Abstract
Objective
To review the use of aromatase inhibitors for the treatment of endometriosis
Design
Literature review
Conclusions
Most studies show that in reproductive aged women, the combination of and AI with conventional therapy does alleviate endometriosis related pain. In post-menopausal women, using an AI alone has been shown to be an effective treatment, although more studies are needed in this subgroup. Side effects using AIs appear to be tolerable in most women, although special consideration should be given to monitoring BMD. More studies need to be done examining pregnancy rates and outcomes following aromatase inhibitor treatment for endometriosis. In addition, larger randomized clinical trials using AIs need to be done. In summary, aromatase inhibitors may be effective in treating endometriosis related chronic pelvic pain in both reproductive aged and postmenopausal women.
Keywords: Endometriosis, endometrioma, endometrium, aromatase inhibitors, estrogen, oral contraceptive, progestin, progesterone, GnRH agonist, letrozole, anastrozole
Endometriosis, an estrogen-dependent inflammatory disease, is defined by the growth of endometrial stroma and glands outside of the uterus (1, 2). Affected women often present with chronic pelvic pain, dysmenorrhea, dyspareunia, and infertility (1, 2). It is often cited that retrograde menstruation is the cause of this condition; however retrograde menstruation occurs in nearly all women yet all women are not afflicted with this condition. Therefore, it has been proposed that women with endometriosis are likely to have underlying molecular abnormalities that allow the continued growth of these endometrial tissues outside of the uterus (3).
We and others have demonstrated that the potent mitogen estradiol (E2), the biologically active form of estrogen, supports the growth and inflammation processes in endometriotic lesions (1, 2, 4–8). Local estrogen content of endometriotic lesions is highly correlated with the expression levels of steroidogenic enzyme aromatase cytochrome P450 (1, 9, 10). Elevated levels of aromatase mRNA have been found in extraovarian endometriotic lesions and ovarian endometriomas (11). Adrenal and ovarian androstenedione function as the primary substrate for aromatase activity in endometriotic tissue, catalyzing the reaction to give rise to estrone, which is further converted to the more active estradiol (9, 12–17). Additionally, peritoneal and ovarian endometriotic tissues express all the genes needed to convert cholesterol to estradiol.
Aromatase is regulated at the levels of transcriptional expression, protein expression, and enzyme activity in endometriosis. (8, 18, 19). It is involved in a positive feedback loop that favors expression of key steroidogenic genes (1). Estrogen stimulates expression of the COX-2 enzyme, resulting in elevated levels of prostaglandin E2 (PGE2), which is a potent stimulator of aromatase activity in endometriosis (1). This leads to continuous local production of E2 and PGE2 in endometriotic tissue (1). In endometriosis, estrogens promote the growth and invasion of endometriotic tissue, while prostaglandins mediate pain, inflammation, and infertility. Because of the integral role of aromatase and estrogens in endometriosis, Aromatase inhibitors (AIs) have been investigated as a potential treatment option for women afflicted with endometriosis (17, 20–23).
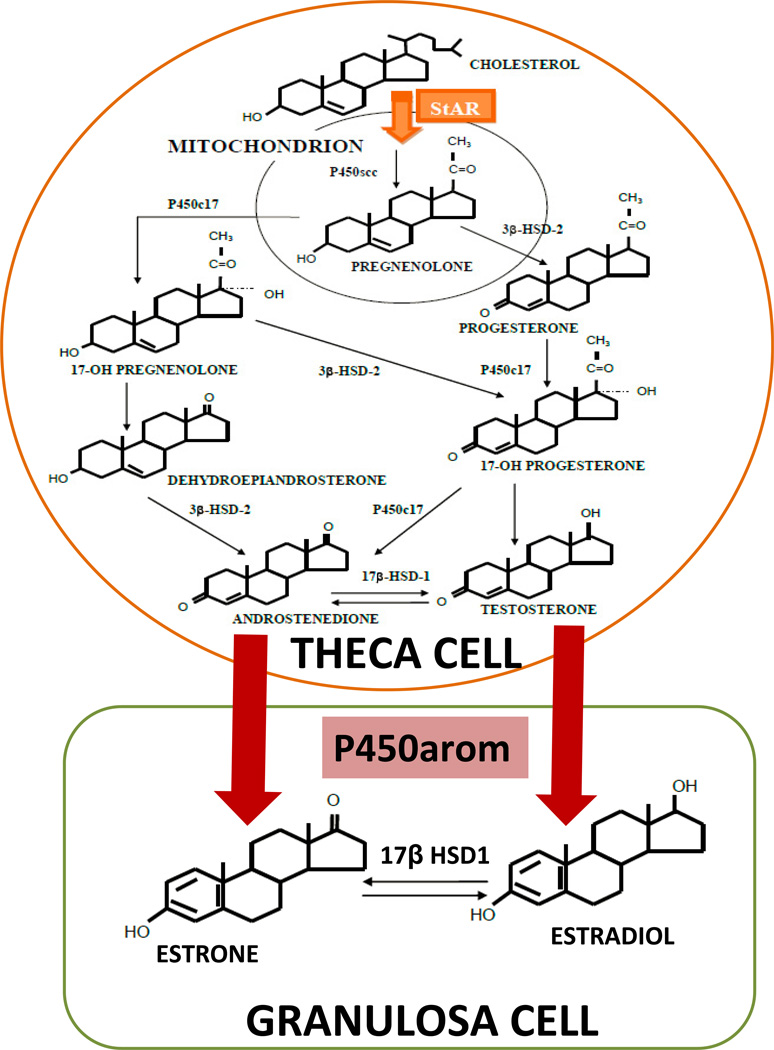
Medical treatment of endometriosis seeks to either mitigating estrogen action or induce a hypoestrogenic state. The inhibition of estrogen synthesis is a rational approach to treatment because of the importance of estrogen in stimulating endometriotic tissues and the in situ presence of aromatase in these tissues. In addition, aromatization is the last step in estradiol biosynthesis, meaning that there are no important downstream enzymes that can be affected (Figure 1). Although aromatase is a P450 enzyme, it is unique in performing the aromatizing reaction, and is therefore amenable to selective inhibition.
Figure 1.
In the ovary, the biologically active estradiol is produced from cholesterol through serial enzymatic actions in two cell types, namely the theca and granulosa cells, which cooperate in a paracrine fashion. There are two rate-limiting steps in this process: (1) entry of cholesterol into the mitochondria of theca cells, regulated by steroidogenic acute regulatory (StAR) protein and (2) conversion of androstenedione to estrone by aromatase in granulosa cells.
This paper will review the role of aromatase in the pathogenesis of endometriosis, discuss the pharmacology of aromatase inhibitors, and examine clinical applications of aromatase inhibitors for the treatment of endometriosis.
ABNORMAL ESTROGEN BIOSYNTHESIS IN ENDOMETRIOSIS
Aromatase is expressed in certain human cells including the ovarian granulosa cell, the placental syncytiotrophoblst, the testicular Leydig cell, and other extraglandular sites such as adipose tissue, the brain, and skin fibroblasts (15, 17). The highest levels of aromatase are in the ovarian granulosa cells in premenopausal women, whereas adipose tissue becomes a major site of aromatase expression in post-menopausal women (24, 25). The principal product of ovarian granulosa cells during the follicular phase of the menstrual cycle is estradiol. In adipose tissue a weaker estrogen, estrone, is produced from androstenedione of adrenal origin in relatively large quantities. At least half of this estrone is eventually converted to estradiol in extra-ovarian tissues (26).
In the ovary, the biologically active estradiol is produced from cholesterol through serial enzymatic actions in two cell types, namely the theca and granulosa cells, which cooperate in a paracrine fashion (Figure 1) (1, 17). There are two rate-limiting steps in this process: entry of cholesterol into the mitochondria of theca cells, regulated by steroidogenic acute regulatory (STAR) protein; and conversion of androstenedione to estrone by aromatase in granulosa cells (Figure 1). Thus, targeting this final step in estradiol production using selective inhibitors effectively eliminates estrogen biosynthesis (17).
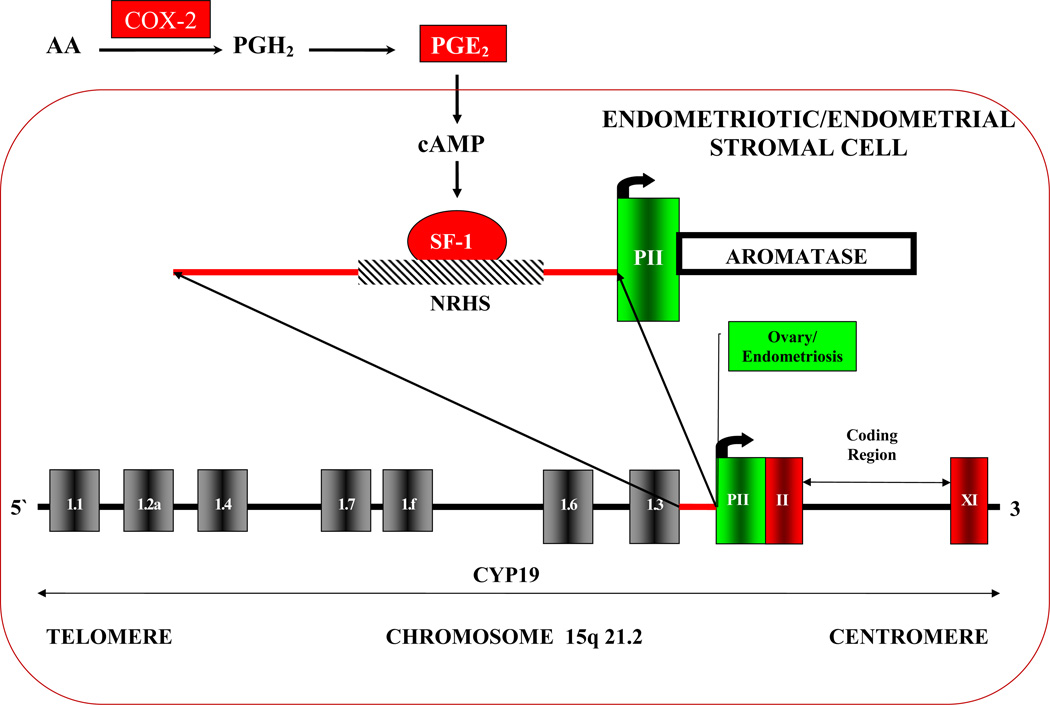
As previously mentioned, estrogen production via aromatase also occurs in tissues throughout the body. In humans, transcription of the aromatase gene is highly regulated under the control of alternatively used, tissue-specific promoters. There are at least 10 distinct promoters in the aromatase gene regulating its transcription (Figure 2) (17, 27). There is a tissue-and-hormone specific activation of promoters via alternative splicing that gives rise to aromatase species with variable first exons but identical coding regions. For example, the adipose tissue uses alternate exon I.3 and I.4 while the brain uses I.f (17, 21). Enhancers can react with upstream elements of these alternate exons to stimulate the rate of transcription of the aromatase gene. Therefore, aromatase expression is highly regulated in a very tissue-specific method.
Figure 2.
The aromatase gene with its promoters. There are at least 10 distinct promoters in the aromatase gene that regulate its transcription. The aromatase gene is transcribed from the telomere to the centromere. The first exon of the aromatase gene is transcribed into the aromatase message but not translated into protein. The translation start site is located in coding exon II. There is a tissue-and-hormone specific activation of promoters via alternative splicing that gives rise to aromatase species with variable first exons but identical coding regions. Endometriotic tissues, both extra-ovarian as well as ovarian endometriomas almost exclusively use Promoter II (PII) for transcription of the aromatase gene. PII is responsive to PGE2 and cAMP. cAMP induces binding of SF-1 to PII of the aromatase gene which starts its transcription.
Endometriotic tissues, both extraovarian as well as ovarian endometriomas, have been shown to almost exclusively use promoter II (labeled PII in Figure 2), which is the proximal promoter responsive to prostaglandin E2 (PGE2) and cyclic adenosine monophosphate to express aromatase. Thus, PII is the likely mediator of abnormal aromatase expression in these endometriotic tissues (17, 21).
In addition, there are other molecular differences between normal endometrium and endometriotic tissues contributing to abnormal exposure to estrogens. More subtle abnormalities also occur in the endometrium of women with endometriosis. As previously mentioned, both the eutopic endometrium of women with endometriosis as well as ectopic endometriotic lesions have been found to express STAR and aromatase (1). Steroidogenic factor 1 (SF1), a transcription factor that is expressed in endometriotic tissue but absent in endometrium, is integral for the expression of STAR and aromatase. SF1, whose expression depends on the presence of prostaglandin E2 in endometriotic cells, assembles enhancer transcriptional complexes, which then bind to the promoters of STAR and aromatase genes to induce their expression (1, 11, 28, 29). Additionally, in normal endometrium, progesterone acts on stromal cells to induce secretion of paracrine factors that act on neighboring epithelial cells to induce the expression of the enzyme 17 beta-hydroxysteroid dehydrogenase type 2 (HSD17B2). This enzyme catalyzes the conversion of E2 to estrone, a less biologically-active estrogen. In endometriotic tissue, progesterone does not induce epithelial HSD17B2 expression due to a defect in stromal cells (30–32). The end result is deficient metabolism of E2 in endometriosis giving rise to high local concentrations of estradiol. Thus, in endometriotic tissues, there is both an overproduction of estradiol and an aberrant conversion to a less biologically active estrogen. In addition, inflammatory and immune responses, angiogenesis, and apoptosis are all altered to favor survival pathways in endometriotic tissue via various mechanisms that are beyond the scope of this review (1).
AROMATASE INHIBITORS: PHARMACOLOGY
Aromatase inhibitors cause a decrease in estrogen concentration, making them useful for treating estrogen-dependent conditions including endometriosis. The aromatase enzyme complex itself is comprised of two polypeptides. The first is aromatase cytochrome P450 (CYP450arom) which is the product of a single gene, CYP19. The second is a flavoprotein, NADPH-cytochrome P450 reductase, and is ubiquitously distributed in most cells (17, 33). There have been three generations of aromatase inhibitors (AI) (Table 1). The first generation inhibitor, glutethimide, induces a medical adrenalectomy, which, in addition to this desired effect, causes many side effects including lethargy, skin rashes and nausea. The second generation inhibitors include fadrozole and formestancel which are more selective and have fewer side effects. The route of administration for these medications is intramuscular. The third generation aromatase inhibitors, including letrozole, anastrazole, and examestande, are triazole derivatives, which are selective, reversible, and potent, making them ideal for use in clinical practice. When these compounds are administered to premenopausal women, the subsequent decrease in estrogen levels causes an increase in FSH secretion from the pituitary gland. This increase in FSH stimulates follicular development (34). At doses of 1–5mg, letrozole and anastrazole inhibit estrogen levels by at least 97–99% (33).
Table 1.
The 3 generations of aromatase inhibitors.
| Generation | Aromatase Inhibitor |
|---|---|
| First Generation | Aminoglutethimide |
| Second Generation | Fadrozol, formestane |
| Third Generation | Letrozole, anastrazole, examestane |
SIDE EFFECTS OF AI
Most side effects associated with the use of third generation AI (e.g. letrozole, anastrazole) are relatively benign, with mild headache, joint stiffness or pain, nausea, and diarrhea as the most common. When compared to GnRH analogues, hot flashes are milder and more infrequent (33, 35). Long-term use may place women at increased risk for developing bone fractures, osteopenia, and osteoporosis. Most of these long-term studies have been done in women using an AI as adjuvant therapy for hormone-receptor positive breast cancer. Fracture rates in patients treated with AI have ranged from 2.5% in one study to 11% in the ATAC (Anastrazole or Tamoxifen Alone or in Combination) study (35, 36).
A number of studies have shown that AI-induced bone loss can be averted or improved by the concomitant use of bisphosphonates (37–41). Because bisphosphonates are not recommended in premenopausal women, studies have also been done looking at add back with progestins and oral contraceptive pills. These studies, which combine AI with either norethindrone acetate (in addition to calcium and vitamin D) or oral contraceptive pills, have shown no significant changes in bone mineral density (BMD) during their use (20, 42). However, a trial using the combination of the GnRH agonist goserelin plus the AI anastrazole showed significant bone loss after six months of treatment (43). It was noted that the observed BMD loss was significantly greater in the goserelin plus anastrazole arm as compared to the goserelin-alone arm and that this effect persisted even after cessation of treatment. However, none of these patients became osteopenic or osteoporotic.
Other side effects include hot flushes and hot flashes, headache, back pain, leg cramps, and arthralgia. Most of these side effects occur with prolonged use (33).
AROMATASE INHIBITORS FOR TREATMENT OF ENDOMETRIOSIS
Based on the molecular observations of increased expression of aromatase P450 in endometriotic tissues, aromatase inhibitors have been used to treat pain associated with endometriosis. Because of the increase in FSH and subsequent follicular development that occurs in premenopausal women, treatment with AI must be combined with additional agents to down regulate the ovaries (34, 44, 45). Vaginal administration of an AI alone has been examined in only one small study with mixed results (46).
COMBINATION OF AN AROMATASE INHIBITOR WITH PROGESTERONE OR PROGESTIN
Several studies have shown favorable results using a combination of AI and a progestin in reproductive-aged women. One of the first studies by Ailawadi et al. was an open-label, phase two nonrandomized prospective pilot study examining 10 women with endometriosis and chronic pelvic pain that persisted after surgical and medical treatment (42). Each patient underwent a diagnostic laparoscopy to confirm the diagnosis of endometriosis and establish the ASRM disease stage. Letrozole 2.5mg/day, norethindrone acetate 2.5mg/day, calcium and vitamin D were given daily for six months. One to two months following treatment, a second-look laparoscopy was performed and the endometriotic lesions were scored and biopsied. This study found that after treatment with letrozole and norethindrone acetate, no histologic evidence of endometriosis was present in any patient. In addition, ASRM and pelvic pain scores significantly decreased in response to treatment, while there was no significant change in bone density, gonadotropin level or circulating E2 and E1 levels (42). A case report using anastrazole 1mg/day and oral progesterone 200mg/day for six months in two reproductive aged women found rapid and progressive decrease in symptoms over three months. Symptoms remained in remission for greater than 24 months after treatment in both cases (47).
A subsequent retrospective study by Abushahin et al. treated 16 women with endometriosis who failed conventional medical and/or surgical therapies with letrozole 2.5mg.day and norethindrone acetate 2.5mg/day (n=14) or letrozole 2.5mg/day and oral contraceptive pills (OCP’s) for an average of six months. The study found that treatment with letrozole significantly improved patients’ pain scores during the course of treatment, with pain recurrence after treatment was completed (48).
Remorgida et al. examined letrozole 2.5mg/day and norethisterone acetate 2.5mg/day for the treatment of colorectal endometriosis. A prospective pilot study of 12 reproductive aged women with pain symptoms including dysmenorrhea, deep dyspareunia and/or chronic pelvic pain that persisted or recurred after one or more previous medical treatments were treated with 2.5mg letrozole and 2.5mg norethisterone acetate plus calcium and vitamin D for six months. The authors found that the intensity of all pain symptoms was significantly lower than at baseline, but that symptoms quickly returned once treatment ceased (49). A subsequent study by the same group of researchers of six women with colorectal endometriosis found that the combined drug regiment with an AI plus norethisterone acetate for six months decreased pain, non-menstrual pelvic pain, deep dyspareunia, dyschezia, and other symptoms mimicking diarrhea-predominant irritable bowel syndrome, with 67% of patients stating that treatment alleviated their GI symptoms (50). These researchers also published a prospective, open-labeled, non-randomized trial of 82 women with pain caused by rectovaginal endometriosis, in which treatment with letrozole 2.5mg/day and norethisterone acetate 2.5mg/day was compared to treatment with norethisterone acetate 2.5 mg/day alone. The authors found that the intensity of chronic pelvic pain and deep dyspareunia was significantly lower using combination therapy compared to mono-therapy with norethisterone acetate alone. However, pain symptoms recurred in both groups after completion of therapy and by six months, there was no difference in the intensity of pain symptoms between the groups (51). In addition, adverse effects were more common in the group treated with letrozole.
Ferrero et al. also randomized 35 women with rectovaginal endometriosis to treatment with letrozole 2.5mg/day and either oral noresthisterone acetate 2.5mg/day or IM triptorelin, a GnRH agonist 11.25mg every three months for six months, and examined pain severity, volume of endometriotic nodules as determined by ultrasonography and virtual organ computer-aided analysis, and adverse effects (23). This group found that both non-menstrual pelvic pain and deep dyspareunia decreased in both study groups. There was a significantly greater reduction in the volume of endometriotic nodules in women treated with AI and triptorelin. However, there was a lower incidence of adverse effects and a lower discontinuation rate when letrozole was combined with oral norethisterone acetate. In addition, mineral bone density was significantly decreased in the AI plus triptorelin group, but not in the AI plus norethisterone acetate group (23).
Remorgida et al. conducted a small open-label prospective study of 12 women with endometriosis-related pain refractory to previous medical and surgical treatments. In this study, all women had laparoscopy documenting stage IV disease. Patients were started on letrozole 2.5mg, desogestrel 75µg as well as calcium and vitamin D. Although patients were supposed to undergo treatment for six months, none of the women could complete the six-month therapy because all developed functional ovarian cysts. The medial length of treatment was 84 days. At interruption of treatment, all women reported significant improvements in dysmenorrhea and dyspareunia. By three months following treatment, recurred (44).
In summary, the combination of AI and progesterone or a progestin may decrease pain and reduce the amount of visible endometriotic lesions. However, the remission in symptoms may not continue beyond the time that treatment is given.
COMBINATION OF AN AROMATASE INHIBITOR WITH COMBINED ORAL CONTRACEPTIVES
Amsterdam et al. published a phase two prospective open-labeled trial involving 15 premenopausal women with documented refractory endometriosis and chronic pelvic pain. In this study, women were given 1mg anastrazole and one tablet of 20µg ethinyl estradiol/0/1mg levonorgestrel daily for six months (20). Pelvic pain, side effects, blood counts, liver and renal function tests, cholesterol levels, and bone density were measured. Fourteen out of 15 patients noted a significant reduction in pain, with average pain scored dropping after only 1 month of treatment. Estradiol levels were significantly suppressed in all patients. No adverse effects on blood counts, liver function, renal function, cholesterol, or bone density were noted and only mild side effects were experienced by participants. This study concluded that combining anastrazole with an oral contraceptive may prove to be useful for the treatment of refractory endometriosis (20).
Lall Seal et al. treated 5 reproductive-aged women with recurrent endometriomas and chronic pelvic pain, who had previously been treated with surgery and hormonal medications with unsatisfactory results, using letrozole 2.5mg desogestrel 0.15mg, ethinyl estradiol 0.03mg and calcium and vitamin D for six months (52). This group found by ultrasound that all women had a disappearance of ovarian endometriomas, with a decrease in size noted by three months of treatment. All women had a reduction in pelvic pain, with pain scores decreasing after one month of treatment. No significant changes in BMD were noted (52).
Thus, the combination of AI with a combined oral contraceptive may alleviate endometriosis-related pelvic pain and decrease the size of ovarian endometriomas without affecting BMD. Further randomized studies should be done to confirm these results.
COMBINATION OF AN AROMATASE INHIBITOR WITH A GnRH-ANALOGUE
A prospective randomized trial was done by Soysal et al. of 80 women to evaluate the efficacy of using either a combination of anastrazole and goserelin, or goserelin alone for six months, after conservative surgery for severe endometriosis (43). Patients were randomized to receive a combination of anastrazole 1 mg/day plus subcutaneous depot injections of 3.6mg goserelin every 4 weeks or goserelin plus a placebo tablet for the same amount of time. Patients were treated for 24 weeks and evaluated at 6, 12, 18, and 24 months after the end of medical treatment. The primary outcomes of this trial were the recurrence rate and impact of allocated treatments on Total Pelvic Symptom Score (TPSS) during the follow-up period of 24 months after the end of medical treatment. Other outcome measures examined were the impact of allocated treatment regimens on menopausal quality of life and on lumbar spine bone mineral density (43).
Both treatment protocols proved to be statistically effective in reducing the TPSS during the study period (43). However, the authors found a statistically significant advantage of goserelin plus anastrazole as compared to goserelin only in terms of median time to detect symptom recurrence. In addition, three cases out of 40 recurred in the goserelin plus anastrazole arm (7.5%), whereas 14 cases of 40 cases recurred in the goserelin only group during the follow up period of 24 months (43).
The authors found that goserelin plus anastrazole did lower E2 concentrations significantly more than goserelin alone. Menopausal quality of life surveys showed no statistically significant differences, which may indicate that this lower E2 level did not cause more climacteric symptoms. Goserelin plus anastrazole did show a greater bone loss at the spine at the completion of six months of therapy. However, by 24 months after therapy, no significant difference was noted (43). Thus, AIs in combination with GnRH analogues have been shown to increase pain-free interval and decrease symptom recurrence rate following surgery in premenopausal women.
AROMATASE INHIBITORS FOR THE TREATMENT OF POST-MENOPAUSAL ENDOMETRIOSIS
Endometriosis in postmenopausal women is a rare condition. Endometriosis is always estrogen-dependent. In premenopausal women, the ovaries are the main source of estrogen production, while in postmenopausal women estrogens are derived either from extra-ovarian production or from exogenous administration. There have been reports linking hormone replacement therapy with postmenopausal endometriosis (33, 53, 54). However, most estrogen production in postmenopausal women originates from extra-ovarian sources including adipose tissue, skin, and the adrenal gland. Adipose tissue likely accounts for the majority of postmenopausal estrogen production via aromatization of androgens produced from the adrenal gland (55).
Treatment for postmenopausal endometriosis should be surgical because there is a potential for malignancy or malignant transformation (56, 57). However, there are recurrences of endometriosis following surgical resection, and some patients may not be candidates for surgery. Therefore, there is a need for medical therapies. Treatments with GnRH analogues, progestins, and danazol have not been as effective for treatment of postmenopausal endometriosis (33). Because of their ability to block extra-ovarian estrogen production, AIs have been used to treat postmenopausal endometriosis.
There have been several case reports published successfully using AIs in postmenopausal women with endometriosis (33, 55, 58–62). All patients had undergone either surgical or natural menopause, with several patients having been exposed to hormone replacement therapy. Most women were previously treated for endometriosis with either surgery, GnRH agonists, or progestins.
In these case reports, administration of either letrozole or anastrazole for 4–18 months improved endometriosis related pain. Subjective symptoms decreased and quantitative parameters, including endometriotic lesion size (by physical exam findings or imaging techniques), were also reduced. Only one patient reported hot flushes (59). Co-administration of bisphosphonates was given in two patients (55, 58), and one reported letrozole associated bone loss, with a slight reduction of BMD after 9 months of anastrazole treatment (55). Although data is limited, AIs may be a promising new therapy for the treatment of postmenopausal endometriosis.
PREGNANCY FOLLOWING AROMATASE INHIBITOR TREATMENT FOR ENDOMETRIOSIS
A prospective randomized trial of 144 infertile patients with laparoscopic and histologic diagnosis of endometriosis was published by Alborzi et al. (63). In this study, patients underwent laparoscopic surgery to diagnose and treat endometriosis. Patients were then randomized to receiving letrozole 2.5mg/day for two months, triptorelin 3.75mg IM every four weeks for two months or no medication for two months. The authors found no statistically significant differences in pregnancy rates between the three groups (23.5% in the letrozole group, 27.5% in the triptorelin group, 28.1% in the no medication group). In addition, there was no significant difference in rates of recurrence for endometriosis, although recurrence was based on patient complaints and sonographic evidence, not laparoscopic evaluation. The rate of functional cyst development was significantly higher in the letrozole treated women (63). Additional studies investigating pregnancy rates and pregnancy outcomes following treatment for endometriosis using an AI are needed.
SUMMARY
Aromatase inhibitors may be effective in alleviating endometriosis-related chronic pelvic pain (Table 4). Treating chronic pelvic pain caused by endometriosis is often challenging for patients and physicians. Both the growth and survival of endometriotic tissue relies on estrogens. Conventional treatment strategies for endometriosis target ovarian E2 production but have little effect on estrogens produced from other sources. AIs target extraovarian E2 production but in doing so stimulate ovarian E2 production by causing an increase in FSH. Therefore, combining AI with conventional therapies should effectively block both ovarian and extraovarian E2 production and be effective in treating endometriosis. The studies reviewed have demonstrated that in reproductive-aged women, the combination of an AI with conventional therapy does alleviate endometriosis related pain. In postmenopausal women, using an AI alone has been shown to be an effective treatment, although more studies are needed in this subgroup. Side effects using AIs appear to be tolerable in most women, although special consideration should be given to monitoring BMD. More studies need to be done examining pregnancy rates and outcomes following aromatase inhibitor treatment for endometriosis. In addition, larger multi-center randomized trials using aromatase inhibitors for the treatment of endometriosis related chronic pelvic pain need to be done. In summary, aromatase inhibitors may be effective in treating endometriosis related chronic pelvic pain in both reproductive-aged and postmenopausal women.
Table 2.
A summary of current clinical reports using aromatase inhibitors in patients with endometriosis
| STUDY (YEAR) |
STUDY TYPE | SAMPLE SIZE | INDICATION | INTERVENTION | TREATMENT TIME |
OUTCOME |
|---|---|---|---|---|---|---|
| Hefler et al. (2005) | Prospective | 10 | Premenopausal rectovaginal endometriosis | Vaginal anastrazole | 6 mths | Improvement in dysmenorrhea, physical and social functioning; no improvement in pelvic pain and dyspareunia |
| Ailawadi et al. (2004) | Prospective | 10 | Premenopausal endometriosis, refractory to surgical or medical management | Letrozole + Norethindrone acetate | 6 mths | Pain relief; reduced lesion size |
| Aubushahin et al (2011) | Retrospective | 16 | Premenopausal endometrosis, failed conventional medical and/or surgical therapies | Letrozole + northindrone acetate (n=14) or Letrozole + OCP (n=2) | 6 mths | Pain relief; pain recurred once treatment stopped |
| Remorgida et al. (2007) | Prospective | 12 | Premenopausal with colorectal endometriosis, failed conventional medical treatments | Letrozole +norethisterone acetate | 6 mths | Pain relief; pain recurred once treatment stopped |
| Remorgida et al. (2007) | Prospective | 12 | Premenopausal with Stage 4 endometriosis, refractory to medical and/or surgical therapies | Letrozole + desogestrel | Median 84 d (range 56-112d) | Pain relief; development of ovarian cysts |
| Ferrero et al. (2010) | Prospective | 6 | Premenopausal with colorectal endometriosis, pain and GI symptoms | Letrozole + norethisterone acetate | 6 mths | Pain relief; GI symptom improvement in 67% |
| Ferrero et al. (2009) | Prospective, non-randomized | 82 | Premenopausal with pain caused by colorectal endometriosis | Letrozole+ norethisterone acetate vs norethisterone acetate alone | 6 mths | Improvement in chronic pelvic pain and deep dyspareunia significantly lower in combination group; pain symptoms recurred once treatment stopped |
| Ferrero et al. (2011) | RCT | 35 | Premenopausal with pain caused by rectovaginal endometriosis | Letrozole+ norethisterone acetate vs letrozole + triptorelin | 6 mths | Improvement in pelvic pain and deep dyspareunia; greater reduction in endometriotic nodules with AI + triptorelin; lower discontinuation rate, less side effects, no change in BMD in AI+norethisterone acetate |
| Amsterdam et al. (2005) | Prospective | 15 | Premenopausal endometrosis, failed conventional medical and/or surgical therapies | Anastrazole + OCP | 6 mths | Improvement in pain in 14 patients |
| Lall Seal et al. (2010) | Prospective | 5 | Premenopausal with chronic pelvic pain, recurrent endometriomas, refractory to medical and surgical treatments | Letrozole + OCP | 6 mths | Improvement in pain; disappearance of ovarian endometrioma |
| Sosyal et al. (2005) | RCT | 80 | Premenopausal endometriosis, s/p conservative surgery | Anastrazole + goserelin vs goserelin | 6 mths | Improvement in pelvic pain |
| Takayama et al. (1998) | Case report | 1 | Post-menopausal endometriosis, refractory to surgical or medical management | Anastrozole | 9 mths | pain relief, reduction in lesion size |
| Fatemi et al. (2005) | Case report | 1 | Post-menopausal endometrioma causing sciatic-like pain | Letrozole | 18 mths | Regression of endometrioma, pain relief |
| Razzi et al. (2004) | Case report | 1 | Post-menopausal endometriosis, refractory to surgical or medical management | Letrozole | 9 mths | pain relief, reduction in lesion size |
| Mousa et al. (2007) | Case report | 1 | Recurrent endometriotic nodule in bladder wall in post-menopausal woman | Letrozole | 8 mths | Pain relief; improvement in urinary symptoms |
| Bohrer et al. (2008) | Case report | 1 | Recurrent ureteral and bowel endometriosis in a post-menopausal woman | Anastrazole | 15 mths | Pain relief; resolution of bowel symptoms |
| Sasson and Taylor (2009) | Case report | 1 | Recurrent abdominal wall endometrioma in a post-menopausal woman | Letrozole + MPA + serial cyst aspirations | 34 d | Decrease in size of cystic mass |
| Alborzi et al. (2011) | RCT | 144 | Premenopausal endometriosis and infertility | Laparoscopy followed by Letrozole vs triptorelin vs nothing | 2 mths | Pregnancy rate and endometriosis recurrence similar in all groups |
Acknowledgments
FUNDING: Supported by K12HD050121, ASRM career development award (to MEP); R37HD038691 (to SEB)
The authors would like to thank Santino Pavone, Irene Pavone, and Christopher J. Novak for their critical review of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors report no conflicts of interest.
References
- 1.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 2.Giudice LC. Clinical practice. Endometriosis. The New England journal of medicine. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucidi RS, Witz CA, Chrisco M, Binkley PA, Shain SA, Schenken RS. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertility and sterility. 2005;84:16–21. doi: 10.1016/j.fertnstert.2004.10.058. [DOI] [PubMed] [Google Scholar]
- 4.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 5.Bulun SE, Cheng YH, Pavone ME, Xue Q, Attar E, Trukhacheva E, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Seminars in reproductive medicine. 2010;28:36–43. doi: 10.1055/s-0029-1242991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith SF, Roberts NJ, Partridge MR. Comparison of a web-based package with tutor based methods of teaching respiratory medicine: subjective and objective evaluations. BMC Med Educ. 2007;7:41. doi: 10.1186/1472-6920-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu MH, Sun HS, Lin CC, Hsiao KY, Chuang PC, Pan HA, et al. Distinct mechanisms regulate cyclooxygenase-1 and -2 in peritoneal macrophages of women with and without endometriosis. Mol Hum Reprod. 2002;8:1103–1110. doi: 10.1093/molehr/8.12.1103. [DOI] [PubMed] [Google Scholar]
- 8.Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–4183. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal VR, Ashanullah CI, Simpson ER, Bulun SE. Alternatively spliced transcripts of the aromatase cytochrome P450 (CYP19) gene in adipose tissue of women. The Journal of clinical endocrinology and metabolism. 1997;82:70–74. doi: 10.1210/jcem.82.1.3655. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal VR, Bulun SE, Leitch M, Rohrich R, Simpson ER. Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients. The Journal of clinical endocrinology and metabolism. 1996;81:3843–3849. doi: 10.1210/jcem.81.11.8923826. [DOI] [PubMed] [Google Scholar]
- 11.Attar E, Tokunaga H, Imir G, Yilmaz MB, Redwine D, Putman M, et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. The Journal of clinical endocrinology and metabolism. 2009;94:623–631. doi: 10.1210/jc.2008-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulun SE. Aromatase deficiency and estrogen resistance: from molecular genetics to clinic. Seminars in reproductive medicine. 2000;18:31–39. doi: 10.1055/s-2000-13481. [DOI] [PubMed] [Google Scholar]
- 13.Fang Z, Yang S, Gurates B, Tamura M, Simpson E, Evans D, et al. Genetic or enzymatic disruption of aromatase inhibits the growth of ectopic uterine tissue. The Journal of clinical endocrinology and metabolism. 2002;87:3460–3466. doi: 10.1210/jcem.87.7.8683. [DOI] [PubMed] [Google Scholar]
- 14.Fazleabas AT, Brudney A, Chai D, Langoi D, Bulun SE. Steroid receptor and aromatase expression in baboon endometriotic lesions. Fertility and sterility. 2003;80(Suppl 2):820–827. doi: 10.1016/s0015-0282(03)00982-8. [DOI] [PubMed] [Google Scholar]
- 15.Simpson ER, Mahendroo MS, Nichols JE, Bulun SE. Aromatase gene expression in adipose tissue: relationship to breast cancer. Int J Fertil Menopausal Stud. 1994;39(Suppl 2):75–83. [PubMed] [Google Scholar]
- 16.Simpson ER, Zhao Y, Agarwal VR, Michael MD, Bulun SE, Hinshelwood M, et al. Aromatase expression in health and disease. Recent Prog Horm Res. 1997;52:185–213. discussion-4. [PubMed] [Google Scholar]
- 17.Attar E, Bulun SE. Aromatase and other steroidogenic genes in endometriosis: translational aspects. Hum Reprod Update. 2006;12:49–56. doi: 10.1093/humupd/dmi034. [DOI] [PubMed] [Google Scholar]
- 18.Kitawaki J, Noguchi T, Amatsu T, Maeda K, Tsukamoto K, Yamamoto T, et al. Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biology of reproduction. 1997;57:514–519. doi: 10.1095/biolreprod57.3.514. [DOI] [PubMed] [Google Scholar]
- 19.Bulun SE, Imir G, Utsunomiya H, Thung S, Gurates B, Tamura M, et al. Aromatase in endometriosis and uterine leiomyomata. J Steroid Biochem Mol Biol. 2005;95:57–62. doi: 10.1016/j.jsbmb.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Amsterdam LL, Gentry W, Jobanputra S, Wolf M, Rubin SD, Bulun SE. Anastrazole and oral contraceptives: a novel treatment for endometriosis. Fertility and sterility. 2005;84:300–304. doi: 10.1016/j.fertnstert.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Attar E, Bulun SE. Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertility and sterility. 2006;85:1307–1318. doi: 10.1016/j.fertnstert.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 22.Bulun SE, Fang Z, Imir G, Gurates B, Tamura M, Yilmaz B, et al. Aromatase and endometriosis. Seminars in reproductive medicine. 2004;22:45–50. doi: 10.1055/s-2004-823026. [DOI] [PubMed] [Google Scholar]
- 23.Ferrero S, Venturini PL, Gillott DJ, Remorgida V. Letrozole and norethisterone acetate versus letrozole and triptorelin in the treatment of endometriosis related pain symptoms: a randomized controlled trial. Reprod Biol Endocrinol. 2011;9:88. doi: 10.1186/1477-7827-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grodin JM, Siiteri PK, MacDonald PC. Source of estrogen production in postmenopausal women. The Journal of clinical endocrinology and metabolism. 1973;36:207–214. doi: 10.1210/jcem-36-2-207. [DOI] [PubMed] [Google Scholar]
- 25.Bulun SE, Mahendroo MS, Simpson ER. Aromatase gene expression in adipose tissue: relationship to breast cancer. J Steroid Biochem Mol Biol. 1994;49:319–326. doi: 10.1016/0960-0760(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald PC, Madden JD, Brenner PF, Wilson JD, Siiteri PK. Origin of estrogen in normal men and in women with testicular feminization. The Journal of clinical endocrinology and metabolism. 1979;49:905–916. doi: 10.1210/jcem-49-6-905. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Pearson EK, Brooks DC, Coon JSt, Chen D, Demura M, et al. A humanized pattern of aromatase expression is associated with mammary hyperplasia in mice. Endocrinology. 2012;153:2701–2713. doi: 10.1210/en.2011-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurates B, Sebastian S, Yang S, Zhou J, Tamura M, Fang Z, et al. WT1 and DAX-1 inhibit aromatase P450 expression in human endometrial and endometriotic stromal cells. The Journal of clinical endocrinology and metabolism. 2002;87:4369–4377. doi: 10.1210/jc.2002-020522. [DOI] [PubMed] [Google Scholar]
- 29.Bulun SE, Utsunomiya H, Lin Z, Yin P, Cheng YH, Pavone ME, et al. Steroidogenic factor-1 and endometriosis. Molecular and cellular endocrinology. 2009;300:104–108. doi: 10.1016/j.mce.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Bulun SE, Cheng YH, Pavone ME, Yin P, Imir G, Utsunomiya H, et al. 17Beta-hydroxysteroid dehydrogenase-2 deficiency and progesterone resistance in endometriosis. Seminars in reproductive medicine. 2010;28:44–50. doi: 10.1055/s-0029-1242992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng YH, Imir A, Fenkci V, Yilmaz MB, Bulun SE. Stromal cells of endometriosis fail to produce paracrine factors that induce epithelial 17beta-hydroxysteroid dehydrogenase type 2 gene and its transcriptional regulator Sp1: a mechanism for defective estradiol metabolism. Am J Obstet Gynecol. 2007;196(391):e1–e7. doi: 10.1016/j.ajog.2006.12.014. discussion e7-8. [DOI] [PubMed] [Google Scholar]
- 32.Cheng YH, Imir A, Suzuki T, Fenkci V, Yilmaz B, Sasano H, et al. SP1 and SP3 mediate progesterone-dependent induction of the 17beta hydroxysteroid dehydrogenase type 2 gene in human endometrium. Biology of reproduction. 2006;75:605–614. doi: 10.1095/biolreprod.106.051912. [DOI] [PubMed] [Google Scholar]
- 33.Polyzos NP, Fatemi HM, Zavos A, Grimbizis G, Kyrou D, Velasco J G, et al. Aromatase inhibitors in post-menopausal endometriosis. Reprod Biol Endocrinol. 2011;9:90. doi: 10.1186/1477-7827-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrero S, Venturini PL, Ragni N, Camerini G, Remorgida V. Pharmacological treatment of endometriosis: experience with aromatase inhibitors. Drugs. 2009;69:943–952. doi: 10.2165/00003495-200969080-00001. [DOI] [PubMed] [Google Scholar]
- 35.Buzdar AU. The ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial: an update. Clin Breast Cancer. 2004;5(Suppl 1):S6–S12. doi: 10.3816/cbc.2004.s.008. [DOI] [PubMed] [Google Scholar]
- 36.Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455–462. doi: 10.1016/S0140-6736(05)67059-6. [DOI] [PubMed] [Google Scholar]
- 37.Ligibel JA, O'Malley AJ, Fisher M, Daniel GW, Winer EP, Keating NL. Patterns of bone density evaluation in a community population treated with aromatase inhibitors. Breast Cancer Res Treat. 2012 doi: 10.1007/s10549-012-2151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llombart A, Frassoldati A, Paija O, Sleeboom HP, Jerusalem G, Mebis J, et al. Immediate Administration of Zoledronic Acid Reduces Aromatase Inhibitor-Associated Bone Loss in Postmenopausal Women With Early Breast Cancer-12-month analysis of the E-ZO-FAST trial. Clin Breast Cancer. 2012;12:40–48. doi: 10.1016/j.clbc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Eidtmann H, de Boer R, Bundred N, Llombart-Cussac A, Davidson N, Neven P, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole-36-month results of the ZO-FAST Study. Ann Oncol. 2010;21:2188–2194. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 40.Bundred NJ, Campbell ID, Davidson N, DeBoer RH, Eidtmann H, Monnier A, et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST Study results. Cancer. 2008;112:1001–1001. doi: 10.1002/cncr.23259. [DOI] [PubMed] [Google Scholar]
- 41.Brufsky AM, Harker WG, Beck JT, Bosserman L, Vogel C, Seidler C, et al. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118:1192–1201. doi: 10.1002/cncr.26313. [DOI] [PubMed] [Google Scholar]
- 42.Ailawadi RK, Jobanputra S, Kataria M, Gurates B, Bulun SE. Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate: a pilot study. Fertility and sterility. 2004;81:290–296. doi: 10.1016/j.fertnstert.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 43.Soysal S, Soysal ME, Ozer S, Gul N, Gezgin T. The effects of post-surgical administration of goserelin plus anastrozole compared to goserelin alone in patients with severe endometriosis: a prospective randomized trial. Hum Reprod. 2004;19:160–167. doi: 10.1093/humrep/deh035. [DOI] [PubMed] [Google Scholar]
- 44.Remorgida V, Abbamonte LH, Ragni N, Fulcheri E, Ferrero S. Letrozole and desogestrel-only contraceptive pill for the treatment of stage IV endometriosis. Aust N Z J Obstet Gynaecol. 2007;47:222–225. doi: 10.1111/j.1479-828X.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 45.Legro RS, Kunselman AR, Brzyski RG, Casson PR, Diamond MP, Schlaff W D, et al. The Pregnancy in Polycystic Ovary Syndrome II (PPCOS II) trial: rationale and design of a double535 blind randomized trial of clomiphene citrate and letrozole for the treatment of infertility in women with polycystic ovary syndrome. Contemp Clin Trials. 2012;33:470–481. doi: 10.1016/j.cct.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hefler LA, Grimm C, van Trotsenburg M, Nagele F. Role of the vaginally administered aromatase inhibitor anastrozole in women with rectovaginal endometriosis: a pilot study. Fertility and sterility. 2005;84:1033–1036. doi: 10.1016/j.fertnstert.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 47.Shippen ER, West WJ., Jr Successful treatment of severe endometriosis in two premenopausal women with an aromatase inhibitor. Fertility and sterility. 2004;81:1395–1398. doi: 10.1016/j.fertnstert.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 48.Abushahin F, Goldman KN, Barbieri E, Milad M, Rademaker A, Bulun SE. Aromatase inhibition for refractory endometriosis-related chronic pelvic pain. Fertility and sterility. 2011;96:939–942. doi: 10.1016/j.fertnstert.2011.07.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Remorgida V, Abbamonte HL, Ragni N, Fulcheri E, Ferrero S. Letrozole and norethisterone acetate in rectovaginal endometriosis. Fertility and sterility. 2007;88:724–726. doi: 10.1016/j.fertnstert.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 50.Ferrero S, Camerini G, Ragni N, Venturini PL, Biscaldi E, Seracchioli R, et al. Letrozole and norethisterone acetate in colorectal endometriosis. Eur J Obstet Gynecol Reprod Biol. 2010;150:199–202. doi: 10.1016/j.ejogrb.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 51.Ferrero S, Camerini G, Seracchioli R, Ragni N, Venturini PL, Remorgida V. Letrozole combined with norethisterone acetate compared with norethisterone acetate alone in the treatment of pain symptoms caused by endometriosis. Hum Reprod. 2009;24:3033–3041. doi: 10.1093/humrep/dep302. [DOI] [PubMed] [Google Scholar]
- 52.Lall Seal S, Kamilya G, Mukherji J, De A, Ghosh D, Majhi AK. Aromatase inhibitors in recurrent ovarian endometriomas: report of five cases with literature review. Fertility and sterility. 2011;95(291):e15, e18. doi: 10.1016/j.fertnstert.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 53.Goumenou AG, Chow C, Taylor A, Magos A. Endometriosis arising during estrogen and testosterone treatment 17 years after abdominal hysterectomy: a case report. Maturitas. 2003;46:239–241. doi: 10.1016/s0378-5122(03)00081-1. [DOI] [PubMed] [Google Scholar]
- 54.Sesti F, Vettraino G, Pietropolli A, Marziali M, Piccione E. Vesical and vaginal recurrent endometriosis in postmenopause following estrogen replacement therapy. Eur J Obstet Gynecol Reprod Biol. 2005;118:265–266. doi: 10.1016/j.ejogrb.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 55.Takayama K, Zeitoun K, Gunby RT, Sasano H, Carr BR, Bulun SE. Treatment of severe postmenopausal endometriosis with an aromatase inhibitor. Fertility and sterility. 1998;69:709–713. doi: 10.1016/s0015-0282(98)00022-3. [DOI] [PubMed] [Google Scholar]
- 56.Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. American journal of obstetrics and gynecology. 1997;176:572–579. doi: 10.1016/s0002-9378(97)70550-7. [DOI] [PubMed] [Google Scholar]
- 57.Borgfeldt C, Andolf E. Cancer risk after hospital discharge diagnosis of benign ovarian cysts and endometriosis. Acta Obstet Gynecol Scand. 2004;83:395–400. doi: 10.1111/j.0001-6349.2004.00305.x. [DOI] [PubMed] [Google Scholar]
- 58.Fatemi HM, Al-Turki HA, Papanikolaou EG, Kosmas L, De Sutter P, Devroey P. Successful treatment of an aggressive recurrent post-menopausal endometriosis with an aromatase inhibitor. Reprod Biomed Online. 2005;11:455–457. doi: 10.1016/s1472-6483(10)61140-6. [DOI] [PubMed] [Google Scholar]
- 59.Mousa NA, Bedaiwy MA, Casper RF. Aromatase inhibitors in the treatment of severe endometriosis. Obstet Gynecol. 2007;109:1421–1423. doi: 10.1097/01.AOG.0000265807.19397.6d. [DOI] [PubMed] [Google Scholar]
- 60.Bohrer J, Chen CC, Falcone T. Persistent bilateral ureteral obstruction secondary to endometriosis despite treatment with an aromatase inhibitor. Fertility and sterility. 2008;2004;90:e7–e9. doi: 10.1016/j.fertnstert.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 61.Sasson IE, Taylor HS. Aromatase inhibitor for treatment of a recurrent abdominal wall endometrioma in a postmenopausal woman. Fertility and sterility. 2009;92(1170):e1–e4. doi: 10.1016/j.fertnstert.2009.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Razzi S, Fava A, Sartini A, De Simone S, Cobellis L, Petraglia F. Treatment of severe recurrent endometriosis with an aromatase inhibitor in a young ovariectomised woman. Bjog. 2004;111:182–184. doi: 10.1046/j.1471-0528.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 63.Alborzi S, Hamedi B, Omidvar A, Dehbashi S, Alborzi M. A comparison of the effect of short-term aromatase inhibitor (letrozole) and GnRH agonist (triptorelin) versus case control on pregnancy rate and symptom and sign recurrence after laparoscopic treatment of endometriosis. Arch Gynecol Obstet. 2011;284:105–110. doi: 10.1007/s00404-010-1599-6. [DOI] [PubMed] [Google Scholar]