Abstract
This review presents an overview of functional magnetic resonance imaging findings in autism spectrum disorders (ASDs), Although there is considerable heterogeneity with respect to results across studies, common themes have emerged, including: (i) hypoactivation in nodes of the “social brain” during social processing tasks, including regions within the prefrontal cortex, the posterior superior temporal sulcus, the amygdala, and the fusiform gyrus; (ii) aberrant frontostriatal activation during cognitive control tasks relevant to restricted and repetitive behaviors and interests, including regions within the dorsal prefrontal cortex and the basal ganglia; (iii) differential lateralization and activation of language processing and production regions during communication tasks; (iv) anomalous mesolimbic responses to social and nonsocial rewards; (v) task-based long-range functional hypoconnectivity and short-range hyper-connectivity; and (vi) decreased anterior-posterior functional connectivity during resting states. These findings provide mechanistic accounts of ASD pathophysiology and suggest directions for future research aimed at elucidating etiologic models and developing rationally derived and targeted treatments.
Keywords: autism spectrum disorder, functional magnetic resonance imaging, fMRI, repetitive behavior, cognitive control, language, reward, connectivity
Abstract
Esta revisión entrega una panoráinica acerca de los hallazgos de la resonancia magnética funcional en los trastornos del espectro autista (TEA), Aunque existe bastante heterogeneidad en los resuliados de los estudios han aparecido aspectos comunes que incluyen: 1) hipoactivación en los nodos del “cerebro social” durante las tareas de procesamiento social, que incluyen regiones dentro de la corteza prefrontal, el sulcus temporal superior posterior, la amígdala y el giro fusiforme, 2) activación frontoestriatal aberrante durante las tareas de control cognitivo, relacionadas con los intereses y las conductas restringidas y repetitivas, y que incluyen regiones dentro de la corteza prefrontal dorsal y los ganglios basales, 3) lateralizatión y activación diferencial de las regiones relacionadas con el procesamiento y la produccion del lenguaje durante las tareas de comunicación, 4) respuestas mesolímbicas anómalas a las recompensas sociales y no sociales, 5) hipoconectividad funcional a largo plazo e hiperconectividad a corto plazo frente a tareas y 6) disminución de la conectividad funcional antero-posterior durante los estados de reposo, Estos hallazgos aportan razones mecanicistas para la fisiopatología de los TEA y sugieren orientaciones para las futuras investigaciones encaminadas a aclarar los modelos etiólogicos y desarrollar tratamientos que puedan ser específicos y obtenerse rationalmente.
Abstract
Cet article présente une synthèse des résultats de l'imagerie par résonance magnétique fonctionnelle dans les troubles autistiques (TA), En dépit d'une grande hétérogénéité due aux résultais des études, des thèmes communs ressortent comme: 1) une hypoactivation des nœuds du « cerveau social » au cours des tâches sociales, qui concerne les régions du cortex préfrontal, du sillon temporal postérosupérieur, de l'amygdale, et du gyrus fusiforme ; 2) une activation froniostriaiale aberrante du cortex dorsal préfrontal et des noyaux gris centraux lors des tâches de contrôle cognitif se rapportant à des intérêts et à des comportements restreints et répétitifs ; 3) une activation et une latéralisation différentielles des régions de production et de traitement du langage au cours des tâches de communication ; 4) des réponses mésolimbiques anormales aux récompenses sociales et non sociales ; 5) une hypoconnectivité fonctionnelle à longue distance et une hyperconnectivité de courte distance basées sur les tâches ; 6) une connectivité fonctionnelle antéropostérieure diminuée pendant les états de repos. Ces résultais donnent un aperçu mécanisie de la physiopathologie des TA et suggèrent des directions pour la recherche future afin d'élaborer des modèles étiologiques et de développer de façon rationnelle des traitements ciblés et dérivés.
Introduction
Autism was first described by Leo Kanner1 and Hans Asperger2 in a series of clinical case studies. Both clinicians suggested that the conditions now referred to as autism spectrum disorders (ASDs) may have a neurobiological basis. With the relatively recent advent of modern brain imaging techniques, translational psychiatric research has embraced the systematic study of ASDs using these measurement tools to gain insight into the pathophysiology and possible etiology of ASDs. The ultimate promise of these approaches is to improve mechanistic accounts of ASDs as well as provide targets for novel intervention approaches.
ASDs emerge early in life and are generally associated with lifelong disability.3 The defining symptoms of the disorder include social and communicative deficits and restricted and repetitive behaviors and interests.4 Individuals with milder constellations of symptoms are classified as having an ASD, a term that reflects the highly heterogenous array of symptom presentations and that will likely be adopted to characterize individuals with a range of intellectual functioning in the next version of the Diagnostic and Statistical Manual of Mental Disorders.5 Geschwind and Levitt6 illustrated the complexity inherent to understanding the neurobiology of ASDs by suggesting that there are likely many “autisms,” each with non-overlapping etiologies and presentations. Given the highly heterogenous nature of ASDs, it is perhaps not surprising that brain imaging studies have yielded a wide array of candidate brain circuits affected by the disorder. This range of brain endophenotypes is consistent with the challenges associated with identifying genes that cause ASDs: although ASDs have a very strong genetic component, with an estimated heritability as high as 90%,7 the identification of reliable genetic markers remains elusive.
Functional magnetic resonance imaging (fMRI) has proven to be a useful tool to investigate aberrant neurobiological function in ASDs because of its excellent contrast properties, spatial resolution, and temporal resolution. fMRI uses specialized pulse sequences to localize metabolic correlates of neural activity linked to relevant neurocognitive processes. Additionally, unlike positron emission tomography (PET) and single-photon emission computed tomography (SPECT), fMRI does not rely on radiotracers and is noninvasive. The past two decades have witnessed a surge in fMRI research in ASDs, and the goal of this review is to provide an overview of the questions addressed by these studies, to identify consistent patterns across investigations, and to suggest directions for future research.
Task-based functional magnetic resonance imaging
Likely due at least in part to the heterogeneity of symptom expression in ASDs, there is no unifying account of brain dysfunction that explains all the core symptoms of ASDs. Instead, the triad of defining ASD symptoms (ie, impaired social functioning, impaired communication, and restricted and repetitive behaviors and interests) suggests distinct neural systems. Additionally, it is common for some cognitive systems to be spared in individuals with ASDs (eg, even severe cases of ASDs may be accompanied by high intelligence and other so-called “islets of ability”8), suggesting that brain dysfunction in ASDs may be domain-specific. Likewise, task-based fMRI studies of ASDs have taken the piecemeal approach of investigating neurocognitive processes linked to specific symptom domains in relative isolation. Therefore, in this review studies are grouped based on these distinct neurocognitive processes. The clear majority of studies have used tasks that map onto the triad of defining ASD symptoms, and thus studies are first presented based on this trichotomy. However, emerging fMRI data addressing reward processing and resting-state functional connectivity do not clearly fit within these three domains, as thus are given separate sections in this review.
Social cognition
Most functional neuroimaging investigations in ASDs have addressed social perception (the automatic and preconscious processing of social information) and social cognition (processing meaning from emotional and social cues). Task-related fMRI studies addressing social functioning in ASDs have focused on nodes of the socalled “social brain,” including the medial prefrontal cortex, implicated in making inferences about others' intentions, the temporoparietal junction, mediating mentalizing, the posterior superior temporal sulcus, activated by biological motion, the inferior frontal gyrus, involved in emotional judgments, the interparietal sulcus, which guides spatial attention in social contexts, the amygdala, involved in recognizing emotions from facial expressions, the fusiform gyrus, critical for face processing, and the anterior insula, involved in understanding internal states and mimicking social expressions (see ref 9 for a review).
Face processing
Perhaps the richest area of inquiry into social cognition deficits in ASDs has been studies of face processing (Table I). Faces are perhaps the quintessential social stimulus, and infants attend to and recognize faces from very early infancy.10 Studies of face processing in ASDs are theoretically grounded by behavioral evidence of impaired joint attention, eye contact, and face recognition and discrimination in ASDs, as well as impaired social emotional judgments about faces, reduced face emotion recognition and perception, and abnormal eye scanpaths when viewing faces.11,12
Table I. Studies investigating face processing in autism spectrum disorders. ASD: Autism Spectrum Disorder; TYP: Neurotypical; †ASD refers to the entire autism sample in a particular study, including high functioning autism, Asperger's syndrome, and pervasive developmental disorder not otherwise specified; *Total number of participants is presented first followed by the number of females in parentheses, if reported; **Not specified; ↓: decreased activation; ↑: increased activation. Abbreviations used in tables: ACC, anterior cingulate cortex; ACG, anterior cingulate gyms; AG, angular gyms; Al, anterior insula; AMY, amygdala; ATL, anterior temporal lobe; BA, Broca's area; BG, basal ganglia; CM, caudate nucleus; DAC, dorsal anterior cingulate; DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; DN, dentate nucleus; FFA, fusiform face area; FG, fusiform gyms; IC, insular cortex; IFA, inferior frontal area; IFC, inferior frontal cortex; IFG, inferior frontal gyrus; IPL, inferior parietal lobe; ITG, inferior temporal gyrus; LG: lingual gyrus; LSTG, left superior temporal gyrus; MCG, >middle cingulate gyrus; MFC, midfrontaI cortex; MFG, midfrontal gryus; MFL, medial frontal lobes; NAC, nucleus accumbens; OFC, orbitofrental cortex; OFG, orbitofrental gyrus; MPFC, medial prefrontal cortex; MTG, medial temporal gyrus; PO, pars opercularis; PCC, posterior cingulate cortex; PFC, prefrontal cortex; PHG, parahippocampal gyrus; PL, parietal lobe; PMC, premotor cortex; PVC, primary visual cortex; RPVC, right primary visual cortex; SFG, superior frontal gyrus; SPL, superior parietal lobe; STG, superior temporal gyrus; STS, superior temporal sulcus; THAL, thalamus; TL, temporal lobe; TPJ, temporoparietal junction; VS, ventral striatium; VLPFC, ventrolateral prefrontal cortex; VOC, ventral occipital cortex; VMPFC, ventromedial prefrontal cortex; WA, Wernicke's Area .
| Citation | ASD*† | TYP*† | ASD age | TYP age | Task(s) | Core findings in ASD group (relative to controls) | Conclusions |
| Ashwin, Baron-Cohen, Wheelwright, O'Riordan, Bullmore, 2007 [163] | 13 (13) | 13 (13) | 31.2 + 9.1 | 25.6 + 5.1 | Viewed facial stimuli known to activate AMY in healthy controls | Differential activation to faces; ↑ACG, superior temporal cortex; No difference in AMY activation between angry and frightened faces | Different activation of social brain during face processing; Absence of response to varying emotional intensity of facial stimuli |
| Bird, Catmur, Silani, Frith, Frith, 2006 [164] | 16 (14) | 16 (14) | 33.3 ± 11.5 | 35.3 + 12.1 | Viewed pairs of stimuli (face/ house) in attended /unattended locations | Attention modulation present only to house images (rather than to both houses and faces) | Social stimuli less salient for individuals with ASD |
| Bookheimer, Wang, Scott, Sigman, Dapretto, 2008 [165] | 12 (12) | 12 (12) | 11.3 ± 40 | 11.9 ± 2.4 | Inverted or upright face matching | ↓Frontal cortex across all conditions, particularly left hemisphere, dorsal IFG (i.e. mirror neurons); ↓AMY; ↑Precuneus | Faces processed as objects; Behavioral differences in processing upright vs inverted faces implicates a social rather that visual processing impairment |
| Corbett, Carmean, Ravizza, et al, 2009 [166] | 12 (12) | 15 (13) | 9.01 ± 13.82 | 9.17 ± 1.44 | Face identify and expression matching | ↓AMY during expression matching; ↓FG during identity matching | ASD recruits frontal and parietal lobes, but not AMY, for face expression matching; ASD processes faces less efficiently and less effectively; AMY fails to provide socio-emotional context during social interactions |
| Coutanche, Thompson-Schill, Schultz, 2011 [167] | 12 (12) | 12 (12) | 13.9 ± 4.48 | 13.6 ± 3.87 | Recognition of emotional facial expressions | Multi-voxel pattern analysis classification negatively correlated with symptom severity (activation levels did not); Searchlight analysis across the ventral TL identified regions with relationships between classification performance and symptom severity | Clinical severity was more classifiable from MVPA than from FG patterns; MVPA can identify regions not found using mean activation, ITG may play a role in ASD face processing |
| Dalton, Nacewicz, Johnstoner, et al, 2005 [168] | Task : 14 (14) Task 2 : 16 (16) | Task 1: 12 (12) Task 2: 16 (16) | 15.9 ± 4.71 | 17.1 ± 2.78 | (1) Facial emotion discrimination (2) Face recognition | ↓Bilateral FG, occipital gyri, MFG; ↑Left AMY, OFG; FG and AMY activation correlated with time fixating on eye regions in the ASD group | Diminished gaze fixation may account for FFG hypoactivation results in the literature |
| Deeley, Daly, Surguladze, et al, 2007 [169] | 18 (18) | 9 (9) | 34 + 10 | 27 ± 5 | Viewed face stimuli with variable emotional expressions | Fusiform, extrastriate hyporesponsiveness across emotion and intensity levels | While fusiform and extrastriate regions are activated to social stimuli in ASD, it is less so than in typical development |
| Greimel, Schulte-Ruther, Kircher, et al, 2010 [170] | 15 (15), 11 (11) (adolescents, fathers) | 15 (15), 9 (9) (adolescents, fathers) | 14.9 ± 1.6, 47.7 ± 5.3 (adolescents, fathers) | 15.0 ± 1.4, 43.9 ± 5.1 (adolescents, fathers) | Emotion identification in facial stimuli and in self | ↓FG correlated with social deficits, ↓IFG during self-task; Fathers of ASD performed similarly to fathers of controls, but showed ↓FG | FG impairment shared between first-degree relatives is a fundamental feature of ASD; FG impairment during face processing related to empathy deficits |
| Hadjikhani, Joseph, Snyder, et al, 2004 [171] | 11** | 10** | 36 ± 12 | 26 ± 6 | Viewed faces, objects, and scrambled images | No FFA activation differences when viewing faces | Face processing abnormalities not due to dysfunction in the FFA, but to abnormalities in surrounding networks involved in social cognition |
| Hadjikhani, Joseph, Snyder, Tager-Husberg, 2007 [172] | 10** | 7** | 34 ± 11 | 35 ± 12 | Viewed unemotional faces | No differences in FFA, inferior occipital gyrus activation; ↓Right AMY, IFC, STS, somatosensory cortex, PMC | Atypical activation in a broader face-processing network outside of FFA and inferior occipital gyrus; Suggests mirror neuron system disturbance during face-processing in ASD |
| Hall, Szeehtman, Nahmias, 2003 [173] | 8 (8) | 8 (8) | ** | ** | Emotion and gender recognition tasks | ↓IFA, FG; ↑right ATL, ACG, THAL | Recognition of emotions in ASD achieved through recruitment of brain regions concerned with attention, perceptual knowledge, and categorization |
| Hall, Doyle, Goldberg, West, Szatman, 2010 [174] | 12 (12) | 12(12) | 31.8** | 32** | Identified gender of subliminally presented images of anxious faces | ↓FFA; No AMY differences between groups | Transmission of social information along subcortical pathways intact, but signaling to downstream structures as well as the mechanisms of subsequent processing are impaired |
| Hubl, Bolte, Feineis-Matthews, et al, 2003 [175] | 10 (10) | 10 (10) | 25.3 ± 6.9 | 27.7 ± 7.8 | Viewed faces and complex patterns | ↓FG, esp during face processing; ↑Medial occipital gyrus, superior parietal lobule, medial frontal gyrus | Deficits in face-specific regions, but overdevelopment in areas of visual search; Predisposed for local processing, rather than global |
| Humphreys, Hasson, Avidan, Minshew, Behrmann, 2008 [176] | 13 (13) | 15 (15) | 27 ± 10 | 29 ± 10 | Viewed faces, buildings, objects and patterns in controlled and naturalistic settings | ↓FFA, occipital face area, STS in response to faces; No group differences in place-related or object-related processing | Differential organization of ventral visual cortex; Developmental effects of lower functional connectivity have a more pronounced effect on later-developing systems, like face-processing, than for early-developing systems, like object- and place-processing |
| Kleinhans, Richards, Sterling, et al,2008 [177] | 19** | 21** | 23.5 ± 7.8 | 25.1 ±7.6 | Viewed familiar faces, houses | Reduced functional connectivity FFA-AMY, FFA-PCC, FFA-THAL; Greater social impairment correlated with worse connectivity FFA-AMY, FFA-right IFC | Abnormal connectivity in limbic system underlies social deficits in ASD |
| Kleinhans, Johnson, Richards, et al, 2009 [178] | 19** | 20** | ** | ** | Viewed neutral faces | Reduced bilateral AMY habituation;No group differences in FG habituation | AMY hyperarousal to socially relevant stimuli; Sustained AMY arousal may contribute to social deficits |
| Kleinhans, Richards, Weaver, et al, 2010 [179] | 31 (29) | 25 (23) | 23.57 ± 6.6 | 23.32 ± 5.15 | Matched facial expressions of fear or anger | ↓Left PFC; ↑Occiptal lobe; Social anxiety correlated with ↑right AMY, ↓left middle temporal gyrus, ↓FFA | Social anxiety mediates emotional face perception |
| Kleinhans, Richards, Johnson, et al, 2011 [180] | 31 (29) | 25 (23) | 23.57 ± 6.6 | 23.32 ± 5.15 | Viewed images of faces and houses | No activation in right AMY, right pulvinar, or bilateral superior colliculi to faces; | Rapid face identification but failure to engage subcortical brain regions involved in face detection and automatic emotional face processing. |
| Koshino, Kana, Keller, et al, 2008 [181] | 11 (11) | 11 (10) | 24.5 ± 10.2 | 28.7 ± 10.9 | Working memory tasks using faces | ↓Inferior left PFC, right posterior temporal; Activation in a different FFA location; Lower FFA-frontal connectivity | Faces processed as objects; Working memory of faces not mediated by typical frontal regions |
| Loveland, Steinberg, Pearson, Mansour, Reddoch, 2008 [182] | 5 (4) | 4 (3) | 18 ± 1.3 | 17 + 1.1 | Auditory and visual emotional congruence task | During emotion trials, ↓OFt, STG, PHG, posterior cingulate gyrus, occipital gyrus | Fronto-limbic and superior temporal activity differences during integration of auditory and visual emotional stimuli |
| Monk, Weng, Wiggins, et al, 2010 [183] | 12** | 12** | 26 ± 6 | 27 ± 6 | Probe detection with different emotional expressions | ↑Right AMY to emotional faces; Greater right AMY and VMPFC coupling; Weaker positive right AMY and TL coupling | Attention must be factored into any model of neural circuitry in ASD; Overconnectivity may underlie greater emotional responses in ASD |
| Morita, Kosaka, Saito, et al, 2011 [184] | 15 (14) | 15 (13) | 23.7 ± 4.3 | 23.3 + 3.6 | Rated photogenicity of faces | ↓Setf-related activity in PCC; ↓Right IC and lateral OFC to embarrassment; ↓IC activity to self-face images associated with weak coupling between cognitive evaluation and emotional responses to self-face | Decoupling between evaluation of self-face images and emotional response; Dysfunction in PCC and IC contributes to lack of self-conscious behaviors in response to self-reflection |
| Ogai, Matsumoto, Suzuki, et al, 2003 [185] | 5** | 9** | 21.8 ± 5.9 | 23.0 ± 5.2 | Facial expression recognition | ↓Left insula, left IFG, left putamen during recognition of disgust and fear | Difficulty understanding facial expressions in others and, therefore, in manipulating social information |
| Pelphrey, Morris, McCarthy, Labar, 2007 [186] | 8 (6) | 8 (6) | 24.5 ± 11.5 | 24.1 ± 5.6 | Dynamic and static face processing | ↓AMY, STS, FG to dynamic faces | Dysfunctions in these component areas may contribute to problems in social and emotional processing |
| Perlman, Hudac, Pegors, Minshew, Pelphrey, 2011 [187] | 12 (11) | 7 (7) | 25.5 ± 7.47 | 28.57 ± 5.74 | Viewed faces while compelled to look at eyes | Right FG activity normalized by following predetermined scan paths to eyes, but AMY response unaffected | Rather than an underdeveloped FFA as a result of not focusing on faces during development, FFA appears functional; Impaired mechanism of appropriately directing gaze |
| Pierce, Muller, Ambrose, Allen, Courchesne, 2001 [188] | 6 (6) | 8 (8) | 29.5 ± 8 | 28.3** | Face perception with gender identification | ↓Bilateral FG, left AMY; 50% of group showed atypical FG activation to faces | ASD is associated with aberrant locations of maximal activations to faces |
| Pierce, Haist, Sedaghat, Courchesne, 2004 [189] | 7 (7) | 9 (9) | 27.1 ± 9.2 | ** | Familiar versus unfamiliar face processing | No group difference in extent of FFA activation to faces; ↑FFA to familiar faces. Right hemisphere dominance to both types of faces; Limited response in the posterior cingulate, AMY, MFL | FFA hypoactivation to faces in ASD may be specific to unfamiliar faces; ASD may be characterized by anomalous FFA modulation by faces, rather than hypoactivation |
| Pierce, Redcay, 2008 [190] | 11 (9) | 11 (9) | 9.9 ± 2.1 | 9.8 ± 1.8 | Matched faces of mothers, other children, adult strangers | Normal FG response to face of mother or other children; ↓FG to stranger adult faces | Selective reduction in FG activity in response to strangers may be a result to reduced attention and interest in those conditions |
| Pinkham, Hopfinger, Peiphrey, Pwen, Penn, 2008 [191] | 12** | 12** | 24.08 ± 5.71 | 27.08 ± 3.99 | Free-viewing face processing | ↓Right AMY, FFA; ↓Left VLPFC compared to non-paranoid individuals with schizophrenia | Potential common substrates of impaired social cognition in ASD and schizophrenia |
| Rudie, Shehzad, Hernandez, et al, 2011 [192] | 23 (21) | 25 (22) | 12.6 ± 2.83 | 13.3 ± 96 | Emotional face processing | Reduced functional integration; AMY-secondary visual areas, PO-parietal cortex, Reduced segregation AMY-DLPFC, PO-VMPFC; Reduced integration PO-FC, within right NAC | Reduced functional integration and segregation of large-scale brain networks during face viewing |
| Scherf, Luna, Minshew, Behrmann, 2010 [193] | 10 (10) | 10 (10) | 12.2 ± 1.1 | 11.2 ± 1.3 | Vignettes of faces, common objects, houses and scenes of navigation | ↓FG occipital face area, STS to faces; ↑Ventral posterior FG to faces | Selective ventral visual pathway disruption; Face-processing alteration present in early adolescence, Face perception in ASD akin to object perception in typical development |
| Schultz, Gauthier, Klin, et aI, 2000 [194] | 14 (14) | 28 (28) (2 groups of 14) | 24.08 ± 5.71 | 27.08 ± 3.99 | Face discrimination | ↓Right FG; ↑Right ITG | Brain activation in the ASD group during face discrimination was consistent with feature-based strategies |
| Uddin, Davies, Scott, et al, 2008 [195] | 18 (18) | 12 (12) | 13.19 ± 2.61 | 12.23+2.10 | Judged “self” or “other” for morphed face images | ↓Right premotor/prefrontal during presentation of “other” faces | Functional dissociation between the representation of self versus others suggests a neural substrate of self-focus and decreased social understanding |
| Wang, Dapretto, Hariri, Sigman, Bookheimer, 2004 [196] | 12 (12) | 12 (12) | 13.91 ± 2.61 | 12.23 ± 2.10 | Emotion matching naming | ↓FG and ↑precuneus during matching facial expressions; Lack of modulation by task demands in the AMY | Recruited different neural networks and relied on different strategies when processing facial emotion |
| Welchew, Ashwm, Berkouk, et al, 2005 [197] | 13 (13) | 13 (13) | 31.2 ± 51 | 25.6± 5.1 | Face processing | Abnormal AMY—parahippocampal connectivity | Difficulty in grasping facial expressions in others and, therefore, in manipulating interpersonally derived information |
| Weng, Carrasco, Swartz, et al, 2011 [198] | 22 (17) | 20 (19) | 14.36 ± 17 | 14.97 ± 1.95 | Emotional face processing | ↑AMY, ventral PFC and striatum, particularly to sad faces; Negative correlation between age, pubertal status, and AMY activation | Greater activation in social-emotional processing regions when viewing faces |
In neurotypical participants, the medial-lateral fusiform gyrus (FG) as well as the superior temporal sulcus, amygdala, and orbitofrontal cortex, activate in response to faces.13 The majority of fMRI studies in ASDs indicate FG hypoactivity to faces14-22 and to facial expressions.15,20,23-25 However, other reports suggest no differences in FG activation to familiar faces,26-29 stranger faces in the presence of an attentional cue,30 or when matching upright with inverted faces.31
These apparently inconsistent findings may be reconciled in a number of ways.32,33 The degree of visual attention to faces appears to be a critical factor moderating FG activation to faces in ASDs, with tasks that guide visual attention to faces or analytic approaches that account for point-of-regard resulting in relatively less FG hypoactivation in ASDs.21,30 This conclusion is supported by research indicating that face familiarity moderates FG responses to faces in ASDs28 and that impaired social cognition in ASDs may be mediated, at least in part, by attention to social cues, rather than by deficits in social cue processing per se.31,35 Similarly, lifelong amotivation to interact with faces may result in reduced perceptual skill when processing faces, and, in turn, cause FG hypoactivation to faces in ASDs that is perhaps a downstream consequence of reduced social experience rather than pathognomonic to ASDs.36 Moreover, the FG encodes not only face percepts, but social knowledge as well,37 suggesting that the FG may mediate: (i) the attribution of social meaning to stimuli: (ii) the retrieval of social semantic information; and (iii) self-referential experiences.28 Thus, the disparate results of the face processing literature in ASDs likely reflect the diverse and subtle social processes mediated by the FG and recruited by diverse fMRI tasks.
Amygdala response to faces in ASDs has also been extensively studied, and results in this area are decidedly mixed. There is evidence of no differences in amygdala activation to faces,18 of amygdala hypoactivation during face viewing15,16,26,31,38 and face matching,16 as well as evidence of amygdala hyperactivation to faces39,40 in ASDs, particularly when accounting for gaze time to faces21 (but see ref 41 for an exception). One study reported decreased amygdala habituation to the repeated presentation of faces, suggesting that social deficits in ASDs may be influenced by hyperarousal to faces due to protracted amygdala activation.42
Theory of mind
Theory of mind and mental inferences have been examine in ASDs via fMRI studies that address the ability to infer feeling states and/or intentions (Table II), skills that typically develop during the first 4 or 5 years of life and that are critical for the development of social skills and for successful navigation of the social world.43 Such tasks include images, stories, and animations designed to elicit the attribution of mental states. Results from typically developing individuals indicate with remarkable consistency that theory of mind is mediated by the posterior superior temporal sulcus at the temporoparietal junction, the temporal poles, the amygdala, and dorsal medial and ventrolateral prefrontal cortex.44
Table II. Studies investigating theory of mind and mental inference-making in autism spectrum disorders. ASD: Autism Spectrum Disorder; TYP: Neurotypical; †ASD refers to the entire autism sample in a particular study, including high functioning autism, Asperger's syndrome, and pervasive developmental disorder not otherwise specified; *Total number of participants is presented first followed by the number of females in parentheses, if reported; **Not specified; ↓: decreased activation; ↑: increased activation. Abbreviations used in tables: ACC, anterior cingulate cortex; ACG, anterior cingulate gyms; AG, angular gyms; Al, anterior insula; AMY, amygdala; ATL, anterior temporal lobe; BA, Broca's area; BG, basal ganglia; CM, caudate nucleus; DAC, dorsal anterior cingulate; DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; DN, dentate nucleus; FFA, fusiform face area; FG, fusiform gyms; IC, insular cortex; IFA, inferior frontal area; IFC, inferior frontal cortex; IFG, inferior frontal gyrus; IPL, inferior parietal lobe; ITG, inferior temporal gyrus; LG: lingual gyrus; LSTG, left superior temporal gyrus; MCG, >middle cingulate gyrus; MFC, midfrontaI cortex; MFG, midfrontal gryus; MFL, medial frontal lobes; NAC, nucleus accumbens; OFC, orbitofrental cortex; OFG, orbitofrental gyrus; MPFC, medial prefrontal cortex; MTG, medial temporal gyrus; PO, pars opercularis; PCC, posterior cingulate cortex; PFC, prefrontal cortex; PHG, parahippocampal gyrus; PL, parietal lobe; PMC, premotor cortex; PVC, primary visual cortex; RPVC, right primary visual cortex; SFG, superior frontal gyrus; SPL, superior parietal lobe; STG, superior temporal gyrus; STS, superior temporal sulcus; THAL, thalamus; TL, temporal lobe; TPJ, temporoparietal junction; VS, ventral striatium; VLPFC, ventrolateral prefrontal cortex; VOC, ventral occipital cortex; VMPFC, ventromedial prefrontal cortex; WA, Wernicke's Area .
| Citation | ASD*† | TYP*† | ASD age | TYP age | Task(s) | Core findings in ASD group (relative to controls) | Conclusions |
| Baron-Cohen, Ring, Wheelwright, et al, 1999 [199] | 6 (4) | 12 (6) | 26.3 ± 2.1 | 25.5 ±2.8 | Inferred mental states from images of eyes | ↑Frontal-temporal regions; ↓AMY | Supports amygdala theory of autism |
| Castelli, Frith, Happe, Frith, 2002 [200] | 10** | 10** | 33 ± 7.6 | 25 ± 4.8 | Viewed animated sequence of geometric shapes | ↓MPFC, STS, temporal poles; Decreased extrastriate functional connectivity | Possible neurofunctional explanation for impaired mentalizing |
| Dapretto, Davies, Pfeifer, et al, 2006 [201] | 10 (9) | 9 (9) | 12.05 ± 2.5 | 12.38 ± 2.22 | Imitation and observation of emotional expressions | ↓IFG; Mirror neuron activity inversely related to social symptom severity | Dysfunctional mirror neuron system may underlie social deficits in autism |
| Kaiser, Hudac, Shultz, et al, 2010 [202] | 25 (20) | 17 (12) (no sibling with ASD); 20 (9) (sibling with ASD) | 11.8 ± 3.6 | 10.9 ± 3.1 (no sibling with ASD); 11.3 ± 28 (sibIing with ASD) | Viewed biological motion clips and scrambled motion clips | Differed in right AMY, VMPFC, left VLPFC, right posterior STS, bilateral FG; Controls without ASD sibling differed from other two groups in left DLPFC, right ITG, bilateral FG, CG; Controls with ASD sibling differed from other two groups in right posterior STS, VMPFC | Identifies non-overlapping regions associated with ASD phenotypes and ASD genetic vulnerability in the absence of ASD symptoms |
| Hadjikhani, Joseph, Manoach, et al, 2009 [203] | 9** | 11 (8) | 30 ± 11 | 31 ± 14 | Emotion processing of body expressions | No differential brain activation to bodies expressing fear compared with neutral bodies; ↓FC, Al to emotionally neutral bodies | Emotion perception deficits in ASD may be due to compromised processing of the emotional component of observed actions |
| Pitskel, Boiling, Hudac et al, 2011 [204] | 15(15) | 14 (13) | 23.4 ± 6.9 | 24.2 ± 7.4 | Viewed direct and averted gaze of virtual human face | ↓Right TPJ, right Al, left lateral OC; ↑ Left DLPFC | Brain mechanisms underlying processing gaze direction in ASD |
| Konishi, Nakajima, Uchida, et al, 1999 [205] | 18 (12) | 18 (12) | 38.6 ± 12.4 | 33.0 ± 10.7 | Imitation inhibition task | Imitation scores correlated with ↓medial PFC, TPJ | Highlights contribution of hyperimitation to reduced social cognition |
| Pelphrey, Morris, McCarthy, 2005 [206] | 10 (9) | 9 (8) | 23.2 ± 9.9 | 23.4 ± 5.8 | Viewing congruent and incongruent eye gaze shifts | ↓STS on incongruent trials | Lack of STS modulation to congruent and incongruent gaze shifts contributes to eye gaze processing deficits |
| Silani, Bird, Brindley, et al, 2008 [207] | 15 (13) | 15 (13) | 36.6 ± 11.7 | 33.7 ± 10.3 | Emotion introspection task | ↓Self-reflection/ mentalizing regions (MPPC, ACC, precuneus, inferior OFC, temporal poles, cerebellum) during self introspection; Al activity predicted alexithymia and empathy in both groups | Alexithymia and empathy deficits linked to anomalous Al actvity |
| Wang, Lee, Sigman, Dapretto, 2007 [208] | 18 (18) | 18 (18) | 12.4 ± 2.9 | 11.8 ± 1.9 | Processed potentially ironic remarks | ↓MPFC, right STG to irony; MPFC activity in ASD modulated by instructions to attend to faces and tones of voice; MPFC activity inversely related to symptom severity in ASD group | MPFC mediates understanding the intentions of others |
| Wicker, Fonlupt, Hubert et al, 2008 [209] | 12 (11) | 14 (14) | 27 ± 11 | 23 ± 10 | Emotion and age discrimination | ↓DMPFC, right VLPFC, right STG; Abnormal connectivity between AMY, VLPFC, DLPFC, posterior occipital-temporal regions | Abnormal connectivity between structures of the social brain could explain social deficits in ASD |
The amygdala plays a critical role in multiple aspects of mentalizing, including determining emotional states of others from facial expressions,45 and a number of studies have reported aberrant amygdala activation in ASDs during tasks requiring inferring mental states from pictures of eyes46,47 and judging facial expressions,23 suggesting that the amygdala may fail to assign emotional relevance to social stimuli in ASDs. Other studies, however, have reported that ASDs are characterized by amygdala hyperactivity during face viewing48 and anticipation,49 suggesting that the so-called “amygdala theory of autism” may reflect impaired amygdala modulation rather than simply hypoactivation in social contexts.
Another brain region that has received scrutiny in fMRI studies of theory of mind in ASDs is the posterior superior temporal sulcus, a region recruited during tasks that involve interpreting other's mental states from biological motion cues.50 There are reports of posterior superior temporal sulcus hypoactivation while processing incongruent eye gaze shifts,51 while viewing direct and averted gaze,52 during intentional attribution to animated sequences of geometric figures,53 and during speech perception.54 A recent study of children with ASDs and their unaffected siblings found that activation in posterior superior temporal sulcus (as well as the amygdala and ventromedial prefrontal cortex) during biological motion perception differentiated children with ASDs both from their unaffected siblings and from matched control participants, suggesting that activation of this region may be related to phenotypic expression of social deficits in ASDs rather than genetic liability.55
Another area of inquiry has been functioning of the mirror neuron system (including, in humans, the pars opercularis in the inferior frontal gyrus). This system is active during imitation, action observation, intention understanding, and understanding emotional states of others.56 The inferior frontal gyrus has been reported to be relatively less active in ASDs during imitation and observation of faces57-59 and during imitation and observation of emotional expressions in ASDs,48,60 suggesting that mirror neuron dysfunction may account for social deficits in ASDs, though this contention has been questioned.61 Additionally, a recent metaanalysis of fMRI studies of social processing in ASDs revealed hypoactivation of the right anterior insula across studies (but see ref 62 for an exception), a region that is believed to be a relay station for projections from the IFG to the amygdala.63
Cognitive control
Restricted and repetitive behaviors and interests constitute a multifaceted symptom domain in ASDs that comprises both lower-order motoric repetitive behaviors (eg, body rocking, hand flapping) as well as higher-order cognitive manifestations (eg, a need for predictability).64 Because fMRI requires minimal motion from research subjects, cognitive manifestations of restricted and repetitive behaviors have been the focus of fMRI research. Such studies have mostly relied on tasks requiring cognitive control because of linkages between deficits on neuropsychological cognitive control tasks and symptoms of restricted and repetitive behaviors and interests in ASDs.65
Animal lesion and nonclinical human neuroimaging studies indicate that cognitive control is mediated by frontostriatal brain systems, including the lateral prefrontal cortex, the inferior frontal cortex (including the insular cortex), the anterior cingulate cortex, the intraparietal sulcus, and the striatum.66 Functional MRI studies of cognitive control in ASDs have revealed anomalous activation in frontostriatal brain regions (Table III), including inferior and middle frontal gyri, dorsal anterior cingulate cortex, and the basal ganglia during cognitive control tasks. Such findings have been reported using go/no-go, Stroop, and switching tasks,67 tasks that require interference inhibition,68-72 response monitoring,73 novelty detection,74-75 spatial attention,68 working memory,76,77 and saccadic eye movements.78 These findings have been interpreted to reflect deficits in behavioral inhibition and/or generation of adaptive behaviors linked to the expression of restricted and repetitive behavior and interests. Although the direction of effects has varied across studies (ie, frontostriatal hyperactivation vs hypoactivation), likely due to task demands and analysis methods, anomalous frontostriatal activation during tasks requiring cognitive control has been a consistent result in ASD samples, with the majority of findings indicating frontostriatal hyperactivation that has been interpreted to reflect a neurof unctional compensatory mechanisms to overcome cortical inefficiency.70
Table III. Studies investigating cognitive control in autism spectrum disorders. ASD: Autism Spectrum Disorder; TYP: Neurotypical; †ASD refers to the entire autism sample in a particular study, including high functioning autism, Asperger's syndrome, and pervasive developmental disorder not otherwise specified; *Total number of participants is presented first followed by the number of females in parentheses, if reported; **Not specified; ↓: decreased activation; ↑: increased activation. Abbreviations used in tables: ACC, anterior cingulate cortex; ACG, anterior cingulate gyms; AG, angular gyms; Al, anterior insula; AMY, amygdala; ATL, anterior temporal lobe; BA, Broca's area; BG, basal ganglia; CM, caudate nucleus; DAC, dorsal anterior cingulate; DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; DN, dentate nucleus; FFA, fusiform face area; FG, fusiform gyms; IC, insular cortex; IFA, inferior frontal area; IFC, inferior frontal cortex; IFG, inferior frontal gyrus; IPL, inferior parietal lobe; ITG, inferior temporal gyrus; LG: lingual gyrus; LSTG, left superior temporal gyrus; MCG, >middle cingulate gyrus; MFC, midfrontaI cortex; MFG, midfrontal gryus; MFL, medial frontal lobes; NAC, nucleus accumbens; OFC, orbitofrental cortex; OFG, orbitofrental gyrus; MPFC, medial prefrontal cortex; MTG, medial temporal gyrus; PO, pars opercularis; PCC, posterior cingulate cortex; PFC, prefrontal cortex; PHG, parahippocampal gyrus; PL, parietal lobe; PMC, premotor cortex; PVC, primary visual cortex; RPVC, right primary visual cortex; SFG, superior frontal gyrus; SPL, superior parietal lobe; STG, superior temporal gyrus; STS, superior temporal sulcus; THAL, thalamus; TL, temporal lobe; TPJ, temporoparietal junction; VS, ventral striatium; VLPFC, ventrolateral prefrontal cortex; VOC, ventral occipital cortex; VMPFC, ventromedial prefrontal cortex; WA, Wernicke's Area .
| Citation | ASD*† | TYP*† | ASD age | TYP age | Task(s) | Core findings in ASD group (relative to controls) | Conclusions |
| Allen, Courchesne, 2003 [210] | 8 (7) | 8 (7) | 26.89 ± 8.59 | 26.77 ± 8.22 | Motor control and attentional control | ↑Motor regions; ↓Cerebellar attention | Developmental cerebellar abnormality has differential functional implications for cognitive and motor systems |
| Allen, MuIIer, Courchesne, 2004 [211] | 8 (7) | 8 (7) | 26.89 ± 8.59 | 26.77 ± 8.22 | Repeated button pressing | ↓Ipsilateral anterior cerebellar hemisphere | Cerebellar dysfunction that is a reflection of abnormal anatomy |
| Agam, Joseph, Barton, Manoach, 2010 [212] | 11** | 14** | 28 ± 10 | 27 ± 8 | Antisaccade task | ↑Frontal eye field, dorsal ACC; Decreased frontal eye field—dorsal ACC connectivity; Both findings associated with repetitive behavior symptoms | Functional neural abnormalities in volitional ocular-motor control linked to repetitive behaviors |
| Belmonte, Yurgelun-Tedd, 2003 [213] | 6 (5) | 6 (5) | 32.7 ± 9.8 | 27.2 ± 4.4 | Bilateral visual spatial attention task | ↓Left VOC; ↑Left IPS | Neurofunctional basis of impaired selective attention |
| Damarla, Keller, Kana, et al, 2010 [214] | 13 (11) | 13 (13) | 19 ± 5.5 | 22.1 ± 4.25 | Embedded figures task | ↓Left DLPFC, inferior parietal areas; ↑Visuospatial areas; Decreased frontal—visuospatial connectivity | Cortical underconnectivity despite preserved visuospatial performance |
| Dichter, Belger, 2007 [215] | 17 (16) | 15 (14) | 22.9 ± 5.2 | 24.6 ± 6.5 | Flanker task (interference inhibition) | ↓Prefrontal, parietal regions during the incongruent social condition only | Social stimuli interfere with brain regions mediating cognitive control |
| Dichter, Belger, 2008 [216] | 12 (12) | 22 (22) | 23.2 ± 5.8 | 25.1 ± 6.0 | Flanker task intermixed with high and low arousal images | ↓Right MFG on conflict trials preceded by high arousal images only | Abnormal modulation of regions mediating cognitive control in context of high arousal |
| Dichter, Felder, Bodf ish, 2009 [217] | 15 (14) | 19 (18) | 23.3 ± 11.1 | 28.0 ± 7.9 | Oddball target detection task with social and non-social targets | ↑Right IFG, DMPFC to social targets, DMPFC activation to social targets predicted severity of social impairments | DMPFC hyper activation during cognitive control of social stimuli contributes to expression of social deficits |
| Gilbert, Bird, Brindley, Frith, Burgess, 2008 [218] | 14 (11) | 18 (13) | 38 ± 13 | 32 ± 8 | (1) Random response generation task (2) Selected stimulus-oriented vs stimulus-independent thought | Task 1: ↓Cerebellum, left lateral temporal cortex; Task 2: ↑MediaI rostral PFC | Impaired cognitive control in is associated with task-specific functional changes |
| Gilbert, Meuwese, Towgood, Frith, Burgess, 2003 [219] | 16 (14) | 16 (12) | 32 ± 7.7 | 31 ±5.7 | (1) Stimulus-oriented spatial task (2) Stimulus-independent spatial task | Similar activation patterns; Multi-voxel similarity analyses revealed found abnormal functional specialization within medial rostral PFC | Abnormal functional specialization within medial rostral PFC |
| Gomot, Belmonte, Bullmore, Bernard, Baron-Cohen, 2008 [220] | 12 (12) | 12 (12) | 13.5 ± 1.6 | 13.8 ± 1 | Auditory novelty detection | ↑Right PFC-premotor, left inferior parietal regions | Cognitive control associated with activation of a more widespread network of regions |
| Haist, Adamo, Westerfield, Courchesne, Townsend, 2005 [221] | 8 (8) | 8 (8) | 23.4 ± 11.4 | 25.6 ± 12.5 | Spatial attention task | ↓Frontal, parietal, occipital, within the IPL; ↑SPL and extrastriate cortex | Deficit in automatic spatial attention abilities and aberrant voluntary spatial attention skills |
| Just, Cherkassky, Keller, Kana, Minshew, 2007 [222] | 18 (17) | 18 (15) | 27.1 ± 11.9 | 24.5 ± 9.9 | Tower of London task | Similar activation in DLPFC between groups; Lower frontal—parietal connectivity | Cognitive control deficits may be preferentially linked to lower cortical integration of information |
| Kana, Keller, Minshew, Just, 2007 [223] | 12 (11) | 12 (11) | 26.8 ± 7.7 | 22.5 ± 3.2 | Go/No-go task | ↓Left ACG, left precuneus, right AG, premotor areas; Lower connectivity between ACS, MCG, right MFG, IFG, inferior parietal regions | Inhibition circuitry is activated atypically and is less synchronized, leaving inhibition to be accomplished by strategic control rather than automatically |
| Keehn, Brenner, Palmer, Lincoln, MuIIer, 2008 [224] | 9 (9) | 13 (13) | 15.1 ± 2.6 | 14.1 ± 2.1 | Visual search task | ↑Occipital and frontoparietal regions | Enhanced discrimination and increased top-down modulation of attentional processes |
| Kennedy, Redcay, Courchesne, 2006 [225] | 12** | 14** | 25.49 ± 9.61 | 26.07 ± 7.95 | Counting Stroop task | Decreased deactivation of resting network regions (MPFC/rostral ACC, PCC) | Lack of deactivation indicates abnormal internally directed processes at rest and may be compensatory |
| Lee, Yerys, Della Rosa, et aI, 2003 [226] | 12 (9) | 12 (8) | 10.17 ± 1.57 | 11.01 ± 1.78 | Go/No-go task | Age-moderated decreased connectivity in IFC, motor planning regions | Atypical developmental connectivity trajectories for IFC with other neural regions supporting response inhibition |
| Lee, Foss-Feig, Henderson et al, 2007 [227] | 17 (12) | 14 (11) | 10.37 ± 1.52 | 10.85 ± 1.47 | Embedded figures task | ↑Dorsomedial premotor, left superior parietal, right occipital cortex | Reduced cortical activation suggests that disembedded visual processing is performed sparingly |
| Liu, Cherkassky, Minshew, Just, 2011 [228] | 15 (14) | 15 (15) | 25.2 ± 7.6 | 26.3 ± 8.2 | (1) Line-counting task (2) Judged whether a 3D object was possible | ↓Medial frontal to possibility task; Decreased frontal—posterior connectivity | Less effort for lower-level processing; Reduced global-to-local interferences |
| Luna, Minshew, Garver, et al, 2002 [229] | 11 (9) | 6 (6) | 32.3 ± 9.3 | 30.3 ± 11.8 | (1) Spatial working memory task (2) Guided saccade task | Task 1: ↓DLPFC, PCC; Task 2: no differences | Neurofunctional basis of impaired working memory |
| Manjaly, Bruning, Neuf ang et al, 2007 [230] | 12** | 12** | 14.4 ± 2.7 | 14.3 ± 2.7 | Embedded figures task | ↑Right PVC, bilateral extrastriate areas | Enhanced local processing in early visual areas rather than impaired global processing |
| Mizuno, Villa lobos, Davies, Dahl, Muller, 2006 [231] | 8 (8) | 8 (8) | 28.4 ± 8.9 | 28.1 ± 8.3 | Visuomotor coordination task | Increased functional connectivity in left insula, right postcentral gyrus, MFG | Underconnectivity hypothesis unsupported; Subcortico-cortical connectivity may be hyperfunctional, potentially compensating for reduced cortico-cortical connectivity |
| Muller, Kleinhans, Kemmotsu, Pierce, Courchesne, 2003 [232] | 8 (8) | 8 (8) | 28.4 ± 8.9 | 28.1 ± 8.3 | 6-digit sequence learning | ↑PFC posterior parietal cortex | Disturbances incerebello-thalamocortical pathways |
| Muller, Cauich, Rabio, Mizuno, Courchesne, 2004 [233] | 8 (8) | 8 (8) | 28.4 ± 8.9 | 28.1 ± 8.3 | 8-digit sequence learning | ↑Right pericentral and PMC; Delayed activation of BA 3, 4, 6 | Atypical use of the primary sensory and premotor cortices during learning |
| Muller, Pierce, Ambrose, Allen, Courchesne, 2001 [234] | 8 (8) | 8 (8) | 28.4 ± 8.9 | 28.1 ± 8.3 | Visual stimulation using finger movements | ↓Contralateral periolandic cortex, BG, THAL, bilateral supplementary; motor area, ipsilateral cerebellum, bilateral DLPFC ↑Postenor cortex, PFC, extrastnrite regions | Abnormal functional variability and less distinct regional activation patterns |
| Noonan, Haist, Muller, 2003 [235] | 10 (10) | 10 (10) | 23 ± 9.9 | 25.8 ± 9.9 | Source recognition task | Increased connectivity between left MFC—left superior parietal regions | An inefficiency in optimizing network connections during task performance |
| Ring, Baron-Cohen, Wheelwright, et al, 1999 [236] | 6 (4) | 12 (6) | 26.3 ± 2.1 | 25.5 ± 2.8 | Embedded figures task | ↓Right DLPFC, bilateral parietal cortex; ↑Right ventral occipitotemporal cortex | Object feature analysis, rather than working memory systems, are used for local processing and visual search in autism |
| Solomon, Ozonoff, Ursu, et al, 2009 [237] | 22 (17) | 23 (18) | 15.2 ± 1.7 | 16.0 ± 2.0 | Preparing to overcome prepotency task | ↓Anterior frontal, parietal occipital regions; Decreased frontal/parietal/occipital connectivity related to ADHD symptoms | Fronto-parietal connectivity deficits contribute to ADHD symptoms in autism |
| Schmitz, Rubia, Daly, et al, 2006 [238] | 10 (10) | 12 (12) | 38 ± 9 | 39 ± 6 | (1) Go/No-go task (2) Stroop task (3) Cognitive set shifting | Task 1: ↑left IFG, OFG Task 2: ↑left insula, AMY-hippocampal junction; Task 3: ↑PL | Cognitive control associated with increased brain activity in multiple regions |
| Shafritz, Dichter, laranek, Belger, 2008 [239] | 18 (16) | 15 (13) | 22.3 ± 8.7 | 24.3 ± 6.2 | Oddball target detection task | ↓Frontal, striatal, and parietal regions; ACC activation correlated with repetitive behavior symptoms | Cognitive control deficits and repetitive behaviors might be associated with dysfunctions in neural circuitry |
| Silk, Rinehart, Bradshaw et al, 2006 [240] | 7 (7) | 9 (9) | 14.7 ± 2.9 | 15.0 ± 1.8 | Mental rotation task | ↓lateral and medial PMC, DLPFC, ACG, CN | Dysfunctional frontostriatal networks during cognitive control |
| Takarae, Minshew, Luna, Sweeney, 2007 [241] | 13** | 14** | 24.5 ± 7.7 | 26.6 ± 7.8 | Saccadic eye movement paradigms | ↑DLPFC, CN, medial THAL, ACC, PCC, right DN | Cognitive control regions may compensate for lower-level processing difficulties |
| Thakkar, Polli, Joseph, et al, 2008 [242] | 12 (10) | 14 (8) | 30 ± 11 | 27 ± 8 | Anti-saccade task | ↑Rostral ACC, Reduced fractional anisotropy in white matter underlying rostral ACC; Repetitive behaviors correlated with rostral ACC activation | Rostral ACC abnormalities contribute to repetitive behaviors |
Communication
Investigations of communication deficits in ASDs have focused predominantly on brain regions mediating language perception, comprehension, and generation. The left hemisphere is typically language-dominant, and speech production is mediated by Broca's area at the junction of the frontal, parietal, and temporal lobes, whereas speech comprehension is mediated by Wernicke's area in the posterior temporal lobe.79 Heschl's gyrus, in the dorsal temporal lobe, contains primary auditory cortex as well as the angular gyrus, involved in higher-order language comprehension and cross-modal integration, and the inferior parietal lobule, involved in processing semantic content.80
fMRI studies of communication functions in ASDs have used tasks requiring listening to speech sounds,54,81,82 sentence comprehension,83-85 verbal fluency,86 pragmatic language comprehension,87 semantic judgments,88 responsenaming,89 and viewing body gestures90-91 (Table IV). Overall, findings indicate differential lateralization patterns in ASDs (ie, reduced left > right lateralization),82,84,86,87,89 decreased synchrony of brain regions processing language,83,92 decreased automaticity of language processing,93 greater neurofunctional deficits for speech than songs,94 and recruitment of brain regions that do not typically process language.83,95-97 A recent methodological innovation in the domain of language-based fMRI studies in ASDs has been to present speech stimuli to veryyoung children with ASDs (as young as 12 months old) while asleep.82,98 Although the diagnostic stability of ASDs for children in this age range must be considered, this approach has the potential to leverage task-based fMRI in far younger children with ASDs to examine altered developmental trajectories associated with impaired receptive language skills. Additionally, sleep fMRI would appear to be well suited to studying early emerging functional brain activation properties linked to speech processing in infant high-risk paradigms.
Table IV. Studies investigating communication in autism spectrum disorders. ASD: Autism Spectrum Disorder; TYP: Neurotypical; †ASD refers to the entire autism sample in a particular study, including high functioning autism, Asperger's syndrome, and pervasive developmental disorder not otherwise specified; *Total number of participants is presented first followed by the number of females in parentheses, if reported; **Not specified; ↓: decreased activation; ↑: increased activation. Abbreviations used in tables: ACC, anterior cingulate cortex; ACG, anterior cingulate gyms; AG, angular gyms; Al, anterior insula; AMY, amygdala; ATL, anterior temporal lobe; BA, Broca's area; BG, basal ganglia; CM, caudate nucleus; DAC, dorsal anterior cingulate; DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; DN, dentate nucleus; FFA, fusiform face area; FG, fusiform gyms; IC, insular cortex; IFA, inferior frontal area; IFC, inferior frontal cortex; IFG, inferior frontal gyrus; IPL, inferior parietal lobe; ITG, inferior temporal gyrus; LG: lingual gyrus; LSTG, left superior temporal gyrus; MCG, >middle cingulate gyrus; MFC, midfrontaI cortex; MFG, midfrontal gryus; MFL, medial frontal lobes; NAC, nucleus accumbens; OFC, orbitofrental cortex; OFG, orbitofrental gyrus; MPFC, medial prefrontal cortex; MTG, medial temporal gyrus; PO, pars opercularis; PCC, posterior cingulate cortex; PFC, prefrontal cortex; PHG, parahippocampal gyrus; PL, parietal lobe; PMC, premotor cortex; PVC, primary visual cortex; RPVC, right primary visual cortex; SFG, superior frontal gyrus; SPL, superior parietal lobe; STG, superior temporal gyrus; STS, superior temporal sulcus; THAL, thalamus; TL, temporal lobe; TPJ, temporoparietal junction; VS, ventral striatium; VLPFC, ventrolateral prefrontal cortex; VOC, ventral occipital cortex; VMPFC, ventromedial prefrontal cortex; WA, Wernicke's Area .
| Citation | ASD*† | TYP*† | ASD age | TYP age | Task(s) | Core findings in ASD group (relative to controls) | Conclusions |
| Anderson, Lange, Froehlich, et al, 2010 [243] | 26 (26) | 15 (15) | 21.5 ± 6.4 | 22.5 ± 6.3 | (1) Thought about a described word (2) Filled in missing word in a sentence | ↓Left posterior insula, bilateral receptive language areas, Receptive language correlated with activation of posterior left WA; Verbal IQ correlated with activation of bilateral BA, PFC, lateral PMC | Posterior insula implicated in receptive language impairments |
| Boddaert, Belin, Chabane, et al, 2003 [244] | 5 (4) | 8 (8) | 19.1 ± 4.5 | 21.9 ± 3.3 | Listened to speech-like sounds | ↑ Right MFG | Abnormal auditory cortical processing implicated in language impairments |
| Catarino, Luke, Waldman, et aI, 2011 [245] | 12 (12) | 12(12) | 27.0 ± 10 | 34.0 ± 13 | Detected semantic incongruities within written sentences | More spatially restricted activation pattern (only left IFG, left ACC, right FG) | impaired integration of multiple neural networks related to difficulties in use of context |
| Eigsti, Schuh, Mend, Schultz, Paul, 2011 [246] | 16** | 11** | ** | ** | Processed linguistic stimuli that varied in emotional and semantic content | Affective and grammatical prosodic cues prompted more generalized activation | Language processing less automatic; Linkages between ToM and language processing deficits; Increased reliance on executive control regions for speech processing |
| Eyler, Pierce, Courchesne, 2012 [247] | 40 (40) | 40 (40) | 32. 0 mo ± 10.2 | 25.6 mo ± 9.6 | Listened to story with complex, simple, or backward speech during sleep | ↓Left hemisphere to speech sounds (worsens with age). Abnormally right-lateralized temporal cortex to language (worsens with age) | Lateralized abnormalities of temporal cortex processing of language in toddlers with autism |
| Grezes, Wicker, Berthoz, de Gelder, 2009 [248] | 12 (10) | 12 (12) | 26.6 ± 10.4 | 21.0 ± 1.6 | Viewed fearful or neutral body language | ↓AMY, IFG, PMC to fearful gestures | Dysfunction in this network may impact the communication deficits present in autism |
| Groen, Tesink, Petersson, et al, 2010 [249] | 16 (12) | 26 (21) | 15.3 ± 1.6 | 15.7 ± 1.7 | Sentences congruent or incongruent to speaker | ↓Left IFG for sentences requiring integration of speaker information; No difference for semantic- and world-knowledge sentences | ASD recruits left IFG atypically in language tasks that demand integration of social information |
| Hadjikhani et al, 2009 [203] | 12 (9) | 11 (11) | 30 ± 11 | 35 ± 12 | Recognition of emotional bodies | ↓lFC, Al in response to emotionally neutral gestures | Identifies neural mechanisms of impaired affect communication |
| Harris, Chabris, Clark, et al, 2006 [250] | 14 (14) | 22 (22) | 36 ± 12 | 31 ± 9 | Semantic and perceptual word processing | During semantic processing, ↓BA, ↑WA; Diminished activation difference between concrete and abstract words | Abnormal Braca's area development that may be linked with language deficits |
| Hesling, Dilharreguy, Peppe, et al, 2010 [251] | 8 (8) | 8 (8) | 23.± 38 ± 2.10 | 23.05 ± 2.02 | Listened to speech stimulus involving variable intonation, rhythm, focus and affect | Abnormal neural network for prosodic speech perception in left supra marginal gyrus; Absence of deactivation patterns in default mode | Prosodic impairments could not only result from activation pattern abnormalities, but also from an inability to inhibit default network |
| Just, Cherkassky, Keller, Minshew, 2004 [252] | 17 (13) | 17 (12) | 28.0 ± 13.3 | 28.6 ± 10.7 | Identified agent or object in each sentence | ↑WA; ↓BA; Decreased functional connectivity between contributing cortical areas | Decreased information synchronization across the language processing network |
| Kana, Keller, Cherkassky, Minshew, Just, 2006 [253] | 12 (11) | 13 (12) | 22.5 ± 8.8 | 20.3 ± 4.0 | Processed sentences with high or low imagery content | Language and spatial centers not as synchronized, ↑Parietal and occipital regions during low-imagery sentences | Under-integration of language and imagery; Reliance on visualization to support language comprehension |
| Kana, Wadsworth, 2012 [254] | 16 (16) | 16 (16) | 20.0 ± 6.43 | 21.6 ± 2.70 | Processed sentences with puns | ↑Overall, particularly in right hemisphere and posterior areas during pun comprehension; ↓Left hemisphere | Altered neural route in language comprehension in general, and figurative language in particular |
| Kleinhans, Muller, Cohen, Courchesne, 2008 [255] | 14 (14) | 14** | 23.79 ± 3.58 | 22.41 ± 8.67 | (1) Letter fluency task; (2) Category fluency task | ↑Right frontal and right superior TL during letter fluency task; Decreased lateralization of activation patterns during letter fluency, but not to category | Reduced hemispheric differentiation for certain verbal fluency tasks; abnormal functional organization may contribute to the language impairments |
| Knaus, Silver, Lindgren, Hadjikhani, Tager-FIusberg, 2008 [256] | 12 (12) | 12 (12) | 15.46 ± 2.48 | 14.94 ± 2.71 | Reading version of response-naming task | ↑BA; Reduced BA left lateralization | Decreased efficiency of semantic processing |
| Knaus, Silver, Kennedy, et aI, 2010 [257] | 14 (14) | 20 (20) | 16.83 ± 2.35 | 14.43 ± 2.47 | (1) Response-naming task; (2) Control letter-judgment task | Atypical language laterality more prevalent in the ASD group | Language laterality may be a novel way to subdivide samples, resulting in more homogenous groups |
| Lai, Schneider, Schwarzenberger, Hirsch, 2011 [258] | 39 (35) | 15 (10) | 12.4 ± 4.7 | 12.13 ± 4.34 | Listened to speech | ↓Mean amplitude and spread of activity in STG | Possible neurofunctional correlate of language impairment |
| Lai, Pantazatos, Schneider, Hirsch, 2012 [259] | 36 (32) | 21 (14) | 9.61 ± 4.04 | 10.72 ± 4.42 | Listened to speech and songs | ↓Left IFG during speech; ↑Left IFG during songs; Increased left IFG-STG connectivity for songs; Increased frontal—posterior connectivity | Functional systems that process speech and song more effectively engaged for song than for speech |
| Mizuno, Liu, Williams, et al, 2011 [260] | 15 (14) | 15 (15) | 24.7 ± 7.8 | 24.7 ± 7.7 | Linguistic perspective-taking task requiring deictic shifting | ↑Right Al, precuneus; Decreased right Al—precuneus connectivity | Higher activation compensates for decreased connectivity during deictic shifting |
| Redcay, Courchesne, 2008 [261] | 12 (12) | 23 (17) | 34.9 mo ± 7.4 | 19.8 mo ± 4.2 | Listened to forward and backward speech | ↓Extended network recruited in typical early language acquisition, ↑Medial, right GC; ↑Right hemisphere to forward speech | Children with ASDs may be on a deviant developmental trajectory characterized by greater recruitment of right hemisphere regions during speech perception |
| Redcay, Dodell-Feder, Mavros, et al, 2012 [262] | 13 (10) | 14 (11) | 28.0 ± 7.05 | 27.0 ± 5.68 | Interactive face-to-face joint attention game | ↓Left posterior STS, DMPFC during joint attention; ↑Posterior STS during solo attention | Failure of developmental neural specialization in STS and DMPFC during joint attention |
| Sahyoun, Belliveau, Soulieres, Schwartz, Mody, 2010 [263] | 12 (10) | 12 (9) | 13.3 ± 2.45 | 13.3 ± 2.07 | Pictorial reasoning with visuospatial processing, semantic processing, or both | ↑Occipito-parietal, ventral temporal areas; Reduced inferior frontal - ventral temporal and middle temporal connectivity | Greater visual mediation of language processing |
| Scott-Van Zeeland, McNealy, Wang, et al, 2010 [264] | 18 (18) | 18 (18) | 12.62 ± 2.5 | 11.64 ± 1.58 | Listened to two artificial languages and a random speech stream | ↑Frorto-temporal-parietal, as number of cues to word boundaries increased; No learning-related increases for artificial languages in BG, left tem poroparietal cortex; Communicative impairment correlated with signal increases in these regions to artificial languages | Abnormalities in neural regions subserving language-related learning; Communicative impairments linked to decreased sensitivity to the statistical and speech cues in language |
| Tesink, Buitelaar, Petersson, et al, 2009 [265] | 24 (16) | 24 (16) | 26.3 ± 6.3 | 26.2 ± 6.0 | Speaker inference task | ↑Right IFG for speaker-incongruent sentences, Absence of VMPFC modulation to incongruent sentences | Compensatory mechanisms during implicit low-level inferential processes in spoken language |
| Tesink, Buitelaar, Petersson, et al, 2011 [266] | 24 (16) | 24 (16) | 26.3 ± 6.3 | 26.2 ± 6.0 | Integrated contextual information during auditory language comprehension | ↓Left, right IFG for sentences with world knowledge anomaly | Reduced integrative capacity of stored knowledge; Difficulties with exception handling |
| Vaidya, Foss-Feig, Shook, et al, 2011 [267] | 15 (11) | 18 (14) | 10.78 ± 1.29 | 10.96 ± 1.26 | Responded to target word in presence of congruent or incongruent arrow or averted gaze | Congruent regions associated with attention to gaze (left STS, PMC) activated to arrows; Incongruent regions associated with arrows (ACC, left DLPFC, right CN) activated to gaze | Atypical functional anatomy to social and nonsocial communicative cues |
Reward processing
The social-communication deficits that characterize ASDs may reflect decreased motivation to engage in social behaviors in early childhood. This decreased motivation may result in fewer experiences with the social environment,99 further compounding social-communicative deficits.100 Reward processing is mediated primarily by dopaminergic projections from the ventral tegmental area to the striatum, orbitofrontal cortex, ventromedial prefrontal cortex, and the anterior cingulate cortex, forming a mesolimbic dopamine reward pathway.101 Emerging evidence suggests that the neural circuits that mediate reward processing may have evolved, at least in part, to facilitate social attachment,102 and reward mechanisms serve to encode and consolidate positive memories of social experiences, facilitating social functioning abilities hypothesized to be impaired in ASDs.103
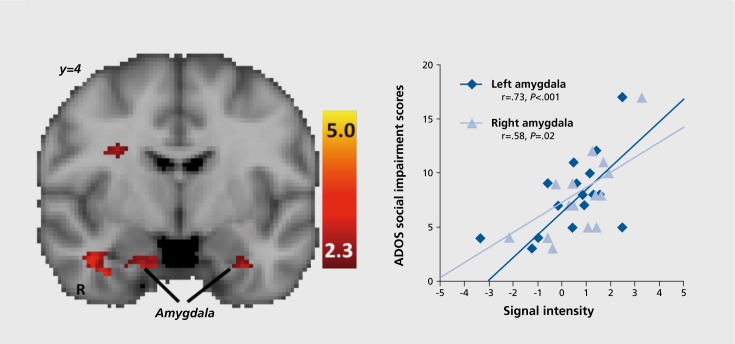
Reward processing deficits in ASDs have been assessed in six fMRI studies to date (Table V). Schmitz and colleagues104 reported decreased left anterior cingulate gyrus and left midfrontal gyrus activation to rewarded trials during a sustained attention task in ASDs and that anterior cingulate gyrus activation predicted social symptom severity. Scott-Van Zeeland and colleagues105 reported ventral striatal hypoactivation during social and nonsocial learning in ASDs. During a rewarded go/no-go paradigm, Kohls and colleagues106 found ventral striatal hypoactivation to monetary rewards and amygdala and anterior cingulate cortex hypoactivation to monetary and social rewards in children with ASDs. Cascio and colleagues107 reported increased bilateral insula and anterior cingulate cortex activation to images of food in children with ASDs who had fasted for at least 4 hours. Two studies by Dichter and colleagues,49,108 using incentive delay tasks, found decreased nucleus accumbens activation during monetary anticipation, bilateral amygdala hyperactivation during face anticipation that predicted social symptom severity (Figure 1), insular cortex hyperactivation during face outcomes, and ventromedial prefrontal cortex hyperactivation while viewing images related to circumscribed interests in ASDs. Taken together, these results suggest that reward network dysfunction in ASDs may not be constrained to responses to social rewards, but rather may be characterized by anomalous responsivity that is contingent on the type of reward processed. When considered in light of empirical findings of dysfunctional reward circuitry in a number of psychiatric conditions, including substance use disorders, schizophrenia, affective disorders, and attention deficit/hyperactivity disorder, abnormal mesolimbic responses to rewards appears to be a common endophenotype that may cut across diagnostic boundaries.109
Figure 1. Individuals with autism spectrum disorders demonstrated bilateral amygdala hyperactivation during the anticipation of social rewards (left), and activation magnitude predicted social impairments (right). This pattern was not evident during the actual presentation of social rewards, or in response to other types of rewards. This and related findings suggest that the functional integrity of brain reward systems in autism spectrum disorders is contingent on both the type of reward processed and the temporal phase of the reward response. ADOS, Autism Diagnostic Observation Schedule. Adapted from ref 49: Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW. Reward circuitry function in autism during face anticipation and outcomes. J Autism Dev Disord. 2012;42:147-160. Copyright © Springer 2012.
Table V. Studies investigating reward processing in autism spectrum disorders. ASD: Autism Spectrum Disorder; TYP: Neurotypical; †ASD refers to the entire autism sample in a particular study, including high functioning autism, Asperger's syndrome, and pervasive developmental disorder not otherwise specified; *Total number of participants is presented first followed by the number of females in parentheses, if reported; **Not specified; ↓: decreased activation; ↑: increased activation. Abbreviations used in tables: ACC, anterior cingulate cortex; ACG, anterior cingulate gyms; AG, angular gyms; Al, anterior insula; AMY, amygdala; ATL, anterior temporal lobe; BA, Broca's area; BG, basal ganglia; CM, caudate nucleus; DAC, dorsal anterior cingulate; DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; DN, dentate nucleus; FFA, fusiform face area; FG, fusiform gyms; IC, insular cortex; IFA, inferior frontal area; IFC, inferior frontal cortex; IFG, inferior frontal gyrus; IPL, inferior parietal lobe; ITG, inferior temporal gyrus; LG: lingual gyrus; LSTG, left superior temporal gyrus; MCG, >middle cingulate gyrus; MFC, midfrontaI cortex; MFG, midfrontal gryus; MFL, medial frontal lobes; NAC, nucleus accumbens; OFC, orbitofrental cortex; OFG, orbitofrental gyrus; MPFC, medial prefrontal cortex; MTG, medial temporal gyrus; PO, pars opercularis; PCC, posterior cingulate cortex; PFC, prefrontal cortex; PHG, parahippocampal gyrus; PL, parietal lobe; PMC, premotor cortex; PVC, primary visual cortex; RPVC, right primary visual cortex; SFG, superior frontal gyrus; SPL, superior parietal lobe; STG, superior temporal gyrus; STS, superior temporal sulcus; THAL, thalamus; TL, temporal lobe; TPJ, temporoparietal junction; VS, ventral striatium; VLPFC, ventrolateral prefrontal cortex; VOC, ventral occipital cortex; VMPFC, ventromedial prefrontal cortex; WA, Wernicke's Area .
| Citation | ASD*† | TYP*† | ASD age | TYP age | Task(s) | Core findings in ASD group (relative to controls) | Conclusions |
| Cascio, Foss-Feig, Heacock, et al, 2012 [268] | 17 (17) | 23** | 12.8 ± 2.5 | 13.2 ± 3.4 | Viewed images of high-calorie foods after fasting | ↑Bilateral insula along anterior-posterior gradient; ↑ACC to food cues | Abnormally enhanced neural response to primary rewards in ASD |
| Dichter, Richey, Rittenberg, 2012 [269] | 16 (14) | 20 (14) | 26.0 ± 9.1 | 25.4 ± 7.0 | Incentive delay task with monetary and social rewards | ↓NAC, OFC during monetary anticipation; ↑Right insula to face incentives; ↑Bilateral AMY during face anticipation that correlated with social symptoms | Domain-general reward circuitry dysfunction, atypical amygdala activation to social rewards may contribute to social symptom severity in ASD |
| Dichter, Felder, Green, et al, 2012 [270] | 15 (15) | 16 (16) | 30.1 ± 11.6 | 27.5 ± 7.5 | Incentive delay task with monetary rewards and rewards related to circumscribed interests | ↓NAC during monetary anticipation and outcomes; ↑VMPFC to circumscribed interests incentives | Reward circuitry hypoactwation to monetary incentives but hyperactivation to circumscribed interests in ASD. Possible neural mechanism of circumscribed interests in ASD |
| Kohls, Schulte-Ruther, Nehrkorn, et al, 2012 [271] | 15 (15) | 17 (17) | 14.6 ± 3.3 | 13.9 ± 3.0 | Go/no-go task with social vs. monetary rewards | ↓Midbrain, THAL, AMY, striatium, ACC to both rewards; ↓NAC to monetary reward, but not social reward | Domain-general reward system dysfunction in ASD |
| Schmitz, Rubia, van Amelswoort, et al, 2008 [272] | 10 (10) | 10 (10) | 37.8 ± 7 | 38.2 ± 6 | Rewarded continuous performance task | ↑Left ACG during reward trials that correlated with social symptom severity; | Reward achievement associated with abnormal activation in areas responsible for attention and arousal in ASD |
| Scott-Van Zeeland, Dapretto, Ghahremani, 2010 [273] | 16 (16) | 16 (16) | 12.4 ± 2.14 | 12.3 ± 1.76 | Implicit learning task with social vs. monetary rewards | ↓VS to both social and monetary rewards (more pronounced to social rewards. | Diminished neural responses during social reward learning may contribute to social learning impairments in ASD |
Functional connectivity
Whereas task-based fMRI studies focus on activity within specific brain regions evoked by cognitive tasks, studies of functional connectivity speak to the temporal dynamics of brain network activity. Hie integrity of brain connections affects integration and synchronization of information processing, and the study of functional connectivity in ASDs addresses circuitry-level questions believed to be central to dysfunction in ASDs.6 There is a confluence of evidence that ASDs are characterized by decreased connectivity, in particular between frontal and posterior-temporal cortical systems that play key roles in processing social-affective information.110 Although initial studies highlighted cortical underconnectivity in ASDs, more recent data suggests that ASDs may be characterized by both local overconnectivity and longdistance underconnectivity. It has been suggested that a cortical underconnectivity account of ASDs may address heterogeneity as well as broad information processing deficits in general, rather than the expression of specific core symptoms.111
Task-based functional connectivity
The majority of task-based studies in ASDs have documented reduced functional connectivity between frontal and parietal regions75,83,112 as well as between frontal and temporal and/or occipital regions.69,113 Tasks have included language comprehension,83,88,97 cognitive control,69,75,114 mentalizing,53,113,115 social processing,113 working memory,116 and visuospatial processing.112 A number of these studies have also indicated smaller and less synchronized cortical networks in ASDs.116-117 It should be noted, however, that some task-based studies have found long-range over-connectivity between subcortical and cortical regions118-119 as well as between frontal and temporal regions.120-122 Other studies have examined connectivity during task-related paradigms by filtering out taskrelated activity to examine connectivity patterns that are task-independent, and found evidence of decreased123-124 and increased118-121 functional connectivity.
Resting-state functional connectivity
Relatively fewer studies have examined brain connectivity in ASDs during resting state fMRI scans (Table VI). Cherkassky and colleagues125 reported decreased frontalposterior default network connectivity during task-based inter-trail intervals (see also refs 126-128) while others have found lower default-mode network connectivity at rest125,128-131 in ASDs. There are also reports of decreased connectivity between the anterior and posterior insula and a number of social processing brain regions in ASDs75,114,116 and less coherent endogenous low-frequency oscillations across multiple cortical and subcortical regions in ASDs.132 von dem Hagen and colleagues133 reported reduced functional connectivity within and between resting state networks incorporating “social brain regions” including the insula and amygdala within the default-mode and salience networks, respectively, and Di Martino and colleagues134 reported increased connectivity between multiple striatal regions and striatal hyperconnectivity with the pons. Monk and colleagues127 reported positive correlations between repetitive behavior symptoms and resting state connectivity between posterior cingulate cortex and the right parahippocampal gyrus in adults with ASDs, despite increased connectivity between the posterior cingulate cortex, the right temporal lobe, and the right parahippocampal gyrus, although Weng and collègues128 found correlations between social and repetitive behavior symptoms and a number of resting connectivity metrics in adolescents with ASDs.
Table VI. Studies investigating resting state connectivity in autism spectrum disorders. ASD: Autism Spectrum Disorder; TYP: Neurotypical; †ASD refers to the entire autism sample in a particular study, including high functioning autism, Asperger's syndrome, and pervasive developmental disorder not otherwise specified; *Total number of participants is presented first followed by the number of females in parentheses, if reported; **Not specified; ↓: decreased activation; ↑: increased activation. Abbreviations used in tables: ACC, anterior cingulate cortex; ACG, anterior cingulate gyms; AG, angular gyms; Al, anterior insula; AMY, amygdala; ATL, anterior temporal lobe; BA, Broca's area; BG, basal ganglia; CM, caudate nucleus; DAC, dorsal anterior cingulate; DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; DN, dentate nucleus; FFA, fusiform face area; FG, fusiform gyms; IC, insular cortex; IFA, inferior frontal area; IFC, inferior frontal cortex; IFG, inferior frontal gyrus; IPL, inferior parietal lobe; ITG, inferior temporal gyrus; LG: lingual gyrus; LSTG, left superior temporal gyrus; MCG, >middle cingulate gyrus; MFC, midfrontaI cortex; MFG, midfrontal gryus; MFL, medial frontal lobes; NAC, nucleus accumbens; OFC, orbitofrental cortex; OFG, orbitofrental gyrus; MPFC, medial prefrontal cortex; MTG, medial temporal gyrus; PO, pars opercularis; PCC, posterior cingulate cortex; PFC, prefrontal cortex; PHG, parahippocampal gyrus; PL, parietal lobe; PMC, premotor cortex; PVC, primary visual cortex; RPVC, right primary visual cortex; SFG, superior frontal gyrus; SPL, superior parietal lobe; STG, superior temporal gyrus; STS, superior temporal sulcus; THAL, thalamus; TL, temporal lobe; TPJ, temporoparietal junction; VS, ventral striatium; VLPFC, ventrolateral prefrontal cortex; VOC, ventral occipital cortex; VMPFC, ventromedial prefrontal cortex; WA, Wernicke's Area .
| Citation | ASD*† | TYP*† | ASD age | TYP age | Task(s) | Core findings in ASD group (relative to controls) | Conclusions |
| Anderson, Nielsen, Froehlich, et al, 2011 [274] | 40 (40) | 40 (40) | 22.7 ± 7.4 | 21.6 ± 7.4 | 8' resting scan with eyes open | Negatively correlated ROI pairs showed decreased anticorrelation in ASD; Greatest connectivity differences in default mode network, superior parietal lobule, FG and Al | Weaker inhibitory connections, particularly for long connections; Resting state fMRI may be feasible as a diagnostic classifier for ASD |
| Cherkassky, Kana, Keller, Just, 2006 [275] | 57 (53) | 57 (52) | 24.0 ± 10.6 | 24.0 ± 9 | Periods of rest during task-based scans (duration not specified) | Decreased connectivity in resting-state networks despite similar volume and organization; Decreased posterior—anterior connectivity | Resting state underconnectivity in ASD |
| Di Martino, Kelly, Grzadzinski, et al, 2011 [276] | 20 (17) | 20 (14) | 10.4 ± 1.7 | 10.9±1.6 | 6' 38'' resting scan with eyes open | Increased connectivity between striatal subregions and heteromodal associative and limbic cortex; Increased pons-striatum and pons-insula connectivity | Increased connectivity in ectopic circuits reflects alternate trajectory of development, rather than immaturity of circuits |
| Kennedy, Courchesne, 2008 [277] | 13 (13) | 12 (12) | 26.9 ± 12.3 | 27.5 ± 10.9 | 7' 10'' resting scan with eyes open | Reduced default mode network connectivity | Altered functional organization of the network involved in social and emotional processing |
| Lai, Lombardo, Chakrabarti, et al, 2010 [278] | 18 (18) | 33 (33) | 26.9 ± 7.4 | 28.4 ± 6.1 | 13' 39'' resting scan with eyes dosed (only last 512 of 625 volumes analyzed). | More randomness in midline structures, medial temporal structures, lateral temporal and parietal structures, insula, AMY, BG, THAL, IFG; Social symptoms negatively correlated with randomness in retrosplenial and right anterior IC | ASD associated with small but significant shift towards randomness in endogenous brain oscillations |
| Monk, Peltier, Wiggins, et aI, 2009 [279] | 12 (11) | 12 (10) | 26 ± 5.93 | 27 ± 6.1 | 10' resting scan with eyes open | Decreased PCC-SFG connectivity; Increased connectivity between PCC and right TL and right PHG; Social symptoms correlated with PCC-SFG connectivity, repetitive behaviors correlated with PCC—right PHG connectivity | Altered intrinsic connectivity that was associated with core symptoms |
| Paakki, Rahko, Long et al, 2010 [280] | 28 (20) | 27 (18) | 14.58 ± 1.62 | 14.49 ± 1.51 | 7' 36'' resting scan with eyes open | Decreased regional homogeneity in right STS, right IFS, right MFG, bilateral cerebellum, right insula, right postcentral gyrus; Increased regional homogeneity in right THAL, left IFG, left anterior subcallosal gyrus, bilateral cerebellar lobule VIII | Right-dominant alterations of resting state activity |
| von dem Hagen, Stoyanowa, Baron-Cohen, Calder,2012 [281] | 18 (18) | 25 (25) | 30 ± 8 | 25 ± 6 | 10' resting scan with eyes open | Decreased default mode network connectivity; Decreased connectivity in salience network (includes insula) and a medial TL network (includes AMY) | Reduced connectivity in networks involved with the “social brain”, May be implicated in difficulties with communication and information integration |
| Weng, Wiggins, Peltier, et al, 2010 [282] | 16 (14) | 15 (14) | 15.0 ± 1.45 | 16.0 ± 1.44 | 10' resting scan with eyes open | Decreased connectivity in 9 of 11 default mode areas; Social and repetitive behavior symptoms correlated with decreased connectivity in parts of default mode network; Communication correlated with increased connectivity in parts of default mode network | Decreased default mode network connectivity in adolescents with ASDs than in adults with ASDs |
| Wiggins, Peltier, Ashmoff et al, 2011[283] | 39 (32) | 41 (33) | 14.0 ± 2.08 | 15.3 ± 2.4 | 10' resting scan with eyes open | Decreased connectivity between posterior hub of default network and right SFG; Less increase in connectivity with age | Different developmental trajectory of default mode network |
Structural MRI
Functional MRI results should ultimately be considered within a broader neuroimaging literature addressing brain structure and white matter connectivity in ASDs. Structural MRI yields information about brain anatomy, including gray- and white-matter volumes as well as gyrus and sulcus development, and this approach is wellsuited for studies seeking to predict future ASDs diagnoses in infants. Very briefly, the structural MRI literature indicates accelerated brain growth during earlydevelopment in ASDs.135,136 There are reports of significantly large head circumference137 and brain volume in children with autism.138 Longitudinal studies indicate that ASDs are characterized by an early transient period of postnatal brain overgrowth evident in 70% of children with ASDs before age 2 that is not present in adolescence and adulthood.139-140 Evidence of enlarged total brain size in ASDs is accompanied by studies showing smaller cerebellar vermis,141,142 amygdala, and hippocampus.138 Increased brain size in young children with ASDs has also been linked to increased frontal lobe white matter143 followed by reduced white matter in early and late adolescence and adulthood.144,145
Diffusion tensor imaging
Because the contrast properties of structural MRI are suboptimal for differentiating still-myelinating white matter from surrounding gray matter in children,146 diffusion tensor imaging (DTI), a measure of microstructural properties of white matter fibers, has emerged as a valuable tool to assess white-matter structure in very young samples.147 There is evidence of widespread abnormalities in white-matter fiber tract integrity in ASDs, but the extent and developmental course of these differences remains unclear.148-151 Two- to three-year-old children with ASDs are characterized by increased fractional anisotropy (an index of white matter fiber density) in the frontal lobes and in the corpus callosum,152 but in 5-year-old children with ASDs fractional anisotropy was reduced in frontal lobe tracts and no different from controls in tracts connecting frontal and posterior regions.153 In 10- to 18-year-old children with ASDs, there is evidence of reduced fractional anisotropy in frontal-posterior tracts154 and in hemispheric fractional anisotropy lateralization in the arcuate fasciculus,155,156 but fractional anisotropy was found to be reduced in adolescents with ASDs in prefrontal cortex and tempoparietal junction.157 It thus appears that young children with ASDs are characterized by increased fractional anisotropy- in brain areas mediating social communication, whereas adolescents and adults with ASDs are characterized by generally lower fractional anisotropy, a pattern that recapitulates patterns of brain overgrowth discussed earlier.
Finally, a prospective DTI study of 6- to 24-month-old infants at high-risk of developing ASDs found that fractional anisotropy trajectories for 12 of 15 fiber tracts examined differed between infants who later were identified as having an ASDs and those who did not. Infants who went on to have a diagnosis of an ASD had fiber tracts characterized by higher fractional anisotropy at 6 months of age, slower change between 6 and 24 months of age, and lower fractional anisotropy at 24 months of age.158
Summary
The goal of this review is to highlight consistencies in the ASD fMRI literature. Given the array of imaging tasks reviewed, it is perhaps not surprising that findings are heterogenous. Despite variations in findings, there is a sufficient degree of consistency to draw a number of substantive conclusions. Studies of social processes have generally found evidence of hypoactivation in nodes of the “social brain,” including the medial prefrontal cortex, the inferior frontal gyrus and the anterior insula, the posterior superior temporal sulcus, the interparietal sulcus, the amygdala, and the fusiform gyrus. Studies addressing cognitive control, designed to address neural mechanisms underlying restricted and repetitive behaviors and interests, have converged on aberrant frontostriatal functioning in ASDs, specifically in inferior and middle frontal gyri, anterior cingulate cortex, and the basal ganglia. Communication impairments in ASDs have been linked to differential patterns of language function lateralization, decreased synchrony- of brain regions processing language, and recruitment of brain regions that do not typically processing language. Reward processing studies have highlighted mesolimbic and mesocortical impairments when processing both social and nonsocial incentives in ASDs. Finally, task-based functional connectivity studies in ASDs have reported local overconnectivity and long-distance (ie, between frontal and posterior regions) underconnectivity-, whereas resting state connectivity studies indicate decreased anterior-posterior connectivity and less coherent endogenous low-frequency oscillations across multiple regions.
Future directions
Most studies reviewed here focus on adulthood or adolescence, yet ASDs are present from very early childhood. It will be critical to address developmental profiles in children with ASDs to disambiguate proximal effects of altered brain function from downstream effects on learning and motivation. There also may be critical periods during early development when brain dysfunction creates a predisposition to develop a number of disorders, and understanding factors that influence these processes will be essential for the prevention of symptom onset. Indeed, emerging techniques allow for functional brain imaging in children as young as 12 months old, and future studies that focus on young samples are needed. Additionally, most studies reviewed here contain small samples, and larger samples will be needed to identify meaningful subgroups and track developmental profiles. Given the high costs associated with brain imaging and challenges recruiting large pediatric patient samples, it will be critical to leverage available bioinformatics tools to facilitate data sharing across research groups. Such tools are under development159 and the National Institutes of Health recently established a database for sharing ASDs neuroimaging data.160
There is also a need to move to designs that incorporate psychiatric comparisons to delineate brain activation patterns in ASDs that diverge and converge with other disorders characterized by social communication impairments and repetitive behaviors. Similarly, ASDs are commonly comorbid with other psychiatric and neurodevelopmental conditions,161 possibly due to shared genetic etiology and common socioenvironmental determinants, and thus it will be important to examine ASD samples with and without comorbid conditions to refine our understanding of neural endophenotypes in ASDs. Finally, the literature reviewed here is cross-sectional. Though these studies have elucidated aberrant patterns of brain activation in ASDs, these paradigms have rarelybeen applied to longitudinal treatment outcome studies aimed at understanding mechanisms of action of treatment response in ASDs. As neuroimaging and data-sharing techniques evolve, functional brain imaging will continue to improve our understanding of the pathophysiology of ASDs, with the ultimate goal of improved ASD identification and treatment.162
Acknowledgments
Preparation of this manuscript was supported by K23 MH081285 and R01 MH073402. I am grateful to Eleanor Hanna for administrative assistance with this manuscript.
REFERENCES
- 1.Kanner L. autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 2.Asperger H. Autistic psychopathy in childhood. In: Frith U, ed. Autism and Asperger's Syndrome. Cambridge, UK: Cambridge University Press, 1944:37–92. [Google Scholar]
- 3.Howlin P., Goode S., Hutton J., Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry. 2004;45:212–229. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 5.American Psychiatric Association. DSM-5 Development. Available at: http://wwwdsm5org. Accessed May 23, 2012. 2012 [Google Scholar]
- 6.Geschwind DH., Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry. 2007;12:2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- 8.Happe F. Autism: cognitive deficit or cognitive style? Trends Cogn Sci. 1999;3:216–222. doi: 10.1016/s1364-6613(99)01318-2. [DOI] [PubMed] [Google Scholar]
- 9.Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- 10.Johnson MH., Dziurawiec S., Ellis H., Morton J. Newborns' preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- 11.Klin A., Sparrow SS., de Bildt A., Cicchetti DV., Cohen DJ., Volkmar FR. A normed study of face recognition in autism and related disorders. J Autism Dev Disord. 1999;29:499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- 12.Klin A., Jones W., Schultz R., Volkmar F., Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 13.Grill-Spector K., Knouf N., Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004;7:555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- 14.Humphreys K., Hasson U., Avidan G., Minshew N., Behrmann M. Cortical patterns of category-selective activation for faces, places and objects in adults with autism. Autism Res. 2008;1:52–63. doi: 10.1002/aur.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelphrey KA., Morris JP., McCarthy G., Labar KS. Perception of dynamic changes in facial affect and identity in autism. Soc Cog Affect Neurosci. 2007;2:140–149. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbett BA., Carmean V., Ravizza S., et al. A functional and structural study of emotion and face processing in children with autism. Psychiatry Res. 2009;173:196–205. doi: 10.1016/j.pscychresns.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherf KS., Luna B., Minshew N., Behrmann M. Location, location, location: alterations in the functional topography of face- but not object- or place-related cortex in adolescents with autism. Front Hum Neurosci. 2010;4:26. doi: 10.3389/fnhum.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall GB., Doyle KA., Goldberg J., West D., Szatmari P. Amygdala engagement in response to subthreshold presentations of anxious face stimuli in adults with autism spectrum disorders: preliminary insights. PloS One. 2010;5:e10804. doi: 10.1371/journal.pone.0010804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubl D., Boite S., Feineis-Matthews S., et al. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61:1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- 20.Pierce K., Muller RA., Ambrose J., Allen G., Courchesne E. Face processing occurs outside the fusiform 'face area' in autism: evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- 21.Dalton KM., Nacewicz BM., Johnstone T., et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz RT., Gauthier I., Klin A., et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- 23.Critchley HD., Daly EM., Bullmore ET., et al. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123:2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- 24.Hall GB., Szechtman H., Nahmias C. Enhanced salience and emotion recognition in Autism: a PET study. Am J Psychiatry. 2003;160:1439–1441. doi: 10.1176/appi.ajp.160.8.1439. [DOI] [PubMed] [Google Scholar]
- 25.Plnkham AE., Hopfinger JB., Pelphrey KA., Piven J., Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99:164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierce K., Haist F., Sedaghat F., Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127:2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- 27.Aylward E., Bernier R., Field A., Grirnme A., Dawson G. Normal activation of fusiform gyrus in adolescents and adults with autism during viewing of familiar, but not unfamiliar, faces. Paper presented at: STAART/CPEA (Studies To Advance Autism Research and Treatment/Collaborative Programs for Excellence in Autism) NIH network meeting. May 17-20. Bethesda, MD; 2004 [Google Scholar]
- 28.Pierce K., Redcay E. Fusiform function in children with an autism spectrum disorder is a matter of “who”. Biol Psychiatry. 2008;64:552–560. doi: 10.1016/j.biopsych.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uddin LQ., Davies MS., Scott AA., et al. Neural basis of self and other representation in autism: an FMRI study of self-face recognition. PloS One. 2008;3:e3526. doi: 10.1371/journal.pone.0003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hadjikhani N., Joseph RM., Snyder J., et al. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22:1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Bookheimer SY., Wang AT., Scott A., Sigman M., Dapretto M. Frontal contributions to face processing differences in autism: evidence from fMRI of inverted face processing. J Int Neuropsychol Soc. 2008;14:922–932. doi: 10.1017/S135561770808140X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Klin A. Three things to remember if you are a functional magnetic resonance imaging researcher of face processing in autism spectrum disorders. Biol Psychiatry. 2008;64:549–551. doi: 10.1016/j.biopsych.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierce K., Glad KS., Schreibman L. Social perception in children with autism: an attentional deficit? J Autism Dev Disord. 1997;27:265–282. doi: 10.1023/a:1025898314332. [DOI] [PubMed] [Google Scholar]
- 35.Dawson G., Meltzoff AN., Osterling J., Rinaldi J., Brown E. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord. 1998;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- 36.Jones W., Carr K., Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65:946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- 37.Schultz RT., Grelotti DJ., Klin A., et al. The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos Trans R Soc Lond B Biol Sci. 2003;358:415–427. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleinhans NM., Richards T., Johnson LC., et al. fMRI evidence of neural abnormalities in the subcortical face processing system in ASD. Neuroimage. 2011;54:697–704. doi: 10.1016/j.neuroimage.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monk CS., Weng SJ., Wiggins JL., et al. Neural circuitry of emotional face processing in autism spectrum disorders. J Psychiatry Neurosci. 2010;35:105–114. doi: 10.1503/jpn.090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weng SJ., Carrasco M., Swartz JR. et al. Neural activation to emotional faces in adolescents with autism spectrum disorders. J Child Psychol Psychiatry. 2011;52:296–305. doi: 10.1111/j.1469-7610.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perlman SB., Hudac CM., Pegors T., Minshew NJ., Pelphrey KA. Experimental manipulation of face-evoked activity in the fusiform gyrus of individuals with autism. Soc Neurosci. 2011;6:22–30. doi: 10.1080/17470911003683185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleinhans NM., Johnson LC., Richards T., et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am J Psychiatry. 2009;166:467–475. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- 43.Baillargeon R., Scott RM., He Z. False-belief understanding in infants. Trends. Cogn Sci. 2010;14:110–118. doi: 10.1016/j.tics.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blakemore SJ. Imaging brain development: the adolescent brain. Neuroimage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- 45.Davis M., Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 46.Baron-Cohen S., Ring HA., Bullmore ET., Wheelwright S., Ashwin C., Williams SC. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 47.Baron-Cohen S., Ring HA., Wheelwright S., et al. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 48.Dapretto M., Davies MS., Pfeifer JH., et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dichter GS., Richey JA., Rittenberg AM., Sabatino A., Bodfish JW. Reward circuitry function in autism during face anticipation and outcomes. J Autism Dev Disord. 2012;42:147–160. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pelphrey KA., Shultz S., Hudac CM., Vander Wyk BC. Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. J Child Psychol Psychiatry. 2011;52:631–644. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pelphrey KA., Morris JP., McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128(Pt 5):1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- 52.Pitskel NB., Boiling DZ., Hudac CM., et al. Brain mechanisms for processing direct and averted gaze in individuals with autism. J Autism Dev Disord. 2011;41:1686–1693. doi: 10.1007/s10803-011-1197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castelli F., Frith C., Happe F., Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- 54.Boddaert N., Belin P., Chabane N., et al. Perception of complex sounds: abnormal pattern of cortical activation in autism. Am J Psychiatry. 2003;160:2057–2060. doi: 10.1176/appi.ajp.160.11.2057. [DOI] [PubMed] [Google Scholar]
- 55.Kaiser MD., Hudac CM., Shultz S., et al. Neural signatures of autism. Proc Natl Acad Sci USA. 2010;107:21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carr L., lacoboni M., Dubeau MC., Mazziotta JC., Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A. 2003 Apr 29;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leslie KR., Johnson-Frey SH., Grafton ST. Functional imaging of face and hand imitation: towards a motor theory of empathy. Neuroimage. 2004;21:601–607. doi: 10.1016/j.neuroimage.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 58.lacoboni M. Imitation, empathy, and mirror neurons. Annu Rev Psychol. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- 59.Williams JH., Whiten A., Suddendorf T., Perrett Dl. Imitation, mirror neurons and autism. Neurosci Biobehav Rev. 2001;25:287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- 60.lacoboni M., Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7:942–51. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- 61.Southgate V., Hamilton AF. Unbroken mirrors: challenging a theory of Autism. Trends Cogn Sci. 2008;12:225–229. doi: 10.1016/j.tics.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Dinstein I., Thomas C., Humphreys K., Minshew N., Behrmann M., Heeger DJ. Normal movement selectivity in autism. Neuron. 2010;66:461–469. doi: 10.1016/j.neuron.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Martine A., Ross K., Uddin LQ., Sklar AB., Castellanos FX., Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lam KS., Bodfish JW., Piven J. Evidence for three subtypes of repetitive behavior in autism that differ in famlllality and association with other symptoms. J Child Psychol Psychiatry. 2008;49:1193–1200. doi: 10.1111/j.1469-7610.2008.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ozonoff S., Cook I., Coon H., et al. Performance on Cambridge Neuropsychological Test Automated Battery subtests sensitive to frontal lobe function in people with autistic disorder: evidence from the Collaborative Programs of Excellence in Autism network. J Autism Dev Disord. 2004;34:139–150. doi: 10.1023/b:jadd.0000022605.81989.cc. [DOI] [PubMed] [Google Scholar]
- 66.Fan J., McCandliss BD., Fossella J., Flombaum JI., Posner Ml. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 67.Schmitz N., Rubia K., Daly E., Smith A., Williams S., Murphy DG. Neural correlates of executive function in autistic spectrum disorders. Biol Psychiatry. 2006;59:7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 68.Gomot M., Belmonte MK., Bullmore ET., Bernard FA., Baron-Cohen S. Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain. 2008;131:2479–2488. doi: 10.1093/brain/awn172. [DOI] [PubMed] [Google Scholar]
- 69.Solomon M., Ozonoff SJ., Ursu S., et al. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47:2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dichter GS., Felder JN., Bodfish JW. Autism is characterized by dorsal anterior cingulate hyperactivation during social target detection. Soc Cogn Affect Neurosci. 2009;4:215–226. doi: 10.1093/scan/nsp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shafritz KM., Dichter GS., Baranek GT., Belger A. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biol Psychiatry. 2008;63:974–980. doi: 10.1016/j.biopsych.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dichter GS., Belger A. Social stimuli interfere with cognitive control in autism. Neuroimage. 2007;35:1219–1230. doi: 10.1016/j.neuroimage.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thakkar KN., Polli FE., Joseph RM., Tuch DS., Hadjikhani N., Barton JJ., et al. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD). Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilbert SJ., Bird G., Brindley R., Frith CD., Burgess PW. Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: an fMRI study of two executive function tasks. Neuropsychologia. 2008;46:2281–2291 . doi: 10.1016/j.neuropsychologia.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Just MA., Cherkassky VL., Keller TA., Kana RK., Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muller RA., Pierce K., Ambrose JB., Allen G., Courchesne E. Atypical patterns of cerebral motor activation in autism: a functional magnetic resonance study. Biol Psychiatry. 2001;49:665–676. doi: 10.1016/s0006-3223(00)01004-0. [DOI] [PubMed] [Google Scholar]
- 77.Allen G., Muller RA., Courchesne E. Cerebellar function in autism: functional magnetic resonance image activation during a simple motor task. Biol Psychiatry. 2004;56:269–278. doi: 10.1016/j.biopsych.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 78.Muller RA., Kleinhans N., Kemmotsu N., Pierce K., Courchesne E. Abnormal variability and distribution of functional maps in autism: An fMRI study of visuomotor learning. Am J Psychiatry. 2003;160:1847–1862. doi: 10.1176/appi.ajp.160.10.1847. [DOI] [PubMed] [Google Scholar]
- 79.Lezak MD. Neuropsychological Assessment. 3rd ed. New York, NY: Oxford University Press; 1995 [Google Scholar]
- 80.Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012. In press. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carmody DP., Moreno R., Mars AE., Seshadri K., Lambert GH., Lewis M. Brief report: brain activation to social words in a sedated child with autism. J Autism Dev Disord. 2007;37:1381–1385. doi: 10.1007/s10803-006-0270-3. [DOI] [PubMed] [Google Scholar]
- 82.Redcay E., Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2-3-year-old children with autism spectrum disorder. Biol Psychiatry. 2008;64:589–598. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kana RK., Keller TA., Cherkassky VL., Minshew NJ., Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muller RA., Behen ME., Rothermel RD., et al. Brain mapping of language and auditory perception in high-functioning autistic adults: a PET study. J Autism Dev Disord. 1999;29:19–31. doi: 10.1023/a:1025914515203. [DOI] [PubMed] [Google Scholar]
- 85.Wang A., Dapretto M., Hariri A., Sigman M., Bookheimer SY. Processing affective and linguistic prosody in autism: an fMRI study. Neuroimage. 2001;13:S621. [Google Scholar]
- 86.Kleinhans NM., Muller RA., Cohen DN., Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 2008;1221:115–125. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tesink CMJY., Buitelaar JK., Petersson KM., et al. Neural correlates of pragmatic language comprehension in autism spectrum disorders. Brain. 2009;132:1941–5192. doi: 10.1093/brain/awp103. [DOI] [PubMed] [Google Scholar]
- 88.Just MA., Cherkassky VL., Keller TA., Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 89.Knaus TA., Silver AM., Lindgren KA., Hadjikhani N., Tager-Flusberg H. fMRI activation during a language task in adolescents with ASD. J int Neuropsychol Soc. 2008;14:967–979. doi: 10.1017/S1355617708081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grezes J., Wicker B., Berthoz S., de Gelder B. A failure to grasp the affective meaning of actions in autism spectrum disorder subjects. Neuropsychologia . 2009;47:1816–1825. doi: 10.1016/j.neuropsychologia.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 91.Hadjikhani N., Joseph RM., Manoach DS., et al. Body expressions of emotion do not trigger fear contagion in autism spectrum disorder. Soc Cogn Affect Neurosci. 2009;4:70–78. doi: 10.1093/scan/nsn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Catarino A., Luke L., Waldman S., Andrade A., Fletcher PC., Ring H. An fMRI investigation of detection of semantic incongruities in autistic spectrum conditions. Eur J Neurosci. 2011;33:558–567. doi: 10.1111/j.1460-9568.2010.07503.x. [DOI] [PubMed] [Google Scholar]
- 93.Eigsti IM., Schuh J., Mend E., Schultz RT., Paul R. The neural underpinnings of prosody in autism. Child Neuropsychol. 2011. In press. doi: 10.1080/09297049.2011.639757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lai G., Pantazatos SP., Schneider H., Hirsch J. Neural systems for speech and song in autism. Brain. 2012;135:961–975. doi: 10.1093/brain/awr335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Groen WB., Tesink C., Petersson KM., et al. Semantic, factual, and social language comprehension in adolescents with autism: an FMRI study. Cereb Cortex. 2010;20:1937–1945. doi: 10.1093/cercor/bhp264. [DOI] [PubMed] [Google Scholar]
- 96.Kana RK., Wadsworth HM. “The archeologist's career ended in ruins”: hemispheric differences in pun comprehension in autism. Neuroimage. 2012;62:77–86. doi: 10.1016/j.neuroimage.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 97.Mizuno A., Liu Y., Williams DL., Keller TA., Minshew NJ., Just MA. The neural basis of deictic shifting in linguistic perspective-taking in high-functioning autism. Brain. 2011;134:2422–2435. doi: 10.1093/brain/awr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eyler LT., Pierce K., Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain. 2012;135:949–960. doi: 10.1093/brain/awr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gervais H., Belin P., Boddaert N., Leboyer M., Coez A., Sfaello I., et al. Abnormal cortical voice processing in autism. Nat Neurosci. 2004;7:801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- 100.Dawson G., Webb SJ., McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol. 2005;27:403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- 101.Berrldge KC., Robinson TE., Aldridge JW. Dissecting components of reward: “liking”, “wanting”, and learning. CurrOpin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Insel TR. Is social attachment an addictive disorder? Physiol Behav. 2003;79:351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 103.Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- 104.Schmitz N., Rubia K., van Amelsvoort T., Daly E., Smith A., Murphy DG. Neural correlates of reward in autism. Br J Psychiatry. 2008;192:19–24. doi: 10.1192/bjp.bp.107.036921. [DOI] [PubMed] [Google Scholar]
- 105.Scott-Van Zeeland AA., Dapretto M., Ghahremani DG., Poldrack RA., Bookheimer SY. Reward processing in autism. Autism Res. 2010;3:53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kohls G., Schulte-Ruther M., Nehrkorn B., et al. Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci. 2012. In press. doi: 10.1093/scan/nss033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cascio CJ., Foss-Feig JH., Heacock JL., et al. Response of neural reward regions to food cues in autism spectrum disorders. J Neurodev Disord. 2012;4 doi: 10.1186/1866-1955-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dichter GS., Felder JN., Green SR., Rittenberg AM., Sasson NJ., Bodfish JW. Reward circuitry function in autism spectrum disorders. Social Cogn Affect Neurosci. 2012;7:160–172. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dichter GS., Damiano CA., Allen JA. Reward circuitry dysfunction in neurodevelopmental and psychiatric disorders: animal models and clinical findings. J Neurodev Disord. In press. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vissers ME., Cohen MX., Geurts HM. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Biobehav Rev. 2012;36:604–625. doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 111.Just MA., Keller TA., Malave VL., Kana RK., Varma S. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev. 2012;36:1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Damarla SR., Keller TA., Kana RK., et al. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: Evidence from an fMRI study of an embedded figures task. Autism Res. 2010;3:273–279. doi: 10.1002/aur.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kana RK., Keller TA., Cherkassky VL., Minshew NJ., Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Soc Neurosci. 2009;4:135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kana RK., Keller TA., Minshew NJ., Just MA. Inhibitory control in highfunctioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mason RA., Williams DL., Kana RK., Minshew N., Just MA. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46:269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koshino H., Kana RK., Keller TA., Cherkassky VL., Minshew NJ., Just MA. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Koshino H., Carpenter PA., Minshew NJ., Cherkassky VL., Keller TA., Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 118.Mizuno A., Villalobos ME., Davies MM., Dahl BC., Muller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006;1104:160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 119.Turner KC., Frost L., Linsenbardt D., Mcllroy JR., Muller RA. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behav Brain Funct. 2006;2:34. doi: 10.1186/1744-9081-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Noonan SK., Haist F., Muller RA. Aberrant functional connectivity in autism: evidence from low-frequency BOLD signal fluctuations. Brain Res. 2009;1262:48–63. doi: 10.1016/j.brainres.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shih P., Shen M., Ottl B., Keehn B., Gaffrey MS., Muller RA. Atypical network connectivity for imitation in autism spectrum disorder. Neuropsychologia. 2010;48:2931–2939. doi: 10.1016/j.neuropsychologia.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Welchew DE., Ashwin C., Berkouk K., et al. Functional disconnectivity of the medial temporal lobe in Asperger's syndrome. Biol Psychiatry. 2005;57:991–998. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 123.Jones TB., Bandettini PA., Kenworthy L., Case LK., Milleville SC., Martin A., et al. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. Neuroimage. 2010;49:401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Villalobos ME., Mizuno A., Dahl BC., Kemmotsu N., Muller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25:916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cherkassky VL., Kana RK., Keller TA., Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- 126.Kennedy DP., Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 127.Monk CS., Peltier SJ., Wiggins JL., et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weng SJ., Wiggins JL., Peltier SJ., et al. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Anderson JS., Nielsen JA., Froehlich AL., DuBray MB., Druzgal TJ., Cariello AN., et al. Functional connectivity magnetic resonance imaging classification of autism. Brain. 2011;134(Pt 12):3742–3754. doi: 10.1093/brain/awr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Assaf M., Jagannathan K., Calhoun VD., et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53:247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wiggins JL., Peltier SJ., Ashinoff S., et al. Using a self-organizing map algorithm to detect age-related changes in functional connectivity during rest in autism spectrum disorders. Brain Res. 2011;1380:187–197. doi: 10.1016/j.brainres.2010.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lai MC., Lombardo MV., Chakrabarti B., et al. A shift to randomness of brain oscillations in people with autism. Biol Psychiatry. 2010;68:1092–1099. doi: 10.1016/j.biopsych.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 133.von dem Hagen EA., Stoyanova RS., Baron-Cohen S., Calder AJ. Reduced functional connectivity within and between 'social' resting state networks in Autism Spectrum Conditions. Soc Cogn Affect Neurosci. 2012. In press. doi: 10.1093/scan/nss053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Di Martino A., Kelly C., Grzadzinski R., Zuo XN., Mennes M., Mairena MA., et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry. 2011;69:847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hazlett HC., Poe MD., Gerig G., et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry. 2011;68:467–476. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hazlett HC., Poe M., Gerig G., et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 137.Vaidya CJ., Foss-Feig J., Shook D., Kaplan L., Kenworthy L., Gaillard WD. Controlling attention to gaze and arrows in childhood: an fMRI study of typical development and Autism Spectrum Disorders. Dev Sci. 2011;14:911–924. doi: 10.1111/j.1467-7687.2011.01041.x. [DOI] [PubMed] [Google Scholar]
- 138.Sparks BF., Friedman SD., Shaw DW., et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 139.Courchesne E., Pierce K. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity, Int J Dev Neurosci. 2005;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 140.Lainhart JE. Advances in autism neuroimaging research for the clinician and geneticist. Am J Med Genet C Semin Med Genet. 2006;142C:33–39. doi: 10.1002/ajmg.c.30080. [DOI] [PubMed] [Google Scholar]
- 141.Akshoomoff N., Lord C., Lincoln AJ., et al. Outcome classification of preschool children with autism spectrum disorders using MRI brain measures. J Am Acad Child Adolesc Psychiatry. 2004;43:349–357. doi: 10.1097/00004583-200403000-00018. [DOI] [PubMed] [Google Scholar]
- 142.Carper RA., Moses P., Tigue ZD., Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- 143.Herbert MR., Ziegler DA., Makris N., et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- 144.Courchesne E., Karns CM., Davis HR., et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 145.Waiter GD., Williams JH., Murray AD., Gilchrist A., Perrett Dl., Whiten A. Structural white matter deficits in high-functioning individuals with autistic spectrum disorder: a voxel-based investigation. Neuroimage. 2005;24:455–461. doi: 10.1016/j.neuroimage.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 146.Levitt JG., Blanton RE., Smalley S., et al. Cortical sulcal maps in autism. Cereb Cortex. 2003;13:728–735. doi: 10.1093/cercor/13.7.728. [DOI] [PubMed] [Google Scholar]
- 147.Cascio CJ., Gerig G., Piven J. Diffusion tensor imaging: application to the study of the developing brain. J Am Acad Child Adolesc Psychiatry. 2007;46:213–223. doi: 10.1097/01.chi.0000246064.93200.e8. [DOI] [PubMed] [Google Scholar]
- 148.Hong S., Ke X., Tang T., et al. Detecting abnormalities of corpus callosum connectivity in autism using magnetic resonance imaging and diffusion tensor tractography. Psychiatry Res. 2011;194:333–339. doi: 10.1016/j.pscychresns.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 149.Alexander AL., Lee JE., Lazar M., et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 150.Barnea-Goraly N., Lotspeich LJ., Reiss AL. Similar white matter aberrations in children with autism and their unaffected siblings: a diffusion tensor imaging study using tract-based spatial statistics. Arch Gen Psychiatry. 2010;67:1052–1060. doi: 10.1001/archgenpsychiatry.2010.123. [DOI] [PubMed] [Google Scholar]
- 151.Cheon KA., Kim YS., Oh SH., et al. Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: a Diffusion Tensor Imaging study. Brain Res. 2011;1417:77–86. doi: 10.1016/j.brainres.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 152.Ben Bashat D., Kronfeld-Duenias V., Zachor DA., et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 153.Sundaram SK., Kumar A., Makki Ml., Behen ME., Chugani HT., Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18:2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sahyoun CP., Belliveau JW., Mody M. White matter integrity and pictorial reasoning in high-functioning children with autism. Brain Cogn. 2010;73:180–188. doi: 10.1016/j.bandc.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fletcher PT., Whitaker RT., Tao R., et al. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage. 2010;51:1117–1125. doi: 10.1016/j.neuroimage.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Knaus TA., Silver AM., Kennedy M., et al. Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain Lang. 2010;112:113–120. doi: 10.1016/j.bandl.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bamea-Goraly N., Kwon H., Menon V., Eliez S., Lotspeich L., Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 158.Wolff JJ., Gu H., Gerig G., Elison JT., Styner M., Gouttard S., et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Glover GH., Mueller BA., Turner JA., et al. Function biomedical informatics research network recommendations for prospective multicenter functional MRI studies. J Magn Reson Imaging. 2012;36:39–54. doi: 10.1002/jmri.23572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hall D., Huerta MF., McAuliffe MJ., Farber GK. Sharing heterogeneous data: The National Database for Autism Research. Neuroinformatics. In press. doi: 10.1007/s12021-012-9151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Holtzheimer PE., Kelley ME., Gross RE., et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69:150–158. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dichter GS., Sikich L., Song A., Voyvodic J., Bodfish JW. Functional neuroimaging of treatment effects in psychiatry: methodological challenges and recommendations. Int J Neurosci. 2012;122:483–493. doi: 10.3109/00207454.2012.678446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ashwin C., Baron-Cohen S., Wheelwright S., O'Riordan M., Bullmore ET. Differential activation of the amygdala and the “social brain” during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2007;45:2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 164.Bird G., Catmur C., Silani G., Frith C., Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. Neuroimage. 2006;31:1614–1624. doi: 10.1016/j.neuroimage.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 165.Bookheimer SY., Wang AT., Scott A., Sigman M., Dapretto M. Frontal contributions to face processing differences in autism: evidence from fMRI of inverted face processing. J Int Neuropsychol Soc. 2008;14:922–932. doi: 10.1017/S135561770808140X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Corbett BA., Carmean V., Ravizza S., et al. A functional and structural study of emotion and face processing in children with autism. Psychiatry Res. 2009;173:196–205. doi: 10.1016/j.pscychresns.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Coutanche MN., Thompson-Schill SL., Schultz RT. Multi-voxel pattern analysis of fMRI data predicts clinical symptom severity. Neuroimage. 2011;57:113–123. doi: 10.1016/j.neuroimage.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dalton KM., Nacewicz BM., Johnstone T., et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Deeley Q., Daly EM., Surguladze S., et al. An event related functional magnetic resonance imaging study of facial emotion processing in Asperger syndrome. Biol Psychiatry. 2007;62:207–217. doi: 10.1016/j.biopsych.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 170.Greimel E., Schulte-Ruther M., Kircher T., et al. Neural mechanisms of empathy in adolescents with autism spectrum disorder and their fathers. Neuroimage. 2010;49:1055–1065. doi: 10.1016/j.neuroimage.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 171.Hadjikhani N., Joseph RM., Snyder J., et al. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22:1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 172.Hadjikhani N., Joseph RM., Snyder J., Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Hum Brain Mapp. 2007;28:441–449. doi: 10.1002/hbm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hall GB., Szechtman H., Nahmias C. Enhanced salience and emotion recognition in Autism: a PET study. Am J Psychiatry. 2003;160:1439–1441 . doi: 10.1176/appi.ajp.160.8.1439. [DOI] [PubMed] [Google Scholar]
- 174.Hall GB., Doyle KA., Goldberg J., West D., Szatmari P. Amygdala engagement in response to subthreshold presentations of anxious face stimuli in adults with autism spectrum disorders: preliminary insights. PloS One. 2010;5:e10804. doi: 10.1371/journal.pone.0010804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hubl D., Boite S., Feineis-Matthews S., et al. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61:1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- 176.Humphreys K., Hasson U., Avidan G., Minshew N., Behrmann M. Cortical patterns of category-selective activation for faces, places and objects in adults with autism. Autism Res. 2008;1:52–63. doi: 10.1002/aur.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kleinhans NM., Richards T., Sterling L., et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(Pt 4):1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- 178.Kleinhans NM., Johnson LC., Richards T., et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am J Psychiatry. 2009;166:467–475. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- 179.Kleinhans NM., Richards T., Weaver K., et al. Association between amygdala response to emotional faces and social anxiety in autism spectrum disorders. Neuropsychologia. 2010;48:3665–3670. doi: 10.1016/j.neuropsychologia.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kleinhans NM., Richards T., Johnson LC., et al. fMRI evidence of neural abnormalities in the subcortical face processing system in ASD. Neuroimage. 2011;54:697–704. doi: 10.1016/j.neuroimage.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Koshino H., Kana RK., Keller TA., Cherkassky VL., Minshew NJ., Just MA. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Loveland KA., Steinberg JL., Pearson DA., Mansour R., Reddoch S. Judgments of auditory-visual affective congruence in adolescents with and without autism: a pilot study of a new task using fMRI. Percep Motor Skills. 2008;107:557–575. doi: 10.2466/pms.107.2.557-575. [DOI] [PubMed] [Google Scholar]
- 183.Monk CS., Weng SJ., Wiggins JL., et al. Neural circuitry of emotional face processing in autism spectrum disorders. J Psychiatry Neurosci. 2010;35:105–114. doi: 10.1503/jpn.090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Morita T., Kosaka H., Saito DN., et al. Emotional responses associated with self-face processing in individuals with autism spectrum disorders: an fMRI study. Soc Neurosci. In press. doi: 10.1080/17470919.2011.598945. [DOI] [PubMed] [Google Scholar]
- 185.Ogai M., Matsurnoto H., Suzuki K., et al. fMRI study of recognition of facial expressions in high-functioning autistic patients. Neuroreport. 2003;14:559–563. doi: 10.1097/00001756-200303240-00006. [DOI] [PubMed] [Google Scholar]
- 186.Pelphrey KA., Morris JP., McCarthy G., Labar KS. Perception of dynamic changes in facial affect and identity in autism. Soc Cogn Affect Neurosci. 2007;2:140–149. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Perlman SB., Hudac CM., Pegors T., Minshew NJ., Pelphrey KA. Experimental manipulation of face-evoked activity in the fusiform gyrus of individuals with autism. Soc Neurosci. 2011;6:22–30. doi: 10.1080/17470911003683185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pierce K., Muller RA., Ambrose J., Allen G., Courchesne E. Face processing occurs outside the fusiform “face area” in autism: evidence from functional MRI. Brain. 2001;124(Pt 10):2059–73. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- 189.Pierce K., Haist F., Sedaghat F., Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127(Pt 12):2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- 190.Pierce K., Redcay E. Fusiform function in children with an autism spectrum disorder is a matter of “who”. Biol Psychiatry. 2008;64:552–560. doi: 10.1016/j.biopsych.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pinkham AE., Hopfinger JB., Pelphrey KA., Piven J., Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99:164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rudie JD., Shehzad Z., Hernandez LM., et al. Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cereb Cortex. In press. doi: 10.1093/cercor/bhr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Scherf KS., Luna B., Minshew N., Behrmann M. Location, location, location: alterations in the functional topography of face- but not object- or place-related cortex in adolescents with autism. Front Hum Neurosci. 2010;4:26. doi: 10.3389/fnhum.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schultz RT., Gauthier I., Klin A., et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- 195.Uddin LQ., Davies MS., Scott AA., et al. Neural basis of self and other representation in autism: an FMRI study of self-face recognition. PloS One. 2008;3:e3526. doi: 10.1371/journal.pone.0003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang AT., Dapretto M., Hariri AR., Sigman M., Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- 197.Welchew DE., Ashwin C., Berkouk K., et al. Functional disconnectivity of the medial temporal lobe in Asperger's syndrome. Biol Psychiatry. 2005;57:991–998. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 198.Weng SJ., Carrasco M., Swartz JR., et al. Neural activation to emotional faces in adolescents with autism spectrum disorders. J Child Psychol Psychiatry. 2011;52:296–305. doi: 10.1111/j.1469-7610.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Baron-Cohen S., Ring HA., Wheelwright S., et al. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 200.Castelli F., Frith C., Happe F., Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(Pt 8):1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- 201.Dapretto M., Davies MS., Pfeifer JH., et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kaiser MD., Hudac CM., Shultz S., et al. Neural signatures of autism. Proc. Natl Acad Sci USA. 2010;107:21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hadjikhani N., Joseph RM., Manoach DS., et al. Body expressions of emotion do not trigger fear contagion in autism spectrum disorder. Soc Cogn Affect Neurosci. 2009;4:70–78. doi: 10.1093/scan/nsn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pitskel NB., Boiling DZ., Hudac CM., et al. Brain mechanisms for processing direct and averted gaze in individuals with autism. J Autism Dev Disord. 2011;41:168616–93. doi: 10.1007/s10803-011-1197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Konishi S., Nakajima K., Uchida I., Kikyo H., Kameyama M., Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122(Pt 5):981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- 206.Pelphrey KA., Morris JP., McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128(Pt 5):1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- 207.Silani G., Bird G., Brindley R., Singer T., Frith C., Frith U. Levels of emotional awareness and autism: an fMRI study. Soc Neurosci. 2008;3:97–112. doi: 10.1080/17470910701577020. [DOI] [PubMed] [Google Scholar]
- 208.Wang AT., Lee SS., Sigman M., Dapretto M. Reading affect in the face and voice: neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders. Arch Gen Psychiatry. 2007;64:698–708. doi: 10.1001/archpsyc.64.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wicker B., Fonlupt P., Hubert B., Tardif C., Gepner B., Deruelle C. Abnormal cerebral effective connectivity during explicit emotional processing in adults with autism spectrum disorder. Soc Cogn Affect Neurosci. 2008;3:135–143. doi: 10.1093/scan/nsn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Allen G., Courchesne E. Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: an fMRI study of autism. Am J Psychiatry. 2003;160:262–273. doi: 10.1176/appi.ajp.160.2.262. [DOI] [PubMed] [Google Scholar]
- 211.Allen G., Muller RA., Courchesne E. Cerebellar function in autism: functional magnetic resonance image activation during a simple motor task. Biol Psychiatry. 2004;56:269–278. doi: 10.1016/j.biopsych.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 212.Agam Y., Joseph RM., Barton JJ., Manoach DS. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. Neuroimage. 2010;52:336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Belmonte MK., Yurgelun-Todd DA. Functional anatomy of impaired selective attention and compensatory processing in autism. Brain Res Cogn Brain Res. 2003;17:651–664. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- 214.Damarla SR., Keller TA., Kana RK., et al. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: evidence from an fMRI study of an embedded figures task. Autism Res. 2010;3:273–279. doi: 10.1002/aur.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dichter GS., Belger A. Social stimuli interfere with cognitive control in autism. Neuroimage. 2007;35:1219–1230. doi: 10.1016/j.neuroimage.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dichter GS., Belger A. Atypical modulation of cognitive control by arousal in autism. Psychiatry Res. 2008;164:185–197. doi: 10.1016/j.pscychresns.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dichter GS., Felder JN., Bodfish JW. Autism is characterized by dorsal anterior cingulate hyperactivation during social target detection. Soc Cogn Affect Neurosci. 2009;4:215–226. doi: 10.1093/scan/nsp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gilbert SJ., Bird G., Brindley R., Frith CD., Burgess PW. Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: an fMRI study of two executive function tasks. Neuropsychologia. 2008;46:2281–2291. doi: 10.1016/j.neuropsychologia.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gilbert SJ., Meuwese JDI., Towgood KJ., Frith CD., Burgess PW. Abnormal functional specialization within medial prefrontal cortex in high-functioning autism: a multi-voxel similarity analysis. Brain. 2009;132:869–878. doi: 10.1093/brain/awn365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gomot M., Belmonte MK., Bullmore ET., Bernard FA., Baron-Cohen S. Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain. 2008;131(Pt 9):2479–2488. doi: 10.1093/brain/awn172. [DOI] [PubMed] [Google Scholar]
- 221.Haist F., Adamo M., Westerfield M., Courchesne E., Townsend J. The functional neuroanatomy of spatial attention in autism spectrum disorder. Dev Neuropsychol. 2005;27:425–458. doi: 10.1207/s15326942dn2703_7. [DOI] [PubMed] [Google Scholar]
- 222.Just MA., Cherkassky VL., Keller TA., Kana RK., Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kana RK., Keller TA., Minshew NJ., Just MA. Inhibitory control in highfunctioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Keehn B., Brenner L., Palmer E., Lincoln AJ., Muller RA. Functional brain organization for visual search in ASD. J int Neuropsychol Soc. 2008;14:990–1003. doi: 10.1017/S1355617708081356. [DOI] [PubMed] [Google Scholar]
- 225.Kennedy DP., Redcay E., Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Nat Acad Sci U S A. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lee PS., Yerys BE., Della Rosa A., et al. Functional connectivity of the inferior frontal cortex changes with age in children with autism spectrum disorders: a fcMRI study of response inhibition. Cereb Cortex. 2009;19:1787–1794. doi: 10.1093/cercor/bhn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lee PS., Foss-Feig J., Henderson JG., et al. Atypical neural substrates of Embedded Figures Task performance in children with Autism Spectrum Disorder. Neuroimage. 2007;38:184–1893. doi: 10.1016/j.neuroimage.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Liu Y., Cherkassky VL., Minshew NJ., Just MA. Autonomy of lower-level perception from global processing in autism: evidence from brain activation and functional connectivity. Neuropsychologia. 2011;49:2105–2111. doi: 10.1016/j.neuropsychologia.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Luna B., Minshew NJ., Garver KE., et al. Neocortical system abnormalities in autism: an fMRI study of spatial working memory. Neurology. 2002;59:834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- 230.Manjaly ZM., Bruning N., Neufang S., et al. Neurophysiological correlates of relatively enhanced local visual search in autistic adolescents. Neuroimage. 2007;35:283–291. doi: 10.1016/j.neuroimage.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mizuno A., Villalobos ME., Davies MM., Dahl BC., Muller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006;1104:160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 232.Muller RA., Kleinhans N., Kemmotsu N., Pierce K., Courchesne E. Abnormal variability and distribution of functional maps in autism: An fMRI study of visuomotor learning. Am J Psychiatry. 2003;160:1847–1862. doi: 10.1176/appi.ajp.160.10.1847. [DOI] [PubMed] [Google Scholar]
- 233.Muller RA., Cauich C., Rubio MA., Mizuno A., Courchesne E. Abnormal activity patterns in premotor cortex during sequence learning in autistic patients. Biol Psychiatry. 2004;56:323–332. doi: 10.1016/j.biopsych.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 234.Muller RA., Pierce K., Ambrose JB., Allen G., Courchesne E. Atypical patterns of cerebral motor activation in autism: a functional magnetic resonance study. Biol Psychiatry. 2001;49:665–676. doi: 10.1016/s0006-3223(00)01004-0. [DOI] [PubMed] [Google Scholar]
- 235.Noonan SK., Haist F., Muller RA. Aberrant functional connectivity in autism: evidence from low-frequency BOLD signal fluctuations. Brain Res. 2009;1262:48–63. doi: 10.1016/j.brainres.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ring HA., Baron-Cohen S., Wheelwright S., et al. Cerebral correlates of preserved cognitive skills in autism: a functional MRI study of embedded figures task performance. Brain. 1999;122(Pt 7):1305–1315. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- 237.Solomon M., Ozonoff SJ., Ursu S., et al. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47:2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Schmitz N., Rubia K., Daly E., Smith A., Williams S., Murphy DG. Neural correlates of executive function in autistic spectrum disorders. Biol Psychiatry. 2006;59:7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 239.Shafritz KM., Dichter GS., Baranek GT., Belger A. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biol Psychiatry. 2008;63:974–980. doi: 10.1016/j.biopsych.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Silk TJ., Rinehart N., Bradshaw JL., et al. Visuospatial processing and the function of prefrontal-parietal networks in autism spectrum disorders: a functional MRI study. Am J Psychiatry. 2006;163:1440–1443. doi: 10.1176/ajp.2006.163.8.1440. [DOI] [PubMed] [Google Scholar]
- 241.Takarae Y., Minshew NJ., Luna B., Sweeney JA. Atypical involvement of frontostriatal systems during sensorimotor control in autism. Psychiatry Res. 2007;156:117–127. doi: 10.1016/j.pscychresns.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Thakkar KN., Polli FE., Joseph RM., et al. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD). Brain. 2008;131(Pt 9):2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Anderson JS., Lange N., Froehlich A., et al. Decreased left posterior insular activity during auditory language in autism. Am J Neuroradiol. 2010;31:131–139. doi: 10.3174/ajnr.A1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Boddaert N., Belin P., Chabane N., et al. Perception of complex sounds: abnormal pattern of cortical activation in autism. Am J Psychiatry. 2003;160:2057–2060. doi: 10.1176/appi.ajp.160.11.2057. [DOI] [PubMed] [Google Scholar]
- 245.Catarino A., Luke L., Waldman S., Andrade A., Fletcher PC., Ring H. An fMRI investigation of detection of semantic incongruities in autistic spectrum conditions. Eur J Neurosci. 2011;33:558–567. doi: 10.1111/j.1460-9568.2010.07503.x. [DOI] [PubMed] [Google Scholar]
- 246.Eigsti IM., Schuh J., Mend E., Schultz RT., Paul R. The neural underpinnings of prosody in autism. Child Neuropsychol. In press. doi: 10.1080/09297049.2011.639757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Eyler LT., Pierce K., Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain. 2012;135(Pt 3):949–960. doi: 10.1093/brain/awr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Grezes J., Wicker B., Berthoz S., de Gelder B. A failure to grasp the affective meaning of actions in autism spectrum disorder subjects. Neuropsychologia. 2009;47:1816–1825. doi: 10.1016/j.neuropsychologia.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 249.Groen WB., Tesink C., Petersson KM., et al. Semantic, factual, and social language comprehension in adolescents with autism: an FMRI study. Cereb Cortex. 2010;20:1937–1945. doi: 10.1093/cercor/bhp264. [DOI] [PubMed] [Google Scholar]
- 250.Harris GJ., Chabris CF., Clark J., Urban T., Aharon I., Steele S., et al. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cognition. 2006;61:54–68. doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 251.Hesling I., Dilharreguy B., Peppe S., Amirault M., Bouvard M., Allard M. The integration of prosodie speech in high functioning autism: a preliminary FMRI study. PloS One. 2010;5:e11571. doi: 10.1371/journal.pone.0011571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Just MA., Cherkassky VL., Keller TA., Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(Pt 8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 253.Kana RK., Keller TA., Cherkassky VL., Minshew NJ., Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129(Pt 9):2484–2893. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kana RK., Wadsworth HM. “The archeologist's career ended in ruins”: Hemispheric differences in pun comprehension in autism. Neuroimage. In press. doi: 10.1016/j.neuroimage.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 255.Kleinhans NM., Muller RA., Cohen DN., Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 2008;1221:115–125. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Knaus TA., Silver AM., Lindgren KA., Hadjikhani N., Tager-Flusberg H. fMRI activation during a language task in adolescents with ASD. J Int Neuropsychol Soc. 2008;14:967–979. doi: 10.1017/S1355617708081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Knaus TA., Silver AM., Kennedy M., et al. Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain Lang. 2010;112:113–120. doi: 10.1016/j.bandl.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lai G., Schneider HD., Schwarzenberger JC., Hirsch J. Speech stimulation during functional MR imaging as a potential indicator of autism. Radiology. 2011;260:521–530. doi: 10.1148/radiol.11101576. [DOI] [PubMed] [Google Scholar]
- 259.Lai G., Pantazatos SP., Schneider H., Hirsch J. Neural systems for speech and song in autism. Brain. 2012;135(Pt 3):961–75. doi: 10.1093/brain/awr335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Mizuno A., Liu Y., Williams DL., Keller TA., Minshew NJ., Just MA. The neural basis of deictic shifting in linguistic perspective-taking in high-functioning autism. Brain. 2011;134(Pt 8):2422–2435. doi: 10.1093/brain/awr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Redcay E., Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2-3-year-old children with autism spectrum disorder. Biol Psychiatry. 2008;64:589–598. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Redcay E., Dodell-Feder D., Mavros PL., et al. Atypical brain activation patterns during a face-to-face joint attention game in adults with autism spectrum disorder. Hum Brain Mapp. In press. doi: 10.1002/hbm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sahyoun CP., Belliveau JW., Soulieres I., Schwartz S., Mody M. Neuroimaging of the functional and structural networks underlying visu-ospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia. 2010;48:86–95. doi: 10.1016/j.neuropsychologia.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Scott-Van Zeeland AA., McNealy K., Wang AT., Sigman M., Bookheimer SY., Dapretto M. No neural evidence of statistical learning during exposure to artificial languages in children with autism spectrum disorders. Biol Psychiatry. 2010;68:345–351. doi: 10.1016/j.biopsych.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tesink CMJY., Buitelaar JK., Petersson KM., et al. Neural correlates of pragmatic language comprehension in autism spectrum disorders. Brain. 2009;132:1941–1952. doi: 10.1093/brain/awp103. [DOI] [PubMed] [Google Scholar]
- 266.Tesink CM., Buitelaar JK., Petersson KM., van der Gaag RJ., Teunisse JP., Hagoort P. Neural correlates of language comprehension in autism spectrum disorders: when language conflicts with world knowledge. Neuropsychology. 2011;49:1095–1104. doi: 10.1016/j.neuropsychologia.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 267.Vaidya CJ., Foss-Feig J., Shook D., Kaplan L., Kenworthy L., Gaillard WD. Controlling attention to gaze and arrows in childhood: an fMRI study of typical development and Autism Spectrum Disorders. Dev Sci. 2011;14:911–924. doi: 10.1111/j.1467-7687.2011.01041.x. [DOI] [PubMed] [Google Scholar]
- 268.Cascio CJ., Foss-Feig JH., Heacock JL., et al. Response of neural reward regions to food cues in autism spectrum disorders. J Neurodev Disord. In press. doi: 10.1186/1866-1955-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Dichter GS., Richey JA., Rittenberg AM., Sabatino A., Bodfish JW. Reward circuitry function in autism during face anticipation and outcomes. J Autism DevDisord. 2012;42:147–160. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Dichter GS., Felder JN., Green SR., Rittenberg AM., Sasson NJ., Bodfish JW. Reward circuitry function in autism spectrum disorders. Soc. Cogn Affect Neurosci. 2012;7:160–172. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kohls G., Schulte-Ruther M., Nehrkorn B., et al. Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci. In press. doi: 10.1093/scan/nss033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Schmitz N., Rubia K., van Amelsvoort T., Daly E., Smith A., Murphy DG. Neural correlates of reward in autism. Br J Psychiatry. 2008;192:19–24. doi: 10.1192/bjp.bp.107.036921. [DOI] [PubMed] [Google Scholar]
- 273.Scott-Van Zeeland AA., Dapretto M., Ghahremani DG., Poldrack RA., Bookheimer SY. Reward processing in autism. Autism Res. 2010;3:53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Anderson JS., Nielsen JA., Froehlich AL., et al. Functional connectivity magnetic resonance imaging classification of autism. Brain. 2011;134(Pt 12):3742–3754. doi: 10.1093/brain/awr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Cherkassky VL., Kana RK., Keller TA., Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- 276.Di Martino A., Kelly C., Grzadzinski R., et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry. 2011;69:847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kennedy DP., Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 278.Lai MC., Lombarde MV., Chakrabarti B., et al. A shift to randomness of brain oscillations in people with autism. Biol Psychiatry. 2010;68:1092–1099. doi: 10.1016/j.biopsych.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 279.Monk CS., Peltier SJ., Wiggins JL., et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Paakki JJ., Rahko J., Long X., et al. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Res. 2010;1321:169–179. doi: 10.1016/j.brainres.2009.12.081. [DOI] [PubMed] [Google Scholar]
- 281.von dem Hagen EA., Stoyanova RS., Baron-Cohen S., Calder AJ. Reduced functional connectivity within and between “social” resting state networks in autism spectrum conditions. Soc Cogn Affect Neurosci. In press. doi: 10.1093/scan/nss053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Weng SJ., Wiggins JL., Peltier SJ., et al. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wiggins JL., Peltier SJ., Ashinoff S., et al. Using a self-organizing map algorithm to detect age-related changes in functional connectivity during rest in autism spectrum di1sorders. Brain Res. 2011;1380:187–197. doi: 10.1016/j.brainres.2010.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]