Abstract
Animal studies have shown that paraoxonase 1 (PON1) genotype can influence susceptibility to the organophosphorus pesticide chlorpyrifos (CPF). However, Monte Carlo analysis suggests that PON1 genotype may not affect CPF-related toxicity at low exposure conditions in humans. The current study sought to determine the influence of PON1 genotype on the activity of blood cholinesterase as well as the effect of CPF exposure on serum PON1 in workers occupationally exposed to CPF. Saliva, blood and urine were collected from agricultural workers (n = 120) from Egypt’s Menoufia Governorate to determine PON1 genotype, blood cholinesterase activity, serum PON1 activity towards chlorpyrifos-oxon (CPOase) and paraoxon (POase), and urinary levels of the CPF metabolite 3,5,6-trichloro-2-pyridinol (TCPy). The PON1 55 (p ≤ 0.05) but not the PON1 192 genotype had a significant effect on CPOase activity. However, both the PON1 55 (p ≤ 0.05) and PON1 192 (p ≤ 0.001) genotype had a significant effect on POase activity. Workers had significantly inhibited AChE and BuChE after CPF application; however, neither CPOase activity nor POase activity was associated with ChE depression when adjusted for CPF exposure (as determined by urinary TCPy levels) and stratified by PON1 genotype. CPOase and POase activity were also generally unaffected by CPF exposure although there were alterations in activity within specific genotype groups. Together, these results suggest that workers retained the capacity to detoxify chlorpyrifos-oxon under the exposure conditions experienced by this study population regardless of PON1 genotype and activity and that effects of CPF exposure on PON1 activity are minimal.
Keywords: Paraoxonase 1, chlorpyrifos, organophosphorus, acetylcholinesterase, butyrylcholinesterase, biomarker
Introduction
Organophosphorus pesticides (OPs) continue to be a human health concern due to their worldwide use and documented human exposures (Alexander et al., 2006; Garabrant et al., 2009; Farahat et al., 2010; Farahat et al., 2011). Human and animal studies consistently identify neurotoxicity as the primary endpoint of concern (Bushnell and Moser, 2006; Costa, 2006). Determining the neurotoxic risks associated with occupational exposure to OPs requires an understanding of not only worker exposure levels, but also genetic susceptibility factors. With regards to the latter, genetic polymorphisms in enzymes that metabolize OPs are widely posited to influence susceptibility to OP toxicity. Phosphorothioate OPs undergo cytochrome P-450 (CYP) mediated metabolism to form an active, highly toxic, oxon intermediate metabolite (Ma and Chambers, 1994) which is the metabolite primarily responsible for the inhibition of not only AChE, but also other B-esterases such as butyrylcholinesterase (BuChE) and carboxylesterase (CE) (Sultatos, 1994). Detoxification of the active oxon metabolite primarily occurs by the A-esterase paraoxonase 1 (PON1) (Sultatos and Murphy, 1983; Pond et al., 1998), an enzyme expressed mainly in the liver and secreted into the blood. It has been proposed that inter-individual differences in PON1 can influence the rate of detoxification of OPs, resulting in differences in susceptibility to OP toxicity (Costa et al., 1999; Furlong et al., 2010).
Two common coding region polymorphisms in PON1 have been identified: (1) a leucine/methionine amino acid polymorphism at position 55 (PON1 L55M); and (2) a glutamine/arginine amino acid polymorphisms at position 192 (PON1 Q192R) (Adkins et al., 1993). The PON1 55 polymorphism affects PON1 mRNA (Leviev et al., 1997) resulting in lower PON1 serum protein concentrations in individuals with the M allele compared to the L allele (Garin et al. 1997). The PON1 192 polymorphism is functional, affecting PON1 enzyme activity towards OPs in a substrate specific manner (Davies et al., 1996). In vitro, the PON1 192R isoform hydrolyzes paraoxon and chlorpyrifos-oxon faster than the PON1 192Q isoform; in contrast, PON1 192R and 192Q hydrolyze diazoxon at similar rates (Li et al., 2000; Furlong et al., 2005). Similarly, in vivo studies demonstrate that intraperitoneal injection of PON1 192R or PON1 192Q into PON1 knockout mice confers a similar degree of protection against diazoxon intoxication, whereas PON1 192R provides better protection against chlorpyrifos-oxon than PON1 192Q (Li et al. 2000).
These observations have led to the proposal that PON1 status, which is determined by the amount of PON1 protein present (influenced by PON1 55 genotype) and the activity of the enzyme (influenced by PON1 192 genotype), impacts individual susceptibility to OP toxicity (Furlong et al. 2010; Hofmann et al. 2009; Li et al. 2000). The relationship between PON1 genotype and symptoms associated with chronic OP toxicity has been investigated in workers in the United Kingdom exposed to sheep dip containing primarily diazinon (Cherry et al., 2002; Mackness et al., 2003,; Povey et al., 2005), farmers in India (Prabhavathy Das and Jamil, 2009), greenhouse workers in Spain (Hernandez et al., 2003) and South African workers exposed to pesticides (Lee et al., 2003). Collectively, these studies present conflicting results regarding an association between PON1 genotype and worker health and in those studies that did find an association, there are discrepancies as to which genotype is more sensitive to OP exposure. While these studies fail to provide a consensus view on the value of PON1 status as a biomarker of susceptibility, it is difficult to interpret what this means since OP exposures were determined largely by job classification and OP toxicity was based on symptoms associated with but not unique to chronic OP toxicity. However, two recent studies (Hofmann et al., 2009; Albers et al., 2010) that employed a more specific biomarker of OP effect, blood cholinesterase activity, to address the question of whether PON1 is a biomarker of susceptibility to OP neurotoxicity yielded conflicting conclusions as well. The Hoffman et al. (2009) study of agricultural pesticide applicators reported an inverse association between PON1 activity and butyrylcholinesterase (BuChE) activity; whereas the Albers et al. (2010) study of chlorpyrifos manufacturing workers failed to find an association between PON1 activity and either BuChE or acetylcholinesterase (AChE) activity. The discrepancy between these two studies may reflect differences in the OP exposure history between the two study populations, but the limited exposure data available from the Hofmann et al. (2009) study precludes rigorous assessment of this possibility. We have been conducting a very detailed exposure assessment of Ministry of Agriculture workers which apply pesticides in the cotton fields in Egypt’s Menoufia Governorate. Data collected during the summer of 2007 (Farahat et al. 2010) and 2008 (Farahat et al. 2011) demonstrate significant exposures to the OP, chlorpyrifos (CPF), in this occupational cohort. In this study, we report data collected from a larger cohort (n=120) recruited in 2009. Urinary 3,5,6-trichloro-2-pyridinol (TCPy) levels were measured as a CPF-specific biomarker of exposure; plasma BuChE and red blood cell (RBC) AChE activities were measured as biomarkers of effect; and PON1 genotype (both Q192R and L55M polymorphisms) and phenotype were investigated as potential biomarkers of susceptibility. Samples were collected prior to, during and after a cycle of daily CPF applications over 15 days, allowing us to not only rigorously test the controversial hypothesis that PON1 status influences human susceptibility to OP neurotoxicity in an occupational cohort with clearly defined exposures to a single OP, but to also test the novel hypothesis that repeated CPF exposure modulates PON1 activity
Materials and Methods
Study setting and population
The study setting has been previously described (Farahat et al. 2010). In brief, the study took place in Menoufia, one of 29 Governorates in Egypt, which is situated in the Nile River delta north of Cairo. The Ministry of Agriculture controls and oversees the use of pesticides and application procedures in cotton fields throughout Egypt. Pesticide application is performed by teams of workers employed by the Ministry of Agriculture consisting of applicators who apply CPF to the cotton field using backpack sprayers, technicians who direct the direction of spray and the rate at which applicators walk through the field, and engineers who oversee pesticide mixing and application to crops, often from the perimeter of the field. It should be noted that none of these workers used personal protective equipment to minimize dermal exposures. The current study took place during the summer of 2009 and included the first cycle of CPF application. CPF was the only OP used during this time period. All participants in the study were male Egyptians, indigenous to the Nile delta region, with the vast majority being born, raised and residing in Menoufia, Egypt at the time of the study. Participants were between 14 to 69 years of age and had an average body mass index of 26.9. Because certain disease states can adversely influence the metabolism and excretion of TCPy, all workers were questioned about prior diagnosis of diabetes mellitus and liver or kidney disease by a physician during the recruitment process. However, no exclusions for medical conditions were necessary.
Participants were asked to provide a urine and blood sample at two different time points to monitor individuals for CPF exposure and cholinesterase effects. The first sample was collected at baseline two to seven days prior to the period of daily CPF application. The second sample was collected post-exposure, one to two days after a period of up to 15 consecutive days of CPF application. For each time period, urine and blood samples were collected on the same day for an individual and all participant samples were collected within five days of each other. A total of 120 participants (96% agricultural workers) donated saliva at enrollment and were included in the present study. Of these 120 participants, cholinesterase activity was determined for 100 participants at baseline and 97 participants following CPF application. PON1 activity was determined for 67 participants who met the following criteria: (1) the individual provided a saliva sample for PON1 genotyping; (2) the individual provided a plasma sample at baseline and post CPF application; (3) the plasma sample was in good condition (i.e., remained frozen and had little/no hemolysis); and (4) all samples were clearly labeled. The protocol and consent forms used in this research were approved by the Oregon Health & Science University (USA) and Menoufia University (Egypt) Institutional Review Boards. Subjects gave written informed consent prior to enrollment in the study.
Urine Collection and TCPy Analysis
A single spot urine specimen was collected at the beginning of a given work day (2 pm) for assessing TCPy as a biomarker of exposure, since previous studies conducted in similar groups of Egyptian agriculture workers found that daily urinary TCPy levels for a given worker were very similar in specimens collected at the beginning (2–3 pm) and end (7–8 pm) of a given work day (Farahat et al., 2010, 2011). After collection, samples were placed on wet ice for transport to Menoufia University (Shebin El-Kom, Egypt). Urine samples were subsequently aliquotted and stored at −20 °C until shipped to the University at Buffalo (Buffalo, NY) on dry ice for analysis. All urine samples were in good condition (i.e., remained frozen) upon arrival to the University at Buffalo (Buffalo, NY). Urine samples were analyzed for TCPy (the primary metabolite of CPF) by negative-ion chemical ionization gas chromatography-mass spectrometry, with 13C-15N-3,5,6-TCPy as an internal standard, as described previously (Farahat et al. 2010). Creatinine concentrations were measured using the Jaffe reaction (Fabiny and Ertingshausen, 1971). Urinary TCPy concentrations were expressed as µg TCPy/g creatinine. The within-run imprecision for TCPy analysis was very low as shown by a < 2% coefficient of variation and an intraclass correlation coefficient between analytical replicates of 0.997 (Farahat et al. 2011).
Blood Collection and Analysis of BuChE and AChE Activity
Blood samples were collected by venipuncture into 10 ml lavender top (15% K3 EDTA) Vacutainer tubes and immediately placed on wet ice. Samples were then transported to Menoufia University (Shebin El-Kom, Egypt) where they were analyzed in triplicate for AChE and BuChE activity using an EQM Test-mate kit™ (Cincinnati, Ohio). The EQM Test-mate kit™, based on the original Ellman method (Ellman et al., 1961), is a portable photometric analyzer developed for the determination of cholinesterase activity in whole blood as a basis for monitoring pesticide exposure (McConnell et al., 1992). The intraclass correlation coefficient for BuChE with this method was 0.987 and for AChE, 0.898, indicating that there was very little within sample variation among replicates relative to total variation (Farahat et al. 2011).
Analysis of PON1 genotype
Approximately 1 – 2 ml of saliva for each subject was collected using an OG-250 saliva collection kit (DNA Genotek Inc., Kanata, ON). Samples were transported to Menoufia University (Shebin El-Kom, Egypt) where they were stored at room temperature and shipped to the University at Buffalo for analysis. Genomic DNA was isolated from saliva following the manufacturer’s instructions (DNA Genotek Inc., Kanata, ON). Purified DNA was stored in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) at −20°C. PON1 55 and PON1 192 polymorphisms were determined by multiplex polymerase chain reaction (PCR) followed by restriction fragment length polymorphism analysis as described previously (Motti et al., 2001). The multiplex PCR reaction (25 µl) contained 12.5 µl of Qiagen Multiplex PCR buffer (Qiagen Inc., Valencia, CA), 0.2 µM of each primer, 9 µl of PCR grade water and approximately 0.5 µg of genomic DNA. Thermocycle conditions were: predenaturation at 95 °C for 15 min, 40 cycles of amplification (94 °C for 1 min, 61 °C for 45 sec, 72 °C for 45 sec) and a final extension at 72 °C for 10 min. PCR products were digested in a restriction endonuclease mixture (20 µl) containing 10 µl of PCR product, 2 µl of REact 2 buffer (Invitrogen Inc., Carlsbad, CA), 5 U of Hinf I (Invitrogen Inc., Carlsbad, CA) and 7.5 µl of distilled water at 37 °C for 3 hours. The digested products were separated by electrophoresis on a 4% agarose gel containing ethidium bromide and visualized on a UV transilluminator. Genotypes were determined by the banding pattern of the digested products, where fragment sizes were: 144bp (PON1 55L), 122bp (PON1 55M), 111bp (PON1 192Q) and 77bp (PON1 192R).
Analysis of PON1 activity
Blood samples were collected by venipuncture into 10 ml red top (no anticoagulant) Vacutainer tubes and immediately placed on wet ice. Samples were then transported to Menoufia University (Shebin El-Kom, Egypt) where clotted samples were centrifuged and serum collected. Serum samples were subsequently aliquotted and stored at −20 °C until shipped to the University at Buffalo (Buffalo, NY) on dry ice for analysis. All serum samples were frozen upon arrival to the University at Buffalo (Buffalo, NY). Frozen serum was visually inspected for sample quality after storage and samples with little/no hemolysis were included in the activity analysis. Serum PON1 activity towards chlorpyrifos-oxon (e.g., chlorpyrifosoxonase activity referred to as CPOase) and paraoxon (e.g., paraoxonase activity referred to as POase) was measured on a Cobas Fara II (Roche, Indianapolis, IN, USA) chemistry analyzer as described previously (Furlong et al., 1989; Browne et al., 2007). Assay conditions for CPOase activity consisted of buffer (0.1 Tris-HCL, 1M NaCl, 2 mM CaCl2, pH 8.5), 320 µM chlorpyrifos-oxon (final concentration), and 5µl of diluted serum (1:100 in 0.1M Tris-HCL, pH 8.5) in a final volume of 303 µl. The composition of the buffer was taken from Furlong et al. (1989). Hydrolysis of chlorpyrifos-oxon by PON1 was monitored by the appearance of TCPy at 310 nm over three minutes. Assay conditions for POase activity consisted of buffer (50 mM glycine, 1 mM CaCl2, pH 10.5), 1 mM paraoxon, and 20 µl diluted serum (1:40 in 20 mM Tris-HCL, pH 8.0) in a final volume of 300 µl. The buffer for the POase assay was adapted from Eckerson et al., 1983 and a pH of 10.5 was confirmed as being the optimal pH for POase activity. Hydrolysis of paraoxon by PON1 was monitored by the appearance of para-nitrophenol at 405 nm over 1.5 minutes. Quality control materials consisting of frozen serum aliquots from two previously genotyped individuals (one PON1 192 QQ and one RR) were replicated in every run. These quality control materials showed coefficients of variation of < 9.1% for CPOase activity and < 6.9% for POase activity assays. All samples were analyzed in triplicate. The Cobas Fara II analyzer automatically conducts a reagent diluent blank with every analytical run. Blank absorbance values are subtracted from all samples automatically by the analyzer software to account for spontaneous hydrolysis of the substrate. PON1 activities were expressed as U/l (U = µmol of substrate hydrolyzed per minute).
Statistical analysis
Allele and genotype frequencies for the SNPs investigated were determined using the SNP and disease association analysis software SNPAlyze (version 7, Dynacom Co. Ltd., Yokohama, Japan). The normalized linkage disequilibrium coefficient D’ was also determined by the SNPAlyze software. The Hardy-Weinberg equilibrium was tested for the study population by the chi-squared test and was not rejected. Differences between baseline and post CPF exposure measures for individuals of the same genotype were determined using the paired t-test for parametric data (plasma BuChE, RBC AChE, CPOase and POase) and the Wilcoxon signed rank test for non-parametric data (urinary TCPy). Differences in PON1 activity between PON1 genotypes for the same time period were determined by ANOVA with Tukey’s post-hoc test. PON1 genotype and activity were investigated as potential modifiers of CPF toxicity by using the percent change in BuChE activity between baseline and post-spray levels as a sensitive biomarker of effect. Percent change was calculated as (BuChEbaseline− BuChEpost/BuChEbaseline)*100. The degree of change in BuChE activity was used in order to eliminate effects of the interindividual variability of baseline BuChE activity. Linear regression analysis was performed with baseline serum PON1 CPOase activity modeled as a continuous predictor of BuChE inhibition after stratification by L55M or Q192R genotype. Linear regression analysis was also performed with baseline serum PON1 POase activity modeled as a continuous predictor of BuChE inhibition after stratification by L55M or Q192R genotypes. The analysis was repeated in all cases after adjustment for urinary TCPy concentration (post exposure TCPy minus pre exposure TCPy; marker of CPF exposure) as a covariate. Analyses were done using SPSS statistical software (Chicago, IL). A p-value ≤ 0.05 was deemed statistically significant unless otherwise noted.
Results
Urinary TCPy and blood ChE
Baseline and post CPF application urinary TCPy concentrations are shown in Table 1. At baseline, there was a wide range in urinary TCPy concentrations (1.5 to 734 µg/g creatinine) with the median urinary TCPy concentration being 7.0 µg/g creatinine. Following the CPF application period, urinary TCPy concentration were significantly increased with the median urinary TCPy concentration being 15.9 µg/g creatinine. There was a broad range in BuChE activity at baseline (0.49 to 3.65 U/ml) and post CPF application (0.39 to 2.82 U/ml). BuChE activity was significantly decreased following CPF application (Table 1 and Supplemental Table 1). There was also a statistically significant decrease in AChE activity following CPF application (Table 1 and Supplemental Table 1). An inverse correlation was observed between urinary TCPy and plasma BuChE but not between urinary TCPy and RBC AChE (Supplemental Figure 1).
Table 1.
Urinary TCPy concentration, blood cholinesterase activity, and plasma PON1 activity from the Egyptian study population before and after CPF application
| Measure | Baseline | Post CPF application | P-value |
|---|---|---|---|
| Urinary TCPy (µg/g creatinine) | |||
| N | 100 | 97 | < 0.001a |
| Mean ± SD | 18.4 ± 73.4 | 49.1 ± 177.8 | |
| Median | 7.0 | 15.9 | |
| Range | 1.5 – 734 | 2.9 – 1664 | |
| Plasma BuChE (U/ml) | |||
| N | 100 | 97 | < 0.001b |
| Mean ± SD | 1.77 ± 0.52 | 1.59 ± 0.52 | |
| Median | 1.75 | 1.64 | |
| Range | 0.49 – 3.65 | 0.36 – 2.82 | |
| RBC AChE (U/g Hgb) | |||
| N | 100 | 97 | 0.018b |
| Mean ± SD | 28.3 ± 3.5 | 27.7 ± 3.2 | |
| Median | 28.4 | 26.8 | |
| Range | 11.7 – 37.3 | 19.0 – 37.5 | |
| CPOase (U/l) | |||
| N | 67 | 67 | 0.398b |
| Mean ± SD | 3680 ± 1140 | 3772 ± 1216 | |
| Median | 3668 | 3829 | |
| Range | 1286 – 6348 | 1329 – 7030 | |
| POase (U/l) | |||
| N | 67 | 67 | 0.053b |
| Mean ± SD | 83.5 ± 39.3 | 78.3 ± 35.2 | |
| Median | 77.4 | 73.4 | |
| Range | 23.8 – 171.2 | 18.0 – 173.4 |
P-value compares measures collected at baseline to post CPF application using
Wilcoxon sign rank test or
paried t-test
PON1 Genotype
PON1 gene frequencies for the Egyptian study population were determined for PON1 55 and PON1 192 (Table 2). All genotypes were consistent with Hardy-Weinberg equilibrium. The haplotype combinations were QR/LM (25%), QQ/LM (18%), QR/LL (17%), RR/LL (13%), QQ/MM (12%), QQ/LL (11%) QR/MM (4%) and RR/LM (1%). The haplotype combination of RR/MM did not occur in the study population. In this population, the 192R allele and the 55L allele are in strong linkage disequilibrium (D’ = −0.7463).
Table 2.
PON1 allele and genotype frequencies in the Egyptian study population
| PON1 L55M | Number of individuals (total = 120) |
Frequency |
| Genotype | ||
| LL | 49 | 40.8 |
| LM | 52 | 43.3 |
| MM | 19 | 15.8 |
| Allele | ||
| L | 62.5 | |
| M | 37.5 | |
| PON1 Q192R | ||
| Genotype | ||
| 48 | 40.0 | |
| QR | 55 | 45.8 |
| RR | 17 | 14.2 |
| Allele | ||
| Q | 62.9 | |
| R | 37.1 |
PON1 activity
Serum PON1 CPOase and POase activities were determined at baseline and post CPF application (Table 1). No significant difference was observed in the total population between PON1 activity (CPOase or POase) at baseline versus post CPF exposure. However, when the population was stratified by genotype, (PON1 55 or PON1 192), a statistically significant (p = 0.048) increase in CPOase activity post CPF exposure was observed for the PON1 192 RR genotype and a statistically significant (p = 0.018) decrease in POase activity was observed for the PON1 192 QR genotype. No significant difference was observed between baseline and post CPF application PON1 activity for all other genotypes.
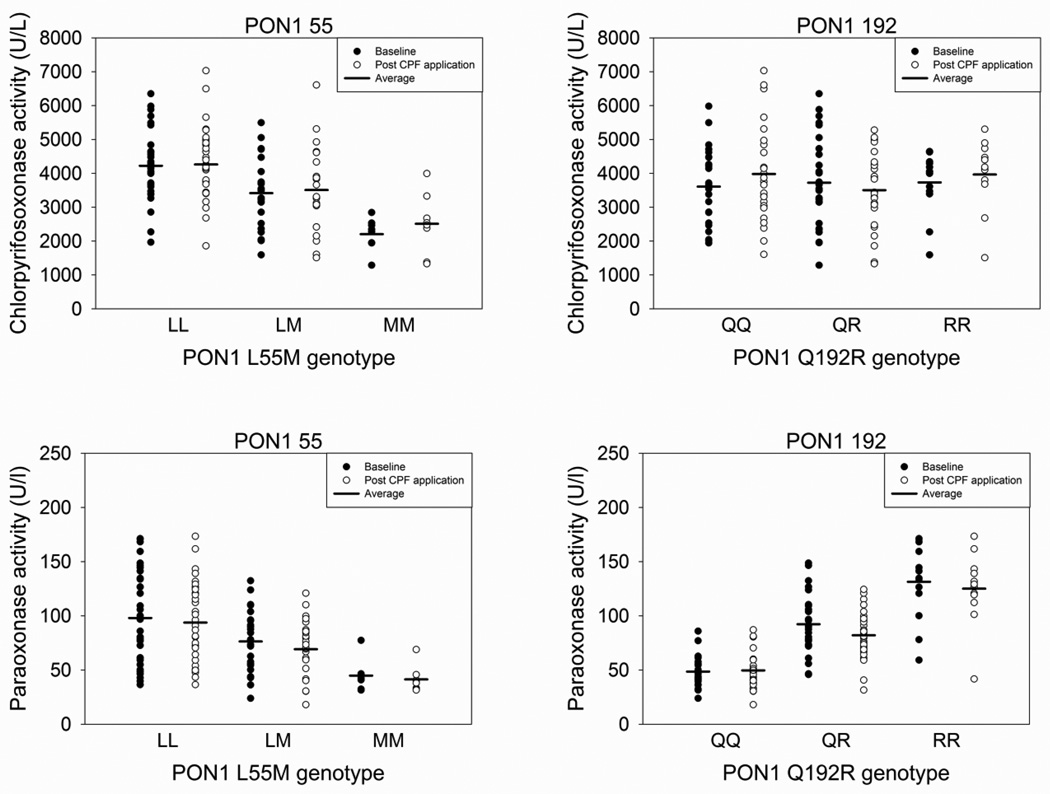
Mean CPOase activity was significantly different between the three different PON1 55 genotypes (Figure 1 and Table 3). Individuals with the PON1 55 LL genotype had the highest average CPOase activity (baseline, 4223 U/l) while individuals with the PON1 55 MM genotype had the lowest average CPOase activity (baseline, 2205 U/l). Even though significant differences in average CPOase activity were seen among the different PON1 55 genotypes, there was still a wide range of activities within a given genotype. No significant difference was observed in average CPOase activity among the different PON1 192 genotypes. When stratifying CPOase activity by PON1 55 and PON1 192 genotype there was still a large range in average activity; however, the highest average activity was observed in individuals with the PON1 LL/QQ and PON1 LL/QR genotypes while the lowest average activity was from individuals with the PON1 MM/QQ and PON1 MM/QR genotypes (Table 4).
Figure 1.
Baseline and post CPF application CPOase (top panels) and POase (bottom panels) activities based on the PON1 polymorphism at position 55 (left panels) and position 192 (right panels).
Table 3.
Serum PON1 activity towards chlorpyrifos-oxon and paraoxon stratified by PON1 genotype
| PON1 genotype | N | CPOase (U/l) | POase (U/l) | ||
|---|---|---|---|---|---|
| Baseline | Post CPF exposure | Baseline | Post CPF exposure | ||
| PON1 L55M | |||||
| LL | 34 | 4223 (1966 – 6348) | 4262 (1858 – 7030) | 98 (36 – 171) | 94 (36 – 173) |
| LM | 25 | 3415 (1592 – 5494)a | 3508 (1507 – 6607)a | 76 (24 – 132) | 69 (18 – 121)a |
| MM | 8 | 2205 (1286 – 2842)b,c | 2511 (1329 – 3992)b | 45 (31 – 77)b | 41 (32 – 69)b,c |
| PON1 Q192R | |||||
| 25 | 3607 (1941 – 5982) | 3981 (1606 – 7030) | 49 (24 – 86) | 50 (18 – 87) | |
| QR | 29 | 3722 (1286 – 6348) | 3504 (1329 – 5277) | 92 (46 – 149)d | 82 (32 – 124)d |
| RR | 13 | 3731 (1592 – 4643) | 3965 (1507 – 5301) | 131 (59 – 171)e | 125 (42 – 173)e |
Data expressed as the mean (range). P-value compares CPOase or POase activity among the different PON1 55 or PON1 192 genotypes for the same time period using ANOVA with Tukey post-hoc test.
P ≤ 0.05 compared to the PON1 55 LL genotype.
P ≤ 0.001 compared to the PON1 55 LL genotype.
P ≤ 0.05 compared to the PON1 55 LM genotype.
P ≤ 0.001 compared to the PON1 192 QQ genotype.
P ≤ 0.001 compared to the PON1 192 QQ and QR genotypes.
Table 4.
Serum PON1 activity towards chlorpyrifos-oxon and paraoxon respective to PON1 55 and PON1 192 genotype
| PON1 genotype | N | CPOase (U/l) | POase (U/l) | ||
|---|---|---|---|---|---|
| Baseline | Post CPF exposure | Baseline | Post CPF exposure | ||
| QQ/LL | 11 | 4196 (2854 – 5982) | 4601 (2977 – 7030) | 54 (36 – 86) | 58 (36 – 81) |
| QQ/LM | 9 | 3552 (2010 – 5494) | 3779 (1606 – 6607) | 47 (24 – 63)a,b | 46 (18 – 87)a,b |
| QQ/MM | 5 | 2411 (1941 – 2842)a,b,c | 2982 (2380 – 3992) | 38 (31 – 42)a,b,d | 38 (32 – 46)a,b,e |
| QR/LL | 11 | 4593 (1966 – 6348) | 4024 (1858 – 5277) | 100 (61 – 149)c | 88 (59 – 124)c |
| QR/LM | 15 | 3455 (2262 – 5053)a | 3478 (2152 – 4924) | 94 (56 – 132)b,c,e | 85 (61 – 121)b,c,e |
| QR/MM | 3 | 1860 (1286 – 2345)a,b,c | 1727 (1329 – 2474)a,b,c | 57 (46 – 77)b | 47 (32 – 69)a,b,e |
| RR/LL | 12 | 3909 (2269 – 4643) | 4170 (2680 – 5301) | 136 (59 – 171)a,c | 132 (101 – 173)a,c |
| RR/LM | 1 | 1592 | 1507 | 78 | 42 |
| RR/MM | 0 | - | - | - | - |
Data expressed as the mean (range). P-value compares CPOase or POase activity among the different genotypes for the same time period using ANOVA with Tukey post-hoc test.
P ≤ 0.05 compared to QRLL genotype.
P ≤ 0.05 compared to RRLL genotype.
P ≤ 0.05 compared to QQLL genotype.
P ≤ 0.05 compared to QRLM genotype
P ≤ 0.05 compared to QQLM genotype.
With regard to POase activity, individuals with the PON1 55 LL genotype had significantly higher average activity compared to individuals with the PON1 55 MM genotype (Figure 1 and Table 3). Furthermore, individuals with the PON1 192 QQ genotype had significantly less average POase activity than those with the PON1 192 RR genotype. Similar to CPOase activity, there was a wide range in POase activity within a given PON1 genotype. The highest and lowest average POase activity was from individuals with the PON1 LL/RR and PON1 MM/QR genotype, respectively.
Relationship between serum PON1 and blood BuChE Activity
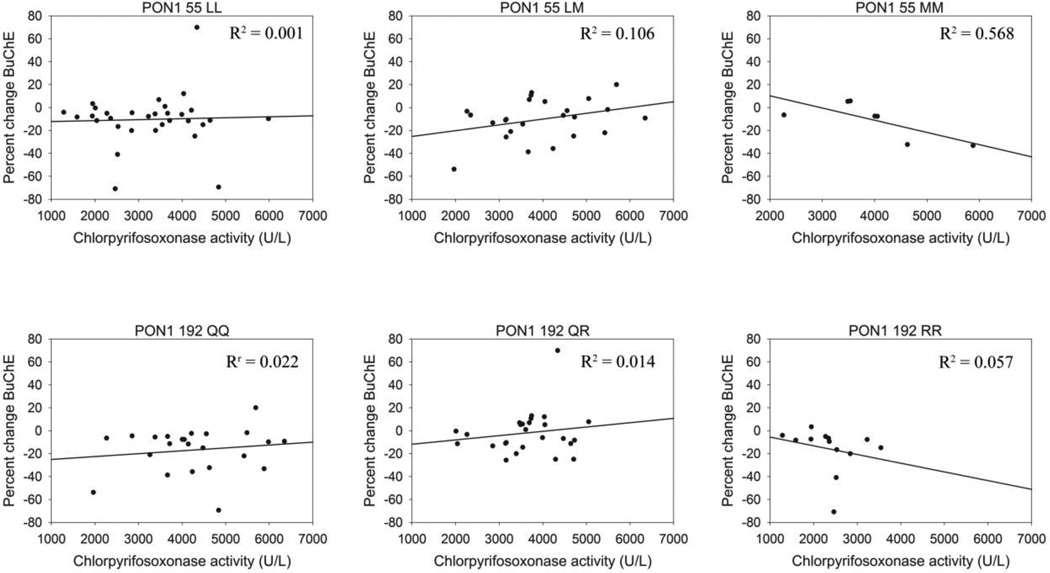
BuChE is more susceptible to chlorpyrifos-oxon inhibition than AChE (Amitai et al. 1998) and greater BuChE inhibition post CPF exposure was observed in our population as compared to AChE (Table 1 and Supplemental Table 1). Therefore, BuChE activity was used as a sensitive marker of potential CPF toxicity. The percentage change in BuChE from baseline to post CPF application was calculated and used to minimize the effects of variability in BuChE activity between individuals. Serum POase activity was modeled as a predictor of BuChE activity after stratifying by PON1 genotype (L55M or Q192R) both with and without adjustment for CPF exposure level (as measured by post exposure TCPy minus pre exposure TCPy). No significant correlation between PON1 POase activity and BuChE inhibition was seen within any of the genotypes with or without adjustment for exposure (data not shown). CPOase activity was similarly modeled against BuChE activity (Figure 2). No significant correlation between PON1 CPOase activity and BuChE inhibition was seen within any of the genotypes with or without adjustment for exposure (Table 5).
Figure 2.
BuChE inhibition in relation to CPOase activity, stratified by PON1 L55M or PON1 Q192R genotype.
Table 5.
Results of linear regression model of CPOase activity as a predictor of BuChE inhibition stratified by PON1 55 or 192 genotype
| PON1 Genotype | N | Unadjusted | Adjusteda | ||
|---|---|---|---|---|---|
| β coefficient | p-value | β coefficient | p-value | ||
| LL | 31 | 0.001 | 0.837 | 0.003 | 0.489 |
| LM | 24 | 0.005 | 0.118 | 0.006 | 0.105 |
| MM | 7 | −0.011 | 0.058 | −0.011 | 0.061 |
| 23 | 0.003 | 0.497 | 0.004 | 0.231 | |
| QR | 26 | 0.004 | 0.435 | 0.006 | 0.186 |
| RR | 13 | −0.008 | 0.432 | −0.010 | 0.351 |
The β coefficient is based on linear regression analysis and represents the unit change in BuChE attributable to a unit change in CPOase activity.
Urinary TCPy included as a covariate.
Discussion
It remains controversial as to whether PON1 genotype influences OP neurotoxicity in humans. Not all the studies performed to date have identified an association between PON1 status and either blood cholinesterase activity (Albers et al., 2010) or symptoms associated with but not necessarily specific to chronic OP neurotoxicity (Hernandez et al., 2003), and amongst those studies that have reported an association between PON1 genotype and worker health, there have been conflicting results as to which genotype is more sensitive to OP exposure (Hofmann et al. 2009; Mackness et al. 2003). It is not clear whether the conflicting findings in the current literature reflect differences in the study populations, differences in exposure histories (including types, duration and levels of OP exposures) or the failure to include both PON1 genotype and phenotype in the analysis. The current study was conducted using an occupational cohort with a clearly defined CPF exposure and longitudinal measures of TCPy, a specific biomarker of exposure, AChE and BuChE activity as biomarkers of effect, and PON1 genotype and phenotype before and after CPF exposure; thereby, enabling conclusions to be drawn about the role of PON1 as a susceptibility factor for OP neurotoxicity in a defined exposure scenario and population.
Application of CPF to cotton fields was associated with a significant exposure of agriculture workers based on urinary TCPy levels, which provide a sensitive and specific biomarker of exposure to CPF (Table 1). Urinary TCPy concentration in study cohort from 2009 ranged from 1.5 to 1,664 µg/g creatinine, while previous studies of Egyptian agricultural workers involved in CPF application have reported concentrations from approximately 20 to 2,000 µg/g creatinine in 2007 (Farahat et al. 2010) and 10 to 10,000 µg/g creatinine in 2008 (Farahat et al. 2011). Some workers had elevated urinary TCPy levels at baseline which has been explained by the episodic use of pesticides outside of their ministry jobs (Farahat et al. 2011).
Application of CPF to the fields was also associated with a statistically significant decrease in blood BuChE and AChE activity (Table 1). It is notable that although the 2009 study population had lower CPF exposures on average relative to the study populations in previous years as documented in Farahat et al. 2010 and Farahat et al. 2011, there remains a consistent decrease in AChE and BuChE activity for these workers. Of the workers for whom samples were collected for cholinesterase measures, CPF application was associated with a significant decrease in BuChE relative to baseline in 70 of 90 while 58 of 90 experienced significantly decreased AChE (Supplemental Table 1). However, few individuals had BuChE depression of more than 40% inhibition and there were no cases of AChE greater than 30% inhibition, which are the levels of cholinesterase inhibition proposed to be biologically significant (Furman, 2010; Ngai et al. 2002). As expected given these findings, none of the workers were clinically symptomatic.
Normal reported values for the EQM Test-Mate™ for whole blood AChE and BuChE are 27.1 ± 2.9 U/g Hgb and 2.03 ± 0.40 U/mL, respectively. Even though AChE in the currently study population displayed a significant decrease post-exposure (27.7 ± 3.2 U/g Hgb), levels remained within the normal range for this assay. In contrast, even at baseline, mean BuChE activity in this study population (1.77 ± 0.52 U/mL) was at the low end of this range. Additionally, there was a wide range in BuChE activity at baseline, which was not unexpected given the normal variation in BuChE activity. BuChE activity can vary with body mass index, age, lipid and cholesterol levels, and serum albumin (Sklan et al., 2004; Calderon-Margalit et al., 2006). In this case, differences in home pesticide use and other outside exposure scenarios are no doubt also contributing to varying baseline activity among individual agricultural workers. This wide range of baseline activity emphasizes the usefulness of monitoring workers over a season relative to their own baseline in order to characterize occupational exposure. Collectively, however, these data indicate that individuals in our study population exhibit variable exposures and responses to CPF exposures, which provides a rich dataset for testing an association between PON1 status and susceptibility to OP toxicity.
The 192R allele and the 55L allele are in linkage disequilibrium to varying degrees in different populations. In Caucasian populations, the 192R/55M genotype is rarely found (Garin et al., 1997) while in Brazilians of African descent, this linkage disequilibrium is not as strong (Allebrandt et al., 2002). In our population, the 192R allele and the 55L allele are in strong linkage disequilibrium (D’ = 0.7463). The allele frequencies for the variant 192R in our study population of Egyptian males is similar to reported frequencies for Egyptian females (N=100) (Hussein et al., 2011), though the frequency for the 55M allele we observed in the male agricultural workers (37%) was lower than that reported in the female Egyptian population (53.5%).
The current study determined PON1 activity prior to and following 15 consecutive days of CPF application. This provided the opportunity to test the hypothesis that repeated CPF exposures modify PON1 activity. There is significant experimental evidence that OPs can alter the activity of diverse enzymes (Casida and Quistad, 2005; Grigoryan et al., 2009; Lockridge and Schopfer, 2010; Ruark et al., 2011), so it is not inconceivable that either CPF or its oxon metabolite might alter the structure or function of PON1 or induce PON1 activity as a compensatory mechanism. If repeated CPF exposure altered PON1 activity, this might suggest not only an explanation of the discrepant findings between the Hofmann et al. (2009) and Albers et al. (2010) studies, but if PON1 activity were upregulated by repeated CPF exposure, it would suggest a compensatory mechanism to explain our earlier observation that Egyptian agricultural workers with repeated high exposures to CPF and greatly inhibited AChE nevertheless show no overt signs of toxicity (Farahat et al., 2011). In the current study, similar PON1 activities (CPOase and POase) were observed at baseline and post CPF exposure for the entire population (Table 1). However, when PON1 activity was stratified by genotype there was a statistically significant increase in CPOase activity post CPF exposure for the PON1 192 RR genotype and a significant decrease in POase activity for the PON1 192 QR genotype. It is not clear whether this is linked to a particular genotype or driven by a few individuals experiencing an increase in CPOase activity or a decrease in POase activity over the CPF application period; however, it is possible that CPF exposure may cause slight variations in PON1 activity within an individual. But, since we were ultimately not able to detect a relationship between CPOase or POase activity and cholinesterase inhibition (see below), it suggests that these subtle changes in an individual may not have a large overall impact on CPF metabolism and toxicity. It should also be noted that PON1 activity can vary up to 40-fold within a given population and protein levels can vary up to 13-fold for a single PON1 192 genotype (Costa et al., 2005b) and the slight detected changes in response to CPF exposure seen in the current study do not alter activity outside the normal range for a particular genotype group.
We found that human serum PON1 activity towards chlorpyrifos-oxon, e.g. CPOase activity, is significantly associated with PON1 55 genotype but not PON1 192 genotype. Our findings for PON1 55 are consistent with results from Holland et al. (2006); whereas the lack of association we observed between PON1 192 genotype and CPOase activity differs from that of Holland et al. (2006) but is consistent with findings from Albers et al. (2010). The Albers population is more similar to our population in that they are predominantly adult males occupationally exposed to CPF while the Holland study population is very different in that it consists of pregnant women and newborns exposed to environmental levels of OPs. With regard to the PON1 55 genotype, even though a significant relationship was observed between genotype and average CPOase activity, a wide range in activity still existed within a genotype. In some cases, individuals with a low activity genotype (PON1 55 MM) had approximately twice as much CPOase activity than an individual with a high activity genotype (PON1 55 LL). This is in agreement with previous reports that genotype alone is insufficient to determine an individual’s potential to hydrolyze chlorpyrifos-oxon (Richter and Furlong, 1999; Brophy et al., 2000; Costa et al., 2005b; Furlong et al., 2010; Povey, 2010).
Our findings support the conclusion that PON1 genotype does not impact BuChE or AChE activity. Furthermore, unlike the previous human studies, we also examined the change in CPOase activity and POase activity from baseline to post exposure which has never been done before and included the PON1 L55M genotype in our analysis in addition to the more commonly studied PON1 Q192R polymorphism. In the current study, even though individuals among the different PON1 55 genotypes had significant differences in CPOase activity, this did not significantly affect BuChE or AChE inhibition at CPF exposure levels experienced by the study population. Monte Carlo analysis suggest that PON1 genotype may not affect CPF- related toxicity at low exposure conditions but may become an important determinant in CPF sensitivity at higher CPF exposures (Timchalk et al., 2002). Future studies with defined exposures are needed to determine if the relative influence of PON1 genotype on CPF-toxicity is greater in a more highly exposed population.
In summary, the present study utilized urinary TCPy concentrations as a biomarker of CPF exposure and confirmed that Egyptian agricultural workers experience occupational exposures to CPF during CPF application to cotton fields. Following CPF exposure, there was a statistically significant decrease in plasma BuChE and RBC AChE activities among the workers, a significant decrease in POase activity in the PON1 192 QR group and a significant increase in CPOase activity in the PON1 192 RR group. However, these changes were relatively subtle given the inherent biological variability of these enzymes. The PON1 55 but not the PON1 192 genotype significantly affected an individual’s ability to hydrolyze chlorpyrifos-oxon but neither genotype nor CPOase activity had a significant effect on plasma BuChE or red blood cell AChE inhibition. Together this suggests that regardless of PON1 genotype and activity the workers retained the capacity to detoxify chlorpyrifos-oxon under the exposure conditions experienced by this study population.
Supplementary Material
Plasma BuChE and RBC AChE activity plotted against urinary TCPy concentration for Egyptian agricultural workers at baseline and post CPF application. Data points represent paired matched values for cholinesterase activity and urine TCPy content from individual workers.
Highlights.
Determine if PON1 status is a biomarker of susceptibility for CPF related toxicity
Agricultural workers involved in CPF application donated saliva, blood, and urine
Occupational CPF exposure resulted in an increase in TCPy and decreases in BuChE and AChE
CPOase activity decreased in subjects with the PON1 55LM and PON1 55MM genotypes
Neither PON1 genotype nor CPOase activity had an effect on BuChE or AChE inhibition
Acknowledgments
We thank Steve Hutton (Dow Agrosciences, Indianapolis, IN) for providing 13C–15N– 3,5,6-TCP, and the Egyptian Ministry of Agriculture. Barb McGarrigle (University of Buffalo) analyzed the urine samples. This work was supported by the National Institute of Environmental Health Sciences (NIEHS) [grant number ES016308]. Corie Ellison was supported by a Research Supplement to Promote Diversity in Health-Related Research from the NIEHS [grant number ES016308-02S]. Alice Crane was supported by a Ruth L. Kirschstein National Research Service Award from the NIEHS [grant number F30 ES020655]. The content is solely the authors’ responsibility and does not necessarily represent official views of NIEHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
There are no conflicts of interest
Contributor Information
Corie A. Ellison, Email: cellison@buffalo.edu.
Alice L. Crane, Email: alcrane@buffalo.edu.
Matthew R. Bonner, Email: mrbonner@buffalo.edu.
James B. Knaak, Email: jbknaak@aol.com.
Richard W. Browne, Email: rwbrowne@buffalo.edu.
Pamela J. Lein, Email: pjlein@ucdavis.edu.
James R. Olson, Email: jolson@buffalo.edu.
References
- Adkins S, Gan KN, Mody M, La Du BN. Molecular basis for the polymorphic forms of human serum paraoxonase/arylesterase: glutamine or arginine at position 191, for the respective A or B allozymes. Am. J. Hum. Genet. 1993;52:598–608. [PMC free article] [PubMed] [Google Scholar]
- Albers JW, Garabrant DH, Berent S, Richardson RJ. Paraoxonase status and plasma butyrylcholinesterase activity in chlorpyrifos manufacturing workers. J. Expo. Sci. Environ. Epidemiol. 2010;20:79–89. doi: 10.1038/jes.2009.9. [DOI] [PubMed] [Google Scholar]
- Alexander BH, Burns CJ, Bartels MJ, Acquavella JF, Mandel JS, Gustin C, Baker BA. Chlorpyrifos exposure in farm families: results from the farm family exposure study. J. Expo. Sci. Environ. Epidemiol. 2006;16:447–456. doi: 10.1038/sj.jes.7500475. [DOI] [PubMed] [Google Scholar]
- Allebrandt KV, Souza RL, Chautard-Freire-Maia EA. Variability of the paraoxonase gene (PON1) in Euro- and Afro-Brazilians. Toxicol. Appl. Pharmacol. 2002;180:151–156. doi: 10.1006/taap.2002.9368. [DOI] [PubMed] [Google Scholar]
- Brophy VH, Jarvik GP, Richter RJ, Rozek LS, Schellenberg GD, Furlong CE. Analysis of paraoxonase (PON1) L55M status requires both genotype and phenotype. Pharmacogenetics. 2000;10:453–460. doi: 10.1097/00008571-200007000-00008. [DOI] [PubMed] [Google Scholar]
- Browne RW, Koury ST, Marion S, Wilding G, Muti P, Trevisan M. Accuracy and biological variation of human serum paraoxonase 1 activity and polymorphism (Q192R) by kinetic enzyme assay. Clin. Chem. 2007;53:310–317. doi: 10.1373/clinchem.2006.074559. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Moser VC. Behavioral toxicity of cholinesterase inhibitors. In: Gupta RC, editor. Toxicology of Organophosphate and Carbamate Compounds. San Diego: Elsevier; 2006. pp. 347–360. [Google Scholar]
- Calderon-Margalit R, Adler B, Abramson JH, Gofin J, Kark JD. Butyrylcholinesterase activity, cardiovascular risk factors, and mortality in middle-aged and elderly men and women in Jerusalem. Clin. Chem. 2006;52:845–852. doi: 10.1373/clinchem.2005.059857. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Serine hydrolase targets of organophosphorus toxicants. Chem. Biol. Interact. 2005:157–158. 277–283. doi: 10.1016/j.cbi.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Cherry N, Mackness M, Durrington P, Povey A, Dippnall M, Smith T, Mackness B. Paraoxonase (PON1) polymorphisms in farmers attributing ill health to sheep dip. Lancet. 2002;359:763–764. doi: 10.1016/s0140-6736(02)07847-9. [DOI] [PubMed] [Google Scholar]
- Costa LG. Current issues in organophosphate toxicology. Clin. Chim. Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Vitalone A, Furlong CE. Measurement of paraoxonase (PON1) status as a potential biomarker of susceptibility to organophosphate toxicity. Clin. Chim. Acta. 2005a;352:37–47. doi: 10.1016/j.cccn.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Costa LG, Li WF, Richter RJ, Shih DM, Lusis A, Furlong CE. The role of paraoxonase (PON1) in the detoxication of organophosphates and its human polymorphism. Chem. Biol. Interact. 1999:119–120. 429–438. doi: 10.1016/s0009-2797(99)00055-1. [DOI] [PubMed] [Google Scholar]
- Costa LG, Richter RJ, Li WF, Cole T, Guizzetti M, Furlong CE. Paraoxonase (PON 1) as a biomarker of susceptibility for organophosphate toxicity. Biomarkers. 2003;8:1–12. doi: 10.1080/13547500210148315. [DOI] [PubMed] [Google Scholar]
- Costa LG, Vitalone A, Cole TB, Furlong CE. Modulation of paraoxonase (PON1) activity. Biochem. Pharmacol. 2005b;69:541–550. doi: 10.1016/j.bcp.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowalla J, Furlong CE. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat. Genet. 1996;14:334–336. doi: 10.1038/ng1196-334. [DOI] [PubMed] [Google Scholar]
- Eckerson HW, Wyte CM, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am. J. Hum. Genet. 1983;35:1126–1138. [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fabiny DL, Ertingshausen G. Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Clin. Chem. 1971;17:696–700. [PubMed] [Google Scholar]
- Farahat FM, Ellison CA, Bonner MR, McGarrigle BP, Crane AL, Fenske RA, Lasarev MR, Rohlman DS, Anger WK, Lein PJ, Olson JR. Biomarkers of chlorpyrifos exposure and effect in Egyptian cotton field workers. Environ. Health. Perspect. 2011;119:801–806. doi: 10.1289/ehp.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat FM, Fenske RA, Olson JR, Galvin K, Bonner MR, Rohlman DS, Farahat TM, Lein PJ, Anger WK. Chlorpyrifos exposures in Egyptian cotton field workers. Neurotoxicology. 2010;31:297–304. doi: 10.1016/j.neuro.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong CE, Cole TB, Jarvik GP, Pettan-Brewer C, Geiss GK, Richter RJ, Shih DM, Tward AD, Lusis AJ, Costa LG. Role of paraoxonase (PON1) status in pesticide sensitivity: genetic and temporal determinants. Neurotoxicology. 2005;26:651–659. doi: 10.1016/j.neuro.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Furlong CE, Richter RJ, Seidel SL, Costa LG, Motulsky AG. Spectrophotometric assays for the enzymatic hydrolysis of the active metabolites of chlorpyrifos and parathion by plasma paraoxonase/arylesterase. Anal. Biochem. 1989;180:242–247. doi: 10.1016/0003-2697(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Furlong CE, Suzuki SM, Stevens RC, Marsillach J, Richter RJ, Jarvik GP, Checkoway H, Samii A, Costa LG, Griffith A, Roberts JW, Yearout D, Zabetian CP. Human PON1, a biomarker of risk of disease and exposure. Chem. Biol. Interact. 2010;187:355–361. doi: 10.1016/j.cbi.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman J. Cholinesterase monitoring for agricultural pesticide handlers: Guidelines for health care providers in Washington state. Department of labor and industries Division of Occupational Safety and Health (DOSH) 2010 [Google Scholar]
- Garabrant DH, Aylward LL, Berent S, Chen Q, Timchalk C, Burns CJ, Hays SM, Albers JW. Cholinesterase inhibition in chlorpyrifos workers: Characterization of biomarkers of exposure and response in relation to urinary TCPy. J. Expo. Sci. Environ. Epidemiol. 2009;19:634–642. doi: 10.1038/jes.2008.51. [DOI] [PubMed] [Google Scholar]
- Garin MC, James RW, Dussoix P, Blanche H, Passa P, Froguel P, Ruiz J. Paraoxonase polymorphism Met-Leu54 is associated with modified serum concentrations of the enzyme. A possible link between the paraoxonase gene and increased risk of cardiovascular disease in diabetes. J. Clin. Invest. 1997;99:62–66. doi: 10.1172/JCI119134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan H, Schopfer LM, Peeples ES, Duysen EG, Grigoryan M, Thompson CM, Lockridge O. Mass spectrometry identifies multiple organophosphorylated sites on tubulin. Toxicol. Appl. Pharmacol. 2009;240:149–158. doi: 10.1016/j.taap.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AF, Mackness B, Rodrigo L, Lopez O, Pla A, Gil F, Durrington PN, Pena G, Parron T, Serrano JL, Mackness MI. Paraoxonase activity and genetic polymorphisms in greenhouse workers with long term pesticide exposure. Hum. Exp. Toxicol. 2003;22:565–574. doi: 10.1191/0960327103ht400oa. [DOI] [PubMed] [Google Scholar]
- Hofmann JN, Keifer MC, Furlong CE, De Roos AJ, Farin FM, Fenske RA, van Belle G, Checkoway H. Serum cholinesterase inhibition in relation to paraoxonase-1 (PON1) status among organophosphate-exposed agricultural pesticide handlers. Environ. Health. Perspect. 2009;117:1402–1408. doi: 10.1289/ehp.0900682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland N, Furlong C, Bastaki M, Richter R, Bradman A, Huen K, Beckman K, Eskenazi B. Paraoxonase polymorphisms, haplotypes, and enzyme activity in Latino mothers and newborns. Environ. Health. Perspect. 2006;114:985–991. doi: 10.1289/ehp.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein YM, Gharib AF, Etewa RL, ElSawy WH. Association of L55M and Q192R polymorphisms in paraoxonase 1 (PON1) gene with breast cancer risk and their clinical significance. Mol. Cell. Biochem. 2011;351:117–123. doi: 10.1007/s11010-011-0718-4. [DOI] [PubMed] [Google Scholar]
- Lee BW, London L, Paulauskis J, Myers J, Christiani DC. Association between human paraoxonase gene polymorphism and chronic symptoms in pesticide-exposed workers. J. Occup. Environ. Med. 2003;45:118–122. doi: 10.1097/01.jom.0000052953.59271.e1. [DOI] [PubMed] [Google Scholar]
- Leviev I, Negro F, James RW. Two alleles of the human paraoxonase gene produce different amounts of mRNA. An explanation for differences in serum concentrations of paraoxonase associated with the (Leu-Met54) polymorphism. Arterioscler. Thromb. Vasc. Biol. 1997;17:2935–2939. doi: 10.1161/01.atv.17.11.2935. [DOI] [PubMed] [Google Scholar]
- Li WF, Costa LG, Richter RJ, Hagen T, Shih DM, Tward A, Lusis AJ, Furlong CE. Catalytic efficiency determines the in-vivo efficacy of PON1 for detoxifying organophosphorus compounds. Pharmacogenetics. 2000;10:767–779. doi: 10.1097/00008571-200012000-00002. [DOI] [PubMed] [Google Scholar]
- Lockridge O, Schopfer LM. Review of tyrosine and lysine as new motifs for organophosphate binding to proteins that have no active site serine. Chem. Biol. Interact. 2010;187:344–348. doi: 10.1016/j.cbi.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Chambers JE. Kinetic parameters of desulfuration and dearylation of parathion and chlorpyrifos by rat liver microsomes. Food. Chem. Toxicol. 1994;32:763–767. doi: 10.1016/s0278-6915(09)80009-4. [DOI] [PubMed] [Google Scholar]
- Mackness B, Durrington P, Povey A, Thomson S, Dippnall M, Mackness M, Smith T, Cherry N. Paraoxonase and susceptibility to organophosphorus poisoning in farmers dipping sheep. Pharmacogenetics. 2003;13:81–88. doi: 10.1097/00008571-200302000-00004. [DOI] [PubMed] [Google Scholar]
- McConnell R, Cedillo L, Keifer M, Palomo MR. Monitoring organophosphate insecticide-exposed workers for cholinesterase depression. New technology for office or field use. J. Occup. Med. 1992;34:34–37. [PubMed] [Google Scholar]
- Motti C, Dessi M, Gnasso A, Irace C, Indigeno P, Angelucci CB, Bernardini S, Fucci G, Federici G, Cortese C. A multiplex PCR-based DNA assay for the detection of paraoxonase gene cluster polymorphisms. Atherosclerosis. 2001;158:35–40. doi: 10.1016/s0021-9150(00)00765-6. [DOI] [PubMed] [Google Scholar]
- Myers DK, Mendel B, Gersmann HR, Ketelaar JA. Oxidation of thiophosphate insecticides in the rat. Nature. 1952;170:805–807. doi: 10.1038/170805b0. [DOI] [PubMed] [Google Scholar]
- Ngai W, Ames RG, Wisniewski J, Fan AM. Guidelines for physicians who supervise workers exposed to cholinesterase-inhibiting pesticides. California Environmental Protection Agency (EPA) office of environmental health hazard assessment. (4 ed) 2002 [Google Scholar]
- Pond AL, Chambers HW, Coyne CP, Chambers JE. Purification of two rat hepatic proteins with A-esterase activity toward chlorpyrifos-oxon and paraoxon. J. Pharmacol. Exp. Ther. 1998;286:1404–1411. [PubMed] [Google Scholar]
- Povey AC. Gene-environmental interactions and organophosphate toxicity. Toxicology. 2010;278:294–304. doi: 10.1016/j.tox.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Povey AC, Mackness MI, Durrington PN, Dippnall M, Smith AE, Mackness B, Cherry NM. Paraoxonase polymorphisms and self-reported chronic ill-health in farmers dipping sheep. Occup. Med. (Lond) 2005;55:282–286. doi: 10.1093/occmed/kqi128. [DOI] [PubMed] [Google Scholar]
- Prabhavathy Das G, Jamil K. Human serum paraoxonase gene polymorphisms and association with chronic symptoms of pesticide toxicity in Indian farmers. Toxicological & Environmental Chemistry. 2009;91:177–185. [Google Scholar]
- Richter RJ, Furlong CE. Determination of paraoxonase (PON1) status requires more than genotyping. Pharmacogenetics. 1999;9:745–753. [PubMed] [Google Scholar]
- Ruark CD, Hack CE, Robinson PJ, Gearhart JM. Quantitative structure-activity relationships for organophosphates binding to trypsin and chymotrypsin. J. Toxicol. Environ. Health. A. 2011;74:1–23. doi: 10.1080/15287394.2010.501716. [DOI] [PubMed] [Google Scholar]
- Sklan EH, Lowenthal A, Korner M, Ritov Y, Landers DM, Rankinen T, Bouchard C, Leon AS, Rice T, Rao DC, Wilmore JH, Skinner JS, Soreq H. Acetylcholinesterase/paraoxonase genotype and expression predict anxiety scores in Health, Risk Factors, Exercise Training, and Genetics study. Proc. Natl. Acad. Sci. U S A. 2004;101:5512–5517. doi: 10.1073/pnas.0307659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultatos LG. Mammalian toxicology of organophosphorus pesticides. J. Toxicol. Environ. Health. 1994;43:271–289. doi: 10.1080/15287399409531921. [DOI] [PubMed] [Google Scholar]
- Sultatos LG, Murphy SD. Kinetic analyses of the microsomal biotransformation of the phosphorothioate insecticides chlorpyrifos and parathion. Fundam. Appl. Toxicol. 1983;3:16–21. doi: 10.1016/s0272-0590(83)80167-5. [DOI] [PubMed] [Google Scholar]
- Timchalk C, Kousba A, Poet TS. Monte Carlo analysis of the human chlorpyrifos-oxonase (PON1) polymorphism using a physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model. Toxicol. Lett. 2002;135:51–59. doi: 10.1016/s0378-4274(02)00233-3. [DOI] [PubMed] [Google Scholar]
- Yang D, Howard A, Bruun D, Ajua-Alemanj M, Pickart C, Lein PJ. Chlorpyrifos and chlorpyrifos-oxon inhibit axonal growth by interfering with the morphogenic activity of acetylcholinesterase. Toxicol. Appl. Pharmacol. 2008;228:32–41. doi: 10.1016/j.taap.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasma BuChE and RBC AChE activity plotted against urinary TCPy concentration for Egyptian agricultural workers at baseline and post CPF application. Data points represent paired matched values for cholinesterase activity and urine TCPy content from individual workers.