Abstract
Autism is a neurodevelopmental disorder whose diagnosis is based on three behavioral criteria: unusual reciprocal social interactions, deficits in communication, and stereotyped repetitive behaviors with restricted interests. A large number of de novo single gene mutations and chromosomal deletions are associated with autism spectrum disorders. Based on the strong genetic evidence, mice with targeted mutations in homologous genes have been generated as translational research tools. Mouse models of autism have revealed behavioral and biological outcomes of mutations in risk genes. The field is now poised to employ the most robust phenotypes in the most replicable mouse models for preclinical screening of novel therapeutics.
Keywords: neurodevelopmental disorder, autism, genetics, mouse model, social behavior, therapeutics, olfactory, ultrasonic vocalization, repetitive behavior, self-grooming, anxiety, cognitive, Fragile X, tuberous sclerosis, mGluRS antagonist
Abstract
El autismo es un trastorno del neurodesarrollo cuyo diagnóstico se basa en tres criterios conductuales: interacciones sociales recíprocas inusuales, déficit en la comunicación y conductas repetitivas estereotipadas con intereses disminuidos. Los trastornos del espectro autista están asociados con un gran número de mutaciones monogénicas de novo y deleciones cromosómicas. En base a la gran evidencia genética, se han generado ratones con mutaciones específicas en genes homólogos como herramientas de investigatión traslacional. Hay modelos de autismo en el ratón que han revelado resultados conductuales y biológicos que corresponden a mutaciones en genes de riesgo en genes de riesgo. Este campo ahora está preparado para emplear los fenotipos más potentes en los modelos de ratón más reproducibles para la evaluatión preclínica de nuevas terapéuticas.
Abstract
L'autisme est un trouble du neurodéveloppement dont le diagnostic se fonde sur 3 critères comportementaux: des interactions sociales réciproques inhabituelles, des déficits de communication et des comportements répétitifs stéréotypés accompagnés d'intérêts restreints. Les troubles autistiques sont associés à de nombreuses mutations monogéniques de novo et à des deletions chromosomiques. Sur la base des arguments génétiques solides, des souris aux mutations ciblées sur des gènes homologues ont été élevées comme outil de recherche translationnelle. Les modèles murins d'autisme ont présenté des mutations biologiques et comportementales correspondant aux mutations des gènes à risque. Ce domaine de recherche est maintenant prêt à employer les phénotypes les plus fiables des modèles murins les plus reproductibles pour le dépistage préclinique de nouveaux traitements.
Genetic causes of autism spectrum disorder
Autism spectrum disorders were originally diagnosed by Kanner and Asperger in the 1930s.1,2 However, the diagnostic criteria were not codified until the 1994 Diagnostic and Statistical Manual of Mental Disorders (DSM).3 Astonishingly high heritability of autism spectrum disorders, reaching 90% concordance for monozygotic twins, as compared with less than 10% concordance for dizygotic twins and siblings, along with a 4:1 male:female ratio of prevalence, quickly led to an major international search for genes causing autism. By assembling large numbers of simplex and familial cases, several research consortia have discovered single gene mutations, rare and common polymorphisms, and epigenetic modifications associated with autism.4,5 Copy number variants, including duplications of a sequence of genes within defined chromosomal loci, were reported to be relatively common in autism.6-9 Clearly, autism is not a single-gene disorder.
To parse the role of each of these many genetic abnormalities in the etiology and symptomology of autism spectrum disorders, and in other neurodevelopmental disorders in which autism is concomitantly diagnosed, homologous genetic mutations have been generated in experimental animals. Because the targeted gene mutation technology was perfected in the mouse, mice are currently used throughout biomedical research as the primary model organism for generating transgenic and knockout mouse models of human genetic disorders. Table I and the descriptions in this review illustrate a small portion of the wealth of available mouse models of autism. In addition, recent advances in generating knockout rats10 are leading to the development of mutant rat models of neurodevelopmental disorders.
Table I. Autism-relevant behavoral phenotypes in selected mouse models with targeted mutations in associated with autism.155-169.
| Gene | Protein | Autism-relevant behavoral phenotypes | |
| Integrin β3 | Integrin β3 | Lack of preference for social novelty | |
| Increased repetitive self-grooming | |||
| Nign1 | Neuroligin 1 | Increased repetitive self-grooming | |
| Nign2 | Neuroligin 2 | Increased repetitive self-grooming | |
| Synaptic | Nign3 | Neuroligin 3 | Reduced pup ultrasonic vocalizations |
| cell-adhesion | Sensory abnormalities | ||
| proteins | Nign4 | Neuroligin 4 | Reduced sociability and vocalizations |
| Neurexin-1α | Neurexin-1α | Increased repetitive self-grooming | |
| Cntnap2 | Contactin-associated protein2 | Seizures, reduced social behaviors | |
| Shank3 | Shank3 | Mild reduction in social interactions and adult vocalizations | |
| . | |||
| En2 | Engrailed-2 | Reduced social behaviors - Impaired learning and memory | |
| Signaling and | Met | Tyrosine kinase/ hepatocyte | Cognitive deficit |
| developmental | growth factor receptor | ||
| proteins | Foxp2 | Forkhead box protein 2 | Reduced pup ultrasonic vocalizations |
| Pten | Phosphatase and tensin homolog | Reduced reciprocal social interactions - Reduced sociability | |
| . | |||
| Avrp1 | Vasopressin receptor | Impaired social recognition - Reduced reciprocal social interaction | |
| Reduced ultrasonic vocalizations | |||
| Cadps2 | Calcium-dependent secretion | Reduced reciprocal social interactions | |
| Neurotransmitters | activator 2 | ||
| and receptors | Gabrb3 | GABA A receptor beta3 subunit | Low sociability - Lack of preference for social novelty |
| Repetitive stereotyped circling behavior | |||
| Oxtr | Oxytocin receptor | Impaired social gecognition - Reduced pup ultrasonic vocalizations | |
| Slc6a4 | Solute carrier family 6, member 4 | Low sociability | |
| (Serotonin transporter) | Lack of preference for social novelty |
Because concordance for autism spectrum disorder is not 100% between identical twins, whose genomes are presumably identical, environmental and epigenetic causes of autism spectrum disorders are also under investigation. Hypotheses about prenatal exposure to toxicological and immunological insults, and neuroanatomical lesions, have been modeled in mice, rats, and monkeys.11-18 The challenge now is to understand the consequences of each of these genetic and environmental perturbations, and their interactions. Animal models employing behavioral assays relevant to the specific symptoms offer excellent translational research tools to identify the biological mechanisms underlying the core features of autism spectrum disorder.
How do we model the behavioral symptoms of autism in mice?
As defined in the DSM-IV,3 the diagnosis of autism requires the presence of at least six symptoms, including a minimum of two measures of qualitative impairment in social interaction, one symptom of qualitative impairment in communication, and one symptom of restricted and repetitive behaviour.19,20 The DSM-5 is expected to redefine Autism Spectrum Disorder into two symptom domains: (i) Social interaction and social communication deficits; (ii) Restricted, repetitive patterns of behavior, interests, or activities (http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=94, January 2011). Associated symptoms that appear in subsets of individuals with autism include seizures, anxiety, intellectual impairment, hyperactivity, hyper-responsiveness and hyporesponsiveness to sensory stimuli, sleep disruption, and gastrointestinal distress.20-26
Given that the defining criteria for autism are behavioral, investigations employing mouse models require considerable insight into which specific behaviors in the mouse repertoire are sufficiently relevant to each category of the diagnostic symptoms of autism. Inclusion of behavioral assays relevant to associated symptoms further enhance the heuristic value of animal models of autism spectrum disorders. We and other behavioral neuroscientists have generated a comprehensive set of assays for social interaction, social communication, and repetitive behaviors in mice, to test hypotheses about the causes of autism.27-45 Social approach, reciprocal social interactions, olfactory communication, ultrasonic vocalizations, motor stereotypies such as circling and vertical jumping, repetitive behaviors such as self-grooming and digging, and perserveration in spatial tasks, are now in routine use for phenotyping mouse and rat models of autism and other neurodevelopmental disorders. Procedures for assaying behaviors relevant to associated symptoms of autism, including neurodevelopmental milestones, cognitive abilities, anxiety-like tendencies, seizures, motor dysfunctions, hyperactivity, responsiveness to sensory stimuli, and altered sleep patterns in mice have been adapted from the available behavioral neuroscience literature.46
In each case, we begin with the human endophenotype. In what ways are mouse behaviors similar to a defining feature of autism? Luckily, Mus musculus is a social species. Laboratory mice display a social repertoire that includes approach to olfactory pheromones emitted by other mice, approach to familiar and new conspecifics, reciprocal social interactions, ultrasonic vocalizations, communal nesting, sexual and parenting behaviors, territorial scent marking, and aggressive behaviors.47-49 Standardized methods for scoring adult social approaches, reciprocal social interactions, nesting, sexual interactions, parental behaviors, and aggressive encounters are available in the behavioral neuroscience literature.28,50-61
First diagnostic category
We employ social assays that have been refined from standard tests in the behavioral neuroscience literature.48,49 These choices are designed to maximize relevance to the types of social deficits specific to autism, including playing alone with inanimate toys rather than engaging in social interactions, and inappropriate responses to social cues. To quantify tendencies to engage in reciprocal social interactions, each subject mouse is paired with a novel partner mouse, inside a testing arena that permits free interactions over a test session of 10 to 30 minutes' duration. Digital videocameras record the session for later scoring of multiple parameters of social interactions. Ratings are performed by investigators who are blind to the genotype or treatment of the subject mice. Parameters routinely scored include sniffing, following, physical contact, and allogrooming.58,62 Automated videotracking systems can accurately score some of the simpler elements of social interaclions.34,35,44,63,64
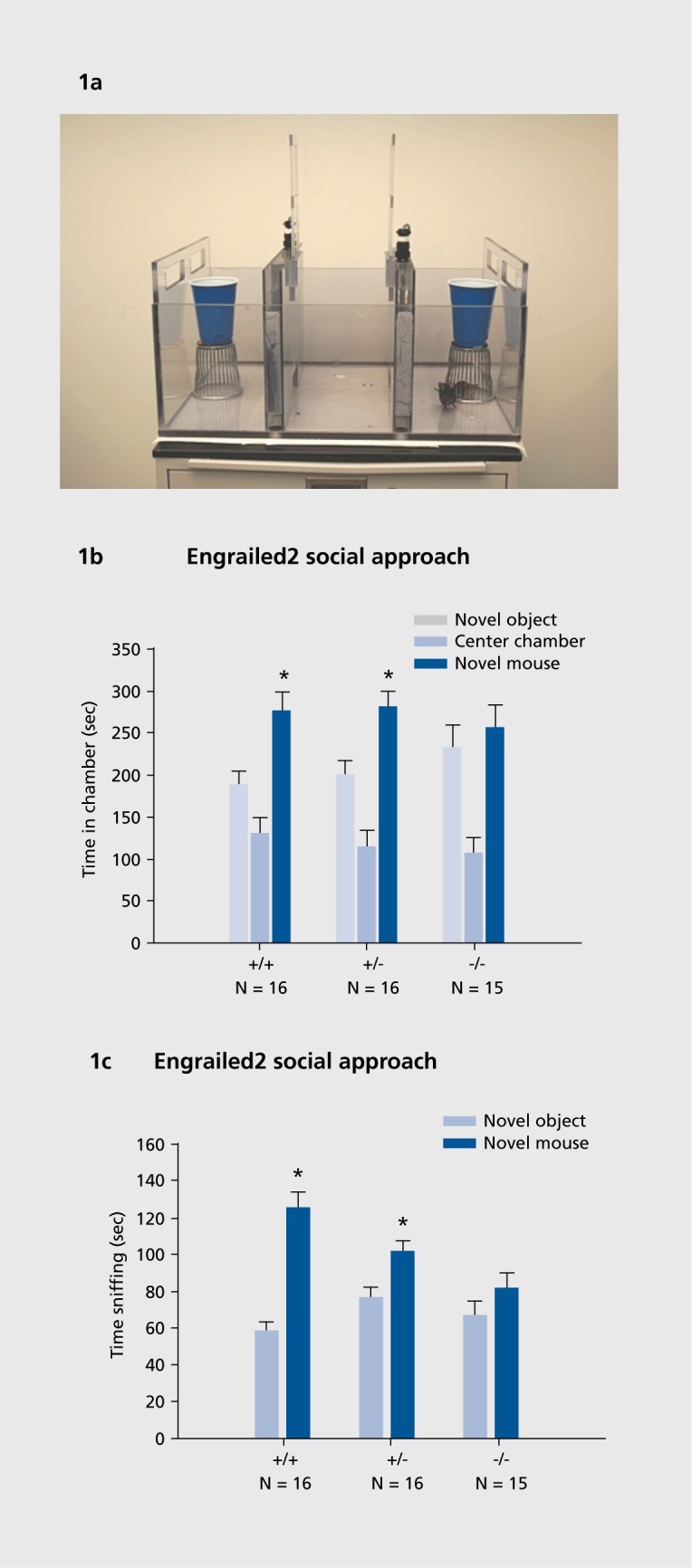
Tendency to spend time with a novel mouse versus a novel nonsocial object is evaluated in a social approach apparatus (Figure 1). This 3-chambered assay, which was developed by our team to provide a simple measure of general sociability,30 is widely used as an initial, highthroughput test for social deficits in mouse models of autism.27,32,34,38,41,64-70
Figure 1. (a) Social approach apparatus for assaying sociability in mice. The subject mouse begins in the empty center chamber of a three-chambered Plexiglas apparatus. A novel object, an inverted wire pencil cup, is placed in one side chamber. A novel mouse, who has not been in visual, olfactory, or tactile contact with the subject, is placed inside an identical wire control cup in the opposite side chamber. Over a 10-minute test session, the automated photocells and software score the amount of time the subject mouse spends in each of the three chambers. The number of entries into each compartment is simultaneously recorded as an internal control for general exploratory activity. Time spent engaged in bone fide social interactions with the novel mouse, and directed exploration of the novel object, are subsequently scored from digital videotapes of the session, by investigators uninformed of the genotype or treatment condition. Photograph by Dr Mu Yang, contributed by the author. (b) Social approach chamber time scores in adult Engrailed2 (En2) knockout mice. Normal sociability is defined as more time spent in the chamber with the novel mouse than in the chamber with the novel object, and more time spent sniffing the novel mouse than sniffing the novel object. Wild-type littermate controls and heterozygotes displayed normal sociability on the chamber time parameter, while En2 null mutants failed to display sociability on this parameter. Reproduced from reference 170: Brielmaier J, Matteson PG, Silverman JL, et al. Autism-relevant social abnormalities and cognitive deficits in engrai!ed-2 knockout mice. PLoSOne. 2012;7:e40914. (c) Social approach sniff time scores in adult En2 mice. Wild-type littermate controls and heterozygotes displayed normal sociability on the sniff time parameter, while En2 null mutants failed to display sociability on this parameter. Time spent sniffing the novel mouse is a more direct measure of social inter actions, and usually more sensitive to mutations. Reproduced from reference 170: Brielmaier J, Matteson PG, Silverman JL, et al. Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS One. 2012;7:e40914.
Second diagnostic category
Social communication in rodents is mostly through the emission and detection of olfactory pheromones, and perhaps to a lesser extent, the emission and detection of ultrasonic vocalizations.40,44,55,57,59,60,71-74 Olfactory communication is assayed by time spent sniffing olfactory stimuli from novel mice, and identification of novel versus familiar mice through olfactory cues.36,75,76
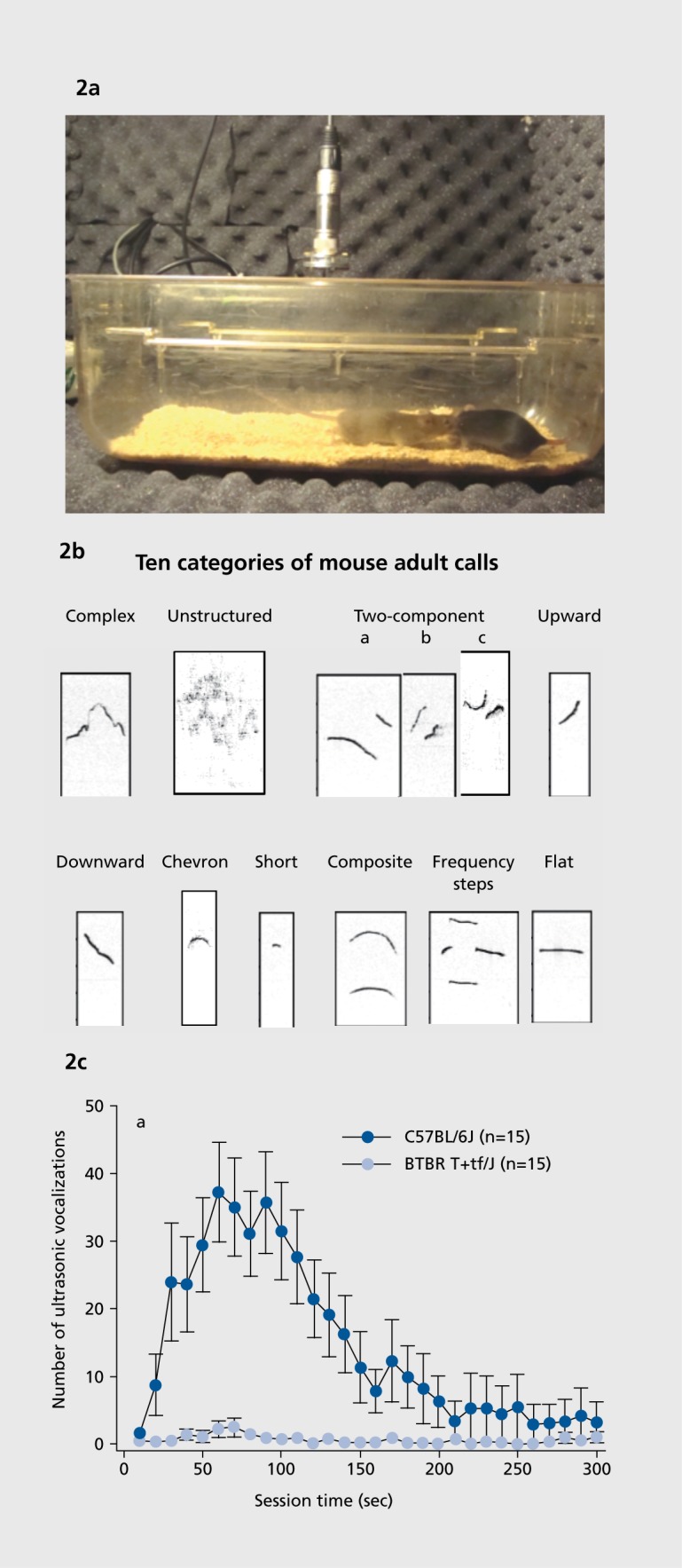
Vocal communication is assayed by recording ultrasonic vocalizations emitted during social interactions77 (Figure 2). Number of calls and their properties are subsequently scored by investigators. Software is available for quantifying some of the simpler parameters. Different patterns of ultrasonic vocalizations are emitted by separated pups, adult males interacting with estrus females or urine from estrus females, adult females interacting with each other, and adult male residents in response to an intruder.72,78
Figure 2. (a) Ultrasonic vocalizations are recorded in adult mice engaged in social interactions, using an ultrasonic microphone and specialized software. Photograph by Dr Jennifer Brielmaier, contributed by the author. (b) Ultrasonic vocalization call categories in adult C57BL/6J mice, an inbred strain with normal sociability. Reproduced from ref 153: Scattoni M, Ricceri L, Crawley J. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10:44-56. Copyright © Munskgaard 2011. (c) C57BL/6J adult male mice emit high numbers of ultrasonic vocalizations, while BTBR adult male mice emit low numbers of ultrasonic vocalizations, during a 5-minute session with an estrus female mouse. BTBR T+tf/J (BTBR) is an inbred strain of mice that displays robust, well-replicated social deficits on multiple tasks,58,62,147 markedly fewer vocalizations during social interaction sessions,59,153,154 and high levels of repetitive self-grooming and marble burying,58,62,83 representing the three diagnostic criteria for autism. Reproduced from ref 154: Wohr M, Roullet Fl, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:35-43. Copyright © Munksgaard 2011.
The anatomical and neurobiological substrates of olfactory and ultrasonic communication in mice do not precisely map onto the biological substrates of language and visual social communication in humans. In addition, considerably more work is needed to fully understand the communication value of ultrasonic vocalizations in mice.
At present, the existing tasks offer a reasonable start for discovering mechanistic similarities between species. Face validity of these mouse social interaction and communication tasks to the tendencies of people with autism to engage in less social approach and interaction, and to respond appropriately to complex social cues, remains inferential. We cannot know what a mouse is thinking, feeling, or intending, but only the quantifiable external expressions of those internai states.
Third diagnostic category
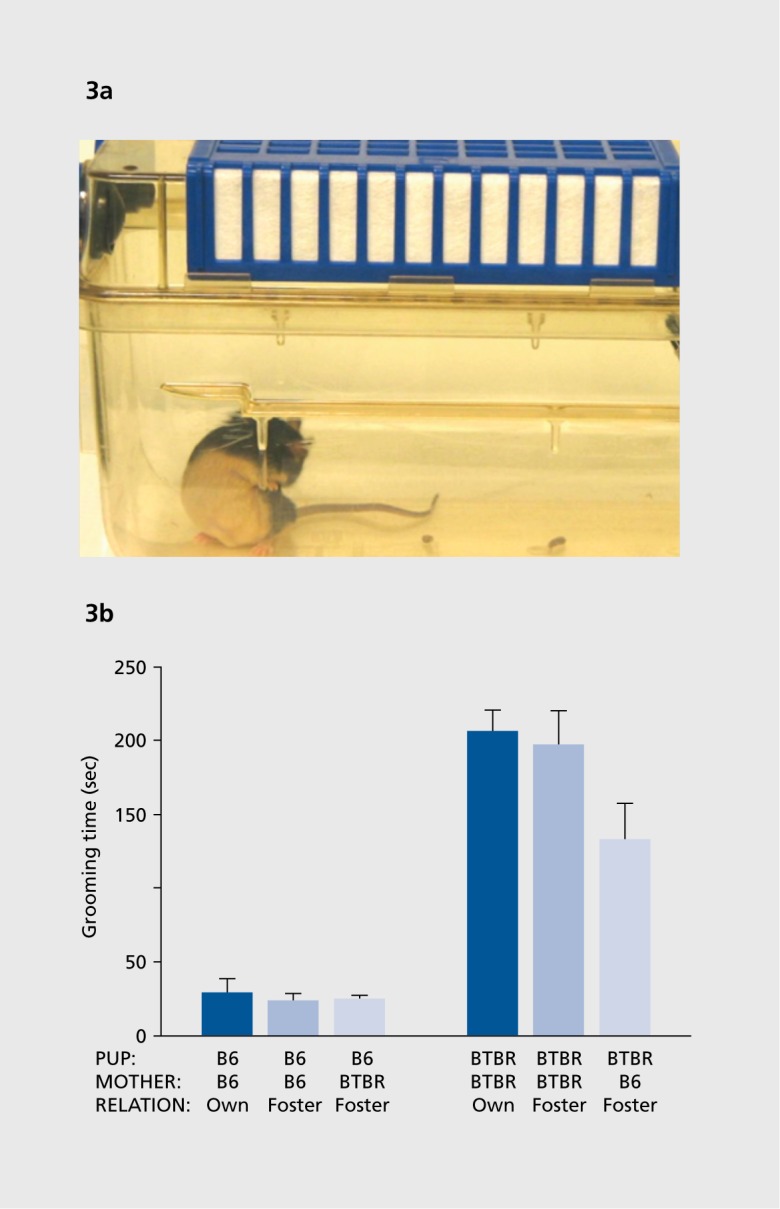
Mice with various genetic mutations exhibit spontaneous motor stereotypies such as circling and vertical jumping, and spontaneous repetitive behaviors such as long bouts of self-grooming and excessive digging in the litter (Figure 3). Assays generally focus on the number of bouts of the behavior, or the cumulative time engaged in the behavior, during a defined test session of 10 minutes or longer.40,58,65,79-81 Restricted interests, insistence on sameness, and special interests are more challenging diagnostic features of autism to model in mice. Perseveration of spatial habits, such as difficulty in learning a new location of a re inforcer in a T-maze or water maze after the initial learning of a first location, has been employed with some success in mouse models of autism.82,83
Figure 3. (a) Unusually high levels of spontaneous repetitive self grooming, in which the normal pattern of grooming behaviors are present but the bouts of grooming are strikingly prolonged, are measured over a 10-minute session in which the subject mouse is in an empty cage. Digital videos of the session are scored by an investigator uninformed of the genotype or treatment condition. Photograph by Dr Mu Yang, contributed by the author. (b) High levels of repetitive self-grooming are displayed by adult BTBR mice as compared with C57BL/6J mice. In this cross-fostering experiment, self-grooming scores in adults were found to be independent of the strain of the dam that raised the pup. Reproduced from ref 55: PankseppJB, Jochman KA, Kim JU, et al. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2:e351. Copyright Public Library of Science 2007.
Associated symptoms
Established, standardized tests are available in the voluminous behavioral neuroscience literature for most of the associated symptoms of autism.46,84-86 Neurodevelopmental milestones are scored from postnatal day 2 to postnatal day 18 on physical attributes such as body length and eye opening, and behavioral attributes such as responses to handling and the righting reflex. Spontaneous seizures are scored with a rating scale or EEG electroencephalography. Learning and memory tests for mice include Morris water maze spatial navigation tasks, contextual and cued fearconditioned freezing after exposure to an aversive footshock, and operant nose-poke and touch-screen reinforcement schedules.
Anxiety-related tests for rodents are primarily approach-avoidance conflicts. Mice are nocturnal. They prefer to be in dimly lit, enclosed environments. The current gold standard for anxiety-like tests for mice is the elevated plus-maze, which consists of two open and two enclosed arms, raised 1 meter from the floor, thus offering the choice between enclosed spaces and a high dropoff ledge.85 The corroborating light-dark test consists of a two-compartment apparatus in which one chamber is dark and enclosed while the other chamber is open and brightly lit.87,88 Mice spend more time in the closed arms of the elevated plus-maze and more time in the dark compartment of the lighte↔dark apparatus. Excessive anxiety-like traits are interpreted when the preference is unusually high for the closed arms and for the dark compartment. Anxiolytic-like treatment responses are interpreted when mice venture out more frequently into the open arms of the elevated plus-maze and the brightly lit chamber of the light↔dark box.
Responses to sensory stimuli include acoustic startle, olfactory habituation and dishabituation to a series of non-social and social odors, and the hot plate and tail flick thermal tests. Hyperactivity is scored from automated parameters of locomotion in a novel open field. Unusual sleep patterns are scored by observations of the home cage during the daylight sleeping hours and during the nighttime active hours, and/or by EEG recordings. Optimal animal models should incorporate: (i) face validity, ie, close analogies to the defining features of the human syndrome; (ii) construct validity, ie, the biological dysfunction that causes the human disease, such as a gene mutation or anatomical abnormality; and (iii) predictive validity, ie, responsiveness to treatments that prevent or reverse symptoms in the human disease. The best animal models of autism and related developmental disorders will maximize face, construct, and predictive validities. At present this combination represents a very small subset of the model systems in use, particularly for neurodevelopmental disorders in which no effective therapeutics exist to test predictive validity of the animal model. The selected examples below are designed to illustrate the progress and promise of the mouse modeling approach in autism basic research and therapeutic development. Our goal is to systematically analyze the wealth of emerging animal models of neurodevelopmental disorders, understand the strengths and weaknesses of each, gain basic knowledge about phenotypic outcomes, and employ the best model systems for treatment discovery.
How do we discover therapeutics using mouse models?
The unmet medical need for effective treatments for neurodevelopmental disorders is striking. The number of reported cases of autism lias risen rapidly over the past décade.89,90 This rapid rise is largely a fonction of better diagnostic instruments and public awareness, allhough possible environmental causes and gene x environment interactions are under investigation.91-93
Personal and financial costs are high, to the affected individuals, their families, schools, and health care providers. At present the only effective interventions are intensive behavioral therapies.26,94 The only pharmacological treatments approved by the US Food and Drug Administration are risperidone and aripiprazole: Risperdol™ and Abilify.™ Their approved use is solely for the associated “irritability,” which includes aggression, self-injury, and tantrums.95
A major revelation from the genetic association studies is that the most frequent mutations in autism are in genes that mediate the formation and maturation of synapses, particularly the postsynaptic densities, dendritic spines, and signaling mechanisms downstream from receptors mediating excitatory neurotransmission.96-100 Pharmacological agents that alter synaptic functions are already available to some extent, and next-generation compounds are under development.101-104 To evaluate the ability of novel drug treatments to reverse and/or prevent the symptoms of diseases, biomedical researchers often begin by testing exploratory compounds in appropriate animal models. Robust behavioral pnenotypes with face validity to autism, In mouse models with construct validity to autism spectrum disorders, hold great promise as predinical tools for discovering effective treatments for components of autism spectrum disorders.
Because rodents are similar to humans in many aspects of biochemistry, physiology, anatomy, and genetics, mice and rats are routinely employed in biomedical research as translational systems. Compounds that reverse behavioral and biological phenotypes in mouse models of autism offer leads which may be worth pursuing in human clinical trials. However, species differences exist in drug metabolism, alternate biochemical pathways, genetic variants, and toxicology. As in any field of biomedical research employing model systems, 100% predictive validity of efficacy and practicality in humans cannot be expected.
Keeping these caveats in mind, we design rigorous methods to evaluate proposed therapeutic interventions for autism spectrum disorders for their ability to reverse and/or prevent the major phenotypes in mouse models.29,43 Behavioral pharmacologists test acute and chronic drug treatments, across a dose range, at various time points after administration, and assay for the most robust autism-relevant behaviors, in the strongest mouse models. Examples of some of the successes are described in the next section.
Mouse models with high translational value
Cell surface adhesion glycoproteins
Cell surface adhesion glycoproteins are a primary mechanism through which connections of presynaptic axons and postsynaptic dendrites are elaborated in neuronal synapses.97,105 Mutations in cell surface protein genes have been reported with comparatively high frequency in neurodevelopmental disorders. Individuals with autism have been identified with mutations in NEUREXIN1, NEUROLIGIN3, NEUROLIGIN4, SHANK2, SHANKS, and CNTNAP2. For each of these rare mutations, a small number of individuals with the mutations who meet the diagnostic criteria for autism spectrum disorder has been identified.106-109 Mice with homologous mutations in these genes are available from several excellent molecular genetics laboratories and from The Jackson Laboratory repository.
Shank3 knockout mice
Shank3 knockout mice present a particularly fascinating example of the importance of the location of the mutation within the gene. The Shank3 gene includes an ankyrin repeat domain, a PDZ domain, and a Homer binding domain.110-112 Five distinct lines of Shank3 knockout mice with mutations at these various sites were generated and phenotyped in the past 2 years.71,81,113,114 Two lines of Shank3 knockouts containing the mutation at the ankyrin domain displayed impairments in excitatory neurotransmission and long-term potentiation, but were predominantly normal on standard measures of sociability, with only small genotype differences detected in ultrasonic vocalizations and repetitive behavior.71,81 Inserting the mutation at the Homer binding site resulted in mice with more social interactions, primarily in the form of aggression, along with mostly normal dendritic spines, reduced long-term potentiation, and enhanced long-term depression.113 When the mutation was in the PDZ domain, Shank3 knockouts displayed much more severe phenotypes, including high spontaneous self-grooming resulting in skin lesions, impaired sociability, reduced corticostriatal excitatory transmission, longer dendritic spines, and lower density of dendritic spines, as compared with wild-type controls.81 These divergent outcomes of mutations at differing sites within the same gene provide a unique opportunity to understand the binding partners and their downstream signaling actions that determine the severity of symptoms in humans. For example, deficits in mGluRS signaling have been reported after Shank3 knockdown in neuronal cultures.115 Augmentation of mGluRS activity could be beneficial in cases of autism with SHANK3 mutations, and in individuals with Phelan-McDermid syndrome, an intellectual disability syndrome in which the SHANK3 mutation is central to the 22q13 chromosomal deletion.108
Contactin associated protein 2 (Cntnap2)
Contactin associated protein 2 (Cntnap2), a member of the neurexin superfamily, plays a role in neuron-glia interactions and neuronal migration during early brain maturation. Mutations in CNTNAP2 are associated with autism in a small number of individuals, particularly with language disabilities.107,116 Cntnap2 knockout mice were generated to understand the actions of this protein on brain development and autism-relevant behaviors.40 Seizures were detected in 9 out of 10 null mutants. Social behaviors were impaired on the 3-chambered task, during reciprocal interactions, and in home cage nesting. Repetitive self-grooming was elevated. Resistance to change was seen in the Morris water maze, in which the initial learning was normal but the Cntnap2 knockouts failed the reversal test when the escape platform location was changed. Less spontaneous alternation in a T-maze was seen in the null mutants, concomitant with moderate hyperactivity Reduced number of GABAergic interneurons and impaired migration of cortical projection neurons in this line of Cntnap2 mice underlie their seizures and some of their behavioral abnormalities. The Geschwind team proceeded to test risperidone, the antipsychotic approved by the US Food and Drug Administration for the treatment of irritability in autism. At 0.2 mg/kg IP daily for 7 days, a dose and regimen which did not affect locomotion in the wildtype controls, risperidone reduced the hyperactivity and repetitive selfgrooming in Cntnapl null mutant mice.40 Social behaviors were unaffected by the treatment with risperidone, which is an atypical antipsychotic.
Single gene mutations, chromosomal deletions, and duplications cause a variety of neurodevelopmental disorders, including Fragile X, Rett, Angelman, PraderWilli, Smith-Lemli-Opitz, Timothy, Williams, and PhelanMcDermid syndromes, and tuberous sclerosis.97,108 A surprisingly large number of these de novo mutations code for signaling proteins that mediate the biochemical events downstream to postsynaptic neurotransmitter receptors. Interactome network analyses revealed convergences in genes that mediate transcriptional and splicing mechanisms that may be dysregulated in autism spectrum disorders.117 Mutant mouse models of many of these syndromes have been generated.43,44,114,118-122 While clinically distinct disorders caused by known single gene mutations suggest straightforward targets, as compared with complex disorders such as cases of autism in which the genetic substrates are unknown, increasing knowledge about the actions of downstream signaling proteins could identify pharmacological interventions which target key mechanistic sites in convergent biochemical cascades. Mice with homologous mutations are being employed as translational tools to evaluate convergent downstream target mechanisms, and to screen compounds that yield useful interventions at those sites.
Tuberous sclerosis
Tuberous sclerosis, caused by a mutation in the Tsc1 or Tsc2 gene, is characterized by benign tubers in the cerebral cortex, seizures, a high incidence of intellectual impairment, and frequent comorbidity with autism.123,124 Tscl and Tsc2, which dimerize, are downstream targets of the PI3K/Akt postsynaptic signaling pathway elements that bind to mTORC2, and regulate mTORCl at a further downstream site.125 mTOR, the mammalian target of rapamycin, is a serine/threonine kinase which regulates many facets of brain development and cytoskeletal organization.126 Deletion of Tsc1 in knockout mice, hippocampal slices, or cortical cultures resulted in enlarged brains, large dysmorphic astrocytes, decreased myelination, reductions in γ-aminobutyric acid (GABA)-ergic interneurons in the cerebral cortex, and loss of mGluR-dependent long-term depression.126,127 Mice with mutations in Tsc2 display neuronal hypertrophy, reduced long-term potentiation in hippocampal slices, impaired hippocampally mediated fear conditioning, and impaired water maze learning.128 Treatment with the mTOR inhibitor rapamycin for 5 days reversed the fear conditioning deficit and improved water maze learning, along with reducing brain weight and increasing survival.128
This early demonstration of a pharmacological rescue of phenotypes in a mouse model of a neurodevelopmental disorder sparked optimism for treating disorders caused by perturbations in signal transduction.129 In a separate mutant line, 4 weeks of treatment with rapamycin reduced the macroencephaly and increased the low social interaction in mice with a mutation in Pten, an upstream regulator of mTOR that is implicated in cancers, seizures, and autism.38 Rapalogs, analogs of mTOR, are in clinical trials for cancers.130 Rapalogs and compounds targeting PI3K and Akt131 present possibilities for therapeutic interventions in neurodevelopmental disorders with underlying mechanisms in the mTOR signaling pathway.
Fragile X syndrome
Fragile X syndrome is the most frequent genetic cause of intellectual disabilities. Constriction at the end of the X chromosome, termed a fragile site, is associated with a dramatic expansion of CGG triplet repeats, which transcriptionally silence the FMR1 gene.132,133 Fragile X mental retardation protein (FMRP) is highly expressed in the brain, where it negatively regulates the synthesis of a large number of downstream proteins.134'1 Mice with a mutation in Fmr1 display impairments in long-term potentiation, unusual social behaviors, and some unusual cognitive and anxiety-related behaviors.135-139
One functional consequence of the FMR1 mutation is upregulation of mGluRS receptors.140 Bear and colleagues discovered that crossing mGluRS knockout mice with Fmrl knockout mice rescued the impaired longterm depression, elevated the dendritic spine densities in the hippocampus, and attenuated seizures.141 Negative allosteric modulators of the mGluR5 receptor were therefore postulated as potential treatments for Fragile X Syndrome. Clinical trials are in progress to test this hypothesis.142 Approximately 30% of individuals with Fragile X syndrome meet the diagnostic criteria for autism.143 Considering this high comorbidity, we reasoned that a treatment effective in Fragile X Syndrome might act through a pathway convergent with other risk genes for autism. 144,145 We discovered that the prototypic mGluR5 antagonist 6-methyl-2-(phenylethynyl) pyridine (MPEP), and GRN-529. a more selective negative allosteric modulators of the mGluRS receptor, reduced the high levels of repetitive self-grooming in BTBR mice,79,146 an inbred strain that displays robust social deficits, low vocalizations in social settings, and high repetitive self-grooming and digging.42,58,60,65,78,79,147,148 In addition, GRN-529 reduced the high levels of stereotyped jumping that characterize another inbred strain, C58/I80,146,149 Further, MPEP reduced marble burying in Fmr1 knockout mice, reduced stereotypies in Swiss-Webster mice, and reduced repetitive self-grooming and marble burying in mice pretreated prenatally with valproic acid.14,150,151 These reports lend credence to the notion that interventions acting through mGluR5 receptors could confer specific benefits for treating repetitive behaviors, a major component of the third diagnostic symptom of autism.
Conclusions
Promising early findings of therapeutic rescues in mouse models have energized the rational search for pharmacological treatments of autism spectrum disorder. While the optimal developmental period for pharmacological intervention remains to be determined, adults with autism will likely be recruited for the first clinical trials,152 since the risks of adverse drug reactions are predicted to be greater in children. Challenges will include discovering the critical window during development and/or adulthood at which interventions are useful, dosages, and treatment regimens which minimize toxicity. We have taken the first step in a long journey.
Acknowledgments
We thank Dr Mu Yang for Figures 1a and 3a, and Dr Jennifer Brielmaier for Figure 1b. Dr Yang was a Research Fellow and Dr Brielmaier was a Postdoctoral Fellow in the author's Laboratory of Behavioral Neuroscience, National Institute of Mental Health Intramural Research Program, Bethesda, MD.
REFERENCES
- 1.Kanner L. Child psychiatry; mental deficiency. Am J Psychiatry. 1946;102:520–522. doi: 10.1176/ajp.102.4.520. [DOI] [PubMed] [Google Scholar]
- 2.Asperger H. Die “Autistischen Psychopathen” im kindesalter. Archiv fur Psychiatrie und Nervenkrankheiten. 1944;117:76–136. [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association. 1994 [Google Scholar]
- 4.El-Fishawy P., State MW. The genetics of autism: key issues, recent findings, and clinical implications. Psychiatr Clin North Am. 2010;33:83–105. doi: 10.1016/j.psc.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook EH., Jr, Scherer SW. Copy-number variations associated with neuropsychiatric conditions. Nature. 2008;455:919–923. doi: 10.1038/nature07458. [DOI] [PubMed] [Google Scholar]
- 6.Sebat J., Lakshmi B., Malhotra D., et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders SJ., Ercan-Sencicek AG., Hus V., et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy D., Ronemus M., Yamrom B., et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70:886–897. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Girirajan S., Brkanac Z., Coe BP., et al. Relative burden of large CNVs on a range of neurodevelopmental phenotypes. PLoS Genet. 2011;7:e1002334. doi: 10.1371/journal.pgen.1002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui X., Ji D., Fisher DA., Wu Y., Briner DM., Weinstein EJ. Targeted integration in rat and mouse embryos with zinc-finger nucleases. Nat Biotechnol. 2011;29:64–67. doi: 10.1038/nbt.1731. [DOI] [PubMed] [Google Scholar]
- 11.Bauman MD., Toscano JE., Babineau BA., Mason WA., Amaral DG. Emergence of stereotypies in juvenile monkeys (Macaca mulatta) with neonatal amygdala or hippocampus lesions. Behav Neurosci. 2008;122:1005–1015. doi: 10.1037/a0012600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith SE., Li J., Garbett K., Mirnics K., Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heo Y., Zhang Y., Gao D., Miller VM., Lawrence DA. Aberrant immune responses in a mouse with behavioral disorders. PLoS One. 2011;6:e20912. doi: 10.1371/journal.pone.0020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta MV., Gandal MJ., Siegel SJ. mGluRS-antagonist mediated reversal of elevated stereotyped, repetitive behaviors in the VPA model of autism. PLoS One. 2011;6:e26077. doi: 10.1371/journal.pone.0026077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berman RF., Pessah IN., Mouton PR., Mav D., Harry J. Low-level neonatal thimerosal exposure: further evaluation of altered neurotoxic potential in SJL mice. Toxicol Sci. 2008;101:294–309. doi: 10.1093/toxsci/kfm265. [DOI] [PubMed] [Google Scholar]
- 16.Bauman MD., Lavenex P., Mason WA., Capitanio JP., Amaral DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci. 2004;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- 17.Martin LA., Ashwood P., Braunschweig D., Cabanlit M., Van de Water J., Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22:806–816. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malkova L., Mishkin M., Suomi SJ., Bachevalier J. Long-term effects of neonatal medial temporal ablations on socioemotional behavior in monkeys (Macaca mulatta). Behav Neurosci. 2010;124:742–760. doi: 10.1037/a0021622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lord C., Petkova E., Hus V., et al. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gen Psychiatry. 2012;69:306–313. doi: 10.1001/archgenpsychiatry.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lord C., Cook EH., Leventhal BL., Amaral DG. Autism spectrum disorders. Neuron. 2000;28:355–363. doi: 10.1016/s0896-6273(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 21.Tuchman R., Moshe SL., Rapin I. Convulsing toward the pathophysiology of autism. Brain Dev. 2009;31:95–103. doi: 10.1016/j.braindev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers SJ., Ozonoff S. Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J Child Psychol Psychiatry. 2005;46:1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- 23.Landa RJ. Diagnosis of autism spectrum disorders in the first 3 years of life. Nat Clin Pract Neurol. 2008;4:138–147. doi: 10.1038/ncpneuro0731. [DOI] [PubMed] [Google Scholar]
- 24.Volk FR., State M., Klin A. Autism and autism spectrum disorders: diagnostic issues for the coming decade. J Child Psychol Psychiatry. 2009;50:108–115. doi: 10.1111/j.1469-7610.2008.02010.x. [DOI] [PubMed] [Google Scholar]
- 25.Amaral D., Dawson G., Geschwind. DH. Autism Spectrum Disorders. New York, NY: Oxford University Press. 2011 [Google Scholar]
- 26.Dawson G., Burner K. Behavioral interventions in children and adolescents with autism spectrum disorder: a review of recent findings. Curr Opin Pediatr. 2011;23:616–620. doi: 10.1097/MOP.0b013e32834cf082. [DOI] [PubMed] [Google Scholar]
- 27.Nakatani J., Tamada K., Hatanaka F., et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang M., Silverman JL., Crawley JN. Automated three-chambered social approach task for mice. Curr Protoc Neurosci. 2011;Chapter 8: Unit 8.:26. doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- 30.Nadler JJ., Moy SS., Dold G., et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 31.Brodkin ES. BALB/c mice: low sociability and other phenotypes that be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 32.DeLorey TM., Sahbaie P., Hashemi E., Homanics GE., Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder. Behav Brain Res. 2008;187:207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan BC., Young NB., Moy SS., Crawley JN. Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57BL/6J mice. Behav Brain Res. 2008;193:235–242. doi: 10.1016/j.bbr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page DT., Kuti OJ., Prestia C., Sur M. Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior. Proc Natl Acad Sci U S A. 2009;106:1989–1994. doi: 10.1073/pnas.0804428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radyushkin K., Hammerschmidt K., Boretius S., et al. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8:416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- 36.Yang M., Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009;Chapter 8: Unit 8:24. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva AJ., Ehninger D. Adult reversal of cognitive phenotypes in neurodevelopmental disorders. J Neurodev Disord. 2009;1:150–157. doi: 10.1007/s11689-009-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou J., Blundell J., Ogawa S., et al. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Etherton M., Foldy C., Sharma M., et al. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc Natl Acad Sci U S A. 2011;16; 108:13764–13769. doi: 10.1073/pnas.1111093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penagarikano O., Abrahams BS., Herman El., et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silverman J., Turner S., Barkan C., et al. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pobbe RL., Pearson BL., Defensor EB., Bolivar VJ., Blanchard DC., Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214:443–449. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silverman JL., Yang M., Lord C., Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SE., Zhou YD., Zhang G., Jin Z., Stoppel DC., Anderson MP. Increased gene dosage of Ube3a results in autism traits and decreased glutamate synaptic transmission in mice. Sci Transl Med. 2011;3:103ra197. doi: 10.1126/scitranslmed.3002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silverman J., Turner S., Barkan C., et al. Sociability and motor functions in Shankl mutant mice. Brain Res. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crawley JN. What's Wrong With My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. 2007;2nd ed. Hoboken, NJ: John Wiley & Sons, Inc. [Google Scholar]
- 47.Grant E., Macintosh J. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21:246–259. [Google Scholar]
- 48.Carter CS., Williams JR., Witt DM., Insel TR. Oxytocin and social bonding. Ann N Y Acad Sci. 1992;652:204–211. doi: 10.1111/j.1749-6632.1992.tb34356.x. [DOI] [PubMed] [Google Scholar]
- 49.Terranova ML., Laviola G. Scoring of Social Interactions and Play in Mice During Adolescence. 2005;Vol 13. Hoboken, NJ: John Wiley & Sons, Inc. doi: 10.1002/0471140856.tx1310s26. [DOI] [PubMed] [Google Scholar]
- 50.Hofer MA., Shair HN. Ultrasonic vocalization, laryngeal braking, and thermogenesis in rat pups: a reappraisal. Behav Neurosci. 1993;107:354–362. doi: 10.1037//0735-7044.107.2.354. [DOI] [PubMed] [Google Scholar]
- 51.Miczek KA., Maxson SC., Fish EW., Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 52.Winslow JT., Hearn EF., Ferguson J., Young LJ., Matzuk MM., Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- 53.Wrenn CC., Harris AP., Saavedra MC., Crawley JN. Social transmission of food preference in mice: methodology and application to galanin-overexpressing transgenic mice. Behav Neurosci. 2003;117:21–31. [PubMed] [Google Scholar]
- 54.Wesson DW., Keller M., Douhard Q., Baum MJ., Bakker J. Enhanced urinary odor discrimination in female aromatase knockout (ArKO) mice. Horm Behav. 2006;49:580–586. doi: 10.1016/j.yhbeh.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panksepp JB., Jochman KA., Kim JU., et al. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wersinger SR., Caldwell HK., Martinez L., Gold P., Hu SB., Young WS. 3rd. Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression. Genes Brain Behav. 2007;6:540–551. doi: 10.1111/j.1601-183X.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 57.Wang H., Liang S., Burgdorf J., Wess J., Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One. 2008;3:e1893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McFarlane HG., Kusek GK., Yang M., Phoenix JL., Bolivar VJ., Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 59.Scattoni ML., Gandhy SU., Ricceri L., Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scattoni M., Ricceri L., Crawley J. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arakawa H., Arakawa K., Blanchard DC., Blanchard RJ. A new test paradigm for social recognition evidenced by urinary scent marking behavior in C57BL/6J mice. Behav Brain Res. 2008;190:97–104. doi: 10.1016/j.bbr.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang M., Clarke AM., Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahern TH., Modi ME., Burkett JP., Young LJ. Evaluation of two automated metrics for analyzing partner preference tests. J Neurosci Methods. 2009;182:180–188. doi: 10.1016/j.jneumeth.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsuo N., Tanda K., Nakanishi K., et al. Comprehensive behavioral phenotyping of ryanodine receptor type 3 (RyR3) knockout mice: decreased social contact duration in two social interaction tests. Front Behav Neurosci. 2009;3:3. doi: 10.3389/neuro.08.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang M., Zhodzishsky V., Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crawley JN., Chen T., Puri A., et al. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Yang M., Scattoni ML., Zhodzishsky V., et al. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor IB mutant mice. Front Behav Neurosci. 2007;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chadman KK., Gong S., Scattoni ML., et al. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jamain S., Radyushkin K., Hammerschmidt K., et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bozdagi O., Sakurai T., Papapetrou D., et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holy TE., Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ey E., LeBlond CS., Bourgeron T. Behavioral profiles of mice carrying synaptic gene mutations associated with autism spectrum disorders. Autism Res. 2011;4:5–16. doi: 10.1002/aur.175. [DOI] [PubMed] [Google Scholar]
- 74.Young DM., Schenk AK., Yang SB., YN, LY Altered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism. Proc Natl Acad Sci U S A. 2010;107:11074–11079. doi: 10.1073/pnas.1005620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bakker J., Honda S., Harada N., Balthazart J. Sexual partner preference requires a functional aromatase (cyp19) gene in male mice. Horm Behav. 2002;42:158–171. doi: 10.1006/hbeh.2002.1805. [DOI] [PubMed] [Google Scholar]
- 76.McGraw LA., Young LJ. The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci. 2010;33:103–109. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scattoni ML., Crawley J., Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33:508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wohr M., Roullet Fl., Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2012;32:6525–6541. [Google Scholar]
- 79.Silverman JL., Tolu SS., Barkan CL., Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluRS antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ryan BC., Young NB., Crawley JN., Bodfish JW., Moy SS. Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain. Behav Brain Res. 2010;208:178–188. doi: 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peca J., Feliciano C., Ting JT., et al. Shank3 mutant mice display autisticlike behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moy SS., Nadler JJ., Young NB., et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arnodeo DA., Jones JH., Sweeney JA., Ragozzino ME. Differences in BTBR T+tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav Brain Res. 2012;227:64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 85.Cryan JF., Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 86.Fox W. Reflex-ontogeny and behavioural development of the mouse. Animal Behavior. 1969;13:234–241. doi: 10.1016/0003-3472(65)90041-2. [DOI] [PubMed] [Google Scholar]
- 87.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. Spring 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 88.Crawley J., Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 89.Rice C., et al. Prevalence of autism spectrum disorders - Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58:1–20. [PubMed] [Google Scholar]
- 90.Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. 2009;65:591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- 91.Charman T., Pickles A., Chandler S., et al. Commentary: Effects of diagnostic thresholds and research vs service and administrative diagnosis on autism prevalence. Int J Epidemiol. 2009;38:1234–1238. author reply 1243–1234.. doi: 10.1093/ije/dyp256. [DOI] [PubMed] [Google Scholar]
- 92.Rice C., Nicholas J., Baio J., et al. Changes in autism spectrum disorder prevalence in 4 areas of the United States. Disabil Health J. 2010;3:186–201. doi: 10.1016/j.dhjo.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 93.Durkin MS., Maenner MJ., Meaney FJ., et al. Socioeconomic inequality in the prevalence of autism spectrum disorder: evidence from a U.S. cross-sectional study. PLoS One. 2010;5:e11551. doi: 10.1371/journal.pone.0011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zwaigenbaum L., Bryson S., Lord C., et al. Clinical assessment and management of toddlers with suspected autism spectrum disorder: insights from studies of high-risk infants. Pediatrics. 2009;123:1383–1391. doi: 10.1542/peds.2008-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wink LK., Erickson CA., McDougle CJ. Pharmacologic treatment of behavioral symptoms associated with autism and other pervasive developmental disorders. Curr Treat Options Neurol. 2010;12:529–538. doi: 10.1007/s11940-010-0091-8. [DOI] [PubMed] [Google Scholar]
- 96.Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 97.Betancur C., Sakurai T., Buxbaum JD. The emerging role of synaptic celladhesion pathways in the pathogenesis of autism spectrum disorders. Trends Neurosci. 2009;32:402–412. doi: 10.1016/j.tins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buxbaum JD. Multiple rare variants in the etiology of autism spectrum disorders. Dialogues Clin Neurosci. 2009;11:35–43. doi: 10.31887/DCNS.2009.11.1/jdbuxbaum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Penzes P., Cahill ME., Jones KA., VanLeeuwen JE., Woolfrey KM. Dendritic spine pathology in neuropsychiatrie disorders. Nat Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hutsler JJ., Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- 101.Ballard TM., Woolley ML., Prinssen E., Huwyler J., Porter R., Spooren W. The effect of the mGluS receptor antagonist MPEP in rodent tests of anxiety and cognition: a comparison. Psychopharmacology (Berl). 2005;179:218–229. doi: 10.1007/s00213-005-2211-9. [DOI] [PubMed] [Google Scholar]
- 102.Wang LW., Berry-Kravis E., Hagerman RJ. Fragile X: leading the way for targeted treatments in autism. Neurotherapeutics. 2010;7:264–274. doi: 10.1016/j.nurt.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lynch G., Palmer LC., Gall CM. The likelihood of cognitive enhancement. Pharmacol Biochem Behav. 2011;99:116–129. doi: 10.1016/j.pbb.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brennand KJ., Gage FH. Concise review: the promise of human induced pluripotent stem cell-based studies of schizophrenia. Stem Cells. 2011;29:1915–1922. doi: 10.1002/stem.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Casey JP., Magalhaes T., Conroy JM., et al. A novel approach of homozygous haplotype sharing identifies candidate genes in autism spectrum disorder. Hum Genet. 2012;131:565–579. doi: 10.1007/s00439-011-1094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alarcon M., Abrahams BS., Stone JL., et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sarasua SM., Dwivedi A., Boccuto L., et al. Association between deletion size and important phenotypes expands the genomic region of interest in Phelan-McDermid syndrome (22q13 deletion syndrome). J Med Genet. 2011;48:761–766. doi: 10.1136/jmedgenet-2011-100225. [DOI] [PubMed] [Google Scholar]
- 109.Jamain S., Quach H., Betancur C., et al. Mutations of theX-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takeuchi M., Hata Y., Hirao K., Toyoda A., Irie M., Takai Y. SAPAPs. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density. J Biol Chem. 1997;272:11943–11951. doi: 10.1074/jbc.272.18.11943. [DOI] [PubMed] [Google Scholar]
- 111.Sheng M., Kim E. The Shank family of scaffold proteins. J Cell Sci. 2000;113(Pt 11):1851–1856. doi: 10.1242/jcs.113.11.1851. [DOI] [PubMed] [Google Scholar]
- 112.Hayashi MK., Tang C., Verpelli C., et al. The postsynaptic density proteins Horner and Shank form a polymeric network structure. Cell. 2009;137:159–171. doi: 10.1016/j.cell.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bangash MA., Park JM., Melnikova T., et al. Enhanced polyubiquitination of Shank3 and NMDA receptor in a mouse model of autism. Cell. 2011;145:758–772. doi: 10.1016/j.cell.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Wang X., McCoy PA., Rodriguiz RM., et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet. 2011;20:3093–3108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Verpelli C., Dvoretskova E., Vicidomini C., et al. Importance of shank3 in regulating metabotropic glutamate receptor 5 (mGluRS) expression and signaling at synapses. J Biol Chem. 2011;286:34839–34850. doi: 10.1074/jbc.M111.258384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Whalley HC., O'Connell G., Sussmann JE., et al. Genetic variation in CNTNAP2 alters brain function during linguistic processing in healthy individuals. Am J Med Genet B Neuropsychiatr Genet. 2011;1566:941–948. doi: 10.1002/ajmg.b.31241. [DOI] [PubMed] [Google Scholar]
- 117.Voineagu I., Wang X., Johnston P., et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hayashi ML., Rao BS., Seo JS., et al. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci USA. 2007;104:11489–11494. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zoghbi HY. Rett syndrome: what do we know for sure? Nat Neurosci. 2009;12:239–240. doi: 10.1038/nn0309-239. [DOI] [PubMed] [Google Scholar]
- 120.Moretti P., Bouwknecht JA., Teague R., Paylor R., Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- 121.Ogier M., Wang H., Hong E., Wang Q., Greenberg ME., Katz DM. Brainderived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J Neurosci. 2007;27:10912–10917. doi: 10.1523/JNEUROSCI.1869-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lauterborn JC., Rex CS., Kra E., et al. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J Neurosci. 2007;27:10685–10694. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Datta AN., Hahn CD., Sahin M. Clinical presentation and diagnosis of tuberous sclerosis complex in infancy. J Child Neurol. 2008;23:268–273. doi: 10.1177/0883073807309250. [DOI] [PubMed] [Google Scholar]
- 124.Cusmai R., Moavero R., Bombardieri R., Vigevano F., Curatolo P. Long-term neurological outcome in children with early-onset epilepsy associated with tuberous sclerosis. Epilepsy Behav. 2011;22:735–739. doi: 10.1016/j.yebeh.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 125.Huang J., Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37(Pt 1):217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carson RP., Van Nielen DL., Winzenburger PA., Ess KC. Neuronal and glia abnormalities in Tsc1-deficient forebrain and partial rescue by rapamycin. Neurobiol Dis. 2012;45:369–380. doi: 10.1016/j.nbd.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Khwaja OS., Sahin M. Translational research: Rett syndrome and tuberous sclerosis complex. Curr Opin Pediatr. 2011;23:633–639. doi: 10.1097/MOP.0b013e32834c9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ehninger D., Han S., Shilyansky C., et al. Reversal of learning deficits in a Tsc2+/- mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ehninger D., Li W., Fox K., Stryker MP., Silva AJ. Reversing neurodevelopmental disorders in adults. Neuron. 2008;60:950–960. doi: 10.1016/j.neuron.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schatz JH. Targeting the PI3K/AKT/mTOR pathway in non-Hodgkin's lymphoma: results, biology, and development strategies. Curr Oncol Rep. 2011;13:398–406. doi: 10.1007/s11912-011-0187-7. [DOI] [PubMed] [Google Scholar]
- 131.Ogita S., Lorusso P. Targeting phosphatidylinositol 3 kinase (PI3K)-Akt beyond rapalogs. Target Oncol. 2011;6:103–117. doi: 10.1007/s11523-011-0176-7. [DOI] [PubMed] [Google Scholar]
- 132.Fu YH., Kuhl DP., Pizzuti A., et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 133.Pieretti M., Zhang FP., Fu YH., et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- 134.Brown V., Jin P., Ceman S., et al. Microarray identification of FMRP-associated brain mRNAsand altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 135.Gantois I., Bakker CE., Reyniers E., et al. Restoring the phenotype of fragile X syndrome: insight from the mouse model. Curr Mol Med. 2001;1:447–455. doi: 10.2174/1566524013363492. [DOI] [PubMed] [Google Scholar]
- 136.Mineur YS., Huynh LX., Crusio WE. Social behavior deficits in the Fmr1 mutant mouse. Behav Brain Res. 2006;168:172–175. doi: 10.1016/j.bbr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 137.Chen LY., Rex CS., Babayan AH., et al. Physiological activation of synaptic RaoPAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome. J Neurosci. 2010;30:10977–10984. doi: 10.1523/JNEUROSCI.1077-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Spencer CM., Graham DF., Yuva-Paylor LA., Nelson DL., Paylor R. Social behavior in Frnrl knockout mice carrying a human FMR1 transgene. Behav Neurosci. 2008;122:710–715. doi: 10.1037/0735-7044.122.3.710. [DOI] [PubMed] [Google Scholar]
- 139.Baker KB., Wray SP., Ritter R., Mason S., Lanthorn TH., Savelieva KV. Male and female Frnrl knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes. Brain Behav. 2010;9:562–574. doi: 10.1111/j.1601-183X.2010.00585.x. [DOI] [PubMed] [Google Scholar]
- 140.Huber KM., Gallagher SM., Warren ST., Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dolen G., Osterweil E., Rao BS., et al. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Krueger DD., Bear MF. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med. 2011;62:411–429. doi: 10.1146/annurev-med-061109-134644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hagerman R., Hoem G., Hagerman P. Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Mol Autism. 2010;1:12. doi: 10.1186/2040-2392-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Auerbach BD., Osterweil EK., Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hagerman R., Lauterborn J., Au J., Berry-Kravis E. Fragile x syndrome and targeted treatment trials. Results Probl Cell Differ. 2012;54:297–335. doi: 10.1007/978-3-642-21649-7_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Silverman JL., Smith DG., Rizzo SJ., et al. Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Sci Transi Med. 2012;4:131ra51. doi: 10.1126/scitranslmed.3003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bolivar VJ., Walters SR., Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pearson BL., Pobbe RL., Defensor EB., et al. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes. Brain Behav. 2011;213:446–451. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moy SS., Nadler JJ., Young NB., et al. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thomas A., Burant A., Bui N., Graham D., Yuva-Paylor LA., Paylor R. Marble burying reflects a repetitive and perseverative behavior more than noveltyinduced anxiety. Psychopharmacology (Berl). 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Burket JA., Herndon AL., Winebarger EE., Jacome LF., Deutsch SI. Complex effects of mGluRS antagonism on sociability and stereotypic behaviors in mice: possible implications for the pharmacotherapy of autism spectrum disorders. Brain Res Bull. 2011;86:152–158. doi: 10.1016/j.brainresbull.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 152.Piven J., Rabins P. Autism spectrum disorders in older adults: toward defining a research agenda. J Am Geriatr Soc. 2011;59:2151–2155. doi: 10.1111/j.1532-5415.2011.03632.x. [DOI] [PubMed] [Google Scholar]
- 153.Scattoni M., Ricceri L., Crawley J. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wohr M., Roullet Fl., Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Carter MD., Shah CR., Muller CL., Crawley JN., Carneiro AM., Veenstra-Vanderweele J. Absence of preference for social novelty and increased grooming in integrin beta3 knockout mice: Initial studies and future directions. Autism Res. 2011;4:57–67. doi: 10.1002/aur.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Blundell J., Blaiss CA., Etherton MR., et al. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Radyushkin K., Hammerschmidt K., Boretius S., et al. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8:416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- 158.Jamain S., Radyushkin K., Hammerschmidt K., et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Scattoni ML., Gandhy SU., Ricceri L., Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Silverman JL., Yang M., Lord C., Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Blundell J., Tabuchi K., Bolliger MF., et al. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes Brain Behav. 2009;8:114–126. doi: 10.1111/j.1601-183X.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Martins GJ., Plachez C., Powell EM. Loss of embryonic MET signaling alters profiles of hippocampal interneurons. Dev Neurosci. 2007;29:143–158. doi: 10.1159/000096219. [DOI] [PubMed] [Google Scholar]
- 163.Shu W., Cho JY., Jiang Y., et al. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci U S A. 2005;102:9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fujita E., Tanabe Y., Shiota A., et al. Ultrasonic vocalization impairment of Foxp2 (R552H) knockin mice related to speech-language disorder and abnormality of Purkinje cells. Proc Natl Acad Sci U S A. 2008;105:3117–3122. doi: 10.1073/pnas.0712298105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Enard W., Gehre S., Hammerschmidt K., et al. A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell. 2009;137:961–971. doi: 10.1016/j.cell.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 166.Bielsky IF., Hu SB., Szegda KL., Westphal H., Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin Via receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- 167.Pobbe RL., Pearson BL., Defensor EB., et al. Oxytocin receptor knockout mice display deficits in the expression of autism-related behaviors. Horm Behav. In press. doi: 10.1016/j.yhbeh.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sala M., Braida D., Lentini D., et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 169.Macbeth AH., Stepp JE., Lee HJ., Young WS., 3rd., Caldwell HK. Normal maternal behavior, but increased pup mortality, in conditional oxytocin receptor knockout females. Behav Neurosci. 2010;124:677–685. doi: 10.1037/a0020799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brielmaier J., Matteson PG., Silverman JL., et al. Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS One. 2012;7:e40914. doi: 10.1371/journal.pone.0040914. [DOI] [PMC free article] [PubMed] [Google Scholar]


