used. However, this needs to be formally tested in future trials.
Clinical factors other than age were independently associated with CNI troughs. For cyclosporine, time posttransplant and age, male recipient, CrCl and prophylactic antiviral use were important factors; however, age had the greatest effect. For tacrolimus, we found that time posttransplant, recipient and donor age, CYP3A5 genotype, male recipient, African American recipient, diabetes at transplant, steroid, calcium channel blocker, antiviral drug use and antibody induction type was associated with troughs. We and others have previously shown that individuals with one or more CYP3A5*1 alleles have lower tacrolimus troughs, lower dose and weight normalized troughs, and higher tacrolimus clearance relative to those without CYP3A5*1.(5, 33, 34) Interestingly, after adjustment for clinical factors and CYP3A5*1, African American race was associated with a higher tacrolimus troughs. Previous studies have shown that African Americans have lower tacrolimus troughs than non-African Americans mostly because African Americans have a high minor allele frequency of CYP3A5*1 relative to non-African Americans. However, there is a subset of African Americans that do not carry a CYP3A5*1 allele, and those individuals most likely have higher troughs than the nonAfrican Americans. When race was removed from the model, the estimates of the other covariates did not qualitatively change; therefore, when genotype is accounted for, the race effect is independent of the other covariates.
Authors have proposed that the impact of genotype on drug phenotypes declines with age and therefore may have limited utility in older individuals.(17) We showed that the effects of CYP3A5 genotype and age are independent and that the combination profoundly affects tacrolimus troughs. Older recipients with the CYP3A5*3/*3 genotype had a median dose and weight normalized trough that was two times higher than troughs of older individuals with one or more CYP3A5*1 alleles (145.9 vs 76.4 ng/mL per mg/kg). In addition, within the older group, the median trough was higher in those with CYP3A5*3/*3 (8.4 vs 7.3 ng/mL) and dose was dramatically lower (4 vs 7 mg/day) compared to those with one or more CYP3A5*1 allele. Therefore, genotype remains important in older individuals. Older individuals with the CYP3A5*3/*3 genotype may be at especially high risk of adverse effects, and this should be evaluated in future studies.
There are several limitations to our study. First this is an observational trial and pharmacokinetic blood draws were not supervised in a research setting. Consequently dose compliance is assumed and trough measurements were not conducted in a centralized laboratory. To address the differences in analytical techniques used by the centers our analyses were adjusted for center. In the future these findings should be validated in patients undergoing a clinical trial using the same analytical methods and compliance formally verified. Second there are also likely comorbidities occurring in the older adult that we have not accounted for and should be examined in future studies. There are also drug interactions with the CNIs that may not be accounted for (such as differences in inhibitory potential of the calcium channel blockers). Finally, because frail elders rarely undergo transplantation this data does not apply to these individuals.
In summary, we have found that transplant recipients 65–84 years of age have significantly higher dose and weight normalized CNI troughs than younger recipients. The use of lower CNI doses in older recipients should be evaluated in future randomized clinical trials. Reducing CNI exposure may lessen CNI related toxicities in the older recipient.
Acknowledgments
Support from National Institutes of Health NIAID Genomics of Transplantation (5U19-AI070119)
Support from National Institutes of Health NIAID Genomics of Transplantation (5U19-AI070119) and DeKAF (5U01-AI058013)
Support from National Institutes of Health NIAID Genomics of Transplantation (5U19-AI070119)
Support from National Institutes of Health NIAID Genomics of Transplantation (5U19-AI070119) and DeKAF (5U01-AI058013)
Support from National Institutes of Health NIAID Genomics of Transplantation (5U19-AI070119)
Received support from National Institutes of Health NIAID Genomics of Transplantation (5U19-AI070119) and DeKAF (5U01-AI058013)
Received support from National Institutes of Health NIAID Genomics of Transplantation (5U19-AI070119)
This project was supported by grant number (5U19-AI070119 and 5U01-AI058013) from the National Institute of Allergy and Infectious Disease. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Disease or the National Institutes of Health.
We acknowledge and appreciate the dedication and hard work of our coordinators: University of Alberta, Nicoleta Bobocea, Tina Wong, Adrian Geambasu and Alyssa Sader; University of Minnesota, Mandi DeGrote and Jill Nagorski; Hennepin County Medical Center, Lisa Berndt; Mayo Clinic, Tom DeLeeuw; University of Iowa, Wendy Wallace and Tammy Lowe; University of Alabama, Catherine V. Barker and Tina Hilario. We also acknowledge the dedicated work of our research scientists: Marcia Brott, Becky Willaert, Brian Kasel and Winston Wildebush.
Abbreviations
- CNI
calcineurin inhibitor
DeKAF Investigators
Arthur Matas, M.D. Department of Surgery University of Minnesota, Minneapolis, MN 55455 Email: matas001@umn.edu
J. Michael Cecka, M.D. UCLA Immunogenetics Center, Los Angeles, CA 90095 Email: mcecka@ucla.edu
John Connett, Ph.D. Division of Biostatistics. University of Minnesota, Minneapolis, MN 55455 Email: john-c@biostat.umn.edu
Fernando G. Cosio, M.D. Division of Nephrology, Mayo Clinic, Rochester, MN 55905 Email: Cosio.Fernando@mayo.edu
Robert Gaston, M.D. Division of Nephrology, University of Alabama, Division of Nephrology, Birmingham, AL 35294 Email: rgaston@uab.edu
Sita Gourishankar M.D. Division of Nephrology and Immunology, University of Alberta, Edmonton, Alberta, Canada Email: sitag@ualberta.ca
Joseph P. Grande, M.D., Ph.D. Mayo Clinic College of Medicine, Rochester MN 55905 Email: Grande.Joseph@mayo.edu
Lawrence Hunsicker, M.D. Nephrology Division, Iowa City, IA 52242-1082 Email: lawrence-hunsicker@uiowa.edu
Bertram Kasiske, M.D. Division of Nephrology, Hennepin County Medical Center, Minneapolis, MN 55415 Email: kasis001@umn.edu
Rosalyn B. Mannon, M.D. Division of Nephrology, University of Alabama at Birmingham, Birmingham, AL 35294 Email: rmannon@uab.edu
David Rush, M.D. Health Sciences Center, Winnipeg MB, Canada Email: drush@exchange.hsc.mb.ca
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Ruiter R, Visser LE, Rodenburg EM, Trifiro G, Ziere G, Stricker BH. Adverse drug reaction-related hospitalizations in persons aged 55 years and over: a population-based study in the Netherlands. Drugs & aging. 2012;29:225–232. doi: 10.2165/11599430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.McLachlan AJ, Pont LG. Drug metabolism in older people--a key consideration in achieving optimal outcomes with medicines. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67:175–180. doi: 10.1093/gerona/glr118. [DOI] [PubMed] [Google Scholar]
- 3.Le Couteur DG, McLachlan AJ, de Cabo R. Aging, drugs, and drug metabolism. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67:137–139. doi: 10.1093/gerona/glr084. [DOI] [PubMed] [Google Scholar]
- 4.Blosser CD, Huverserian A, Bloom RD, Abt PD, Goral S, Thomasson A, et al. Age, exclusion criteria, and generalizability of randomized trials enrolling kidney transplant recipients. Transplantation. 2011;91:858–863. doi: 10.1097/TP.0b013e31820f42d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson PA, Oetting WS, Brearley AM, Leduc R, Guan W, Schladt D, et al. Novel Polymorphisms Associated With Tacrolimus Trough Concentrations: Results From a Multicenter Kidney Transplant Consortium. Transplantation. 2011;91:300–308. doi: 10.1097/TP.0b013e318200e991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson PA, Schladt D, Oetting WS, Leduc R, Guan W, Matas AJ, et al. Genetic Determinants of Mycophenolate-Related Anemia and Leukopenia After Transplantation. Transplantation. 2011;91:309–316. doi: 10.1097/TP.0b013e318200e971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaston RS, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, Gourishankar S, et al. Use of cardioprotective medications in kidney transplant recipients. Am J Transplant. 2009;9:1811–1815. doi: 10.1111/j.1600-6143.2009.02696.x. [DOI] [PubMed] [Google Scholar]
- 8.Gourishankar S, Leduc R, Connett J, Cecka JM, Cosio F, Fieberg A, et al. Pathological and clinical characterization of the ‘troubled transplant’: data from the DeKAF study. Am J Transplant. 2010;10:324–330. doi: 10.1111/j.1600-6143.2009.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Israni A, Leduc R, Holmes J, Jacobson PA, Lamba V, Guan W, et al. Single-nucleotide polymorphisms, acute rejection, and severity of tubulitis in kidney transplantation, accounting for center-to-center variation. Transplantation. 2010;90:1401–1408. doi: 10.1097/TP.0b013e3182000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Israni I, Holmes J, Chen J, DS, Leduc R, Oetting W, et al. Transplant Center Influence on Single Nucleotide Polymorphism and Acute Kidney Rejection. Am J Transplant. 2010;10:184. [Google Scholar]
- 11.Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90:68–74. doi: 10.1097/TP.0b013e3181e065de. [DOI] [PubMed] [Google Scholar]
- 12.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 13.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 14.Hesselink DA, van Schaik RH, van der Heiden IP, van der Werf M, Gregoor PJ, Lindemans J, et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74:245–254. doi: 10.1016/S0009-9236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 15.Tang HL, Ma LL, Xie HG, Zhang T, Hu YF. Effects of the CYP3A5*3 variant on cyclosporine exposure and acute rejection rate in renal transplant patients: a meta-analysis. Pharmacogenetics and genomics. 2010;20:525–531. doi: 10.1097/FPC.0b013e32833ccd56. [DOI] [PubMed] [Google Scholar]
- 16.Anglicheau D, Thervet E, Etienne I, Hurault De Ligny B, Le Meur Y, Touchard G, et al. CYP3A5 and MDR1 genetic polymorphisms and cyclosporine pharmacokinetics after renal transplantation. Clin Pharmacol Ther. 2004;75:422–433. doi: 10.1016/j.clpt.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 17.McLachlan AJ, Hilmer SN, Le Couteur DG. Variability in response to medicines in older people: phenotypic and genotypic factors. Clinical pharmacology and therapeutics. 2009;85:431–433. doi: 10.1038/clpt.2009.1. [DOI] [PubMed] [Google Scholar]
- 18.Staatz CE, Tett SE. Pharmacokinetic considerations relating to tacrolimus dosing in the elderly. Drugs & aging. 2005;22:541–557. doi: 10.2165/00002512-200522070-00001. [DOI] [PubMed] [Google Scholar]
- 19.Miura M, Satoh S, Kagaya H, Saito M, Inoue T, Tsuchiya N, et al. No impact of age on dose-adjusted pharmacokinetics of tacrolimus, mycophenolic acid and prednisolone 1 month after renal transplantation. Eur J Clin Pharmacol. 2009;65:1047–1053. doi: 10.1007/s00228-009-0721-9. [DOI] [PubMed] [Google Scholar]
- 20.Wang CX, Meng FH, Chen LZ, Ren B, Li SX, Fei JG, et al. Population pharmacokinetics of mycophenolic acid in senile Chinese kidney transplant recipients. Transplantation proceedings. 2007;39:1392–1395. doi: 10.1016/j.transproceed.2007.02.082. [DOI] [PubMed] [Google Scholar]
- 21.Sotaniemi EA, Arranto AJ, Pelkonen O, Pasanen M. Age and cytochrome P450-linked drug metabolism in humans: an analysis of 226 subjects with equal histopathologic conditions. Clinical pharmacology and therapeutics. 1997;61:331–339. doi: 10.1016/S0009-9236(97)90166-1. [DOI] [PubMed] [Google Scholar]
- 22.Marchesini G, Bua V, Brunori A, Bianchi G, Pisi P, Fabbri A, et al. Galactose elimination capacity and liver volume in aging man. Hepatology. 1988;8:1079–1083. doi: 10.1002/hep.1840080516. [DOI] [PubMed] [Google Scholar]
- 23.Woodhouse KW, James OF. Hepatic drug metabolism and ageing. British medical bulletin. 1990;46:22–35. doi: 10.1093/oxfordjournals.bmb.a072387. [DOI] [PubMed] [Google Scholar]
- 24.Woodhouse KW, Wynne HA. Age-related changes in liver size and hepatic blood flow. The influence on drug metabolism in the elderly. Clinical pharmacokinetics. 1988;15:287–294. doi: 10.2165/00003088-198815050-00002. [DOI] [PubMed] [Google Scholar]
- 25.Toornvliet R, van Berckel BN, Luurtsema G, Lubberink M, Geldof AA, Bosch TM, et al. Effect of age on functional P-glycoprotein in the blood-brain barrier measured by use of (R)-[(11)C]verapamil and positron emission tomography. Clinical pharmacology and therapeutics. 2006;79:540–548. doi: 10.1016/j.clpt.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Warrington JS, Greenblatt DJ, von Moltke LL. Age-related differences in CYP3A expression and activity in the rat liver, intestine, and kidney. The Journal of pharmacology and experimental therapeutics. 2004;309:720–729. doi: 10.1124/jpet.103.061077. [DOI] [PubMed] [Google Scholar]
- 27.Meier-Kriesche H, Ojo AO, Arndorfer JA, Leichtman AB, Lake K, Cibrik DM, et al. Need for individualized immunosuppression in elderly renal transplant recipients. Transplantation proceedings. 2001;33:1190–1191. doi: 10.1016/s0041-1345(00)02380-0. [DOI] [PubMed] [Google Scholar]
- 28.Meier-Kriesche HU, Kaplan B. Immunosuppression in elderly renal transplant recipients: are current regimens too aggressive? Drugs & aging. 2001;18:751–759. doi: 10.2165/00002512-200118100-00004. [DOI] [PubMed] [Google Scholar]
- 29.de Fijter JW. The impact of age on rejection in kidney transplantation. Drugs Aging. 2005;22:433–449. doi: 10.2165/00002512-200522050-00007. [DOI] [PubMed] [Google Scholar]
- 30.Kuypers DR, de Jonge H, Naesens M, Vanrenterghem Y. A prospective, open-label, observational clinical cohort study of the association between delayed renal allograft function, tacrolimus exposure, and CYP3A5 genotype in adult recipients. Clinical therapeutics. 2010;32:2012–2023. doi: 10.1016/j.clinthera.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Meier-Kriesche HU, Ojo AO, Hanson JA, Kaplan B. Exponentially increased risk of infectious death in older renal transplant recipients. Kidney international. 2001;59:1539–1543. doi: 10.1046/j.1523-1755.2001.0590041539.x. [DOI] [PubMed] [Google Scholar]
- 32.Danovitch GM, Gill J, Bunnapradist S. Immunosuppression of the elderly kidney transplant recipient. Transplantation. 2007;84:285–291. doi: 10.1097/01.tp.0000275423.69689.dc. [DOI] [PubMed] [Google Scholar]
- 33.Passey C, Birnbaum AK, Brundage RC, Oetting WS, Israni AK, Jacobson PA. Dosing Equation for Tacrolimus Using Genetic Variants and Clinical Factors. British journal of clinical pharmacology. 2011 doi: 10.1111/j.1365-2125.2011.04039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin Pharmacokinet. 2010;49:141–175. doi: 10.2165/11317350-000000000-00000. [DOI] [PubMed] [Google Scholar]