Abstract
Introduction
Alcohol dependence in aging populations is seen as a public health concern, most recently because of the significant proportion of heavy drinking among “Baby Boomers.” Basic animal research on the effects of aging on physiological and behavioral regulation of ethanol (EtOH) intake is sparse, since most of this research is limited to younger models of alcoholism. Here, EtOH drinking and preference were measured in groups of aged Syrian hamsters. Further, because voluntary exercise (wheel-running) is a rewarding substitute for EtOH in young adult hamsters, the potential for such reward substitution was also assessed.
Methods
Aged (24 month-old) male hamsters were subjected to a three-stage regimen of free-choice EtOH (20% v/v) or water and unlocked or locked running wheels to investigate the modulatory effects of voluntary wheel running on EtOH intake and preference. Levels of fluid intake and activity were recorded daily across 60 days of experimentation.
Results
Prior to wheel running, levels of EtOH intake were significantly less than levels of water intake, resulting in a low preference for EtOH (30%). Hamsters with access to an unlocked running wheel had decreased EtOH intake and preference compared with hamsters with access to a locked running wheel. These group differences in EtOH intake and preference were sustained for up to 10 days after running wheels were re-locked.
Discussion
These results extend upon those of our previous work in young adult hamsters, indicating that aging dampens EtOH intake and preference. Voluntary wheel running further limited EtOH intake, suggesting that exercise could offer a practical approach for managing late-life alcoholism.
INTRODUCTION
Chronic ethanol (EtOH) intake disrupts the regulation of physiological and behavioral processes, which can lead to a self-reinforcing cycle of EtOH dependence [1, 2, 3]. Like EtOH, aging can also perturb these regulatory processes [4, 5, 6]. Thus, aging could exacerbate the adverse effects of EtOH drinking on these processes [7, 8]. Further, high rates of late-life alcohol dependence compounded by the increasing longevity of aging populations are viewed as public health concerns [9, 10, 11]. In this regard, it is notable that little is known of the physiological effects of aging on EtOH intake and dependence, since most basic research on alcoholism is limited to young animals.
With the goal of developing therapeutic interventions for EtOH dependence, recent studies in humans and animal models suggest that aerobic exercise may offer a practical substitute for EtOH. Access to a running wheel, which is a rewarding stimulus [12,13], decreases EtOH intake in several young adult rodent models of alcoholism, including the C57BL/6J mouse [14], Syrian hamster [15], and the P (but not NP) rat [16]. Wheel running can also suppress cocaine and methamphetamine self-administration [17, 18]. In young adults with a family history of alcoholism, aerobic exercise can result in less frequent alcohol use and can decrease relapse risk [19, 20]. In the elderly, exercise can also improve general health and well-being [21], but the potential, therapeutic effects of daily exercise on alcohol intake are unknown.
Basic information on the physiological aspects of EtOH drinking and its related pathologies in aging populations is sparse. This is largely due to the difficulties inherent in assessing drinking problems in this population; the lack of standardized tests for assessing alcohol dependence in humans is problematic, as are the distortive effects of cognitive impairment associated with aging and long-term drinking on self-reported drinking practices [22]. Other confounds include geographic and socioeconomic differences between populations studied, as well as differences in the age range used to define an elderly population [22]. In view of these limitations, the use of inbred strains of laboratory animals is useful for reducing genetic and environmental variables to better understand the effects of aging on behavioral and physiological aspects of EtOH intake and preference. Our previous work has focused on the effects of EtOH on circadian behavioral processes and interactions between exercise and EtOH intake in young adult, male Syrian hamsters [15], owing, in part, to their high levels of EtOH intake and ~80% preference for an EtOH solution over pure water [15, 23, 24]. Here we extend this work to explore how voluntary exercise might modulate this first-time assessment of chronic EtOH intake in aged hamsters. This information would increase our understanding of age-related behavioral and physiological changes underlying EtOH dependence and possibly point to practical strategies for managing or preventing alcoholism in the elderly.
METHODS AND MATERIALS
2.1. Animals
Aged (24-month) male, Syrian hamsters (Mesocricetus auratus) raised from breeders purchased from Harlan Sprague-Dawley (Madison, IL) were individually housed in cages with a 14” running wheel in a temperature-controlled vivarium (23°C). Males were strictly used to reduce any endocrinal confounds on daily wheel running rhythms and EtOH intake as reported in females [25, 26]. Animals were maintained on a 14-hour light (light intensity: ~270 lux): 10-hour dark photocycle (LD). Food (Prolab 3000; PMI Feeds, St. Louis, MO) and water were provided ad libitum. The experiments were approved by the Kent State University Institutional Animal Care and Use Committee and were conducted under the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.2. Combined Effects of Aging and Exercise on EtOH Intake and Preference
This experiment was designed to test the modulatory effects of wheel-running on EtOH intake and preference in aged hamsters (n=7, experimental group; n=8, control group, due to one animal that died mid-experimentation). All animals received free-choice access to 20% EtOH (v/v; Sigma-Aldrich, St. Louis, MO) or water in 50 ml canonical tubes (Fisher Scientific, Pittsburgh, PA) across three experimental phases: Phase I: All running wheels were locked across the 10-day EtOH introductory period; Phase II: Running wheels were unlocked for the experimental group or remained locked for the control group across 30 days; Phase III: Running wheels were re-locked for the experimental group or remained locked for the control group across the final 20 days of the experiment. Schematic representation of this experimental protocol is shown in Fig. 1.
Figure 1.
This experimental protocol was designed to test the potential of voluntary wheel running to serve as a reward substitute for free-choice ethanol (EtOH) in 24-month old, male Syrian hamsters. Free-choice intake of a 20% EtOH solution or water was measured across three, separate experimental phases. Phase I: EtOH introductory period (all wheels locked; 10 days). Phase II: Wheels unlocked for the experimental group (30 days). Phase III: Wheels re-locked for the experimental group (20 days).
Wheels and overhead infrared sensors were interfaced to a Clocklab behavioral analysis system (Coulbourn Instruments, Whitehall, PA) to monitor nighttime levels and onsets of wheel running and general locomotor activity. Daily total distances run were calculated from revolutions per minute (RPMs). Nighttime onsets of general locomotor activity and wheel running were characterized as activity near the light-dark photocycle transition that: 1) exceeded 10% of the maximum rate for the day; 2) was preceded by at least 4 hr of activity quiescence; and 3) was followed by periods of sustained activity. Daily amounts of fluids consumed were measured at midday to the nearest 0.25 ml. Preference was calculated as the percentage of volume of EtOH solution consumed over total volume of fluid consumed.
2.3. Statistical Analyses
Repeated measures ANOVAs and subsequent Student Newman-Keuls post-hoc mean comparison tests were used for between-treatment statistical comparisons of fluid intake and EtOH preference. A one-way ANOVA was used for between-treatment statistical comparisons of nighttime general locomotor activity onset across experimentation. Paired t-tests were used for within-treatment statistical comparisons of fluid intake, EtOH preference, and distances run between phases I-III and the first, second, and third 10-day periods of phase II. A Pearson's correlation of mean fluid (EtOH or water) intake or EtOH preference against mean distance run across each 10-day period of phase II was also undertaken. All statistical analyses were completed with SPSS 19.0 (Chicago, IL). Significance level was set at p<0.05, in all cases.
RESULTS
3.1. Runners Have Lower Levels of EtOH Intake and Preference
Phase I (all wheels locked). Means±SE of EtOH intake and preference across the three-stage regimen of free-choice EtOH (20% v/v) or water are presented in Fig. 2. Means±SE of EtOH intake and preference for phase I represent stable levels of EtOH intake across the last 5 days. EtOH intake for the designated experimental (running) group averaged 11.4±0.4 g/kg/d with a 28.2±3.4% preference for the EtOH solution over water. EtOH intake for the designated control (non-running) group averaged 11.5±0.2 g/kg/d with a 30.3±1.1% EtOH preference. No between-group differences in fluid intake and EtOH preference were found (both, p>0.05). Phase II (wheels unlocked for the experimental group). Nighttime activity onset averaged 23.7±3.1 min after lights-off for the experimental group and 20.1±6.2 min after lights-off for the control group, with wheel running in the experimental group beginning 13.3±3.0 min after activity onset. Wheel running averaged 4.4±0.2 km/d. Further analysis revealed a decrease in wheel running from 4.9±0.4 km/d across the first 20 days of phase II to 3.5±0.3 km/d across the last 10 days of phase II (p<0.05; Fig. 3). Runners had reduced levels of free-choice EtOH intake (F1,13=9.0; p<0.05) and EtOH preference (F1,13=12.9; p<0.05) compared with non-runners, such that EtOH intake for the runners averaged 11.3±0.4 g/kg/d with a 26.3±2.1% EtOH preference and EtOH intake for the non-runners averaged 12.9±0.4 g/kg/d with a 34.4±2.2% EtOH preference. No between-group differences in water intake were found (p>0.05). Within-treatment comparisons revealed lower levels of EtOH intake and preference across the first 10 days of phase II compared with the remaining 20 days of phase II for both treatment groups (all, p<0.05). There were no detections of significant correlations between the extent of wheel running and fluid (EtOH or water) intake or EtOH preference across each 10 day period of access to an unlocked running wheel (all, p>0.05).
Figure 2.
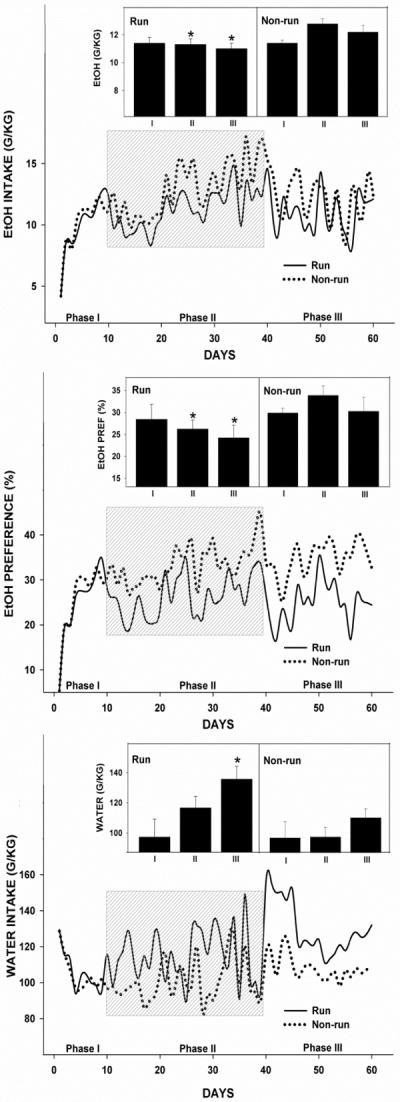
Wheel running limits ethanol (EtOH) intake and preference. Line graphs: Daily fluid (EtOH and water) intake and EtOH preference (calculated as the percentage of volume of EtOH solution consumed over total volume of fluid consumed) in the experimental (solid line) and control (dotted line) groups across three, separate phases. Phase I: free-choice EtOH introductory period (wheels locked). Phase II (shaded): Wheels were unlocked for the experimental group and locked for the control group. Phase III: All wheels locked. Bar graphs: Fluid (EtOH and water) intake and preference for runners and non-runners averaged over each phase. Bars represent means±SEM. Among all phases, bars with different letters are significantly different (p<0.05).
Figure 3.
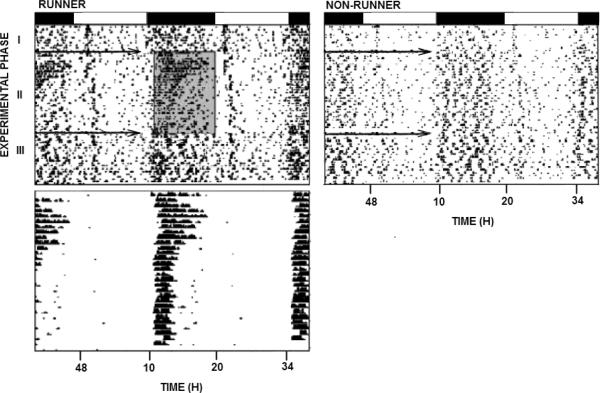
Representative, double-plotted actograms of general locomotor activity (top) and wheel running rhythms (bottom) across phases I–III in an experimental (running wheel unlocked; shaded region) and control (running wheel locked) 24-month old, male Syrian hamster. White-black bars represent the light (14 h)-dark (10 h) photoperiods.
Phase III (all wheels locked). EtOH intake and preference remained lower in the runners compared with non-runners (Intake: F1,13=4.1; Preference: F1,13=12.9; both, p<0.05; Fig. 2), such that EtOH intake for the runners averaged 11.0±0.4 g/kg/d with a 24.2±2.9% EtOH preference, and EtOH intake for the non-runners averaged 12.3±0.5 g/kg/d with a 30.7±3.3% EtOH preference. Water intake for the runners was higher vs. non-runners (135.7±7.7 g/kg/d vs. 100.9±6.1 g/kg/d, respectively; F1,13=6.2; p<0.05; Fig. 2). Between-phase comparisons revealed an increase in water intake for the runners across phase III compared with levels found across phases I (97.3±11.8 g/kg/d) and II (116.6±6.6 g/kg/d; both, p<0.05; Fig. 2).
DISCUSSION
EtOH and aging are independently disruptive to physiologic and behavioral rhythmic processes in mammals [27, 28, 29]. Thus, it is conceivable that the adverse effects of one of these processes could exacerbate the effects of the other. However, little is known about the effects of aging combined with environmental enrichment, such as wheel running, on EtOH intake, since most basic research on alcoholism is limited to studies in younger models. Nevertheless, young adult models of addiction have been useful to reveal the suppressive effects of voluntary exercise on reward intake and seeking, including that of EtOH, cocaine, and methamphetamine [14, 15, 17, 18]. This suppression is thought to manifest from the ability of exercise to offer an alternative neurochemical reward state [12, 13]. In aging individuals, exercise has been found to improve general health [21], but the direct, therapeutic benefits of exercise on alcohol intake, particularly for “Baby Boomers”; with their high rates of alcohol dependence are unknown [9, 10, 11]. Here, we report that the aging process dampens high levels of free-choice EtOH intake and preference found in young adult hamsters from Hammer et al. 2010 [15] by 20% and 50%, respectively. We also report that the aged hamsters with access to unlocked running wheels had lower levels of free-choice EtOH intake and preference compared with non-runners, and that these lower levels of EtOH intake and preference in runners persisted for up to 10 days after running wheels were re-locked.
4.1. Aging Reduces EtOH Intake
Compared with baseline levels of EtOH intake and preference in all treatment groups of young adult hamsters in Hammer et al. 2010 [15], which ranged from 15–17 g/kg/day with an 80% preference for the 20% EtOH solution over water, aged hamsters under the same experimental protocol in this study had a 20% reduction in free-choice EtOH intake concurrent with a nearly 400% increase in water intake and a subsequent 50% decrease in EtOH preference (data not shown, Hammer). Further, it is noteworthy that aging-related declines in EtOH intake and preference are not associated with any modulatory effect of daily wheel running on EtOH intake and preference in young adult vs. aged hamsters (4.3±0.3 km/d vs. 4.4±0.2 km/d; data not shown, Hammer).
To date, few studies have undertaken any long-term assessment of daily EtOH intake in aged animal models of alcoholism or have shown aging-related reductions in EtOH intake. However, there is prior evidence of differential sensitivities to the hypothermic, ataxic, and soporic properties of EtOH between young adult and aged rodent models of alcoholism [7, 8, 30], which can affect overall levels of subsequent EtOH intake independently of age [31, 32]. Daily profiles of systemic EtOH levels achieved with 24-hr of continuous microdialysis sampling were not measured in this study due to the higher risk of infection and poorer recovery from anesthesia with advanced aging. In a previous study, we have found that systemic EtOH concentrations consistently peak at 40 mM following a drinking episode in young adult Syrian hamsters consuming ~ 15 g/kg of EtOH per day with an 80% preference for a 20% EtOH solution over water [24]. Based on the findings of Ruby et al. 2009 [24], we predict daily systemic EtOH peaks to have been significantly lower in the aged Syrian hamsters in this study.
Previous studies that have independently investigated the underlying physiological mechanisms of elevated EtOH intake and the effects of aging on EtOH intake may also provide a physiological context for the present results. For example, it has been shown that EtOH intake and craving are tightly gated by central ghrelin signaling; upregulation of central ghrelin signaling in the ventral tegmental area increases EtOH intake and its reward threshold. In contrast, knockdown or antagonism of central ghrelin signaling decreases EtOH intake and its reward threshold [33]. It has also been shown that aging and vigorous exercise can independently dampen central ghrelin signaling [34, 35], which could, hypothetically, suppress EtOH intake and its reward threshold. An aging-related decline in central reward dopamingeric tonus, including a decrease in the number of dopamine receptors, a decline in receptor binding affinities, and enhanced degradation of dopamine [36, 37], could also, hypothetically, have contributed to the reduction in EtOH intake in the aged hamsters compared with the young adults of Hammer et al. 2010 [15] through a reconstitution of dopamine homeostasis. Thus, future studies ought to examine the physiological mechanisms that underlie the reported aging- and exercise-related reductions in EtOH intake, including changes in central neuroendocrine processes and dopaminergic tonus. Further, it would be notable to quantify aging- and exercise-related influences on EtOH reward through experimental paradigms of EtOH self-administration and conditioned place preference, which are stronger measures of the motivating and self-reinforcing properties of EtOH compared with two-bottle, free-choice access to an EtOH solution or water [38].
The substantially higher levels of water intake at ~100 g/kg reported in the aged hamsters in this study reflect a 330% increase from levels of water intake at ~30 g/kg in young adult hamsters of Hammer et al. 2010 [15; data not shown, Hammer]. This difference in daily water intake in young adult hamsters of Hammer et al. 2010 compared with the aged hamsters under the same experimental protocol in this study is possibly due to aging-related declines in rhythmic expression and central circulation of arginine-vasopressin (AVP) signaling [39, 40] compounded by the dehydrating effects of chronic EtOH intake [41]. There was also a trend towards increased water intake in runners compared with non-runners across phase II (repeated measures ANOVA; p=0.08), although there was no detection of a significant correlation between the extent of wheel running and water intake. Despite this, there was a significant and prolonged increase in water intake in the runners compared with non-runners once running wheels were re-locked across phase III, suggesting that the hamsters were mildly dehydrated during the 30-day regimen of wheel running. Further, while higher levels of water intake in runners compared with non-runners, particularly after re-locking of the running wheels across phase III, may have contributed to a concomitant treatment difference in EtOH preference, it is important to emphasize that levels of daily EtOH intake were still lower in the runners compared with non-runners across this time as well.
4.2. Wheel Running Limits EtOH Intake
The rewarding and self-reinforcing properties of natural and chemical rewards, including wheel-running and EtOH, neurochemically manifest from increases in dopaminergic signaling within the mesocorticolimbic and mesopontine reward systems of the brain [12, 42, 43]. Hence, reciprocal interactions between dopamine neuromodulatory systems, wheel-running, and EtOH, allow one of these rewards (wheel-running) to offer an alternative neurochemical reward state for another (EtOH). Reward substitution in this manner has been found in young adult hamsters [15] and C57BL/6J mice [14]. An additional study in mice observed that voluntary wheel running markedly increased during EtOH withdrawal and subsequently decreased following EtOH re-introduction [44]. Here, we found a reduction in free-choice EtOH intake in aged runners compared with non-runners across phase II as well as for the first 10 days across phase III. We did not, however, detect a significant correlation between the extent of wheel running and fluid (EtOH or water) intake or EtOH preference, suggesting that even low levels of wheel running are sufficient to limit free-choice EtOH intake. In both treatment groups, there was also a progressive increase in daily EtOH intake across the second-third compared with the first-third of phase II (Fig. 2). This change in EtOH intake may be a sign of EtOH tolerance, which, at a behavioral level, increases EtOH intake through a reduction of motoric intoxication [45]. Nevertheless, access to a running wheel did have a modest and prolonged impact on levels of EtOH intake in this first-time assessment of chronic EtOH drinking behavior in aged hamsters.
CONCLUSIONS
We found that the aging process, in general, dampens free-choice EtOH intake and concurrently heightens water intake, likely though aging-related declinations in neuroendocrine processes. The results of this study are also in agreement with previous studies in young adult models of alcoholism illustrating the therapeutic benefits of wheel running as a means of limiting the intake of EtOH [14, 15, 44] and other drugs of abuse [17, 18]. Further, this study extends beyond these previous studies by showing the residual benefits of exercise on levels of EtOH intake in an aged animal model of alcoholism. Collectively, these results illustrate the therapeutic potential of exercise as a non-pharmacological, naturally-rewarding treatment strategy for alcohol dependence and relapse prevention in young adult and aging populations. Thus, the sustainability of an exercise program in young adults points to its therapeutic value for individuals with a family history of alcoholism. In aging populations, the relative cost of an exercise program compared with alcoholism pharmaceuticals and the general physical and mental benefits of exercise [21] make exercise a desirable and supplemental treatment strategy for late-life, heavy alcohol use
Highlights
Aging dampens high levels of free-choice EtOH intake found in young hamsters.
Wheel running further lowers levels of free-choice EtOH intake.
Dampening of free-choice EtOH intake by wheel running is residual.
ACKNOWLEDGMENTS
The authors would like to thank Dr. J. David Glass in the Department of Biological Sciences at Kent State University for providing experimental animals, facilities, and funding to conduct the study, and Drs. Rebecca A. Prosser in the Department of Molecular and Cellular Biology and Biochemistry at University of Tennessee-Knoxville, Drs. J. Christopher Ehlen and Jennifer Evans in the Department of Neurobiology at Morehouse School of Medicine, and Dr. Glass for assistance with manuscript preparation. This study was funded by National Institute on Alcohol Abuse and Alcoholism grants AA-019821 to AJB and JDG, AA-015948 to JDG and RAP, and AA-017898 to JDG and RAP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Brower KJ. Alcohol's effects on sleep in alcoholics. Alcohol Res Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- [2].Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001;5:287–297. doi: 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- [3].Kühlwein E, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biol Psychiatry. 2003;54:1437–1443. doi: 10.1016/s0006-3223(03)00005-2. [DOI] [PubMed] [Google Scholar]
- [4].Dijk DJ, Duffy JF. Circadian regulation of human sleep and age-related changes in its timing, consolidation, and EEG characteristics. Ann Med. 1999;31:130–40. doi: 10.3109/07853899908998789. [DOI] [PubMed] [Google Scholar]
- [5].Czeisler CA, Dumont M, Duffy JF, Steinberg JD, Richardson GS, Brown EN, Sánchez R, Rìos CD, Ronda JM. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–6. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- [6].Hida A, Kusanagi H, Satoh K, Kato T, Matsumoto Y, Echizenya M, Shimizu T, Higuchi S, Mishima K. Expression profiles of PERIOD 1, 2, and 3 in peripheral blood mononuclear cells from older subjects. Life Sci. 2009;84:33–7. doi: 10.1016/j.lfs.2008.10.012. [DOI] [PubMed] [Google Scholar]
- [7].York CL, Chan AW. Age-related differences in sensitivity to alcohol in the rat. Alcohol Clin Exp Res. 1993;17:864–9. doi: 10.1111/j.1530-0277.1993.tb00855.x. [DOI] [PubMed] [Google Scholar]
- [8].Hervonen A, Jaatinen P, Sarviharju M, Kilanmaa K. Interaction of aging and lifelong ethanol ingestion on ethanol-related behaviors and longevity. Exp Gerontol. 1992;27:335–45. doi: 10.1016/0531-5565(92)90060-d. [DOI] [PubMed] [Google Scholar]
- [9].Boeri MW, Sterk CE, Elifson KW. Baby boomer drug users: career phases, social control, and social learning theory. Sociol Inquiry. 2006;76:264–291. [Google Scholar]
- [10].Patterson TL, Jeste DV. The potential impact of the baby-boom generation on substance abuse among elderly persons. Psychiatr Serv. 1999;50:1184–9. doi: 10.1176/ps.50.9.1184. [DOI] [PubMed] [Google Scholar]
- [11].Gfroerer J, Penne M, Pemberton M, Folsom R. Substance abuse treatment need among older adults in 2020: the impact on the aging baby-boom cohort. Drug Alcohol Depend. 2003;69:127–35. doi: 10.1016/s0376-8716(02)00307-1. [DOI] [PubMed] [Google Scholar]
- [12].Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fieshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217:354–62. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thorén P, Floras JS, Hoffmann P, Seals DR. Endorphins and exercise physiological mechanisms and clinical implications. Med Sci Sports Exerc. 1990;22:417–28. [PubMed] [Google Scholar]
- [14].Ehringer MA, Hoft NR, Zunhammer M. Reduced alcohol consumption in mice with access to a running wheel. Alcohol. 2009;43:443–52. doi: 10.1016/j.alcohol.2009.06.003. [DOI] [PubMed] [Google Scholar]
- [15].Hammer SB, Ruby CL, Brager AJ, Prosser RA, Glass JD. Environmental modulation of alcohol intake in hamsters: effects of wheeling and constant light exposure. Alcohol Clin Exp Res. 2010;34:1651–8. doi: 10.1111/j.1530-0277.2010.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McMillan DE, McClure GY, Hardwick WC. Effects of access to a running wheel on food, water, and ethanol intake in rats bred to accept ethanol. Drug Alcohol Depend. 1995;40:1–7. doi: 10.1016/0376-8716(95)01162-5. [DOI] [PubMed] [Google Scholar]
- [17].Lukaszyk A, Buczko W, Wisniewski K. The effect of strenuous exercise on the reactivity of the central dopaminergic system in the rat. Pol J Pharmacol Pharm. 1983;35:29–36. [PubMed] [Google Scholar]
- [18].Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98:129–35. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Field T, Diego M, Sanders CE. Ad olescent depression and risk factors. Adolescence. 2001;36:491–8. [PubMed] [Google Scholar]
- [20].Collingwood TR, Sunderlin J, Reynolds R, Kohl HW. Physical training as a substance abuse prevention intervention for youth. J Drug Educ. 2000;30:435–451. doi: 10.2190/RVUE-9XW7-TYRQ-EJR8. [DOI] [PubMed] [Google Scholar]
- [21].Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry. 2005;18:189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- [22].Adams WL, Cox NS. Epidemiology of problem drinking among elderly people. Int J Addict. 1995;30:1693–716. doi: 10.3109/10826089509071053. [DOI] [PubMed] [Google Scholar]
- [23].Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. Am J Physiol Regul Integr Comp Physiol. 2009;297:R29–37. doi: 10.1152/ajpregu.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Seggio JA, Logan RW, Rosenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacol Biochem Behav. 2007;87:297–305. doi: 10.1016/j.pbb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Morin LP, Forger NG. Endocrine control of ethanol intake by rats or hamsters: relative contributions of ovaries, adrenals, and steroids. 1982 doi: 10.1016/0091-3057(82)90315-x. [DOI] [PubMed] [Google Scholar]
- [26].Young-Janik L, Janik D. Nonphotic phase shiting in female Syrian hamsters: interactions with the estrous cycle. J Biol Rhythms. 2003;18:307–17. doi: 10.1177/0748730403254005. [DOI] [PubMed] [Google Scholar]
- [27].Brager AJ, Ruby CL, Prosser RA, Glass JD. Chronic ethanol disrupts circadian photic entrainment and daily locomotor activity in the mouse. Alcohol Clin Exp Res. 2010;34(7):1266–1273. doi: 10.1111/j.1530-0277.2010.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gorman MR, Yellon S. Lifespan daily locomotor activity rhythms in a mouse model of amyloid-induce neuropathology. Chronobiol Int. 2010;27:1159–77. doi: 10.3109/07420528.2010.485711. [DOI] [PubMed] [Google Scholar]
- [29].Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS, Block GD. Age-related decline in circadian output. J Neurosci. 2011;31:10201–5. doi: 10.1523/JNEUROSCI.0451-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pietrzak ER, Wilce PA, Ward LC, Shanley BC. A feeding regime for the study of the interaction of ethanol and aging. Drug Alcohol Depend. 1989;23:171–5. doi: 10.1016/0376-8716(89)90024-0. [DOI] [PubMed] [Google Scholar]
- [31].Wasielewski JA, Holloway FA. Alcohol's interactions with circadian rhythms. Alcohol Res Health. 2001;25:94–100. [PMC free article] [PubMed] [Google Scholar]
- [32].Leasure JL, Nixon K. Exercise neuroprotection in a rat model of binge alcohol consumption. Alcohol Clin Exp Res. 2010;34:404–14. doi: 10.1111/j.1530-0277.2009.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jerlhag E, Egecioglu E, Landgren S, Salomé N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci USA. 2009;106:11318–23. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kizaki T, Maegawa T, Sakurai T, Ogasawara JE, Ookawara T, Oh-ishi S, Izawa T, Haga S, Ohno H. Voluntary exercise attenuates obesity-associated inflammation through ghrelin expressed in macrophages. Biochem Biophys Res Commun. 2011;413:454–9. doi: 10.1016/j.bbrc.2011.08.117. [DOI] [PubMed] [Google Scholar]
- [35].Kmiec Z. Aging and peptide control of food intake. Curr Protein Pept Sci. 2011;12:271–9. doi: 10.2174/138920311795906718. [DOI] [PubMed] [Google Scholar]
- [36].Memo M, Lucchi L, Spano PF, Trabucchi M. Aging process affects a single class of dopamine receptors. Brain Res. 1980;202:488–492. doi: 10.1016/0006-8993(80)90161-4. [DOI] [PubMed] [Google Scholar]
- [37].Morgan DG. The dopamine and serotonin systems during aging in human and rodent brain. A brief review. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11:153–7. doi: 10.1016/0278-5846(87)90053-4. [DOI] [PubMed] [Google Scholar]
- [38].Shippenberg TS, Koob GF. Recent progress in animal models of drug addiction. In: David KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacolgy-5th Generation of Progress. Lippincott, Williams, and Wilkins; Philadelphia: 2002. pp. 1383–1397. [Google Scholar]
- [39].Duncan MJ, Herron JM, Hill SA. Aging selectively suppresses vasoactive intestinal peptide messenger RNA expression in the suprachiasmatic nucleus of the Syrian hamster. Brain Res Mol Brain Res. 2001;87:196–203. doi: 10.1016/s0169-328x(01)00015-8. [DOI] [PubMed] [Google Scholar]
- [40].Geelen G, Corman B. Relationship between vasopressin and renal concentrating ability in aging rats. Am J Physiol. 1992;262:R826–33. doi: 10.1152/ajpregu.1992.262.5.R826. [DOI] [PubMed] [Google Scholar]
- [41].Madeira MD, Andrade JP, Lieberman AR, Sousa N, Almeid a OF, Paula-Barbosa MM. Chronic alcohol consumption and withdrawal do not induce cell death in the suprachiasmatic nucleus, b ut lead to irreversible depression of peptide immunoreactivity and mRNA levels. J Neurosci. 1997;17:1302–1319. doi: 10.1523/JNEUROSCI.17-04-01302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang DV, Tsien JZ. Conjunctive processing of locomotor signals by the ventral tegmental area neuronal population. PLoS One. 2011;6:e16528. doi: 10.1371/journal.pone.0016528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- [44].Ozburn AR, Harris RA, Blednov YA. Wheel running, voluntary ethanol consumption, and hedonic substitution. Alcohol. 2008;42:417–24. doi: 10.1016/j.alcohol.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84(1):53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]