Abstract
The tachykinin, neurokinin 3 receptor (NK3R) is a g-protein coupled receptor that is broadly distributed in the nervous system and exerts its diverse physiological actions through multiple signaling pathways. Despite the role of the receptor system in a range of biological functions, the effects of NK3R activation on chromatin dynamics and gene expression have received limited attention. The present work determined the effects of senktide, a selective NK3R agonist, on chromatin organization, acetylation, and gene expression, using qRT-PCR, in a hypothalamic cell line (CLU 209) that expresses the NK3R. Senktide (1 nM, 10 nM) caused a relaxation of chromatin, an increase in global acetylation of histone H3 and H4, and an increase in the expression of a common set of genes involved in cell signaling, cell growth, and synaptic plasticity. Pretreatment with histone acetyltransferase (HAT) inhibitor (Garcinol and 2-methylene y-butylactone), that inhibits p300, p300/CREB Binding Protein (CBP) associated factor (PCAF), and GCN 5, prevented the senktide-induced increase in expression of most, but not all, of the genes upregulated in response to 1 nM and 10 nM senktide. Treatment with 100 nM had the opposite effect: a reduction in chromatin relaxation and decreased acetylation. The expression of four genes was significantly decreased and the HAT inhibitor had a limited effect in blocking the upregulation of genes in response to 100 nM senktide. Activation of the NK3R appears to recruit multiple pathways, including acetylation, and possibly histone deactylases, histone methylases, or DNA methylases to affect chromatin structure and gene expression.
Keywords: hypothalamus, neurokinin 3 receptor, receptor signaling, chromatin, acetylation
1. Introduction
The tachykinin, neurokinin 3 receptor (NK3R) is a g-protein coupled receptor that has a widespread distribution in brain and spinal cord [1, 15, 58, 59]. Its endogenous ligand, neurokinin B (NKB), is similarly dispersed in brain with NKB soma present at all levels of the neuraxis [49]. The distribution of NKB terminals and the NK3R overlap with a variety of systems, including those that process reward, fluid balance and vasopressin release, cardiovascular function, locomotion, pain, psychiatric disorders, temperature regulation, and reproduction [10, 13, 18, 22, 24, 42, 46, 54, 61, 73]. Furthermore, dysfunction in the signaling function of the NK3R has profound effects. Mutations in the TACR3 gene encoding NK3R causes hypogonadotropic hypogonadism and infertility [65, 72]. NK3R’s are also implicated in the hypertension [42] and blockade of the receptor prevents the systemic release of vasopressin and has a anti-hypertensive action [25, 42]. In addition, NK3R’s are a potential therapeutic target to treat gastrointestional pain [57], anxiety, and psychotic disorders, particularly schizophrenia [22, 48, 56, 61].
The NK3R is coupled to pertussis-toxin-insensitive Gq/G11, which activates phospholipase Cβ (PLC) and results in the generation of the second messengers, diacylglycerol (DAG) and inositol triphosphate (IP3). These two second messengers facilitate the release of Ca++ from intracellular stores and mediate a number of signaling cascades [27, 39]. In addition to PLC, higher doses of NK3R agonists increase cAMP formation, suggesting a linkage of NK3R and Gs, but PLC activation occurs at a lower agonist concentration than that required for stimulation of cyclic AMP formation [52].
In addition to the traditional signaling pathways through which NK3R activation may affect neuronal function, recent in vivo and in vitro data show an activity-dependent translocation of NK3R to the nucleus of hypothalamic and ventral tegmental area neurons [23, 28, 30, 42]. Transport of NK3R through the nuclear pore complex and into the nucleus is mediated by importin beta [31]. Once within the nucleus, co-immunoprecipitation and double immune-electron microscopy show that nuclear NK3R associates with acetylated histone H4 and H3 [17]. Thus, in addition to traditional second messenger signaling cascades, ligand-induced trafficking of NK3R to the nucleus may directly regulate gene expression.
In spite of its broad distribution, involvement in multiple physiological systems, and multiple signaling pathways that are invoked by activation of the NK3R, the effects of NK3R activation on gene expression and the means by which these gene expression changes occur have received limited attention. Gene expression in eukaryotes is regulated, in part, through changes in chromatin structure and post-translational modifications, such as acetylation of histones. Acetylation of the histones relaxes chromatin structure and is generally linked to transcriptional activation [3, 26, 45]. In the following experiments the effects of doses of an NK3R agonist, senktide, on chromatin compaction, global acetylation, and gene expression were determined.
2. Materials and methods
2.1 Cell culture
CLU209 cells, a rat immortalized neuronal hypothalamic cell line collected on embryonic day 18, were purchased from Cedarlane (Ontario, Canada). Cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS), 25 mM glucose, and 1% penicillin/streptomycin for the first three passes. After the third pass, the cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS) and 25 mM glucose but without 1% penicillin/streptomycin. CLU209 cells were incubated at 37 °C with 5% CO2 and were grown as a monolayer in a T-75 flask. When the cells were 70% confluent they were split at a 1:7 ratio by trypsinization to get single cells in suspension. Cells were generally passed every 3rd day.
2.2 Chromatin condensation assay
Cells were grown to ~70 % confluency, and separate flasks of cells were incubated for 45 minutes in media, or media containing 1nM, 10nM, or 100nM senktide, a selective NK3R agonist. At that point the cells were lysed to obtain nuclei using ChIP-IT express (Active Motif, Carlsbad, CA, USA), following the manufacturer’s protocol. Briefly, cells were fixed by adding formaldehyde (0.27 ml per 10 ml cell culture media) to the flasks for 10 min at room temperature. After pouring off the fixation solution, the cells were rinsed with PBS and the fixation reaction was stopped by adding Glycine Stop-Fix Solution (Active Motif) and incubating the cells at room temperature on a shaking platform for 5 min. Once the reaction was stopped the cells were rinsed with PBS. Cell Scraping Solution (30 μl 100 mM phenylmethanesulfonylfluoride [PMSF] added to 6 ml PBS) was added, and cells were scraped and transferred to 15 ml conical tubes. Cells were centrifuged at 2500 rpm for 10 min at 4°C. The pellet was subjected to lysis by adding 1 ml ice-cold Lysis buffer (Active Motif) containing 5 μl Protease Inhibitor Cocktail (Thermo Scientific, Rockford, IL, USA) and 5 μl 100 mM PMSF (Sigma). After incubation for 30 min on ice, cells were transferred to an ice-cold dounce homogenizer. Nuclei release was aided by stroking the cells 15 times each using loose and tight pestles. After douncing, the cells were transferred to 1.7 ml microcentrifuge tubes and centrifuged at 5000 rpm for 10 min at 4°C to pellet the nuclei. The nuclear pellet was resuspended in Shearing Buffer (Active Motif) containing 1.75 μl Protease Inhibitor Cocktail and 1.75 μl 100 mM PMSF. Chromatin was sheared using a sonicator (Qsonica, Newtown, CT, USA) to obtain fragments of about 500 bp size. Sonication was carried out at an amplitude of 25 for 21 cycles of a 20 second pulse followed by a 30 second pause on ice. Sheared chromatin was centrifuged at 15,000 rpm for 10 min at 4°C and the supernatant was transferred to a microtube and then subjected to a phenol/chloroform treatment to purify open chromatin [19]. The phenol-chloroform extraction procedure allows the quantification of relaxed/open DNA. DNA segments that are heavily compacted and cross-linked are retained in the interphase, while DNA segments that are not protein associated accumulate in the aqueous phase. Purified DNA was precipitated by adding 0.03 M sodium acetate (pH 5.2) and 100% ethanol (2.5 ×the sample volume), and incubating overnight at −20° C. The pellet was washed with 70% ethanol and air dried. The dried pellet was resuspended in dH2O and the DNA was quantified at 260 nm (optical density) using a Nanodrop spectrophotometer (Thermo Scientific Nanodrop Products, Wilmington, DE, USA). The samples were also quantified at 280 nm to check the purity. The 260/280 ratio of the samples were in the range of 1.7 – 1.9. The ratio of ~1.8 is considered to be pure for DNA.
2.3 Assays of Histone H3 and H4 acetylation
Cells were grown to ~70% confluency and then treated with either media, 1 nM (4 replications), 10 nM (5 replications), or 100 nM senktide (5 replications) for 45 min at 37°C. Histones were extracted by acetone precipitation using EpiQuikTM Global Histone H3/H4 Acetylation Assay Kit (EPIGNTEK, Brooklyn, NY, USA) following the manufacturer’s instructions. Senktide-treated or untreated (control) cells were trypsinized, collected, and counted. Cells (approximately 5×106) were centrifuged (1000 rpm for 5 min at 4°C) and the pellets were collected. The pellets were washed with PBS and centrifuged again at 1000 rpm for 5 min at 4°C. The cell pellets were re-suspended in buffer (provided by manufacturer), incubated on ice for 5 min, and centrifuged at 12000 rpm to collect a the cell debris. Buffer was added to the pellet (1:3), mixed, and incubated for 5 min on ice. After the incubation, nucleic debris were pelleted by centrifugation (12,000 rpm for 5 min at 4°C). Then 100% TCA was added to the supernatant (1:4) and samples were kept on ice for 30 min. Samples were centrifuged (12,000 rpm for 10 min at 4°C) and the precipitates were collected. Histone proteins were extracted by acidified acetone precipitation. Acetone (1 ml), containing 0.1% HCl, was added to the collected precipitate. After mixing and incubating the sample on ice for 1–2 min, the pellet was collected by centrifugation at 12,000 rpm for 5 min at 4°C. The pellet was washed with 1 ml acetone and incubated on ice for 1 min. The pellet was collected by centrifugation at 12,000 rpm for 5 min at 4°C, air dried, and dissolved in dH2O. Extracted histones were subjected to Enzyme Linked Immunosorbent Assay (ELISA) for quantification. Acetylated histones H3/H4 were recognized with a high-affinity antibody (EPIGNTEK, USA) and were quantified using a HRP conjugated secondary antibody color development system. Acetylation % was calculated using OD (sample – blank)/OD (untreated control – blank) X 100%. Acetylation of H3 and H4 was calculated for each replication and then averaged across the replications for each treatment. These data were then analyzed using ANOVA.
2.4 Gene expression
Cells were grown to ~70% confluency and incubated in media or in media containing 1 nM, 10 nM or 100 nM senktide for 45 min. A separate sample of cells were pretreated with histone acetyltransferase (HAT) inhibitors, 10 μM Garcinol and 100 μM 2 Methylene γ – Butyrolactone, (Sigma) for 10 min before senktide treatment. Garcinol inhibits PCAF and p300 while 2-methylene γ-butyrolactone inhibits GCN5 [11]. After the treatment the cells were lysed using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) and the RNA was extracted for qRT-PCR. After removing the cell culture media from the cells, 2 ml of Trizol was added to the flask. The cells were lysed by vortexing for 5 min. Next, chloroform (0.4 ml) was added to Trizol cell lysate, mixed, and incubated at room temperature for 5 min. The samples were then centrifuged at 12,000 g for 15 min. The aqueous phase was transferred to a fresh tube. Isopropyl alcohol (1 ml) was added and incubated for 10 min and then the sample was centrifuged at 12,000 g for 10 min. The supernatant was discarded, and the RNA pellet was washed in 70% ethanol and centrifuged at 12,000 g for 5 min. The resulting supernatant was discarded and the pellet was air dried to remove any ethanol. The RNA pellet was dissolved in 50 μl of RNAse free dH2O. The RNA was quantified using a Nanodrop (Thermo Scientific).
For gene expression studies, qRT-PCR was carried out using the extracted RNA and the GPCR Signaling PathwayFinder Array (SABiosciences, Valencia, CA, USA). This Array targets 84 genes involved in several GPCR-mediated signal transduction pathways. Genomic DNA contamination was removed from the sample. RNA (5 μg) was incubated with genomic DNA Elimination Buffer for 5 min at 42 °C in a Minicycler (Bio-Rad, Hercules, CA, USA). RT Cocktail (RT buffer, Primers, RT enzyme mix and Rnase free H20 from Qiagen, Valencia, CA, USA) was added to DNA Elimination mixture and incubated at 42°C for 15 min. The reaction was stopped immediately by heating at 95°C for 5 min and dH20 (91 μl) were added to each cDNA synthesis reaction. The first cDNA strand was mixed with 2X SABiosciences RT2 qPCR Master MIX and Rnase free H20. This mixture (25 μl) was added to each well of the PCR array and the plate was placed in real-time thermal cycler. The samples were heated at 95°C for 10 min and then 40 cycles at: 95°C for 15 min, 55°C for 40 sec, and 72°C for 30 sec, as per the manufacturer’s instructions (SABiosciences, USA) on a Minicycler (Bio-Rad). The Data were collected using Bio-Rad CFX manager and analyzed using PCR Array software, http://sabiosciences.com/pcrarraydataanalysis.php.
3. Results
3.1 Senktide affects chromatin condensation
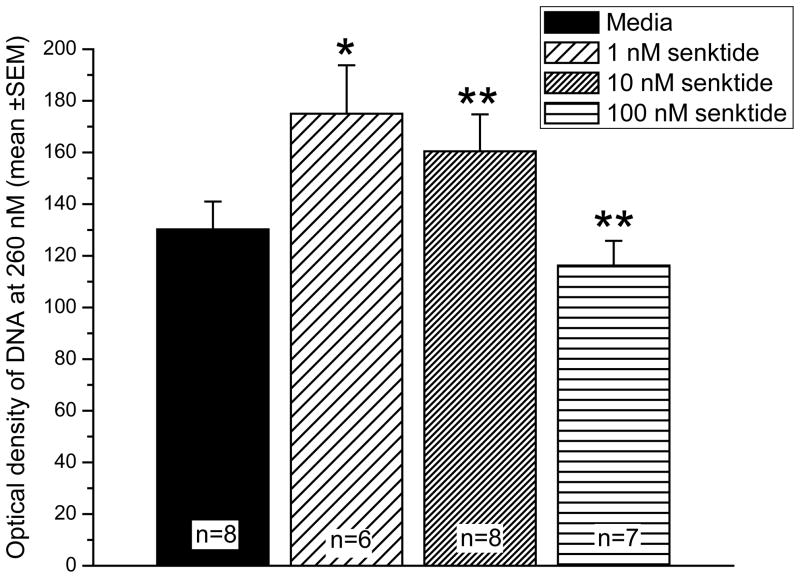
The experiment was run with 6–8 replications (shown on Figure 1). The data were compared across treatments (control/media, 1 nM, 10 nM, and 100 nM senktide) with an ANVOA and additional comparisons were made using a Bonferroni t-test. Compared to media, 1 nM and 10 nM senktide caused a significant relaxation in chromatin structure, P<0.02 and P<0.01, respectively. These two doses of senktide caused approximately a 34% and 23% increase in chromatin relaxation, respectively. In contrast, incubation of cells in 100 nM senktide reversed this pattern and condensed chromatin. The highest dose resulted in a slight (10%), but significant decrease in chromatin relaxation compared to media, P<0.01 (Figure 1).
Figure 1.
Dose dependent changes in chromatin structure in response to 1 nM, 10 nM, and 100 nM senktide. Condensation or relaxation of chromatin was determined by Formaldehyde-Assisted Isolation of Regulatory Elements (FAIRE). Numbers (n=6–8) indicate the number of replications for each treatment. Asterisk indicates significantly different from media, * P<0.02 **P<0.01.
The possibility that changes in chromatin condensation were due to senktide triggering apoptosis was tested by a caspase assay (data not shown). Caspase-3 is a member of group II effector caspases, which are important apoptotic signaling proteins [41]. Caspase-3 exists as an inactive 32 kDa protein and during apoptosis forms an active dimer after being cleaved into two subunits of 14 kDa and 10 kDa. There was no evidence of this result; we could only detect the 32 kDa protein following senktide treatment.
3.2 Senktide effects on global acetylation
The previous data show that 1 nM and 10 nM senktide facilitated the relaxation of chromatin structure. Acetylation relaxes chromatin structure and the effects of senktide on global acetylation were determined. As shown in Figure 2, acetylation of H3 and H4 varied in response to senktide treatment. Incubation in 1 nM or 10 nM senktide caused a 57% and 94%, respectively, increase in the acetylation of histone H3, and a 17% and 57% increase in the acetylation of H4, respectively, compared to control conditions. The highest dose of senktide, 100 nM, had the reverse effect and acetylation of both H3 and H4 were significantly decreased compared to 1 nM and 10 nM senktide, P’s<0.02.
Figure 2.
Global acetylation of histone H3 (left panels) and H4 (right panels) normalized to media in response to 1 nM, 10 nM, and 100 nM senktide. Acetylation % was calculated using OD (sample – blank)/OD (untreated control – blank) X 100%.
3.3 Gene expression
Acetylation of the histone tail and relaxation of chromatin structure are generally linked to transcriptional activation. Treatment with 1 nM and 10 nM senktide relaxed chromatin and increased histone acetylation. The high dose, 100 nM, had the opposite effect. Gene expression in response to senktide paralleled the changes observed in chromatin and acetylation levels. The effects of 1 nM, 10 nM, and 100 nM senktide on gene expression were measured in hypothalamic cells. Table 1 presents the gene, the category of its function in brain, the fold change, and probability level. As shown in Table 1, 1 nM and 10 nM senktide caused a twofold or greater increase in a common set of 11 genes. Of these genes, there was a near significant change for: FOS, P<0.06; VEGFA, encoding vascular endothelial growth factor a, P<0.07; and YWHAZ, encoding tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide, P<0.06. Treatment with 10 nM senktide additionally caused a significant increase in the expression of BCL2, the gene that encodes the pro survival, B-cell lymphoma 2 apoptosis regulator protein, P<0.02.
Table 1.
Genes over expressed by at least 2-fold upon senktide treatment in neuronal hypothalamic cells.
| 1 nM Senktide | 10 nM Senktide | ||||
|---|---|---|---|---|---|
| Gene | Function in CNS | Fold Regulation | p-value | Fold Regulation | p-value |
| CALCRL (calcitonin like receptor) | CS | 3.8 | 0.20 | 3.7 | 0.18 |
| * CCNE2 (cyclin E2) | CDP | 5.0 | 0.09 | 5.4 | 0.07 |
| * CTGF (connective tissue growth factor) | CDP | 7.0 | 0.35 | 7.6 | 0.3 |
| LPAR1 (lysophosphatidic acid receptor 1) | CDP, CS | 4.2 | 0.11 | 5.2 | 0.08 |
| * EDN1 (endothelin 1) | CDP | 2.1 | 0.27 | ||
| FOS (FBJ murine osteosarcoma viral oncogene homolog) | CDP, CS | 4.1 | 0.06 | 4.6 | 0.10 |
| * GNAQ (guanine nucleotide binding protein (G protein), q polypeptide) | CS | 2.8 | 0.11 | 2.6 | 0.22 |
| * PTGS2 (prostaglandin-endoperoxide synthase 2) | CDP, CS | 6.6 | 0.08 | 6.5 | 0.22 |
| RGS2 (regulator of G-protein signaling 2) | CS | 7.8 | 0.24 | 10.5 | 0.24 |
| * VEGFA (vascular endothelial growth factor A) | CDP, NP | 2.5 | 0.07 | 2.1 | 0.10 |
| * YWHAZ (tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide) | CS | 2.2 | 0.06 | 2.4 | 0.08 |
| HPRT1 (hypoxanthine phosphoribosyltransferase 1) | CDP | 2.6 | 0.09 | 3.4 | 0.15 |
| BCL2 (B-cell CLL/lymphoma 2) | CDP, NP | 2.4 | 0.02 | ||
| ADORA2A (adenosine A2a receptor) | CS | ||||
| BAI1 (brain-specific angiogenesis inhibitor 1) | CS | ||||
| CCL2 (chemokine (C-C motif) ligand 2) | CS | ||||
| GALR2 (galanin receptor 2) | CS | ||||
| IL1R2 (interleukin 1 receptor, type II) | CS | ||||
| IL2 (interleukin 2) | CS | ||||
| PIK3CG (phosphoinositide-3-kinase, catalytic, gamma polypeptide1) | CS | ||||
| SERPINE1(serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1), member 1) | NP | ||||
| UCP1 (uncoupling protein 1) | T | ||||
Genes marked were suppressed in response to 100 nM senktide.
Treatment with 100 nM senktide caused a very different pattern of gene expression (Table 1). None of the genes that were over expressed by 2 fold in response to 1 nM and 10 nM senktide were changed in response to 100 nM senktide. Ten genes were over expressed by at least 2 fold and a significant or marginally significant change was seen in expression of 3 genes: ADORA2a, encoding the adenosine A2A receptor, P<0.04; CCL2, encoding Chemokine (C-C motif) ligand 2, P<0.07; and IL1R2, encoding the interleukin 1 receptor type 2, P<0.01.
Senktide had a dose dependent effect on the suppression of genes (Table 2). The 1 nM senktide dose significantly inhibited the expression of NOS, encoding nitric oxide synthase, P<0.01; UCP1, encoding uncoupling protein 1, P<0.01; and GRM5, encoding metabotropic glutamate receptor 5, P<0.01. The 10 nM dose also inhibited NOS and GRM5 gene expression (P’s <0.01) and two additional genes: DRD2, the gene encoding the dopamine D2 receptor, P<0.01, and SOCS1, the gene encoding the suppressor of cytokine signaling 1, P<0.01 (Table 2). Overall, treatment with 100 nM senktide gave a different pattern. Genes that were under expressed in response to 1 nM and 10 nM were neither under or over expressed following 100 nM senktide treatment. However, a new set of 10 genes were under expressed by at least 2 fold in response to 100 nM senktide (Table 2). Seven of the 10 genes that were suppressed were genes that had shown an upregulation in response to the 1 nM or 10 nM dose of senktide (identified by asterisk in Table 1).
Table 2.
Genes under expressed by at least 2-fold upon senktide treatment in neuronal hypothalamic cells.
| 1 nM Senktide | 10 nM Senktide | ||||
|---|---|---|---|---|---|
| Gene | Function in brain | Fold Regulation | p-value | Fold Regulation | p-value |
| ADORA2A (adenosine A2a receptor) | CS | −2.0 | 0.27 | ||
| NOS2 (nitric oxide synthase 2, inducible) | CS | −3.9 | 0.01 | −3.9 | 0.01 |
| SERPINE1 (serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1), member 1) | NP | −3.3 | 0.18 | −3.4 | 0.08 |
| UCP1 (uncoupling protein 1) | T | −2.0 | 0.01 | ||
| GRM5 (glutamate receptor, metabotropic 5) | CS | −2.1 | 0.01 | −2.1 | 0.01 |
| SOCS1 (suppressor of cytokine signaling 1) | CS | −2.9 | 0.01 | ||
| DRD2 (dopamine receptor D2) | CS | −2.7 | 0.01 | ||
| CCNE2 (cyclin E2) | CDP | ||||
| CDKN1B (cyclin-dependent kinase inhibitor 1B) | CDP, NP | ||||
| CTGF (connective tissue growth factor) | CDP | ||||
| EDN1 (endothelin 1) | CDP | ||||
| GNAQ (guanine nucleotide binding protein (G protein), q polypeptide) | CS | ||||
| S1PR3 (sphingosine-1-phosphate receptor 3) | CS, CDP, NP | ||||
| PDPK1 (3-phosphoinositide dependent protein kinase-1) | CS | ||||
| PTGS2 (prostaglandin-endoperoxide synthase 2) | CDP, CS | ||||
| VEGFA (vascular endothelial growth factor A) | CDP, NP | ||||
| YWHAZ (tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide) | CS | ||||
CDP= Cellular and Developmental Processes (genes regulating neuronal differentiation, neurite outgrowth and metabolism); T= Thermogenesis; CS= Cell Signaling (genes regulating receptors, ligand and cell-cell communication); NP= (gene associated with neuroprotection and are anti-apoptotic)
3.4 Histone acetyltransferase inhibitor pretreatment
Pretreatment with the HAT inhibitor Garcinol and 2 Methylene γ – Butyrolactone prevented or reduced the 1 nM senktide-induced increase in the expression of 11 out of 12 genes (Figure 3) by greater than 50%. These genes were: FOS, CALCRL, CCNE2, CTGF, LPAR1, EDN1, GNAQ, PTGS2, RGS2, VEGFA and YWHAZ.
Figure 3.
Graphs show the genes that were up regulated by two fold or greater after 1 nM (upper panel), 10 nM (middle panel), and 100 nM senktide (lower panel). Superimposed on the histograms is effect of pretreatment with HAT inhibitor cocktail prior to senktide treatment on the expression of that gene. Values above the bars indicate the percent change (suppression or enhancement) in gene expression.
The HAT inhibitor appeared less potent in blocking the effects of 10 nM senktide on gene expression. The HAT inhibitor cocktail reduced by 50% or more the expression of 6 out of the 12 genes that were increased by 10 nM senktide (Figure 3). As in the case of 1 nM senktide, the HAT inhibitor was highly potent (greater than 70%) in suppressing the increase in the expression of RGS2, FOS, and CALCR1. Unlike the effects of the HAT inhibitor on gene expression in response to 1 nM senktide, the HAT inhibitor did not attenuate the expression of LPAR1, GNAQ, VEGFA, and HPRT1 in response to 10 nM senktide. In additional BCL2, which was over expressed at 10 nM and not at 1 nM senktide, was inhibited by 29%.
The gene profile in response to 100 nM senktide was different from that of the two other doses. The HAT inhibitor blocked the 100 nM senktide-induced increase in SERPINE 1 and UCP1 by more than 50%; whereas, ADORA2A and CCL2 were only blocked by 24% and 18%, respectively (Figure 3). Pretreatment with the HAT inhibitor prior to senktide increased the expression of BAI1, GALR2, IL2, PIK3CG, and IL1R2 over that seen in response to senktide alone.
4. Discussion
NK3Rs are widely distributed in brain and are linked to many physiological functions. Yet, there is limited information on the effects of NK3R activation on cellular gene function. The objective of this study was to describe the role of NK3R activation on chromatin structure and gene expression in immortalized neuronal hypothalamic cells. This cell line mimics the gene profile of magnocellular PVN neurons and expresses the NK3R, vasopressin (VP), and importin beta, and functionally the activity-dependent translocation of NK3R to the nucleus of CLU cells mimics that seen in vivo in hypothalamic neurons [23, 31].
4.1 Chromatin condensation after senktide treatment is related to histone acetylation
Chromatin structure plays a dominant role in the control of gene regulation. Relaxation or condensation of chromatin either allows or restricts transcription factor access to DNA. Changes in chromatin structure may be due to a number of interconnected mechanisms, including ATP-dependent chromatin remodeling complexes [16], changing histone stoichiometry in the nucleosome [75], and posttranslational modifications of histones [5, 40]. The main post translational modification affecting gene expression is acetylation. Acetylation of histones decreases the structural interaction between DNA and histones, and acetylation of core histones is associated with transcriptionally active sites [3, 26].
Activation of NK3R could potentially affect chromatin and gene expression by at least two pathways. First, we showed using immuno-electron microscopy and immunoprecipitation that NK3R is translocated to the nuclei of hypothalamic neurons in an activity-dependent fashion, and there it associates with acetylated H3 and H4 [17]. Second, NK3R activates the IP3 and adenylate cyclase signaling pathway [39] that affect acetylation and chromatin structure [43]. The direct association of NK3R and acetylated histones, in addition to its cytopasmic signaling pathways, lead to the prediction that the activation of NK3R by an agonist, senktide, would lead either directly or indirectly, by second messenger systems, to the relaxation of chromatin and increased acetylation of H3 and H4. The present data show that senktide treatment causes a dose-related change in chromatin organization. Low doses of senktide (1 nM and 10 nM) caused an increase in chromatin relaxation, while the 100 nM dose caused chromatin compaction. The relaxation in chromatin structure seen in response to 1 and 10 nM senktide was accompanied by an increase in acetylation of H3 and H4. In contrast, treatment of the cells with 100 nM senktide for the same amount of time had the opposite effect. The high dose of senktide resulted in a significant chromatin compaction and a corresponding decrease in acetylation compared to 1 nM and 10 nM senktide, and media.
Treatment with 1 nM and 10 nM increased acetylation and relaxed chromatin while global acetylation in response to 100 nM senktide was half that seen in response to 1 nM and 10 nM senktide. This suggests that the 100 nM dose recruited an additional pathway that acted to deacetylate H3 and H4. Agonists for the NK3R activate PLC and adenylate cyclase in a dose dependent manner. The threshold dose of the NK3R agonist for IP3 formation is 1 nM, and increasing the concentration to 10 nM and 100 nM further increases IP3 formation [52]. In contrast, neither 1 nM nor 10 nM doses facilitate cyclic AMP formation and only when concentration is increased to 100 nM does the NK3R agonist facilitate cyclic AMP formation [52]. cAMP signaling can induce the nuclear accumulation of histone deacetylase [62] and may be a pathway by which the 100 nM dose of senktide causes decreased histone acetylation.
Cellular stress and death can lead to changes in chromatin structure and gene expression. However, several lines of evidence indicate that the effects of 100 nM senktide were not secondary to the treatment stimulating apoptotic pathways. First, as mentioned above, Caspase-3 assay showed an intact 32 kDa protein. Second, no cell toxicity is reported after incubation of neuronal cells in doses as high as 8 μM senktide for 24 h [8]. Third, senktide caused a decreased expression of the CDKN1B gene, which encodes cyclin-dependent kinase inhibitor 1B. Apoptosis is associated with an increased, not decreased, expression of cyclin-dependent kinase inhibitor 1B [53].
4.2 Increased gene expression following senktide treatment
Acetylation of histones is generally linked to transcriptional activation of genes while chromatin condensation is associated with a repression of gene transcription. Treatment with 1 nM and 10 nM relaxed chromatin structure and were associated with elevated levels of acetylated H3 and H4. Correspondingly, incubation of cells in 1 nM and 10 nM senktide resulted in a two fold increase in the expression of a total of 13 genes; 11 genes were upregulated by both doses of senktide. Several of the genes upregulated in response to 1 nM and 10 nM senktide treatments, including FOS, CCNE2, VEGFA, and YWHAZ, affect neurite outgrowth, cell cycle and proliferation, and cell signaling. Senktide-induced acetylation played a dominant role in the upregulation of these four genes because pretreatment with the HAT inhibitor cocktail greatly reduced their expression.
Furthermore, three of these genes, FOS, VEGFA, and YWHAZ, are upregulated in hypothalamic neurons in response to dehydration and/or hyperosmolarity. In animal studies, hyperosmolarity causes NK3R to be translocated to the nucleus of hypothalamic neurons where it associates with acetylated H3 and H4 [17]. The induction of c-fos involves the acetylation of H3 and H4 at the c-fos promoter [66]. Similarly, in vivo, injection of senktide induces c-FOS expression in brain and particularly in the paraventricular and supraoptic nuclei of the hypothalamus [14, 37, 60]. Also, hyperosmolarity, dehydration, and hypovolemia induces c-Fos in hypothalamic neurons [47, 50]. Fos expression in response to these treatments in vitro is mediated by NK3R because pretreatment with a NK3R antagonist prevents the induction of c-Fos in response to hypovolvemia [25]. The present data are consistent linking the activation of NK3R and Fos in hypothalamic neurons.
In addition to FOS, treatment with 1 nM and 10 nM senktide caused an increase in the expression of VEGFA, encoding vascular endothelial growth factor A. This growth factor has a neuroprotective effect and enhances neurite outgrowth [33, 38]. Also, increased VEGF gene expression facilitates excitatory synaptic transmission and promotes neuronal plasticity [29, 44]. Previous studies link another tachykinin, substance P (SP) and the NK1 receptor, with VEGF. In mast cells, application of 100 nM SP induces VEGF mRNA [64]. Interestingly, hypothalamic magnocellular neurons express VEGF, and VEGF expression in these neurons is increased in response to hyperosmolarity or dehydration [4]. Similar to VEGA, YWHAZ expression affects neural function [36], and YWHAZ message and protein increase in hypothalamic neurons in response to hyperosmolarity resulting from dehydration [20]. The neurotransmitter mediators of this change in expression were not identified. Hyperosmolarity and hypovolemia activate NK3R expressed on hypothalamic neurons [23, 28] and the present data show that treatment with an NK3R agonist increases VEGFA and YWHAZ gene expression. As such, the increase in VEGF and YWHAZ expression in hypothalamic neurons in response to hyperosmolarity or dehydration may be mediated, at least in part, by activation of NK3R.
The ability of the HAT inhibitor to reduce or block up regulation of 12/12 genes and 8/12 genes in response to 1 nM and 10 nM senktide, respectively, indicates that these treatments cause the selective acetylation of promoter sites by p300, PCAF, and/or GCN5. Additional support for the notion that activation of NK3R by 1 nM and 10 nM senktide recruits these HATs to promoter sites is provided by results linking these genes to the specific HATs. For example, FOS and RGS2 expression depend upon p300 [7, 35]. In addition, CTGF gene expression requires PCAF [68] and p300 is essential for PTGS2 and EDN1 activation [9, 12]. The HAT inhibitor decreased the senktide-induced upregulation of SERPINE 1 and UCP1, and both SERPINE 1 and UCP1 are regulated by p300 and CBP [55, 63]. The effect of the HAT inhibitor on the senktide-induced expression of these genes identifies that activation of NK3R by low doses of senktide (1 nM and 10 nM) recruits p300, PCAF, and/or GCN5 to promoter specific sites.
The 100 nM dose of senktide produced a very different profile compared to the lower doses. The higher dose condensed chromatin and resulted in significantly reduced levels of H3 and H4 acetylation when compared to media and the levels seen in response to 1 nM and 10 nM senktide. Treatment with 100 nM senktide resulted in a new set of 10 genes that were over expressed by at least 2 fold. None of the genes that were over expressed by two fold or more in response to 1 nM or 10 nM senktide were over expressed in response to 100 nM senktide. This suggests that there is a dose-dependent recruitment of different signaling pathways by senktide leading to different gene expression. Furthermore, whereas acetylation was a key modification leading to the upregulation of genes in response to 1 nM and 10 nM senktide, that was not the case with 100 nM senktide. Treatment with 100 nM senktide significantly increased the expression of two receptor-encoding genes: ADORA2A and IL1R2, but the HAT cocktail pretreatment only decreased the expression of ADORA2A by ~20% and caused an increase in the expression of IL1R2. Also, the increased expression of BAI1, GALR2, IL1R2, IL2, PIK3CG, that was seen in response to 100 nM senktide was not blocked by the HAT inhibitor, and rather, their expression increased. Indeed, many of the genes upregulated in response to 100 nM senktide are regulated by mechanisms other than hyperacetylation, including DNA methylation, demethylation, deacetylation, or over expression of proteins binding to methylated molecules. For example, IL1R2, BAI1, IL2, GALR2, and ADORA2A are regulated by DNA methylation [6, 32, 34, 51, 74]. CCLl2 transcription is regulated by multiple mechanisms, including phosphorylation of histone H3 and the recruitment of histone deacetylase 3 (HDAC3) leading to hypoacetylation [70]. The mechanisms by which phosphorylation promotes transcription are poorly defined but can be recruited by a variety of ligands [21]. As such, not only were the genes that were upregulated by 100 nM senktide different from those upregulated by 1 nM and 10 nM senktide, but the modifications leading to the upregulation were different as well. Through downstream pathways 100 nM senktide may recruit or direct DNA methyltransferases or demethylating enzymes to specific target sites.
4.3 Decreased expression of genes
Senktide had a dose dependent effect on the suppression of gene expression. The low dose of senktide significantly inhibited the expression of NOS, UCP1, and GRM5. The 10 nM dose also inhibited NOS and GRM5, as well as two additional genes encoding the dopamine D2 receptor (DRD2) and suppressor of cytokine signaling (SOCS1). Overall, the effect of senktide on gene silencing was highly significant. Noteworthy is the decrease in highly significant signaling pathways: Nos, D2, MGlu5 receptor, while the decrease in SOCS1, a highly regulated negative regulator of signaling pathways for cytokines among other ligands [69], would presumably support or facilitate cytokine and growth factor signaling. D2 and mGluR5 often function as heterodimers [2] and we observe that the two are regulated in parallel in response to senktide. Thus, activation of NK3R is negatively regulating dopamine and glutamate signaling by down regulating their respective receptors while potentially up regulating the responses to cytokines and several hormones.
Treatment with 100 nM senktide gave a different pattern. Treatment with 100 nM senktide caused the compaction of chromatin and a decrease in acetylation, and gene suppression. Genes that were under expressed by 1 nM and 10 nM were not under expressed following 100 nM senktide. Rather, a new set of 10 genes were under expressed by at least 2 fold following 100 nM senktide. Of these 10 genes, 7 of the genes that were under expressed in response to 100 nM senktide had been over expressed in response to the lower doses of senktide. The silencing of the genes can be due to methylation of DNA and a compaction of chromatin and/or a decrease in acetylation. Several genes that were significantly suppressed by the high dose of senktide, including EDN1, are regulated by methylation at the promoter area [67, 71]. Activation of NK3R by high doses of senktide may recruit different or additional signaling pathway, histone deactylases, histone methylases, or DNA methylases to suppress gene expression.
4.4 Conclusion
NK3R’s are widely distributed in both the nervous system and peripheral tissues. Previous results show that injections of senktide cause the activation and internalization of NK3R to the cytoplasm and cell nucleus [23, 28]. Furthermore, activation of NK3R by hyperosmolarity causes the translocation of NK3R expressed by hypothalamic neurons to the nucleus where it associates with acetylated H3 and H4 [17, 23]. The present results show that NK3R activation plays a role in the regulation of transcription by modifying chromatin structure through the acetylation of core histones. Low doses of senktide have a pro-gene transcription effect by promoting global acetylation of H3 and H4 and by relaxing chromatin. Treatment with 1 nM and 10 nM senktide provided a consistent pattern of gene activation and the increase in gene expression was largely attributable to acetylation. Many of the genes that were upregulated affect cellular signaling, either the expression of receptors, ligands, or factors in various signaling pathways, neuron growth, and neurite or synapse formation.
The higher dose of senktide recruited a different pathway because global acetylation was decreased and chromatin was compacted. These effects would serve to suppress gene transcription and indeed gene suppression dominated the profile in response to 100 nM senktide. Higher doses are not pro apoptotic but do suppress a number of the genes that were upregulated by 1 nM and 10 nM senktide, perhaps by deacetylation since overall acetylation of H3 and H4 decreased in response to 100 nM senktide. The results suggest that, depending on dose or occupancy of the receptor, activation of NK3R can direct different downstream cascades to affect histone acetylation and gene expression.
Highlights.
Application of senktide, a NK3R agonist, modified chromatin and gene expression.
Senktide (1 or 10 nM) increased histone acetylation and gene expression.
Increased gene expression was largely prevented by pretreatment with HAT inhibitors.
Senktide (100 nM) decreased acetylation and gene expression, and compacted chromatin.
Acknowledgments
This project was supported by grants for the National Institutes of Health R01 NS53728 and grants from the National Center for Research Resources (P30 RR32128) and the National Institute of General Medical Sciences (P30 GM10398) awarded to F.W.F. We are grateful to Dr. Dane D. Jensen for his insightful discussions of the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adam SA, Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991;66:837–47. doi: 10.1016/0092-8674(91)90431-w. [DOI] [PubMed] [Google Scholar]
- 2.Albizu L, Moreno JL, Gonzalez-Maeso J, Sealfon SC. Heteromerization of G protein-coupled receptors: relevance to neurological disorders and neurotherapeutics. CNS Neurol Disord Drug Targets. 2010;9:636–50. doi: 10.2174/187152710793361586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allfrey VG, Faulkner R, Mirsky AE. Acetylation and Methylation of histones and their possible role in the regulation of RNA sythesis. Proc Natl Acad Sci USA. 1964;51:786–94. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso G, Galibert E, Duvoid-Guillou A, Vincent A. Hyperosmotic stimulus induces reversible angiogenesis within the hypothalamic magnocellular nuclei of the adult rat: a potential role for neuronal vascular endothelial growth factor. BMC Neurosci. 2005;6:20. doi: 10.1186/1471-2202-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–81. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 6.Buira SP, Dentesano G, Albasanz JL, Moreno J, Martin M, Ferrer I, et al. DNA methylation and Yin Yang-1 repress adenosine A2A receptor levels in human brain. J Neurochem. 2010;115:283–95. doi: 10.1111/j.1471-4159.2010.06928.x. [DOI] [PubMed] [Google Scholar]
- 7.Byun JS, Wong MM, Cui W, Idelman G, Li Q, De Siervi A, et al. Dynamic bookmarking of primary response genes by p300 and RNA polymerase II complexes. Proc Natl Acad Sci U S A. 2009;106:19286–91. doi: 10.1073/pnas.0905469106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen LW, Wang YQ, Bian GL, Wei LC, Yung KL. Neurokinin-3 peptide instead of neurokinin-1 synergistically exacerbates kainic acid-inducing degeneration of neurons in the substantia nigra of mice. J Neurochem. 2008;105:203–16. doi: 10.1111/j.1471-4159.2007.05132.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Feng B, George B, Chakrabarti R, Chen M, Chakrabarti S. Transcriptional coactivator p300 regulates glucose-induced gene expression in endothelial cells. Am J Physiol Endocrinol Metab. 2010;298:E127–37. doi: 10.1152/ajpendo.00432.2009. [DOI] [PubMed] [Google Scholar]
- 10.Dacks PA, Krajewski SJ, Rance NE. Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology. 2011;152:4894–905. doi: 10.1210/en.2011-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dekker FJ, Haisma HJ. Histone acetyl transferases as emerging drug targets. Drug Discov Today. 2009;14:942–8. doi: 10.1016/j.drudis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Deng WG, Zhu Y, Wu KK. Role of p300 and PCAF in regulating cyclooxygenase-2 promoter activation by inflammatory mediators. Blood. 2004;103:2135–42. doi: 10.1182/blood-2003-09-3131. [DOI] [PubMed] [Google Scholar]
- 13.Ding YQ, Lu BZ, Guan ZL, Wang DS, Xu JQ, Li JH. Neurokinin B receptor (NK3)-containing neurons in the paraventricular and supraoptic nuclei of the rat hypothalamus synthesize vasopressin and express Fos following intravenous injection of hypertonic saline. Neuroscience. 1999;91:1077–85. doi: 10.1016/s0306-4522(98)00643-5. [DOI] [PubMed] [Google Scholar]
- 14.Ding YQ, Shi J, Su LY, Xu JQ, Su CJ, Guo XE, et al. Intracerebroventricular injection of senktide-induced Fos expression in vasopressin-containing hypothalamic neurons in the rat. Brain Research. 2000;882:95–102. doi: 10.1016/s0006-8993(00)02836-5. [DOI] [PubMed] [Google Scholar]
- 15.Ding YQ, Shigemoto R, Takada M, Ohishi H, Nakanishi S, Mizuno N. Localization of the neuromedin K receptor (NK3) in the central nervous system of the rat. J Comp Neurol. 1996;364:290–310. doi: 10.1002/(SICI)1096-9861(19960108)364:2<290::AID-CNE8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Flaus A, Owen-Hughes T. Mechanisms for ATP-dependent chromatin remodelling: farewell to the tuna-can octamer? Curr Opin Genet Dev. 2004;14:165–73. doi: 10.1016/j.gde.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Flynn FW, Jensen DD, Thakar A, Xu X, Flynn SW, Zhang Z. Neurokinin 3 receptor forms a complex with acetylated histone H3 and H4 in hypothalamic neurons following hyperosmotic challenge. Am J Physiol Regul Integr Comp Physiol. 2011;301:R822–31. doi: 10.1152/ajpregu.00254.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaudreau GA, Plourde V. Role of tachykinin NK1, NK2 and NK3 receptors in the modulation of visceral hypersensitivity in the rat. Neurosci Lett. 2003;351:59–62. doi: 10.1016/s0304-3940(03)00414-2. [DOI] [PubMed] [Google Scholar]
- 19.Giresi PG, Kim J, McDaniell RM, Iyer VR, Lieb JD. FAIRE (Formaldehyde-Assisted Isolation of Regulatory Elements) isolates active regulatory elements from human chromatin. Genome Res. 2007;17:877–85. doi: 10.1101/gr.5533506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouraud SS, Yao ST, Heesom KJ, Paton JF, Murphy D. 14-3-3 proteins within the hypothalamic-neurohypophyseal system of the osmotically stressed rat: transcriptomic and proteomic studies. J Neuroendocrinol. 2007;19:913–22. doi: 10.1111/j.1365-2826.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- 21.Grant PA. A tale of histone modifications. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-4-reviews0003. REVIEWS0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griebel G, Beeske S. Is there still a future for neurokinin 3 receptor antagonists as potential drugs for the treatment of psychiatric diseases? Pharmacol Ther. 2012;133:116–23. doi: 10.1016/j.pharmthera.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Haley GE, Flynn FW. Agonist and hypertonic saline-induced trafficking of the NK3-receptors on vasopressin neurons within the paraventricular nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1242–R50. doi: 10.1152/ajpregu.00773.2005. [DOI] [PubMed] [Google Scholar]
- 24.Haley GE, Flynn FW. Tachykinin NK3 receptor contribution to systemic release of vasopressin and oxytocin in response to osmotic and hypotensive challenge. Am J Physiol Regul Integr Comp Physiol. 2007;293:R931–R7. doi: 10.1152/ajpregu.00196.2007. [DOI] [PubMed] [Google Scholar]
- 25.Haley GE, Flynn FW. Blockade of NK3R signaling in the PVN decreases vasopressin and oxytocin release and c-Fos expression in the magnocellular neurons in response to hypotension. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1158–R67. doi: 10.1152/ajpregu.90402.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hebbes TR, Thorne AW, Crane-Robinson C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 1988;7:1395–402. doi: 10.1002/j.1460-2075.1988.tb02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho MK, Su Y, Yeung WW, Wong YH. Regulation of transcription factors by heterotrimeric G proteins. Curr Mol Pharmacol. 2009;2:19–31. doi: 10.2174/1874467210902010019. [DOI] [PubMed] [Google Scholar]
- 28.Howe HE, Somponpun SJ, Sladek CD. Role of neurokinin 3 receptors in supraoptic vasopressin and oxytocin neurons. J Neurosci. 2004;24:10103–10. doi: 10.1523/JNEUROSCI.3164-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang YF, Yang CH, Huang CC, Tai MH, Hsu KS. Pharmacological and genetic accumulation of hypoxia-inducible factor-1alpha enhances excitatory synaptic transmission in hippocampal neurons through the production of vascular endothelial growth factor. J Neurosci. 2010;30:6080–93. doi: 10.1523/JNEUROSCI.5493-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen D, Zhang Z, Flynn FW. Trafficking of tachykinin neurokinin 3 receptor to nuclei of neurons in the paraventricular nucleus of the hypothalamus following osmotic challenge. Neuroscience. 2008;155:308–16. doi: 10.1016/j.neuroscience.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen DD, Sundstrom K, Flynn FW. Expression of the nuclear transport protein importin ss-1 and its association with the neurokinin 3 receptor in the rat hypothalamus following acute hyperosmotic challenge. Neuroscience. 2010;170:1020–7. doi: 10.1016/j.neuroscience.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin B, Yao B, Li JL, Fields CR, Delmas AL, Liu C, et al. DNMT1 and DNMT3B modulate distinct polycomb-mediated histone modifications in colon cancer. Cancer Res. 2009;69:7412–21. doi: 10.1158/0008-5472.CAN-09-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci U S A. 2000;97:10242–7. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanazawa T, Kommareddi PK, Iwashita T, Kumar B, Misawa K, Misawa Y, et al. Galanin receptor subtype 2 suppresses cell proliferation and induces apoptosis in p53 mutant head and neck cancer cells. Clin Cancer Res. 2009;15:2222–30. doi: 10.1158/1078-0432.CCR-08-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasper LH, Lerach S, Wang J, Wu S, Jeevan T, Brindle PK. CBP/p300 double null cells reveal effect of coactivator level and diversity on CREB transactivation. EMBO J. 2010;29:3660–72. doi: 10.1038/emboj.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawaai K, Tominaga-Yoshino K, Urakubo T, Taniguchi N, Kondoh Y, Tashiro H, et al. Analysis of gene expression changes associated with long-lasting synaptic enhancement in hippocampal slice cultures after repetitive exposures to glutamate. J Neurosci Res. 2010;88:2911–22. doi: 10.1002/jnr.22457. [DOI] [PubMed] [Google Scholar]
- 37.Kawasaki M, Ponzio TA, Yue C, Fields RL, Gainer H. Neurotransmitter regulation of c-fos and vasopressin gene expression in the rat supraoptic nucleus. Exp Neurol. 2009;219:212–22. doi: 10.1016/j.expneurol.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khaibullina AA, Rosenstein JM, Krum JM. Vascular endothelial growth factor promotes neurite maturation in primary CNS neuronal cultures. Brain Res Dev Brain Res. 2004;148:59–68. doi: 10.1016/j.devbrainres.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 39.Khawaja AM, Rogers DF. Tachykinins: receptor to effector. Int J Biochem Cell Biol. 1996;28:721–38. doi: 10.1016/1357-2725(96)00017-9. [DOI] [PubMed] [Google Scholar]
- 40.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest. 2005;115:2665–72. doi: 10.1172/JCI26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lessard A, Laurin M, Yamaguchi N, Couture R. Central anti-hypertensive effect of tachykinin NK3 receptor antagonists in rat. Eur J Pharmacol. 2004;486:75–83. doi: 10.1016/j.ejphar.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Levine AA, Guan Z, Barco A, Xu S, Kandel ER, Schwartz JH. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci U S A. 2005;102:19186–91. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, et al. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci U S A. 2011;108:5081–6. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo RX, Dean DC. Chromatin remodeling and transcriptional regulation. J Natl Cancer Inst. 1999;91:1288–94. doi: 10.1093/jnci/91.15.1288. [DOI] [PubMed] [Google Scholar]
- 46.Massi M, Panocka I, de Caro G. The psychopharmacology of tachykinin NK-3 receptors in laboratory animals. Peptides. 2000;21:1597–609. doi: 10.1016/s0196-9781(00)00291-6. [DOI] [PubMed] [Google Scholar]
- 47.McKinley MJ, Hards DK, Oldfield BJ. Identification of neural pathways activated in dehydrated rats by means of Fos-immunohistochemistry and neural tracing. Brain Res. 1994;653:305–14. doi: 10.1016/0006-8993(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 48.Meltzer HY, Arvanitis L, Bauer D, Rein W. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161:975–84. doi: 10.1176/appi.ajp.161.6.975. [DOI] [PubMed] [Google Scholar]
- 49.Merchenthaler I, Maderdrut JL, O’Harte F, Conlon JM. Localization of neurokinin B in the central nervous system of the rat. Peptides. 1992;13:815–29. doi: 10.1016/0196-9781(92)90192-6. [DOI] [PubMed] [Google Scholar]
- 50.Morien A, Garrard L, Rowland NE. Expression of Fos immunoreactivity in rat brain during dehydration: effect of duration and timing of water deprivation. Brain Res. 1999;816:1–7. doi: 10.1016/s0006-8993(98)00828-2. [DOI] [PubMed] [Google Scholar]
- 51.Murayama A, Sakura K, Nakama M, Yasuzawa-Tanaka K, Fujita E, Tateishi Y, et al. A specific CpG site demethylation in the human interleukin 2 gene promoter is an epigenetic memory. EMBO J. 2006;25:1081–92. doi: 10.1038/sj.emboj.7601012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakajima Y, Tsuchida K, Negishi M, Ito S, Nakanishi S. Direct linkage of three tachykinin receptors to stimulation of both phosphatidylinositol hydrolysis and cyclic AMP cascades in transfected Chinese hamster ovary cells. J Biol Chem. 1992;267:2437–42. [PubMed] [Google Scholar]
- 53.Naruse I, Hoshino H, Dobashi K, Minato K, Saito R, Mori M. Over-expression of p27kip1 induces growth arrest and apoptosis mediated by changes of pRb expression in lung cancer cell lines. Int J Cancer. 2000;88:377–83. [PubMed] [Google Scholar]
- 54.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, et al. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300:E202–10. doi: 10.1152/ajpendo.00517.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramos YF, Hestand MS, Verlaan M, Krabbendam E, Ariyurek Y, van Galen M, et al. Genome-wide assessment of differential roles for p300 and CBP in transcription regulation. Nucleic Acids Res. 2010;38:5396–408. doi: 10.1093/nar/gkq184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salome N, Stemmelin J, Cohen C, Griebel G. Selective blockade of NK2 or NK3 receptors produces anxiolytic- and antidepressant-like effects in gerbils. Pharmacol Biochem Behav. 2006;83:533–9. doi: 10.1016/j.pbb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 57.Sanger GJ. Neurokinin NK1 and NK3 receptors as targets for drugs to treat gastrointestinal motility disorders and pain. Br J Pharmacol. 2004;141:1303–12. doi: 10.1038/sj.bjp.0705742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seybold VS, Grkovic I, Portbury AL, Ding YQ, Shigemoto R, Mizuno N, et al. Relationship of NK3 receptor-immunoreactivity to subpopulations of neurons in rat spinal cord. J Comp Neurol. 1997;381:439–48. doi: 10.1002/(sici)1096-9861(19970519)381:4<439::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 59.Shughrue PJ, Lane MV, Merchenthaler I. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. J Comp Neurol. 1996;372:395–414. doi: 10.1002/(SICI)1096-9861(19960826)372:3<395::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 60.Smith ME, Flynn FW. Distribution of Fos-like immunoreactivity within the rat brain following intraventricular injection of the selective NK(3) receptor agonist senktide. J Comp Neurol. 2000;426:413–28. doi: 10.1002/1096-9861(20001023)426:3<413::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 61.Spooren W, Riemer C, Meltzer H. Opinion: NK3 receptor antagonists: the next generation of antipsychotics? Nat Rev Drug Discov. 2005;4:967–75. doi: 10.1038/nrd1905. [DOI] [PubMed] [Google Scholar]
- 62.Taniguchi M, Carreira MB, Smith LN, Zirlin BC, Neve RL, Cowan CW. Histone deacetylase 5 limits cocaine reward through cAMP-induced nuclear import. Neuron. 2012;73:108–20. doi: 10.1016/j.neuron.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tateishi K, Okada Y, Kallin EM, Zhang Y. Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature. 2009;458:757–61. doi: 10.1038/nature07777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, Angelidou A, et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010;107:4448–53. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–8. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–10. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vallender TW, Lahn BT. Localized methylation in the key regulator gene endothelin-1 is associated with cell type-specific transcriptional silencing. FEBS Lett. 2006;580:4560–6. doi: 10.1016/j.febslet.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 68.Wang B, Haldar SM, Lu Y, Ibrahim OA, Fisch S, Gray S, et al. The Kruppel-like factor KLF15 inhibits connective tissue growth factor (CTGF) expression in cardiac fibroblasts. J Mol Cell Cardiol. 2008;45:193–7. doi: 10.1016/j.yjmcc.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J, Campbell IL. Cytokine signaling in the brain: putting a SOCS in it? J Neurosci Res. 2002;67:423–7. doi: 10.1002/jnr.10145. [DOI] [PubMed] [Google Scholar]
- 70.Wolter S, Doerrie A, Weber A, Schneider H, Hoffmann E, von der Ohe J, et al. c-Jun controls histone modifications, NF-kappaB recruitment, and RNA polymerase II function to activate the ccl2 gene. Mol Cell Biol. 2008;28:4407–23. doi: 10.1128/MCB.00535-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang MY, Liu TC, Chang JG, Lin PM, Lin SF. JunB gene expression is inactivated by methylation in chronic myeloid leukemia. Blood. 2003;101:3205–11. doi: 10.1182/blood-2002-05-1598. [DOI] [PubMed] [Google Scholar]
- 72.Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95:2287–95. doi: 10.1210/jc.2009-2600. [DOI] [PubMed] [Google Scholar]
- 73.Zhang X, Andren PE, Chergui K, Svenningsson P. Neurokinin B/NK3 receptors exert feedback inhibition on L-DOPA actions in the 6-OHDA lesion rat model of Parkinson’s disease. Neuropharmacology. 2008;54:1143–52. doi: 10.1016/j.neuropharm.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 74.Zhu D, Hunter SB, Vertino PM, Van Meir EG. Overexpression of MBD2 in glioblastoma maintains epigenetic silencing and inhibits the antiangiogenic function of the tumor suppressor gene BAI1. Cancer Res. 2011;71:5859–70. doi: 10.1158/0008-5472.CAN-11-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zlatanova J, Bishop TC, Victor JM, Jackson V, van Holde K. The nucleosome family: dynamic and growing. Structure. 2009;17:160–71. doi: 10.1016/j.str.2008.12.016. [DOI] [PubMed] [Google Scholar]