Abstract
Extracorporeal porcine liver perfusion is being developed as a bridge to liver allotransplantation for patients with fulminant hepatic failure. This strategy is limited by porcine Kupffer cell destruction of human erythrocytes, mediated by lectin binding of a sialic acid motif in the absence of antibody and complement. Sialoadhesin, a macrophage restricted lectin that binds sialic acid, was originally described as a sheep erythrocyte binding receptor. Given similarities between sialoadhesin and the unidentified macrophage lectin in our model, we hypothesized porcine sialoadhesin contributed to recognition of human erythrocytes. Two additional types of macrophages were identified to bind human erythrocytes - spleen and alveolar. Expression of sialoadhesin was confirmed by immunofluorescence in porcine tissues and by flow cytometry on primary macrophages. A stable transgenic cell line expressing porcine sialoadhesin (pSn CHO) bound human erythrocytes, while a sialoadhesin mutant cell line did not. Porcine macrophage and pSn CHO recognition of human erythrocytes was inhibited approximately 90% by an anti-porcine sialoadhesin monoclonal antibody and by human erythrocyte glycoproteins. Furthermore, this binding was substantially reduced by sialidase treatment of erythrocytes. These data support the hypothesis that porcine sialoadhesin is a xenogeneic receptor that mediates porcine macrophage binding of human erythrocytes in a sialic acid-dependent manner.
Keywords: Macrophages, liver, lectin, sialic acid, xenotransplantation
INTRODUCTION
Within the last decade, innate immune cells, specifically macrophages, have gained attention in the field of xenotransplantation due to their contribution to a new form of xenograft rejection not seen in allotransplantation—a process known as delayed xenograft rejection (1). Delayed xenograft rejection, or acute vascular rejection, is not completely understood, but it is composed of both innate and adaptive immune responses in the organ recipient (2). The innate cellular immune response found in porcine hearts rejected by baboons consists of injuries inflicted by baboon natural killer cells and macrophages (3). The best evidence for baboon macrophage destruction of porcine cells comes from studies infusing porcine peripheral blood progenitor cells into baboons (4). In these studies, depletion of baboon recipient macrophages with medronate liposomes resulted in delayed clearance of porcine cells, suggesting that baboon macrophages bind and eliminate porcine cells by either antibody-dependent cell-mediated cytotoxicity or direct recognition by specific macrophage receptors. Human Kupffer cells, macrophages of the liver, directly recognize porcine erythrocytes without the need for opsonization, suggesting that primate macrophages bind porcine cells using innate immune cell surface receptors (5). Thus, it appears that abrogation of macrophage recognition and destruction of xenogeneic cells will be an important accomplishment in the goal of achieving long-term xenograft survival.
Our laboratory studies how macrophages recognize non-self—specifically how porcine macrophages recognize human erythrocytes. This focus developed from efforts to design a treatment for patients in fulminant liver failure using extracorporeal porcine liver perfusion (6). This treatment option could provide for patients in fulminant liver failure a bridge to liver transplantation or allow recovery without a liver transplantation. During a 72-hour porcine liver perfusion with human blood, a decrease in hematocrit is observed when perfusing livers from both wild-type and human decay accelerating factor transgenic pigs (7), resulting from porcine Kupffer cells binding human erythrocytes (8). In extracorporeal porcine liver perfusion experiments lasting 72 hours, porcine Kupffer cells bind and destroy more than 90% of the perfused human erythrocytes (7). This observation was the opposite of what was expected. Traditionally in solid organ allotransplantation and xenotransplantation, the main risk is that the host destroys the graft; but in this model, the graft was attacking host cells. This mechanism of destruction does not appear to be mediated through complement or antibody mediated opsonization (9), but rather, involves direct recognition by a macrophage receptor (8). Further studies demonstrated that porcine Kupffer cells bind a human erythrocyte carbohydrate ligand (10); specifically, these cells recognize a sialic acid motif on human glycophorin A (11). We hypothesized that macrophages have carbohydrate-specific lectin receptors that mediate direct recognition of unopsonized xenogeneic cells (12).
Sialoadhesin, or siglec-1, is a macrophage restricted lectin that binds sialic acid (13, 14). Historically, sialoadhesin was identified as a bone marrow macrophage receptor that mediated recognition of sheep erythrocytes in attempts to understand the presence of resident bone marrow macrophages and their cellular functions (14). Although characterization of the exact biological function of sialoadhesin remains uncertain, more general roles for sialoadhesin have been identified in the areas of cancer biology (15, 16), erythropoiesis(17), inflammatory conditions (18, 19) and clearance of pathogens (20–22). Neisseria meningitides is one of the few pathogens where sialoadhesin has been shown to mediate macrophage recognition of non-self (20). Sialoadhesin has been shown to mediate internalization (23) and endocytosis (24) of PRRSV, porcine reproductive and respiratory syndrome virus, into macrophages. In an interesting twist, PRRSV directs its host cell to glycosylate viral surface glycoproteins so that PRRSV is bound by sialoadhesin expressed on porcine alveolar macrophages; the virus thereby targets these cells for infection (21). It is worth noting that the ability of sialoadhesin to mediate macrophage recognition of non-self was the defining characteristic for which this molecule was originally named – the sheep erythrocyte binding receptor (25). This cellular interaction is a form of xenogeneic recognition – mouse macrophage recognition of sheep erythrocytes. While most researchers have viewed this interaction as an oddity limited to the laboratory, we propose that this xenogeneic interaction contributes to the understanding of how macrophages recognize xenogeneic epitopes in the field of xenotransplantation.
Given the work identifying porcine sialoadhesin as a sialic acid-binding porcine macrophage receptor involved in PRRSV infection, we hypothesized that this same receptor might be responsible for mediating porcine Kupffer cell recognition of human erythrocytes. We provide evidence that sialoadhesin mediates porcine macrophage recognition of human erythrocytes and that inhibitors of this process block both porcine macrophage binding of human erythrocytes and PRRSV infection of porcine alveolar macrophages.
MATERIALS AND METHODS
All animal experiments were approved by the University of Toledo IACUC. Large white pigs were obtained from a local pig farm (15–20 kg) and treated in accordance with the ILAR and the Animal Welfare Act (26). Blood was collected from either piglets or blood group O human volunteers. Written informed consent was obtained for all human volunteers under a University of Toledo IRB approved protocol.
Virus
The European prototype PRRSV strain Lelystad virus (kindly provided by G. Wensvoort) and the Belgian PRRSV strain, 94V350 (27) were used in these experiments (28). Details regarding passaging and infection rates in porcine alveolar macrophages are previously described (27).
Cells
After macrophage isolation, preparations were incubated overnight to select for adherent cells. Flasks were then washed with the appropriate medium and returned to the incubator for one week before utilization. Generally, macrophage cultures were viable for 2–3 weeks. Unless specified, all mediums and supplements were obtained from Life Technologies (Carlsbad, CA).
Kupffer cells and erythrocytes
Porcine Kupffer cells, human erythrocytes, and porcine erythrocytes were isolated as previously described (8).
Spleen macrophages
Splenectomy was followed by hepatectomy. Residual blood was removed by perfusion of the splenic artery with ice cold saline (Baxter, Deerfield, IL). The spleen was minced and processed with 1 L of cold PBS (Oxoid Inc., Ogdensburg, NY) through 500, 212, and 106 micron metal sieves (CSC Scientific Inc., Fairfax, VA). This cellular solution was equally distributed into six 250 mL bottles and brought up to a final volume of 200 mL with PBS + 10% FBS. The resulting cellular solution was incubated on ice for 30 min. The supernatant was then centrifuged at 600 × g for 5 min. The resulting pellets were combined and brought up to a final volume of 225 mL with PBS + 10% FBS. This cellular solution was layered over Ficoll-Paque PLUS, (GE Healthcare Life Sciences, Piscataway, NJ) and centrifuged for 45 min at 3007 × g. The interface was carefully removed and washed in HBSS + 10% FBS, and centrifuged at 469 × g for 7 min. Finally, the pellet was washed with medium and placed in culture. Spleen macrophages were maintained in RPMI (Cell Gro, Herndon, VA), 1% penicillin/streptomycin (100 U/mL, 100 µg/mL), 10% FBS, and 2.7% mM L-glutamine, 200 mM.
Alveolar macrophages
Porcine alveolar macrophages were collected by performing a broncho-alveolar lavage on adult pigs and frozen for long-term use as previously described (29). Thawed alveolar macrophages were used approximately 4 days after being in culture.
pSn CHO, pSnRE CHO, and wild type CHO cells
CHO-K1 cells (ATCC, CCL-61) were maintained in F12 Medium supplemented with 5% FBS, 1% sodium pyruvate, and 1% penicillin/streptomycin (100 U/mL, 100 µg/mL). The construction of the full-length sialoadhesin mutant, which lacks sialic acid binding activity (pSnRE), and the generation of stable CHO cell line that expresses porcine sialoadhesin (pSn CHO) are described previously (30).
Chromium51 Rosetting Assay
Binding
Quantification of porcine macrophage recognition of human erythrocytes has been described previously (8). Briefly, potential inhibitors were applied to macrophages prior to the addition of 51Chromium labeled erythrocytes. Unbound erythrocytes were washed away and gamma radiation measured. This binding assay was adapted to measure binding between CHO cell lines and human erythrocytes. CHO cells were applied to glass cover slips and incubated at 37°C for 48 hrs. Prior to the application of potential inhibitors, all CHO cell samples were treated with Vibrio cholerae sialidase (Roche, Indianapolis, IN). Sialidase removes cell surface sialic acid that contributes to cis-inhibition of sialoadhesin. 8 mU of sialidase, diluted in RPMI medium, was applied to each cover slip for 30 min and washed twice.
Inhibition of binding with human erythrocyte glycoproteins (hEGP)
Both hEGP and porcine erythrocyte glycoproteins (pEGP) were prepared previously (10).
Inhibition of binding with pSn mAb
The murine monoclonal antibody 41D3 against porcine sialoadhesin (pSn mAb) was described previously (31, 32). A purified mouse IgG monoclonal antibody served as the isotype control (BD Biosciences, Franklin Lakes, NJ).
Sialidase treatment of human erythrocytes
Human erythrocytes were treated with sialidase (Roche, Indianapolis, IN). 1.0 µL of packed erythrocytes was re-suspended in 400 µl of RPMI medium and treated with 20 mU of sialidase to remove terminal sialic acid. Erythrocytes were incubated with sialidase for 1 hr at 37°C. Erythrocytes were washed once to remove released sialic acid prior to 51Chromium labeling.
Flow Cytometry
All treatments were done for 30 min on ice. Since macrophages contain Fc receptors, cells were blocked with 20 µL of neat horse serum per sample. Primary antibodies included pSn mAb and the isotype control mouse anti-T-2 mycotoxin monoclonal antibody (Southern Biotech, Birmingham, AL). A goat anti-mouse IgG Alexa Flour 647 (Life Technologies, Carlsbad, CA) secondary antibody was used. Samples were washed twice between treatments with PBS. Samples were analyzed on a Cytometrics FC 500 flow cytometer (Beckman Coulter, Brea, CA). Data was analyzed using FlowJo, version 7.6.4
Quantification of PRRSV Infection of Alveolar Macrophages
Macrophages were fixed and stained as previously described (28). Briefly, infected cells were detected by immunoperoxidase staining with a monoclonal antibody (P3/27) against the PRRSV nucleocapsid protein (33) followed by incubation with horseradish peroxidase-labeled goat anti-mouse secondary antibody and then developed with 3-amino-9-ethylcarbazole as a substrate.
Immunohistochemistry
Porcine macrophages were incubated with human and porcine erythrocytes as described for the 51Chromium rosetting assay, except that the erythrocytes were not radioactively labeled. Macrophages were fixed in a 1:1 acetone/methanol solution. The primary antibody mouse anti-pig monocyte/granulocyte (BD Biosciences, Franklin Lakes, NJ) was used to identify porcine macrophages. Porcine erythrocytes were identified with the primary antibody mouse anti-pig CD235a monoclonal antibody, (BD Biosciences, Franklin Lakes, NJ) and human erythrocytes were identified with a mouse anti-human glycophorin A monoclonal antibody (DakoCytomation, Carpinteria, CA). Images were taken with an Olympus BHS (Center Valley, PA) microscope equipped with an Evolution MP 5.0 Mega-pixel Color Real Time Viewing digital camera (Media Cybernetics, Silver Spring, MD) and analyzed with Q Capture Pro 5.0 software (Mager Scientific, Dexter, MI).
Confocal Microscopy
Frozen sections were cut with a cryostat and prepared on positively charged glass microscope slides. All incubations were done in a humidified box at 37°C. pSn mAb was labeled with FITC (Zenon Alexa Fluro 488 labeling kit) according to the manufacturer’s instructions (Life Technologies). A comparable mouse IgG1 isotype control was labeled to serve as a negative control. Slides were analyzed on a TCS SP5 multiphoton laser scanning confocal microscope (Leica Microsystems, Buffalo Grove, IL).
Statistical Analysis
Data collected for the quantitative 51Chromium rosetting assay was analyzed using descriptive statistics. Each sample for each experiment was performed in triplicate and the standard error for each sample calculated from the standard deviation of the three values obtained. Each experiment was completed a minimum of three times. The average values of each experiment were then used to calculate the standard error for all three experiments.
RESULTS
Porcine spleen and alveolar macrophages bind human erythrocytes
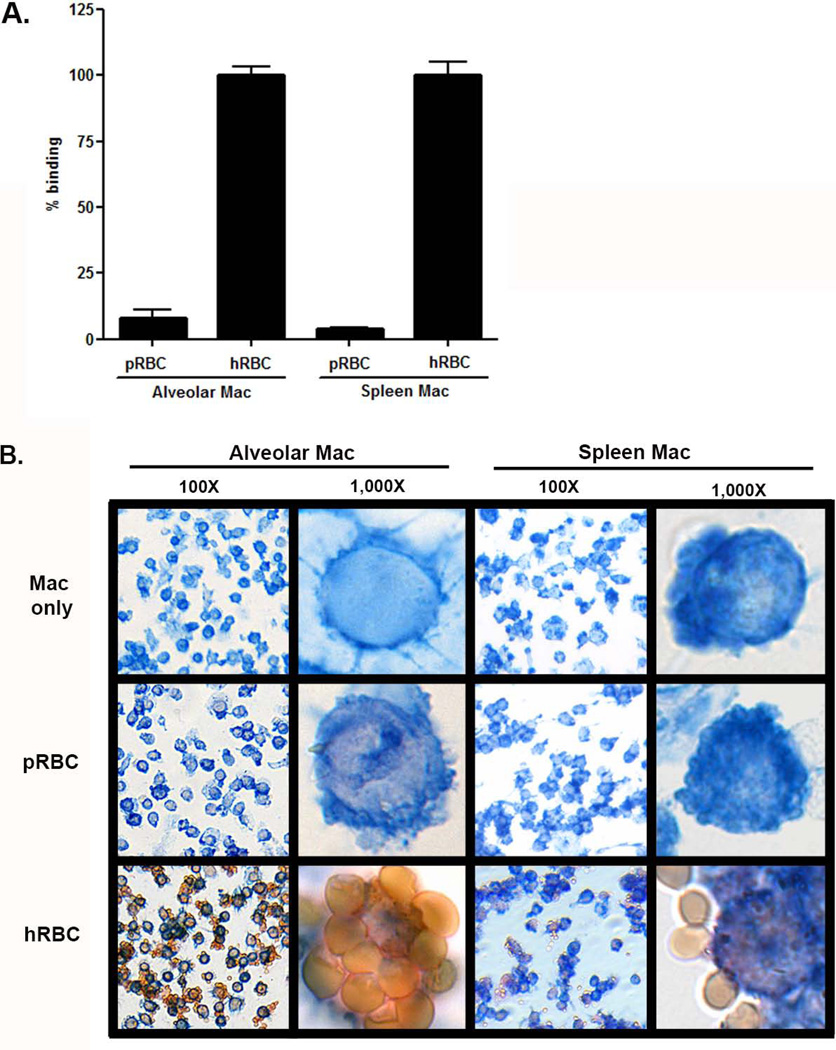
While porcine Kupffer cells have previously been demonstrated to bind human erythrocytes (8), we evaluated the ability of porcine spleen and alveolar macrophages to bind human erythrocytes using both a quantitative 51Chromium rosetting assay (Figure 1A) and a qualitative immunohistochemistry assay (Figure 1B). Porcine spleen and alveolar macrophages bound human erythrocytes, but not porcine erythrocytes (Figure 1 A and B). These data demonstrate that at least three resident porcine tissue macrophages are capable of binding human erythrocytes. Given the difficultly and expense of procuring, and the limited yield of macrophages obtained from the liver and spleen, alveolar macrophages were utilized as a substitute for Kupffer cells given their ease of procurement and large yields (~107 Kupffer cells obtained per pig liver vs. 3 × 109 alveolar macrophages per broncho-alveolar lavage).
Figure 1. Porcine alveolar and spleen macrophages bind human erythrocytes and not porcine erythrocytes.
Spleen macrophages (spleen mac) and alveolar macrophages (alveolar mac) were incubated with porcine erythrocytes (pRBC) and human erythrocytes (hRBC). A. Porcine macrophage binding of human erythrocytes was quantified using a 51Chromium rosetting assay. Samples were prepared in triplicate and repeated 3 times, N=9. The means are shown. The standard error is calculated; however, for some samples it is very small and obscured by the bar. B. Macrophage recognition of human erythrocytes was visualized using two-color immunohistochemistry at 100X and 1,000X magnification. Porcine macrophages were identified with a primary antibody, anti-porcine macrophage antibody 74-22-15A, followed by a biotinylated secondary antibody, and developed blue with a Vector Blue alkaline phosphatase substrate. Erythrocytes were identified using anti-porcine or anti-human glycophorin A followed by a biotinylated secondary antibody, and developed red with Vector Red alkaline phosphatase substrate. Samples were prepared in triplicate. Data is representative of two independent experiments.
Porcine sialoadhesin is expressed in porcine liver and lung tissues, in vivo and in vitro
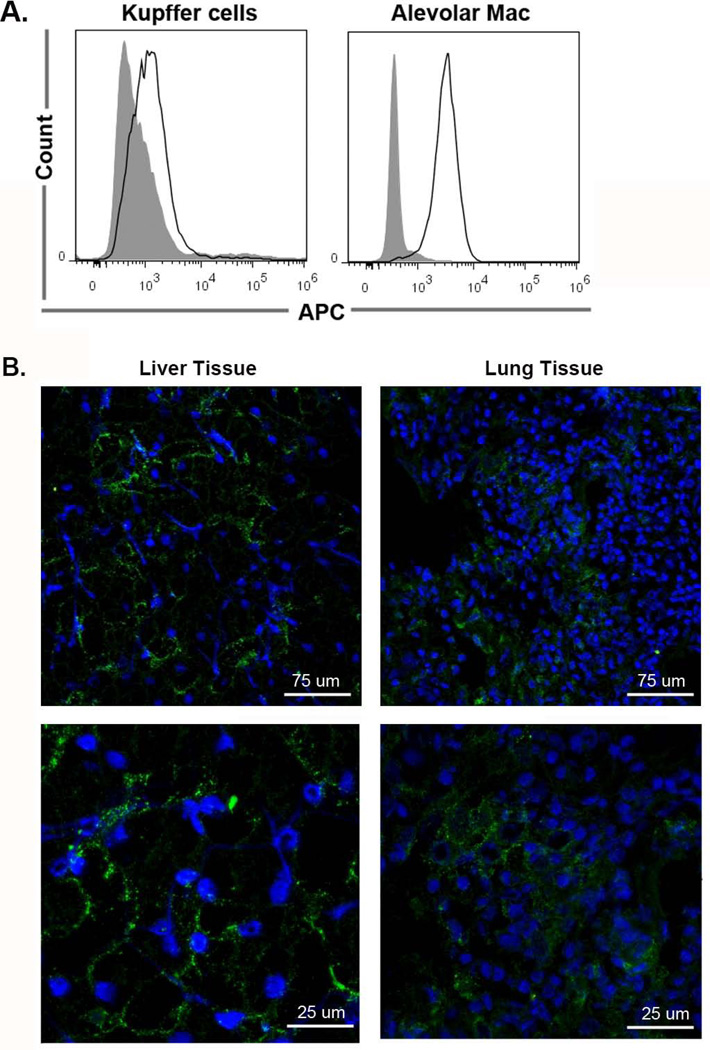
We hypothesized that porcine sialoadhesin is a macrophage receptor that mediates porcine macrophage recognition of human erythrocytes. Thus, we wanted to confirm that porcine sialoadhesin is expressed in porcine liver and lung tissues and in primary macrophages. In Figure 2A, flow cytometry analysis of primary macrophages demonstrated surface expression of sialoadhesin on porcine Kupffer cells (MFI of 2,700.3 ± 609.6) and alveolar macrophages (MFI of 3,586.3 ± 572.6) as compared to the isotype control (478.5 MFI ± 27.1). In Figure 2B, qualitative immunofluorescence of liver and lung tissues demonstrated varied levels of sialoadhesin expression at low and high levels of magnification. It’s not surprising that we found sialoadhesin expression levels vary among resident macrophages as this has been demonstrated with other species of macrophages (14, 25, 34, 35). In regards to how levels of sialoadhesin expression contribute to porcine Kupffer cell recognition of human erythrocytes, about 20 erythrocytes are bound per macrophage; therefore, levels of sialoadhesin expression are not indicative of binding. More likely though, binding is limited by steric hindrance. These data confirm that porcine sialoadhesin is naturally expressed in vivo and that expression in vitro is not an artifact of isolation.
Figure 2. Confirmation that porcine sialoadhesin is expressed in vitro in primary cultures of Kupffer cells and alveolar macrophages and in vivo in liver and lung tissues.
A. Porcine Kupffer cells and alveolar macrophages were evaluated by flow cytometry for expression of sialoadhesin. Macrophages were stained with an appropriate isotype control (filled, gray) and stained with the pSn mAb (outlined, black). 5 × 105 cells were used per sample. Data is plotted FSC (y axis) verses APC (x axis). This experiment was repeated three times. B. 1 cm2 blocks of porcine liver and lung tissues were evaluated for expression of porcine sialoadhesin. Porcine tissues were placed in Tissue-Tek O.C.T. and prepared for cryosectioning. Porcine sialoadhesin was detected with Alexa 488 (green) and the nucleus was stained with DAPI (blue). Isotype control samples were negative (not shown). Images were taken on a multiphoton laser scanning confocal microscope and representative z-stacks are shown. The upper panel images were taken at 40X and the lower panel images were taken at 40X zoomed in at a factor of 3.8. Samples were prepared in duplicate. Data is representative of two independent experiments. White measurement bars are included in each image for estimation of size.
PRRSV infection of porcine alveolar macrophages and porcine alveolar macrophage recognition of human erythrocytes share two common inhibitors: human erythrocyte glycoproteins (hEGP) and an anti-porcine sialoadhesin monoclonal antibody (pSn mAb)
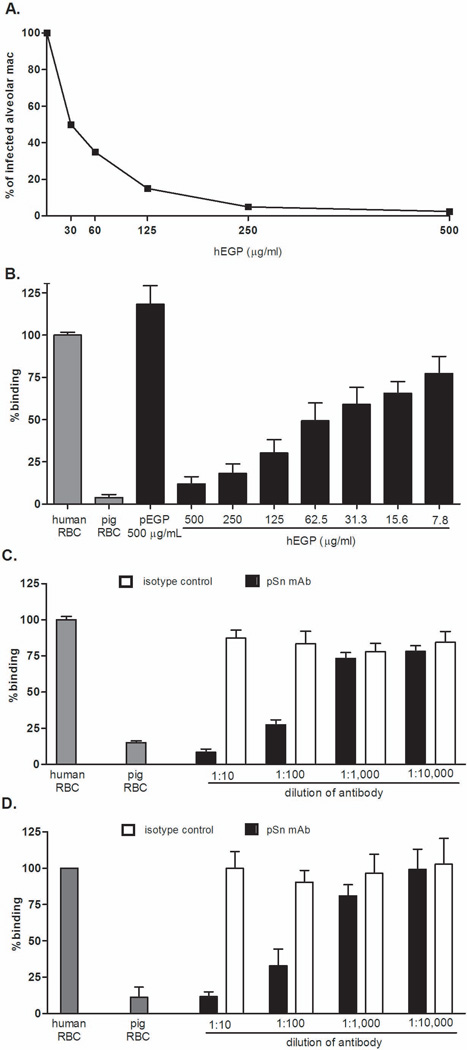
Given our previous observation that porcine Kupffer cells bind human erythrocytes via the terminal sialic acid on human glycophorin A (10, 11), and knowing that the sialic acid-binding lectin responsible for PRRSV entry into porcine alveolar macrophages is sialoadhesin (32), we examined whether two known inhibitors of each model (hEGP and pSn mAb) would reciprocally inhibit the other model in hopes of identifying the porcine macrophage receptor that mediates recognition of human erythrocytes. As demonstrated in Figure 3A, hEGP inhibited infection of porcine macrophages by PRRSV in a concentration dependent manner. Complete inhibition of infection was observed at a concentration of 500 µg/mL. To verify this same observation with porcine alveolar macrophages, alveolar macrophages were incubated with a reciprocal dilution of hEGP. As with porcine Kupffer cells (10), hEGP inhibited porcine alveolar macrophage recognition of human erythrocytes in a concentration dependent manner resulting in approximately 90% inhibition at a concentration of 500 µg/mL (Figure 3B). Porcine erythrocyte glycoproteins were used as a negative control at the highest concentration tested and binding was not inhibited. The pSn mAb at a 1:10 dilution inhibited porcine alveolar macrophage binding of human erythrocytes by nearly 95% (Figure 3C) and porcine Kupffer cell recognition of human erythrocytes by nearly 90% (Figure 3D). These data suggest that sialoadhesin is the porcine macrophage lectin responsible for the binding of human erythrocytes and further support sialoadhesin as the viral receptor for PRRSV.
Figure 3. PRRSV infection of porcine alveolar macrophages and porcine alveolar macrophage recognition of human erythrocytes share two common inhibitors: human erythrocyte glycoproteins (hEGP) and an anti-porcine sialoadhesin monoclonal antibody (pSn mAb).
A. At a range of 500 to 0 µg/ml, hEGP was tested as a potential inhibitor of PRRSV infection of porcine alveolar macrophages. Inhibition was measured using immunofluorescence. Viral antigen-positive cells and total cells were counted with an Olympus light microscope and the percentage of infected cells calculated. Three microscope fields and a minimum of 100 cells per field were counted for each experimental condition. B–D. Potential inhibitors were evaluated using the 51Chromium rosetting assay. Samples were prepared in triplicate. Data is representative of three independent experiments.. The means are shown. The standard error is calculated; however, for some samples it is very small and obscured by the bar. Human and porcine erythrocytes in the absence of a potential inhibitor served as positive and negative controls (gray bars). B. hEGP was tested as a potential inhibitor of alveolar macrophage recognition of human erythrocytes at a range from 500 to 7.8 µg/ml. hEGP was prepared in RPMI medium. The negative control used in this experiment was pEGP, porcine erythrocyte glycoproteins. C. Inhibition of porcine alveolar macrophage recognition of human erythrocytes by pSn mAb was tested. A serial dilution of the pSn mAb ranging from 1:10 – 1:10,000 was prepared in RPMI medium and incubated with porcine alveolar macrophages. Mouse IgG was used as an isotype control. D. Inhibition of Kupffer cell recognition of human erythrocytes by pSn mAb was tested. pSn mAb was incubated with porcine Kupffer cells at a range of dilutions from 1:10 to 1:10,000. Mouse IgG was used as an isotype control.
Porcine sialoadhesin-expressing stable cell line (pSn CHO) binds human erythrocytes and binding is inhibited by both pSn mAb and hEGP
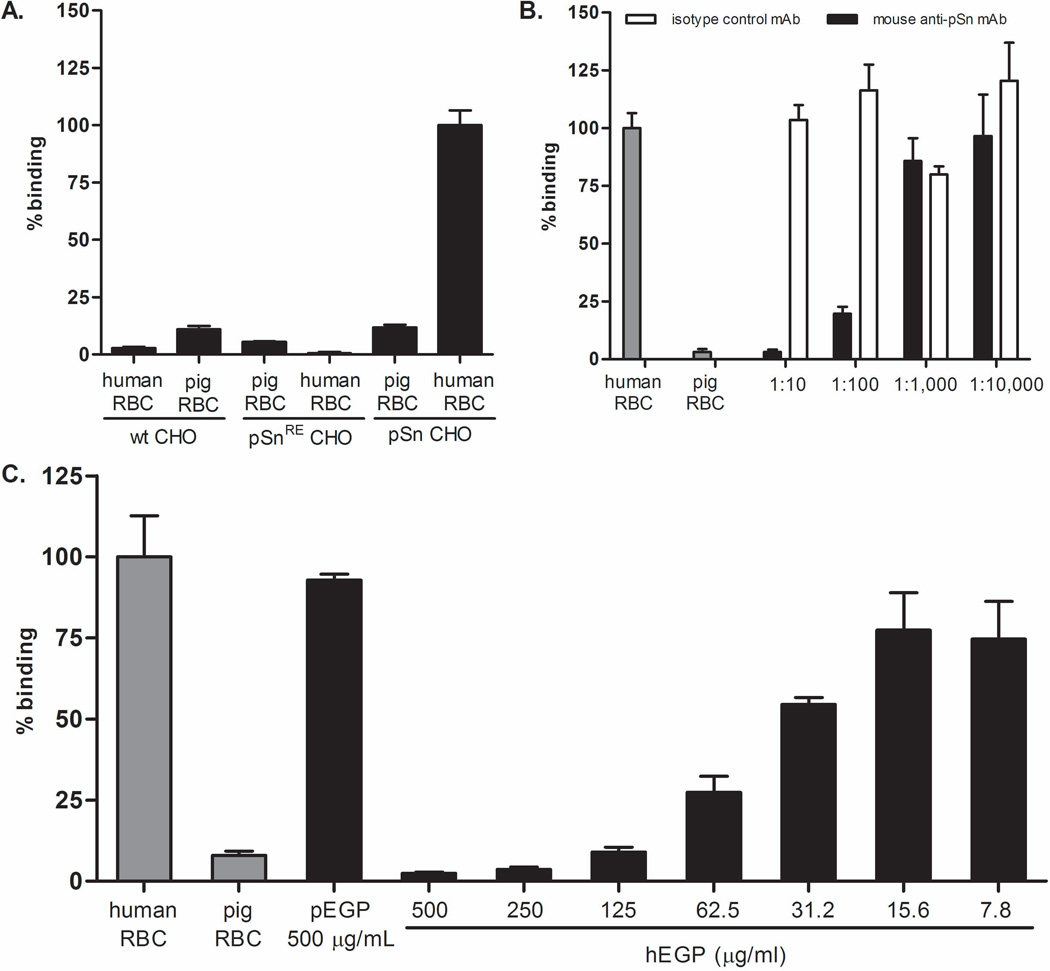
To examine the role of porcine sialoadhesin in porcine macrophage recognition of human erythrocytes, pSn CHO and a stable cell line expressing a mutant form of sialoadhesin (pSnRE CHO; containing a sialic acid-binding domain that is disrupted) were tested for their ability to bind human erythrocytes. Prior to performing rosetting assays, sialoadhesin expression was confirmed on pSn CHO and pSnRE CHO cell lines. In addition, absence of porcine sialoadhesin expression was confirmed in wild type CHO cells by flow cytometry, data not shown. The pSn CHO bound human erythrocytes (represented as 100% binding), but only 11.8% of porcine erythrocytes were bound (Figure 4A). Neither the negative control cell line (pSnRE) nor wild type CHO-K1 cells bound human or porcine erythrocytes, < 12% (Figure 4A) which would be expected since CHO-K1 cells are epithelial cells and sialoadhesin is a macrophage-restricted lectin.
Figure 4. The sialoadhesin stable cell line (pSn CHO) binds human erythrocytes and this binding is inhibited by both the monoclonal antibody against porcine sialoadhesin (pSn mAb) and human erythrocyte glycoproteins (hEGP).
The pSn CHO cell was evaluated for its ability to bind human erythrocytes. For a negative control, a stable cell line expressing a mutant form of sialoadhesin (pSnRE CHO) was used. Briefly, a sialic acid-binding mutant of sialoadhesin was generated by modifying the amino acid arginine 116 (which is by analogy to mouse sialoadhesin critical for the sialic acid-binding activity of porcine sialoadhesin) to a lysine residue by site directed mutagenesis (32). Binding in these experiments was quantified using the 51Chromimum binding assay. Samples were prepared in triplicate. Data is representative of three independent experiments.. The means are shown. The standard error is calculated; however, for some samples it is very small and obscured by the bar. A. pSn CHO cells were evaluated for their ability to bind human erythrocytes. Wild type CHO cells and pSnRE CHO served as negative controls. B. A pSn mAb was tested as a potential inhibitor of binding. A comparable isotype control was used to show that inhibition of binding wasn’t an artifact of steric hindrance. C. hEGP were tested as a potential inhibitor of binding at a range of 500 ug/mL to 7.8 ug/mL. Porcine erythrocyte glycoproteins (pEGP) were used as a negative control.
To further test the hypothesis that porcine sialoadhesin mediates porcine macrophage recognition of human erythrocytes, the inhibitors that blocked binding in the in vitro macrophage experiments were repeated with the pSn stable cell line. Both pSn mAb and hEGP inhibited pSn CHO recognition of human erythrocytes (Figures 4B and 4C). In Figure 4B, the pSn mAb inhibited binding in a serial dilution manner such that at a 1:10 dilution, binding was inhibited by approximately 95% compared to isotype control treated samples. Likewise, hEGP inhibited erythrocyte binding in a concentration dependent manner with 97.6% inhibition at a concentration of 500 µg/mL compared to 7.2% inhibition with pEGP at the same concentration.
pSn CHO and porcine alveolar macrophages do not bind sialidase treated human erythrocytes
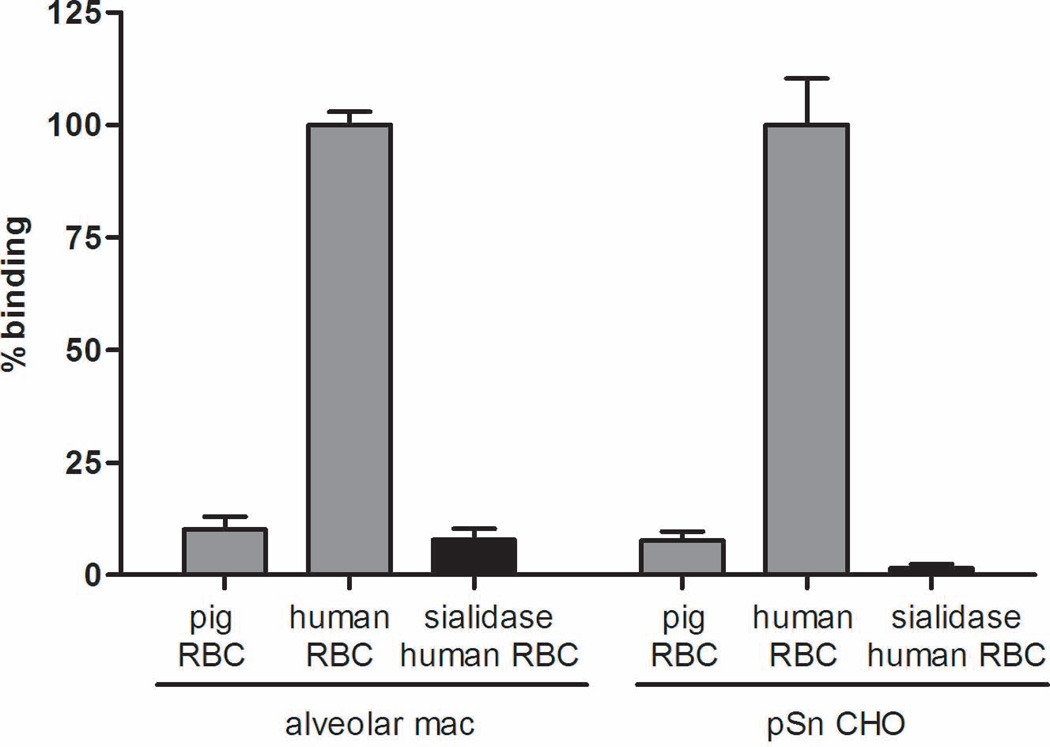
Previously, we demonstrated the importance of sialic acid in porcine Kupffer cell recognition of human erythrocytes by showing that sialidase treatment of human erythrocytes eliminates binding (10). To verify this same observation with porcine alveolar macrophages and pSn CHO, human erythrocytes were sialidase treated and binding was measured using the 51Chromium rosetting assay. Similar to porcine Kupffer cells, sialidase treatment eliminated the binding of human erythrocytes by both porcine alveolar macrophages and pSn (Figure 5). Binding of sialidase treated erythrocytes by alveolar macrophages was reduced by 92.2% and 98.4% for pSn CHO.
Figure 5. Sialic acid on human erythrocytes is necessary for the sialoadhesin stable cell line (pSn CHO) to recognize human erythrocytes.
Human erythrocytes were treated with sialidase to remove terminal sialic acid and were then labeled with 51Chromium. Untreated human and porcine erythrocytes were used as negative and positive controls. Erythrocytes were incubated with either alveolar macrophages (alveolar mac) or the porcine sialoadhesin stable cell line (pSn CHO). Binding in these experiments was quantified using the 51Chromium Binding Assay. Samples were prepared in triplicate. Data is representative of three independent experiments.. The means are shown. The standard error is calculated; however, for some samples it is very small and obscured by the bar.
DISCUSSION
These data suggest that porcine macrophage binding of human erythrocytes is mediated by sialoadhesin. In vitro experiments demonstrated that porcine macrophage recognition of human erythrocytes is inhibited by both human erythrocyte glycoproteins (hEGP) and a monoclonal antibody directed against porcine sialoadhesin (pSn mAb). A porcine sialoadhesin stable cell line (pSn CHO) bound human erythrocytes in contrast to the mutant cell line pSnRE; this binding to pSn CHO was inhibited by both pSn mAb and hEGP. In addition, these inhibitors not only block the xenogeneic model of porcine macrophage recognition of human erythrocytes, but also viral infection of porcine macrophages by PRRSV.
The critical role of carbohydrate recognition in xenotransplantation was first recognized with the discovery of the importance of the carbohydrate epitope galactose α1,3 galactose or Galα1→3Gal (36–38). The Galα1→3Gal epitope is present on all porcine endothelial cells (38, 39), but absent on human and other Old World primate cells (40). Hyperacute rejection has largely been overcome by our understanding of the role of preformed natural antibodies directed against Galα1→3Gal and the production of Galα1→3Gal deficient pigs (41). Humans differ from pigs not only in lacking the Galα1→3Gal epitope, but also in lacking the most common form of sialic acid expressed in all other mammals. Humans have lost the ability to express N-glycolylneuraminic acid due to a deletion mutation in the gene of the enzyme CMP-N-acetylneuraminic acid hydroxylase which converts N-acetylneuraminic acid to N-glycolylneuraminic acid (42). Thus, whereas all mammals other than humans (including chimpanzees) express more N-glycolylneuraminic acid than N-acetylneuraminic acid, humans only express N-acetylneuraminic, leading to humans recognizing N-glycolylneuraminic acid as a foreign antigen. Some have suggested that this difference in sialic acid usage between humans and pigs accounts for the non-Galα1→3Gal epitope recognized by non-Galα1→3Gal antibodies contributing to xenograft rejection (43). What we have shown is the corollary—that pigs have innate cellular immune receptors capable of recognizing the difference in N-acetylneuraminic acid expression in pigs versus humans.
While the destruction of human erythrocytes during extracorporeal porcine liver xenoperfusion was the impetus for elucidating the underlying molecular mechanism, the identification of a lectin-carbohydrate recognition event in innate cellular xenogeneic recognition mechanisms has broader implications (6, 7, 12). For example, these graft vs. host recognition mechanisms will also play a role in bridging liver xenografts (44) and in lung xenotransplantation (45). While we studied a graft versus host response when viewed from the perspective of extracorporeal porcine liver perfusion as a treatment of fulminant hepatic failure, we study this model being cognizant that the knowledge obtained may one day provide insight into host versus graft delayed xenograft rejection. We propose that our observation plays an important role in understanding how host macrophages recognize xenogeneic tissue. Supporting this idea are many studies showing that in the absence of macrophages, xenograft survival is increased (4, 46–56). Elucidating the mechanism(s) by which macrophages or other innate immune cells contribute to xenograft rejection, will enable the development of strategies to overcome this barrier.
For example, human monocytes have been shown to bind porcine endothelium via the interaction of galectin-3 and the xenoantigen Gal-α-(1,3)Gal-β(1,4)GlcNAc-R (57). Kwiatkowski and Itescu demonstrated that human monocytes use unidentified receptors to bind carbohydrates on xenogeneic porcine endothelial cells (58) capable of directly recognizing both a terminally sialylated porcine carbohydrate epitope (59) and the commonly expressed Galα1→3Gal epitope found on porcine endothelium (60). In addition, human natural killer cells have been shown to bind the Galα1→3Gal epitope on porcine endothelial cells in the absence of antibody and complement (61–63). In other species, rat Kupffer cells have been shown to bind human erythrocytes through the GalNAc/Gal-particle receptor in the absence of antibody and complement (64).
Given the role that we have established in this manuscript for sialoadhesin (siglec-1) and the established role for siglecs to bind sialic acid motifs, it is possible that other siglecs or other innate immune receptors contribute to xenogeneic recognition leading to delayed xenograft rejection. To date, sixteen human siglec proteins have been identified. Some bind N-acetylneuraminic sialic acid while others have specificities to N-glycolylneuraminic acid sialosides (65). The specificity we have identified in this study has highlighted one of the unique genetic differences between humans and chimpanzees (and all other animals), so that humans express only the precursor form of sialic acid that is used by all other mammals (42). This study shows how the porcine innate immune system capitalized on this difference to allow the recognition of human erythrocytes and illustrates a limitation of using non-human primates studies to model human extracorporeal porcine liver perfusion (66).
Even so, while pigs express primarily N-glycolylneuraminic acid, the precursor N-acetylneuraminic acid is also present, for example on the surface of porcine aortic endothelial cells (67) and in the porcine liver (68). Therefore, it is not clear why porcine macrophages do not bind self N-acetylneuraminic acid on the surface of porcine cells, yet recognize this structure on human erythrocytes. We provide three possible explanations for this observation. First, porcine sialoadhesin may recognize the different density or spacing of N-acetylneuraminic acid on human versus porcine erythrocytes. Second, porcine sialoadhesin may not bind self-sialic acid due to regulation of sialoadhesin binding through cis interactions with the macrophage glycocalyx (69). Third, porcine macrophages may not bind “self” because a negative signal is communicating, “do not eat me” signals. Sialoadhesin may deliver a positive signal that tells macrophages to bind and eliminate xenogeneic cells, or more likely pathogens such as PRRSV, while other receptor-ligand interactions such as CD47-SIRPα provide a negative signal communicating “do not phagocytose.”
It has been demonstrated in mice that erythrocytes lacking CD47 are readily cleared by splenic macrophages (70, 71). CD47 serves as an erythrocyte marker of self since erythrocytes lack MHC Class I expression. The CD47-SIRPα interaction has been studied in the field of xenotransplantation and molecular incompatibilities have been demonstrated between porcine CD47 and human SIRPα resulting in the phagocytosis of porcine erythrocytes by human macrophages (72). If the CD47/SIRPα interaction in the reverse combination – porcine SIRPα interaction with human CD47 – was comparable, then porcine sialoadhesin would recognize and bind xenogeneic cells because the macrophage would not receive a negative signal inactivating phagocytosis due to species-specific incompatibilities.
A porcine graft versus host response against humans is not limited to erythrocytes. Porcine enriched liver sinusoidal endothelial cells have been shown to mediate phagocytosis of human platelets by the asialoglycoprotein receptor-1 (73). Interestingly, fetuin (a glycoprotein composed of sialic acid-terminated β-glucans) did not prevent human platelet uptake by porcine endothelial cells, a distinction that might be due to the differences in glycosylation between platelets and erythrocytes or receptor expression on endothelial cells versus macrophages. While it is clear that lectins play an important role in innate cellular immunity against xenografts, recent data demonstrating that porcine Kupffer cells bind human platelets using CD18 emphasize that innate cellular recognition of xenografts is not limited to lectin-carbohydrate interactions (74).
We have observed a graft versus host innate cellular immune response to xenogeneic targets during extracorporeal porcine liver perfusion. Some may argue that Kupffer cell binding of human erythrocytes could be overcome by simply removing porcine macrophages. However, deletion of the macrophage progenitor cells has never been successfully accomplished, suggesting that it is an embryonic lethal knock-out. Alternatively, the macrophages could be removed prior to xenotransplantation by treating the organ with clodronate liposomes that when ingested by macrophages, release clodronate into the cytoplasm and induce apoptosis, killing the macrophage. While this approach may prevent loss of erythrocytes during extracorporeal liver perfusion, such pre-treatment may compromise the immune and physiological function of the liver, which may be one of the important benefits of extracorporeal liver perfusion. Another possible solution during extracorporeal liver perfusion would be to block porcine macrophage recognition of human erythrocytes using an inhibitor of sialoadhesin. Solid organ xenografting of liver or lung would require a permanent solution to sialoadhesin-mediated binding of human erythrocytes. Possibly, sialoadhesin knock-out pigs would provide the best solution for extracorporeal liver perfusion. Mice deficient in sialoadhesin have already been prepared and are viable and healthy (75).
Acknowledgements
The authors wish to thank the following individuals at the University of Toledo for their contributions to this study: Karen Domenico and Tom Sawyer, for flow cytometry guidance; Lisa Twining, for technical reading of the manuscript; Katherine Goans and Richard Rulman, for animal surgical assistance; and Andrea Kalinoski, for help with confocal microscopy. The authors wish to thank Christopher Burlak at the Indiana University School of Medicine for his mentoring of J.P.W and for providing the hEGP and pEGP used in these studies. Lastly, the authors wish to thank C. Vanmaercke and C. Bracke from Ghent University for their assistance in isolation of porcine alveolar macrophages and the monoclonal antibody studies.
This work was supported by the National Institutes of Health (R01-DK066160), monies provided by Life Connection of Ohio, and start-up funds from the University of Toledo.
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Bach FH, Winkler H, Ferran C, Hancock WW, Robson SC. Delayed xenograft rejection. Immunol Today. 1996;17(8):379–384. doi: 10.1016/0167-5699(96)10024-4. [DOI] [PubMed] [Google Scholar]
- 2.Dorling A. Are anti-endothelial cell antibodies a pre-requisite for the acute vascular rejection of xenografts? Xenotransplantation. 2003;10(1):16–23. doi: 10.1034/j.1399-3089.2003.01134.x. [DOI] [PubMed] [Google Scholar]
- 3.Goddard MJ, Dunning J, Horsley J, Atkinson C, Pino-Chavez G, Wallwork J. Histopathology of cardiac xenograft rejection in the pig-to-baboon model. J Heart Lung Transplant. 2002;21(4):474–484. doi: 10.1016/s1053-2498(01)00402-8. [DOI] [PubMed] [Google Scholar]
- 4.Basker M, Alwayn IP, Buhler L, Harper D, Abraham S, Kruger Gray H, et al. Clearance of mobilized porcine peripheral blood progenitor cells is delayed by depletion of the phagocytic reticuloendothelial system in baboons. Transplantation. 2001;72(7):1278–1285. doi: 10.1097/00007890-200110150-00017. [DOI] [PubMed] [Google Scholar]
- 5.Ide K, Ohdan H, Kobayashi T, Hara H, Ishiyama K, Asahara T. Antibody- and complement-independent phagocytotic and cytolytic activities of human macrophages toward porcine cells. Xenotransplantation. 2005;12(3):181–188. doi: 10.1111/j.1399-3089.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 6.Rees MA, Butler AJ, Chavez-Cartaya G, Wight DG, Casey ND, Alexander G, et al. Prolonged function of extracorporeal hDAF transgenic pig livers perfused with human blood. Transplantation. 2002;73(8):1194–1202. doi: 10.1097/00007890-200204270-00003. [DOI] [PubMed] [Google Scholar]
- 7.Rees MA, Butler AJ, Davies HF, Bolton E, Wight DG, Skepper J, et al. Porcine livers perfused with human blood mount a graft-versus-"host" reaction. Transplantation. 2002;73(9):1460–1467. doi: 10.1097/00007890-200205150-00016. [DOI] [PubMed] [Google Scholar]
- 8.Rees MA, Butler AJ, Brons IG, Negus MC, Skepper JN, Friend PJ. Evidence of macrophage receptors capable of direct recognition of xenogeneic epitopes without opsonization. Xenotransplantation. 2005;12(1):13–19. doi: 10.1111/j.1399-3089.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 9.Rees MA, Butler AJ, Negus MC, Davies HF, Friend PJ. Classical pathway complement destruction is not responsible for the loss of human erythrocytes during porcine liver perfusion. Transplantation. 2004;77(9):1416–1423. doi: 10.1097/01.tp.0000121135.24688.a3. [DOI] [PubMed] [Google Scholar]
- 10.Burlak C, Twining LM, Rees MA. Carbohydrates borne on human glycophorin A are recognized by porcine Kupffer cells. Transplantation. 2005;80(1):66–74. doi: 10.1097/01.tp.0000162975.88938.d2. [DOI] [PubMed] [Google Scholar]
- 11.Burlak C, Twining LM, Rees MA. Terminal sialic acid residues on human glycophorin A are recognized by porcine kupffer cells. Transplantation. 2005;80(3):344–352. doi: 10.1097/01.tp.0000162974.94890.9f. [DOI] [PubMed] [Google Scholar]
- 12.Rees MA. A novel role for lectins in xenotransplantation. Xenotransplantation. 2005;12(1):7–12. doi: 10.1111/j.1399-3089.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 13.May AP, Robinson RC, Vinson M, Crocker PR, Jones EY. Crystal structure of the N-terminal domain of sialoadhesin in complex with 3' sialyllactose at 1.85 A resolution. Mol Cell. 1998;1(5):719–728. doi: 10.1016/s1097-2765(00)80071-4. [DOI] [PubMed] [Google Scholar]
- 14.Crocker PR, Gordon S. Properties and distribution of a lectin-like hemagglutinin differentially expressed by murine stromal tissue macrophages. J Exp Med. 1986;164(6):1862–1875. doi: 10.1084/jem.164.6.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nath D, Hartnell A, Happerfield L, Miles DW, Burchell J, Taylor-Papadimitriou J, et al. Macrophage-tumour cell interactions: identification of MUC1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor, sialoadhesin. Immunology. 1999;98(2):213–219. doi: 10.1046/j.1365-2567.1999.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee A, Chick JM, Kolarich D, Haynes PA, Robertson GR, Tsoli M, et al. Liver membrane proteome glycosylation changes in mice bearing an extra-hepatic tumor. Molecular & cellular proteomics : MCP. 2011;10(9) doi: 10.1074/mcp.M900538-MCP200. M900538MCP900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser IP, Gordon S. Murine erythroleukemia (MEL) cells bear ligands for the sialoadhesin and erythroblast receptor macrophage hemagglutinins. Eur J Cell Biol. 1994;64(2):217–221. [PubMed] [Google Scholar]
- 18.Jiang HR, Hwenda L, Makinen K, Oetke C, Crocker PR, Forrester JV. Sialoadhesin promotes the inflammatory response in experimental autoimmune uveoretinitis. J Immunol. 2006;177(4):2258–2264. doi: 10.4049/jimmunol.177.4.2258. [DOI] [PubMed] [Google Scholar]
- 19.Crocker PR, Hartnell A, Munday J, Nath D. The potential role of sialoadhesin as a macrophage recognition molecule in health and disease. Glycoconj J. 1997;14(5):601–609. doi: 10.1023/a:1018588526788. [DOI] [PubMed] [Google Scholar]
- 20.Jones C, Virji M, Crocker PR. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol Microbiol. 2003;49(5):1213–1225. doi: 10.1046/j.1365-2958.2003.03634.x. [DOI] [PubMed] [Google Scholar]
- 21.Delputte PL, Costers S, Nauwynck HJ. Analysis of porcine reproductive and respiratory syndrome virus attachment and internalization: distinctive roles for heparan sulphate and sialoadhesin. J Gen Virol. 2005;86(Pt 5):1441–1445. doi: 10.1099/vir.0.80675-0. [DOI] [PubMed] [Google Scholar]
- 22.Seyerl M, Kirchberger S, Majdic O, Seipelt J, Jindra C, Schrauf C, et al. Human rhinoviruses induce IL-35-producing Treg via induction of B7-H1 (CD274) and sialoadhesin (CD169) on DC. European journal of immunology. 2010;40(2):321–329. doi: 10.1002/eji.200939527. [DOI] [PubMed] [Google Scholar]
- 23.Delputte PL, Nauwynck HJ. Porcine arterivirus entry in macrophages: heparan sulfate-mediated attachment, sialoadhesin-mediated internalization, and a cell-specific factor mediating virus disassembly and genome release. Adv Exp Med Biol. 2006;581:247–252. doi: 10.1007/978-0-387-33012-9_41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delputte PL, Van Gorp H, Favoreel HW, Hoebeke I, Delrue I, Dewerchin H, et al. Porcine sialoadhesin (CD169/Siglec-1) is an endocytic receptor that allows targeted delivery of toxins and antigens to macrophages. PLoS ONE. 2011;6(2) doi: 10.1371/journal.pone.0016827. e16827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crocker PR, Gordon S. Mouse Macrophage Hemagglutinin (Sheep Erythrocyte Receptor) With Specificity For Sialylated Glycoconjugates Characterized By A Monoclonal Antibody. J Exp Med. 1989 Apr;169:1333–1346. doi: 10.1084/jem.169.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guide for the Care and Use of Laboratory Animals. The National Academies Press; 1996. Research IoLA, Sciences CoL, Council NR. [PubMed] [Google Scholar]
- 27.Duan X, Nauwynck H, Pensaert M. Effects of origin and state of differentiation and activation of monocytes/macrophages on their susceptibility to PRRSV. Arch Virol. 1997;142:2483–2497. doi: 10.1007/s007050050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delputte PL, Nauwynck HJ. Porcine arterivirus infection of alveolar macrophages is mediated by sialic acid on the virus. J Virol. 2004;78(15):8094–8101. doi: 10.1128/JVI.78.15.8094-8101.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wensvoort G, Terpstra C, Pol JM, ter Laak EA, Bloemraad M, de Kluyver EP, et al. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet Q. 1991;13(3):121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- 30.Delputte PL, Van Breedam W, Delrue I, Oetke C, Crocker PR, Nauwynck HJ. Porcine arterivirus attachment to the macrophage-specific receptor sialoadhesin is dependent on the sialic acid-binding activity of the N-terminal immunoglobulin domain of sialoadhesin. J Virol. 2007;81(17):9546–9550. doi: 10.1128/JVI.00569-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan X, Nauwynck HJ, Favoreel HW, Pensaert MB. Identification of a putative receptor for porcine reproductive and respiratory syndrome virus on porcine alveolar macrophages. J Virol. 1998;72(5):4520–4523. doi: 10.1128/jvi.72.5.4520-4523.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanderheijden N, Delputte PL, Favoreel HW, Vandekerckhove J, Van Damme J, van Woensel PA, et al. Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J Virol. 2003;77(15):8207–8215. doi: 10.1128/JVI.77.15.8207-8215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wieczorek-Krohmer M, Weiland E, Conzelmann KK, Visser N, Van Woensel P, Thiel C, et al. Porcine reproductive and respiratory syndrome virus (PRRSV): monoclonal antibodies detect common epitopes on two viral proteins of European and U.S. isolates. Vet Microbiol. 1996;51:257–266. doi: 10.1016/0378-1135(96)00047-8. [DOI] [PubMed] [Google Scholar]
- 34.Ducreux J, Crocker PR, Vanbever R. Analysis of sialoadhesin expression on mouse alveolar macrophages. Immunol Lett. 2009;124(2):77–80. doi: 10.1016/j.imlet.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Frei K, Steger C, Samorapoompichit P, Lucas T, Forster O. Expression and function of sialoadhesin in rat alveolar macrophages. Immunol Lett. 2000;71(3):167–170. doi: 10.1016/s0165-2478(99)00180-7. [DOI] [PubMed] [Google Scholar]
- 36.Galili U. Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today. 1993;14(10):480–482. doi: 10.1016/0167-5699(93)90261-i. [DOI] [PubMed] [Google Scholar]
- 37.Good AH, Cooper DK, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc. 1992;24(2):559–562. [PubMed] [Google Scholar]
- 38.Sandrin MS, Vaughan HA, Dabkowski PL, McKenzie IF. Anti-pig IgM antibodies in human serum react predominantly with Gal(a1-3)Gal epitopes. Proc Natl Acad Sci U S A. 1993;90:11391–11395. doi: 10.1073/pnas.90.23.11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oriol R, Ye Y, Koren E, Cooper DK. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation. Transplantation. 1993;56(6):1433–1442. doi: 10.1097/00007890-199312000-00031. [DOI] [PubMed] [Google Scholar]
- 40.Galili U, Shohet S, Kobrin E, Stults C, Macher B. Man, apes and old world monkeys differ from other mammals in the expression of a-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. [PubMed] [Google Scholar]
- 41.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci U S A. 1998;95(20):11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ezzelarab M, Ayares D, Cooper DK. Carbohydrates in xenotransplantation. Immunology and cell biology. 2005;83(4):396–404. doi: 10.1111/j.1440-1711.2005.01344.x. [DOI] [PubMed] [Google Scholar]
- 44.Chari RS, Collins BH, Magee JC, DiMaio JM, Kirk AD, Harland RC, et al. Brief report: treatment of hepatic failure with ex vivo pig-liver perfusion followed by liver transplantation. The New England journal of medicine. 1994;331(4):234–237. doi: 10.1056/NEJM199407283310404. [DOI] [PubMed] [Google Scholar]
- 45.Pfeiffer S, Zor GL, 3rd, Zhang JP, Giorgio TD, Robson SC, Azimzadeh AM, et al. Hyperacute lung rejection in the pig-to-human model. III. Platelet receptor inhibitors synergistically modulate complement activation and lung injury. Transplantation. 2003;75(7):953–959. doi: 10.1097/01.TP.0000058517.07194.90. [DOI] [PubMed] [Google Scholar]
- 46.Koyamada N, Sato A, Takayama J, Usuda M, Kawagishi N, Doi H, et al. Macrophage depletion prevents anti-graft antibody production and results in long-term survival in xenotransplantation. Transplant Proc. 2005;37(1):514–515. doi: 10.1016/j.transproceed.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 47.Van Rijn RS, Simonetti ER, Hagenbeek A, Hogenes MC, de Weger RA, Canninga-van Dijk MR, et al. A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2−/− gammac−/− double-mutant mice. Blood. 2003;102(7):2522–2531. doi: 10.1182/blood-2002-10-3241. [DOI] [PubMed] [Google Scholar]
- 48.Yi S, Hawthorne WJ, Lehnert AM, Ha H, Wong JK, van Rooijen N, et al. T cell-activated macrophages are capable of both recognition and rejection of pancreatic islet xenografts. J Immunol. 2003 Mar;1:2750–2758. doi: 10.4049/jimmunol.170.5.2750. [DOI] [PubMed] [Google Scholar]
- 49.Gaca JG, Palestrant D, Lukes DJ, Olausson M, Parker W, Davis RD., Jr Prevention of acute lung injury in swine: depletion of pulmonary intravascular macrophages using liposomal clodronate. J Surg Res. 2003;112(1):19–25. doi: 10.1016/s0022-4804(03)00142-2. [DOI] [PubMed] [Google Scholar]
- 50.Omer A, Keegan M, Czismadia E, De Vos P, Van Rooijen N, Bonner-Weir S, et al. Macrophage depletion improves survival of porcine neonatal pancreatic cell clusters contained in alginate macrocapsules transplanted into rats. Xenotransplantation. 2003;10(3):240–251. doi: 10.1034/j.1399-3089.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 51.Takayama J, Koyamada N, Abe T, Hatsugai K, Usuda M, Ohkohchi N, et al. Macrophage depletion prevents accelerated rejection and results in long-term survival in hamster to rat cardiac xenotransplantation. Transplant Proc. 2000;32(5):1016. doi: 10.1016/s0041-1345(00)01090-3. [DOI] [PubMed] [Google Scholar]
- 52.Wu G, Korsgren O, Zhang J, Song Z, van Rooijen N, Tibell A. Pig islet xenograft rejection is markedly delayed in macrophage-depleted mice: a study in streptozotocin diabetic animals. Xenotransplantation. 2000;7(3):214–220. doi: 10.1034/j.1399-3089.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- 53.Fox A, Koulmanda M, Mandel TE, van Rooijen N, Harrison LC. Evidence that macrophages are required for T-cell infiltration and rejection of fetal pig pancreas xenografts in nonobese diabetic mice. Transplantation. 1998;66(11):1407–1416. doi: 10.1097/00007890-199812150-00002. [DOI] [PubMed] [Google Scholar]
- 54.Andres A, Toso C, Morel P, Bosco D, Bucher P, Oberholzer J, et al. Macrophage depletion prolongs discordant but not concordant islet xenograft survival. Transplantation. 2005;79(5):543–549. doi: 10.1097/01.tp.0000151764.39095.ca. [DOI] [PubMed] [Google Scholar]
- 55.Cantu E, Gaca JG, Palestrant D, Baig K, Lukes DJ, Gibson SE, et al. Depletion of pulmonary intravascular macrophages prevents hyperacute pulmonary xenograft dysfunction. Transplantation. 2006;81(8):1157–1164. doi: 10.1097/01.tp.0000169758.57679.2a. [DOI] [PubMed] [Google Scholar]
- 56.Cantu E, Balsara KR, Li B, Lau C, Gibson S, Wyse A, et al. Prolonged function of macrophage, von Willebrand factor-deficient porcine pulmonary xenografts. Am J Transplant. 2007;7(1):66–75. doi: 10.1111/j.1600-6143.2006.01603.x. [DOI] [PubMed] [Google Scholar]
- 57.Jin R, Greenwald A, Peterson MD, Waddell TK. Human monocytes recognize porcine endothelium via the interaction of galectin 3 and alpha-GAL. J Immunol. 2006;177(2):1289–1295. doi: 10.4049/jimmunol.177.2.1289. [DOI] [PubMed] [Google Scholar]
- 58.Itescu S, Kwiatkowski P, Artrip JH, Wang SF, Ankersmit J, Minanov OP, et al. Role of natural killer cells, macrophages, and accessory molecule interactions in the rejection of pig-to-primate xenografts beyond the hyperacute period. Hum Immunol. 1998;59(5):275–286. doi: 10.1016/s0198-8859(98)00026-3. [DOI] [PubMed] [Google Scholar]
- 59.Kwiatkowski P, Artrip JH, Michler RE, Wang SF, McKenzie IF, Sandrin MS, et al. Human monocytes bind to ligands on porcine endothelium containing the gal alpha (1,3)-Gal antigen. Transplant Proc. 2000;32(5):931. doi: 10.1016/s0041-1345(00)01043-5. [DOI] [PubMed] [Google Scholar]
- 60.Kwiatkowski P, Artrip JH, Wang SF, Michler RE, Itescu S. Human monocytes bind to two distinct carbohydrate capping structures on porcine endothelium. Transplant Proc. 2000;32(5):927. doi: 10.1016/s0041-1345(00)01040-x. [DOI] [PubMed] [Google Scholar]
- 61.Miyagawa S, Nakai R, Yamada M, Tanemura M, Ikeda Y, Taniguchi N, et al. Regulation of natural killer cell-mediated swine endothelial cell lysis through genetic remodeling of a glycoantigen. J Biochem (Tokyo) 1999;126(6):1067–1073. doi: 10.1093/oxfordjournals.jbchem.a022551. [DOI] [PubMed] [Google Scholar]
- 62.Artrip JH, Kwiatkowski P, Michler RE, Wang SF, Tugulea S, Ankersmit J, et al. Target cell susceptibility to lysis by human natural killer cells is augmented by alpha(1,3)-galactosyltransferase and reduced by alpha(1-2)-fucosyltransferase. J Biol Chem. 1999;274(16):10717–10722. doi: 10.1074/jbc.274.16.10717. [DOI] [PubMed] [Google Scholar]
- 63.Inverardi L, Clissi B, Stolzer AL, Bender JR, Sandrin MS, Pardi R. Human natural killer lymphocytes directly recognize evolutionarily conserved oligosaccharide ligands expressed by xenogeneic tissues. Transplantation. 1997;63(9):1318–1330. doi: 10.1097/00007890-199705150-00021. [DOI] [PubMed] [Google Scholar]
- 64.Mohr M, Kolb H, Kolb-Bachofen V, Schlepper-Schafer J. Recognition of xenogeneic erythrocytes: the GalNAc/Gal-particle receptor of rat liver macrophages mediates or participates in Recognition. Biology of the Cell. 1987;60:217–224. doi: 10.1111/j.1768-322x.1987.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 65.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 66.Ekser B, Long C, Echeverri GJ, Hara H, Ezzelarab M, Lin CC, et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. American journal of transplantation. 2010;10(2):273–285. doi: 10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 67.Kobayashi T, Yokoyama I, Suzuki A, Abe M, Hayashi S, Matsuda H, et al. Lack of antibody production against Hanganutziu-Deicher (H-D) antigens with N-glycolylneuraminic acid in patients with porcine exposure history. Xenotransplantation. 2000;7(3):177–180. doi: 10.1034/j.1399-3089.2000.00056.x. [DOI] [PubMed] [Google Scholar]
- 68.Zeleny R, Kolarich D, Strasser R, Altmann F. Sialic acid concentrations in plants are in the range of inadvertent contamination. Planta. 2006;224(1):222–227. doi: 10.1007/s00425-005-0206-8. [DOI] [PubMed] [Google Scholar]
- 69.Hartnell A, Steel J, Turley H, Jones M, Jackson DG, Crocker PR. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood. 2001;97(1):288–296. doi: 10.1182/blood.v97.1.288. [DOI] [PubMed] [Google Scholar]
- 70.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 71.Hagmann M. A new way to keep immune cells in check. Science. 2000;288(5473):1945–1946. doi: 10.1126/science.288.5473.1945. [DOI] [PubMed] [Google Scholar]
- 72.Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007;104(12):5062–5066. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paris L, Chihara R, Reyes L, Sidner R, Estrada J, Downey S, et al. ASGR1 expressed by porcine enriched liver sinusoidal endothelial cells mediates human platelet phagocytosis in vitro. Xenotransplantation. 2011;18(4):245–251. doi: 10.1111/j.1399-3089.2011.00639.x. [DOI] [PubMed] [Google Scholar]
- 74.Chihara R, Paris L, Reyes L, Sidner R, Estrada J, Downey S, et al. Primary porcine Kupffer cell phagocytosis of human platelets involves the CD18 receptor. Transplantation. 2011;92(7):739–744. doi: 10.1097/TP.0b013e31822bc986. [DOI] [PubMed] [Google Scholar]
- 75.Oetke C, Vinson MC, Jones C, Crocker PR. Sialoadhesin-deficient mice exhibit subtle changes in B- and T-cell populations and reduced immunoglobulin M levels. Mol Cell Biol. 2006;26(4):1549–1557. doi: 10.1128/MCB.26.4.1549-1557.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]