Abstract
Cross-talk between hematopoietic stem cells (HSCs) and the cells comprising the niche is critical for maintaining stem cell activities. Yet little evidence supports the concept that HSCs regulate development of the niche. Here the ability of HSCs to directly regulate endosteal development was examined. Marrow was isolated 48h after ‘stressing’ mice with a single acute bleed or from control non-stressed animals. ‘Stressed’ and ‘non-stressed’ HSCs were co-cultured with bone marrow stromal cells to map mesenchymal fate. The data suggest that HSCs are able to guide mesenchymal differentiation towards the osteoblastic lineage under basal conditions. HSCs isolated from animals subjected to an acute stress were significantly better at inducing osteoblastic differentiation in vitro and in vivo than from control animals. Importantly, HSC-derived BMP-2 and BMP-6 were responsible for these activities. Furthermore, significant differences in the ability of HSCs to generate a BMP response following stress were noted in aged and in osteoporotic animals. Together these data suggest a coupling between HSC functions and bone turnover as in aging and in osteoporosis. For the first time, these results demonstrate that HSCs do not rest passively in their niche. Instead, they directly participate in bone formation and niche activities.
Keywords: HSCs, Niche, Osteoblasts, MSCs, Endosteal
Introduction
Hematopoietic stem cells (HSCs) are capable of both self-renewal and multilineage differentiation. These activities reside almost exclusively within niches located in the bone marrow in post-fetal life. Morphologic and in vitro studies suggest that the HSC niches result from the contributions of a number of bone marrow stromal cells (BMSCs) with similar or restricted function(s) [1–11]. Significant progress has been made in determining the role that niche-derived cytokines and adhesion molecules play in stem cell renewal [12]. Whether HSCs themselves regulate the maintenance or development of the niche has not been established. Yet it is a commonly held belief that cross-talk between HSCs and the niche regulates each others function [13–15].
It is now well accepted that cells in the osteoblastic (OBs) lineage play a central role in establishing the HSC niche [1–5;12]. Essential to these observations are demonstrations that OB-expressed cell-to-cell receptors (e.g., N-Cadherin, Jagged, VCAM-1), soluble and cell-surface associated cytokines and growth factors regulate HSC functions. Each of these factors, are in turn influenced by mechanical, hormonal (e.g., PTH) and local signals (e.g., BMPs, Ang-1). Whether HSCs themselves regulate lineage decisions and commitment of OBs from mesenchymal precursors remains unclear. While reciprocal cooperation in establishing the niche has been proposed, there is little direct experimental evidence for its occurrence. Indirect evidence such as the recurring trabeculation of the medullary cavity during ovulation [16], the formation of primitive marrow in bone resorption centers [17], the influence of non-adherent marrow on osteogenesis [18] or the coordinated activities of osteoblasts and bone resorbing osteoclasts in HSC mobilization suggest such a possibility [19]. Nevertheless, there are few if any direct clues that HSCs participate in the development of the niche. If true, it may explain why many hematopoietic defects are accompanied by changes in the osseous architecture and provide new therapeutic targets for regulating bone formation.
To determine if HSCs regulate the formation of their niche, we employed a co-culture system in which HSCs were co-cultured with mesenchymal precursors. The fate of the mesenchymal cells was subsequently mapped. The data suggest that HSCs are able to direct mesenchymal differentiation towards the osteoblastic lineage under basal conditions. HSCs isolated from animals subjected to an acute stress (e.g., bleeding or 5-FU) were significantly better at inducing osteoblastic differentiation in vitro and in vivo than were HSCs obtained from control animals. Importantly, HSC-derived BMP-2 and BMP-6 were responsible for these activities. Critically, the ability of HSCs to generate a BMP response was found to change over time and in an animal model of osteoporosis. These observations prove that HSCs are able to direct stromal cell fate and lineage decisions, an activity which heretofore has largely been relegated to the activity of the niche itself.
Materials and Methods
Induction of Hematopoietic Stresses
C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were bled by jugular venipuncture under a protocol approved by the University of Michigan Committee for the Use and Care of Animals (UCUCA) at the University of Michigan. The mice were anesthetized and approximately 20–30% of the calculated blood volume (~ 0.55 ml for a 20g mouse) was removed. Control mice were also anesthetized and subjected to puncture without hemorrhage. In some cases, mice received a single injection of 150 mg/kg 5-fluroruracil (American Pharmaceutical Partners Inc.) or an equal volume of 0.9% sodium chloride vehicle solution to induce hematopoietic stress.
In separate experiments 12 week old female mice were sham-operated (n=10) or ovariectomized (OVX, n=20) via a lateral approach under anesthesia with ketamine cocktail (80 mg/kg ketamine and 15 mg/kg xylazine). Animals were fed ad libitum a standard mouse chow diet (Diet No. 5015; Harlan Teklad), housed 3–4 per cage in autoclaved filter-top cages with autoclaved water, and kept on a 12-hour light/dark cycle in a conventional clean room facility. At 4 weeks post surgery a group of OVX mice (n=10) were implanted with 17 β-estradiol pellets s.c. (containing 0.18 mg each, 60-day release (Innovative Research of America, Sarasota, Florida)). The mice were euthanized two months after surgery and serum, marrow and bones were collected immediately.
HSC Isolation
Forty-eight hours after the induction of an acute stress, the animals were euthanized and the bone marrows flushed from the femurs and tibias with Hanks buffered salt solution without calcium or magnesium, supplemented with 2% heat-inactivated calf serum HBSS (Gibco, Grand Island, NY). Cells were triturated and filtered through a nylon screen (40 μm; BD Falcon, Bedford, MA) to obtain single-cell suspensions. Cells were incubated first with an antibody cocktail of anti-CD150PE (Clone TC15-12F12.2, BioLengend, San Diego, CA), CD48FITC (Clone BCM-1) and CD41FITC (Clone MWReg30), cKitBIO (Clone 2B8) and SCA-1APC (Clone E13-161.7) for twenty minutes on ice, then rinsed and stained with anti-Biotin MicroBeads (Miltenyl Biotec, Auburn, CA) and streptavidin-APC-Cy7 conjugated secondary antibody for another twenty minutes. Cells were enriched for cKit+ using an AutoMACS machine (Miltenyl Biotec, Auburn, CA). The enriched cells were resuspended in 2 mg/ml 7-AAD (eBioscience, San Diego, CA) to discriminate live from dead cells. Only live (7-AAD) cells were included in analyses and sorts. Hematopoietic stem cells were sorted on a FACS Vantage dual laser flow-cytometer (Becton Dickinson, San Jose, CA) by gating on cells that are Sca-1+cKit+CD150+CD41−CD48−. A typical FACs plot of the recovered cells is presented in Supplemental Figure 1.
In some cases, murine Lin−Sca-1+cKit+ (LSK) cells were obtained using first a Lineage Cell Depletion Kit magnetic labeling system with biotinylated (CD5, CD45R (B220), CD11b, Gr-1 (Ly-6G/C), and Ter-119) and anti-Biotin MicroBeads (Miltenyi Biotec). Positive immunoselection was performed with an anti-Sca-1-PE and anti-cKit-FITC (BD Pharmingen) and sorted on a FACS Vantage dual laser flow-cytometer (Becton Dickinson, San Jose, CA).
Murine Osteoblasts (OBs)
Murine OB cultures were obtained from frontal and parietal bones of newborn mice by sequential digestions with bacterial collagenase (CLS II; Worthington Biochemical, Freehold, NJ). Cells were pooled from the third to the fifth digestions: previous studies have shown that these cells have osteoblastic characteristics and have been extensively used to map OB maturation [20;21]. When the cultures reached confluence, the cells were washed and placed in differentiation medium (α-MEM, supplemented with 10% FBS and 10 μg/ml L-ascorbate and 10 mM β-glycerol phosphate) [22].
Murine Bone Marrow Stromal Cells (BMSCs)
Marrow flushed from the femurs and tibia of non-manipulated animals was used to generate BMSCs. After flushing the marrow into α-MEM medium with 2% FBS and 5 U/ml heparin (Sigma), the low density cells were collected by density centrifugation (Histopaque-1083 (Sigma, St. Louis, MO)) and cultured in α-MEM containing 10% fetal calf serum and antibiotics. Once confluent, the cells were passaged 2–3 times with trypsin to minimize macrophage contamination.
Co-Culture of Hematopoietic Cells with Target Adherent Cells
Non-adherent bone marrow (NABM) cells (2×104) or 200 LSKs or HSCs isolated on the basis of the SLAM family of receptors were placed into the top chambers of 24-well Transwell™ plates (0.4 μm, polycarbonate, Costar). Adherent BMSCs or OBs were plated at a final density of ~ 2 × 104/well in α-MEM containing 10% heat inactivated FBS, antibiotics, 10 μg/ml L-ascorbate and 10 mM β-glycerol phosphate into the bottom chambers of the Transwell™ plates. In some cases adherent cells were used from BMSC isolated from the heterozygous Runx2+/− mice containing the bacterial Lacz gene in the Runx2 locus or wild-type littermates for measuring Lacz activities in vitro [23]. The previously described heterozygous Runx2+/− mice with C57BL/6 genetic background were obtained from Dr. P. Ducy (Baylor College of Medicine, Houston, TX) [23]. Heterozygous mice were interbred and time mated to produce homozygous mutant animals. Where indicated, monoclonal anti-human BMP-2 and BMP-6 antibodies were added daily to the co-cultures (BMP2/4 & BMP6 blocking antibodies) (R&D Systems). The antibodies were added at 5 ng/ml, at a ND50 as reported by the manufacturer to the co-cultures.
Colony Enumeration
For CFU-F enumeration, the cultures were washed, fixed with 100% methanol and stained with an aqueous solution of saturated methyl violet. Colonies with greater than 50 cells are counted. For CFU-OB enumeration, the cultures were fixed in 10% normal buffered formalin and stained for bound phosphate by the Von Kossa technique using 5% (w/v) silver nitrate in PBS [24]. The black nodules (~1 mm2) were enumerated under a dissecting microscope (4X).
Protein Levels
Co-culture conditioned medium was collected and stored at −80 °C until assayed for cytokine levels by double-antibody sandwich method with commercially available ELISA kits according to the directions of the manufacturer; IL-6 (sensitivity 0.7 pg/ml, range 3.33 to 300 pg/ml; R&D Systems, Minneapolis, MN.) and SDF-1 (sensitivity 0.014 ng/ml, range 0.156 to 10 ng/ml, R&D Systems). Cytokine levels are presented as mean ± standard error for triplicate determinations. Osteocalcin levels were determined by RIA (Biomedical Technologies Inc., Stoughton, MA) [25].
Vertebral body transplant (vossicle) implantation
Lumbar vertebrae (vossicle) were isolated from the heterozygous Runx2+/− or wild-type littermates within 4 days of birth. Soft tissues were dissected and the vertebrae were sectioned into single vertebral bodies (vossicles) with a scalpel blade. Two 1 cm incisions were made along the backs of 6- to 7-week old C57BL/6 mice. Pouches were made on either side of the incision by blunt dissection. Four vossicles were placed in each five mice and the surgical sites were closed with surgical clips. One month later, the vossicles were exposed whereupon a small hole was made into the vossicles with a 30 gauge needle under a dissecting microscope. The marrow of ten stressed or non-stressed animals was isolated, pooled, and stained for HSCs using the SLAM family of markers (~ 400–500 HSCs were recovered per animal). Individual vossicles were either sham injected, or injected with five hundred HSCs from stressed or non-stressed animals using a fine tipped glass tube (n=5 per group). The animals were then allowed to recover. One week later the vossicles were dissected and fixed in 10% formalin, processed, and embedded in paraffin. In some cases, fresh frozen sections were generated using cold formalin (4°C) for 10 minutes, washing the tissues 3 times, and then embedding the tissues in Optimal Cutting Temperature (O.C.T.) (Sekura Finetek, Torrance, CA).
Immunohistochemistry
Murine bones were harvested and fixed in 10% neutral buffered formalin overnight, decalcified in 10% EDTA pH 7.5 for 10 days at 4°C. Paraffin embedded slides were prepared (5–7 μm), and stained with antibody to BMP-2 (rabbit polyclonal, ab14944, 5 μg/ml, Abcam Inc., Cambridge, MA), antibody to BMP-6 (mouse monoclonal, ab15640, 2.5 μg/ml, Abcam Inc., Cambridge, MA) or an IgG control (Sigma) in conjunction with a HRP-AEC staining kit following the manufactures protocols (R&D Systems). Vossicles were stained with an antibody to β-galactosidase (rabbit polyclonal, ab616, Abcam Inc., Cambridge, MA). Cryostat sections were stained with X-gal substrate at 37°C for 24 hours. Counter staining was achieved using nuclear fast red for 3–5 minutes. Images were acquired on a Zeiss LSM510 microscope. The percentage of the field stained by immunohistochemistry or β-galactosidase enzyme activity were quantified using an Image Pro Plus v.5.1 image analysis system.
Micro-CT evaluations
Lumbar vertebrae were harvested two months after OVX and fixed in aqueous buffered zinc formalin for 24 hours at 4 °C. For microcomputed tomography (micro-CT) analysis, specimens were scanned at 8.93 μm voxel resolution on an EVS Corp., micro-CT scanner (London, Ontario, Canada), with a total of 667 slices per scan. GEMS MicroView® software was used to make a 3-D reconstruction from the set of scans. A fixed threshold (1, 000) was used to extract the mineralized bone phase, bone volume fraction (BVF), bone mineral density (BMD) and trabecular number (Th.N.) were calculated.
RNA analysis and real-time RT-PCR
Total RNA was harvested from cells using with RNeasy Mini or Micro Kit (Qiagen, Valencia, CA). First-strand cDNA synthesis and real-time PCR were performed by the manufacturer’s directions (Applied Biosystems) or using MessageBooster™ cDNA synthesis kit when evaluating mRNA levels from isolated HSCs (Epicentre Biotechnologies, Madison, WI). TaqMan® predeveloped assay reagents (FAM/ MGB probe; Applied Biosystems) were used for detection of mouse BMPs 2 & 6, FGF-23, bone sialoprotein (BSP), Runx2, osteocalcin (OCN), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Universal mouse reference RNA (Stratagene) was used to generate a relative standard curve. Real-time detection of the PCR products was performed using an ABI PRISM 7700 sequence detector (Applied Biosystems) with assistance from School of Dentistry’s Molecular Biology Core Facility. mRNA expression levels were calculated based on a standard curve, and normalized to GAPDH.
Statistical Analyses
Numerical data were expressed as means ± standard deviation. Statistical differences between the means for the different groups were evaluated with Instat 4.0 (GraphPAD software) using either a Student’s T-test or an analysis of variance (ANOVA) with the level of significance at p<0.05. Where indicated, a Kruskal-Wallis test and Dunn’s multiple comparisons tests were utilized with the level of significance set at p<0.05.
Results
Non-Adherent Marrow Bone Cells Regulate Stromal Cell Fate
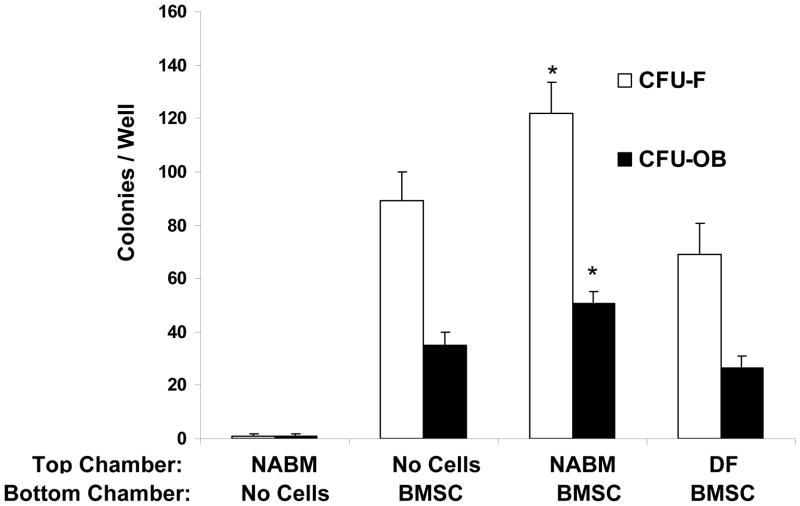
As an initial test to determine if hematopoietic cells influence mesenchymal cell fate, co-cultures were established in dual chambered culture wells containing either non-adherent bone marrow (NABM) fractions or dermal fibroblasts together with pre-established BMSC cultures. After 21 days, the fate of the BMSC layers was mapped. It was observed that the total number of adherent cell colonies increased significantly in the presence of the NABM cells. Segregation of the colonies using methyl violet to identify fibroblastic colonies (CFU-F) or von Kossa staining to identify osteoblastic colonies showed increases in both populations in the presence of the NABM cells. Except under rare conditions, osseous tissues are not normally formed under the dermis of mammals (Figure 1) [26]. Therefore we reasoned that dermal fibroblasts would provide an appropriate negative control for our studies. When these cells were included in the investigation, it was observed that soluble products derived from dermal fibroblasts may inhibit CFU-F or CFU-OB formation (Figure 1), suggesting that the stimulating activity was specific to the NABM cell population. Yet the changes in the mesenchymal cell population were not due to the NABM cells migrating through from the top chamber culture of the Transwell™ plates into the bottom chamber (Figure 1). This suggests that components of the NABM population are able to influence CFU-F and CFU-OB induction in mixed BMSC populations.
Figure 1. Non-Adherent Bone Marrow Fractions Alter CFU-F/CFU-OB Formation.
Non-adherent bone marrow cell fractions (NABM) were evaluated for their effects on the generation of fibroblastic (CFU-F) and osteoblastic (CFU-OB) colonies from BMSCs in vitro. Cells from primary bone marrow stromal cell (BMSC) cultures were trypsinized, resuspended and replated at 100 cells/well. NABM cells were collected from marrow and plated on plastic for 24 hours, recovered and washed, and placed into the top chamber of a chambered culture plate to exclude the possibility that cells from the non-adherent bone marrow fraction may contribute to colony formation. Newborn dermal fibroblasts (DF) were placed into the top chamber of the culture to served as negative controls. Cultures of just the NABM were also included. After 21 days the colonies (>50 cells) were quantified as either CFU-F or CFU-OB using methyl violet or Von Kossa staining. The data are presented as mean ± s.d. for n=6 determinations in three independent investigations. *p <0.05 compared to no cells in the top well (Students t-test).
Lin− Sca-1+ cKit+ (LSK) Bone Marrow Cells Regulate Stromal Cell Fate
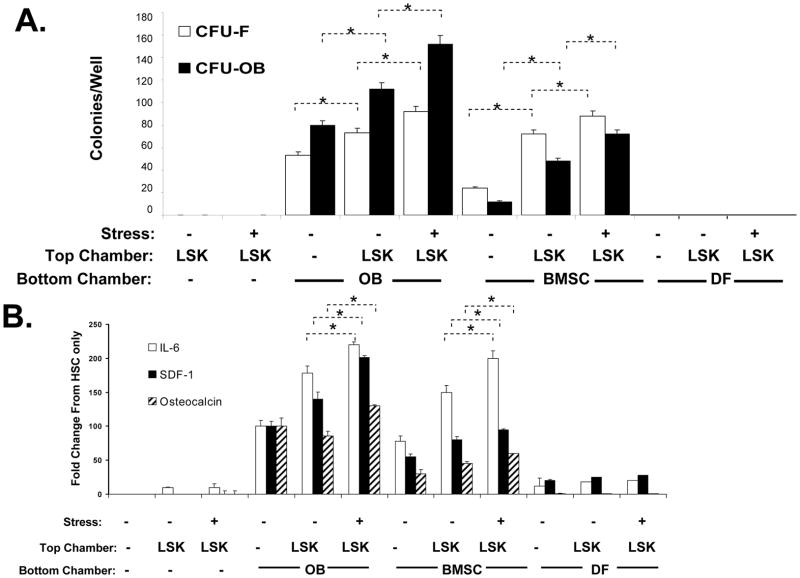
Based upon these observations it was hypothesized that HSCs in the NABM fractions were responsible for the effects observed on BMSCs. If true, two mechanisms may be envisioned in which HSCs could regulate the formation of the niche. The first mechanism may involve the signaling from HSCs to existing cellular populations to change the cytokine environment. A second or indirect mechanism may be that HSCs direct cytokine secretion indirectly by first regulating BMSC fate. To test this hypothesis, mice were phlebotomized to provide a hematopoietic stress by removing approximately 20–30% of their blood volume by jugular vein puncture and aspiration. Control animals were subjected to a transdermal puncture alone (non-stressed). Two days later, LSK cells were isolated and placed into the top chamber of the dual chamber culture wells. BMSCs or purified OBs derived from calvaria digests were placed into the bottom chambers and to serve as the target populations. There were no mesenchymal colonies observed when the LSK cells were placed in the top chambers alone (Figure 2A). The majority of colonies derived from OBs exhibited an OB phenotype, whereupon the number of these colonies increased in the presence of the LSK cells (Figure 2A). In the presence of LSK cells derived from the stressed animals, the number of CFU-OB increased even further (Figure 2A). Cultures established from BMSCs had a smaller percentage of OB colonies than those found in cultures established from OBs themselves. The presence of LSK cells expanded the total number of both CFU-F and CFU-OB, and these were further expanded when LSK cells were obtained from stressed animals.
Figure 2. Hematopoietic Stress Induces Cytokine and Mesenchymal Changes in the HSC niche.
In (A) Lin−Sca-1+cKit+ (LSK) cells were isolated from hematopoetically ‘stressed’ (removing ~20–30% of the calculated blood volume by jugular vein venipucture) (+) and ‘non-stressed’ (puncture only) (−) at 48 hour and were added to murine osteoblasts (OBs), bone marrow stromal cells (BMSC) or dermal fibroblasts (DF) in dual chambered culture plates. At 21 days, the cultures were examined for fibroblastic (CFU-F, methylviolet staining) or osteoblastic (CFU-OBs, Von Kossa staining) colonies. In (B) the CM was assayed for IL-6, SDF-1 by ELISA (R&D Systems), or the bone specific protein osteocalcin by RIA (Biomedical Technologies, Inc. Stoughton, MA), and are expressed as % change from OB levels alone for each assay,where *p <0.05 (ANOVA). The data indicate that HSCs from hematopoietically stressed animals are able to direct the formation of a microenvironment directly and influence cytokine secretion of stroma itself.
To determine if the alteration in mesenchymal phenotype results in functional changes, the levels of secreted proteins in the co-culture conditioned medium were evaluated. As shown in Figure 2B, the LSK cells themselves secreted little or no IL-6, SDF-1 or the OB specific protein, osteocalcin (OCN). All three factors were secreted by OBs, and overall their production increased in the presence of the LSK cells (Figure 2B). Most notably, the levels of all three factors increased significantly when the OBs were co-cultured with LSK cells derived from the stressed animals. Similar findings were observed when the studies were repeated on stromal cells, but not when dermal fibroblasts were used as the cellular targets (Figure 2B).
Sca-1+cKit+CD150+ CD41−CD48− Bone Marrow Cells Regulate Stromal Cell Fate
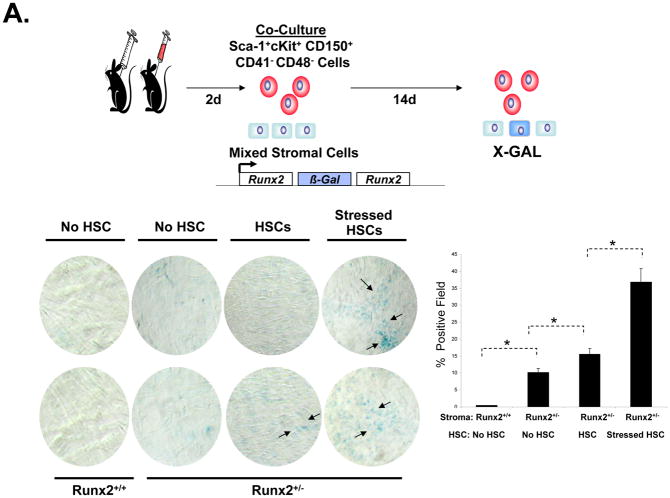
Recently Kiel et al have demonstrated that HSCs could be isolated on the basis of the expression of the SLAM family of receptors [27]. To determine if HSCs themselves are able to alter mesenchymal differentiation, Sca-1+cKit+CD150+CD41−CD48− cells were isolated from the bone marrow of phlebotomized or control animals (Figure 3A and Supplemental Figure 1). HSCs (n= 200) were then placed into co-culture for 14 days with BMSCs. In this case, the BMSCs were derived from animals in which β-galactosidase was knocked into the bone specific transcription factor, Runx2. Runx2/β-galactosidase expression was not seen in cultures of BMSCs derived from wild-type animals (Figure 3A). Low levels of Runx2/ β-galactosidase expression was observed in the BMSCs derived from the knock-in animals in the absence of HSCs, which increased in the presence of HSCs. Significantly, more Runx2/ β-galactosidase induction was observed when the HSCs were derived from phlebotomized animals (Figure 3A).
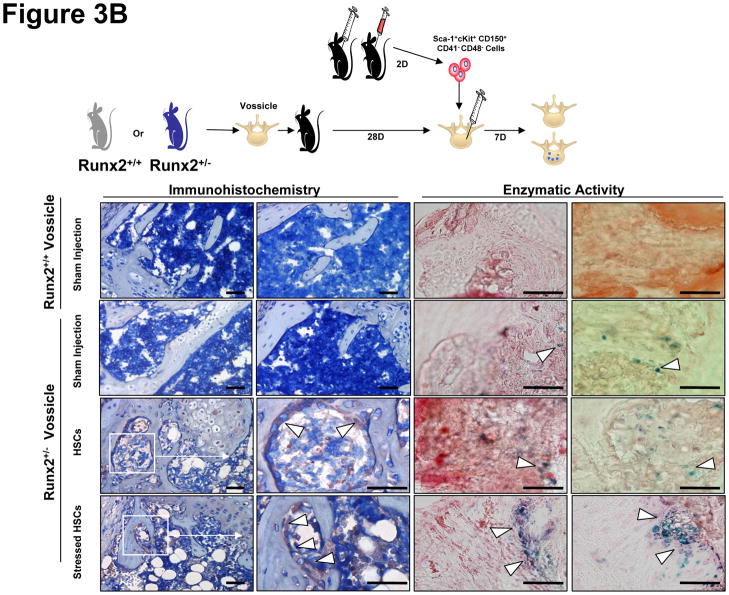
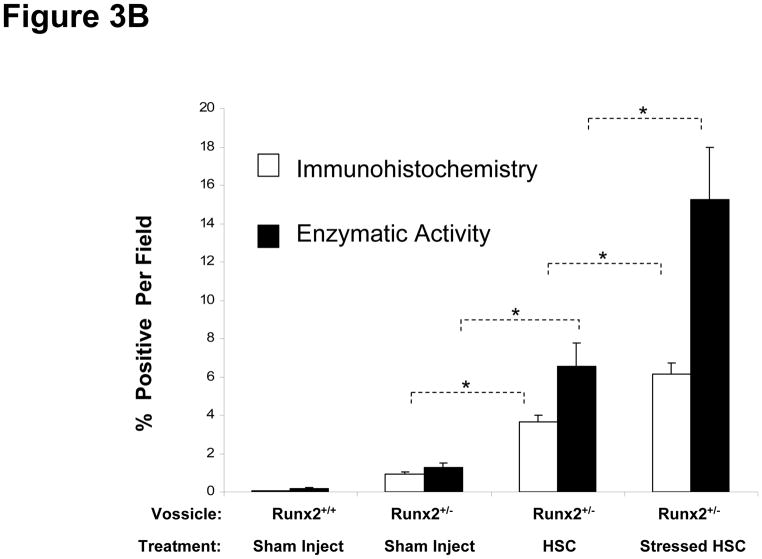
Figure 3. Sca-1+cKit+CD150+CD41−CD48− Bone Marrow Cells Regulate Stromal Cell Fate In Vitro and In Vivo.
In (A) Sca-1+cKit+CD150+CD41−CD48− bone marrow cells were isolated from hematopoetically ‘stressed’ and ‘non-stressed’ (puncture only) (−) animals at 48 hours and were directly added to pre-established murine BMSC derived from Runx2 knock-in animals. FACs profiles of the sorted HSCs are presented in Supplemental Figure 1. After 14 days, β-galactosidase activity was detected. The data are presented as mean ± s.d. for n=5 determinations in three independent investigations. In (B), vossicles derived from Runx2 knock-in animals or littermate controls were implanted into wild-type animals. Four vossicles (n=4) were in of each five mice (n=5). At one month, the vossicles were exposed and either injected with 500 Sca-1+cKit+CD150+CD41−CD48− bone marrow cells isolated from hematopoetically stressed or ‘non-stressed’ animals, or sham injected with PBS. One week later, the vossicles were harvested and tissues stained for β-galactosidase by immunohistochemistry or for enzymatic activity (Bottom Left). Bar=50 microns. IgG control stained tissues are not presented but did not differ from sham injected knock-in tissues. Quantification of the data is presented in the Bottom Right panel. The data demonstrate that Sca-1+cKit+CD150+CD41−CD48− induce Runx2 expression in vitro and in vivo.
Our group has developed an osteogenic assay in which neonatal skeletal elements form bone in vivo (e.g., vertebral body or ‘vossicles’), creating an ectopic bone marrow environment where bone niches are generated and can be studied [28;29]. In order to determine if HSCs are able to direct MSC fate in vivo, Sca-1+cKit+CD150+CD41−CD48− cells were isolated from marrow of phlebotomized or control animals and injected directly into pre-established vossicles derived from the Runx2 knock-in animals. After 1 week, the vossicles were harvested and the induction of Runx2 was examined for β-galactosidase by immunohistochemistry or enzymatic activity (Figure 3B, Bottom Right and Left). No Runx2/β-galactosidase activity was noted in vossicles derived from wild-type animals. Low levels of Runx2/β-galactosidase expression were observed in the sham injected Runx2/ β-galactosidase vossicles in the absence of HSCs. The levels of β-galactosidase increased in the presence of HSCs (Figure 3B). Significantly, more Runx2/β-galactosidase expression was observed when the vossicles were injected with HSCs derived from phlebotomized animals (Figure 3B, Right).
HSCs Regulate Stromal Cell Fate Through BMPs
To determine what the differences are between cells derived from ‘stressed’ versus ‘non-stressed’ animals, LSK cells were isolated and cDNA microarrays were performed on the recovered cells. From a list of over 200 transcripts that were significantly increased in the LSK cells derived from stressed animals (not shown), three of the transcripts were selected for further study based upon their known regulation of osteoblast development including BMP-2, BMP-6 and FGF-23.
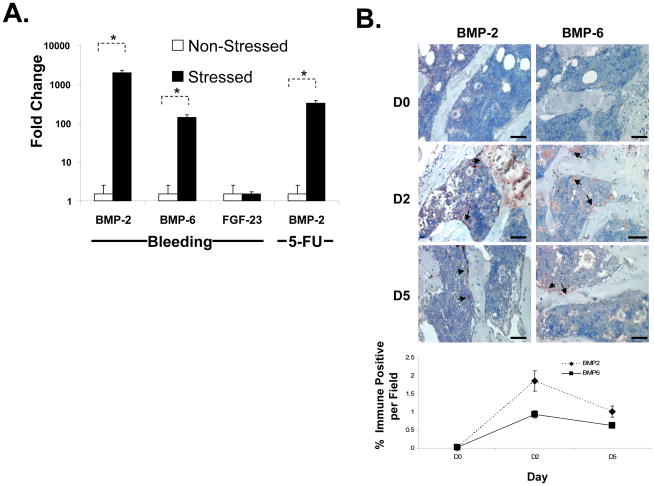
These findings were validated by real-time RT-PCR on HSCs isolated based upon their expression of the SLAM family of receptors (Sca-1+cKit+CD150+CD41−CD48−) as described by Kiel et al [27]. As shown in Figure 4A, the expression of BMP-2 and BMP-6 were significantly induced in HSCs following an acute stress. Yet no induction of FGF-23 was observed. To determine if other hematopoietic stresses induce a BMP response, mice were exposed to 5-fluorouracil (5-FU) or a vehicle control and the HSCs were isolated at 48 hours. As with an acute bleed, induction of a BMP response was observed in the HSCs of mice exposed to 5-FU compared to vehicle alone (Figure 4A). Immunohistologic evaluations of the bone marrows of mice following an acute stress confirmed the induction of BMP2 and BMP6 in vivo (Figure 4B).
Figure 4. Hematopoietic Stress Induces BMP-2 and BMP-6 Expression in the Marrow and HSCs.
In (A) real-time RT-PCR was used to evaluate mRNA levels for BMP-2, BMP-6 and FGF-23 from HSCs (Sca-1+cKit+CD150+CD41−CD48− cells) isolated 2 days following an acute stress (jugular vein puncture and aspiration, versus puncture only, or 5-FU versus vehicle). The cells were lysed and RNA prepared for evaluation of mRNA levels by reai-time RT-PCR. The data is presented as fold change normalized to GAPDH, where the expression level derived from the non-stressed animals was set as the standard. *p < 0.05 versus the non-stressed animals (Krusal-Wallis test, and Dunn’s multiple comparisons test). In (B) bones were harvested at 0, 2 and 5 days following an acute stress, fixed, decalcified and stained with an antibody to BMP-2 and BMP-6 or an IgG control (not shown) in conjunction with a HRP-AEC staining system and counter stained with hematoxylin and eosin. Bar=100 microns. The data demonstrate that BMP-2 and BMP-6 are expressed by HSCs and the marrow following stress. Quantification of the data is presented in the Bottom Right panel.
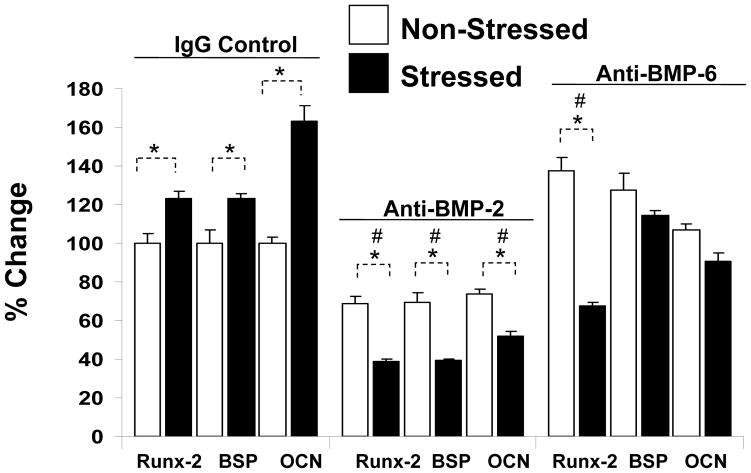
To substantiate that HSC-derived BMPs regulate BMSC fate, the co-culture investigations were repeated by isolating HSCs from stressed and non-stressed 12 week old animals. In this case, the cultures contained IgG or neutralizing antibody to BMP-2 or -6. After 21 days, the OB phenotype was examined by real-time RT-PCR for the expression of Runx2 (an OB specific transcription factor), or the OB-specific proteins bone sialoprotein (BSP) or osteocalcin (OCN). As expected, BMSCs co-cultured with HSCs derived from stressed animals expressed enhanced levels of each of the bone specific markers in the presence of HSCs. These levels were further enhanced following an acute stress (Figure 5). Neutralizing antibody to BMP-2 prevented the increase in the expression of the OB markers when BMSCs were co-cultured with HSCs derived from animals that were stressed. Similar results were seen when antibody to BMP-6 was used (Figure 5), or when OBs were used as the cellular targets (data not shown).
Figure 5. HSCs Regulate Mesenchymal Fate Through BMPs.
Co-culture investigations were established by placing HSCs (Sca-1+cKit+CD150+CD41−CD48−) derived from stressed or non-stressed animals in the top chamber of a dual culture plate, and mixed BMSCs in the bottom well, in the presence or absence of neutralizing antibody to BMP-2 or BMP-6, or an IgG isotype matched control each added daily at 5 ng/ml. After 21 days the OB phenotype was examined by real-time RT-PCR for the expression of the Runx2 (an OB specific transcription factor), or the OB specific proteins bone sialoprotein (BSP) or osteocalcin (OCN). The data is presented as % change normalized to GAPDH, where the expression levels of co-cultures of BMSCs/HSCs (from the non-stressed animals) was set as the standard. *p <0.05 between HSCs derived from stressed and non-stressed animals, and # signifies differences between anti-BMP and IgG treated controls (p<0.05, Krusal-Wallis test, and Dunn’s multiple comparisons test). The data demonstrate that blockade of BMP-2 or BMP-6 activities modifies lineage differentiation.
Aging Causes Dysregulation of BMP Expression by HSCs
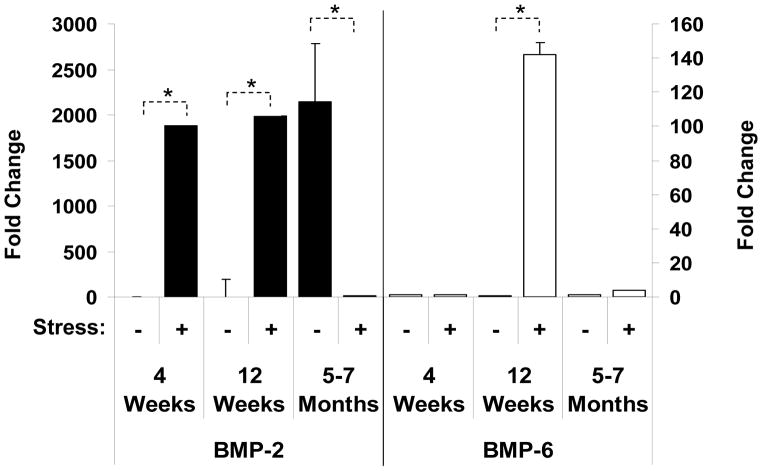
We next examined whether aging altered stem cell function. HSCs were isolated from young, sexually mature and aged mice, and their ability to generate a BMP response to stress was explored. Surprisingly, in animals where the bones were actively forming, BMP-2 and -6 mRNA expression levels in the HSCs were very low under basal conditions, but BMP-2 mRNA increased significantly in the stressed animals (Figure 6). These observations suggest that early cellular pathways for bone formation may not be dominated by hematopoietic stem cell activities. As seen before, sexually mature 12 week old animals responded to a hematopoietic stress by generating a BMP response. Unexpectedly, it was observed that HSCs of aged mice normally express high levels of BMP-2 mRNA, an expression pattern more in line with the stress response. However, stressing the aged animals resulted in decreased BMP expression. These findings suggest that for aged animals, which may already have a stem cell niche under stress, are unable to meet the physiologic demands of additional stressful conditions.
Figure 6. Aging Alters the BMP Response in HSCs.
HSCs (Sca-1+cKit+CD150+CD41−CD48−) were isolated 2 days following an acute stress from young (4 weeks), sexually mature (12 weeks) and aged (5–7 months) mice. The cells were lysed and RNA prepared for evaluation by real-time RT- PCR. The data are presented as fold change normalized to GAPDH, where the expression levels of the HSCs derived from the non-stressed animals was set as the standard. *p < 0.05 versus the non-stressed animals.
Dysregulation of BMP Expression by HSCs in an Osteoporosis Animal Model
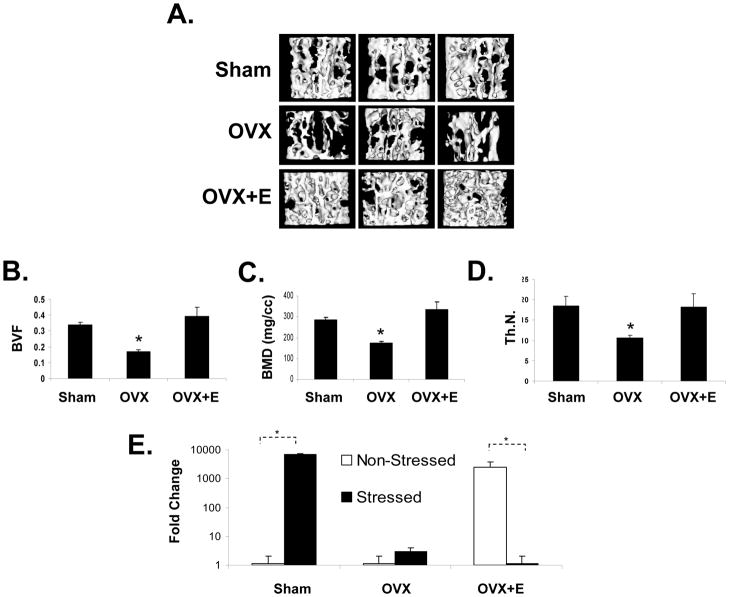
Osteoporosis is a disease of bone that leads to an increased risk of fracture, resulting from reductions in bone mineral density (BMD) and alterations bone microarchitecture. It is largely considered to be a metabolic bone disease that results from defects in osteoblastic and osteoclastic function (e.g., bone remodeling) [30–32]. To explore the possibility that bone loss in osteoporosis may be a consequence of defective or alterations in HSC function, an osteoperosis model generated by ovariectomy (OVX) in mice as used. Mice were randomized into three groups and treated as follows: sham operated, OVX, and OVX + hormone replacement (β-estradiol or E). At two months micro-CT measurements of the vertebrae revealed lower density in the OVX treated animals compared with controls or OVX + hormone replacement (Figure 7A). Likewise bone mineral density, volume, trabecular number and thickness were significantly reduced in the OVX treated animals compared to controls or OVX +E (Figure 7B–D and supplemental Figure 2). When controlled for body weight, the difference in BMD between groups OVX mice still remained significant (not shown). Sham treated animals responded to a hematopoietic stress by generating a BMP-2 response in response to a hematopoietic stress (Figure 7E). OVX treated animals in failed to generate a BMP response following phlebotomy. Under non-stressed conditions, the HSCs of OVX + hormone replacement treated animals exhibited an increase in BMP-2 mRNA. However this response was reduced under stressed conditions reminiscent of the observations made in the aging model (Figure 6). These data suggest that hormone replacement alone was not sufficient to reverse the stem cell defect.
Figure 7. Ovarectomy Alters the BMP response in HSCs.
To explore the possibility that bone loss in osteoporosis may be a consequence of defective HSC function, mice were either sham operated (n=10), ovariectomized (OVX, n=10), or treated with OVX with hormone replacement (β-estradiol or E, n=10). At two months, (A) 3-dimensional micro-CT measurements of the first lumbar vertebrate were performed. (B) Bone volume fraction (BVF), (C) bone mineral density (BMD), (D) trabecular number (Th.N.) were also significantly reduced in the OVX treated animals compared to controls or OVX + E treated groups. (E) HSCs derived from sham treated animals responded to a hematopoietic stress by generating a BMP-2 response. The data are presented as fold change normalized to GAPDH, where the expression level of derived from the non-stressed animals was set as the standard. * *p < 0.05 versus the non-stressed animals (Krusal-Wallis test, and Dunn’s multiple comparisons test).
Discussion
Previous work suggests an interdependent relationship between HSCs and OBs, whereby HSCs induce the expression of hematopoietic-supportive activities by OBs [25]. Therefore it was hypothesized that cross-talk between HSCs-OBs is essential for the development of both cellular populations. The data presented here suggest that HSCs are able to direct mesenchymal differentiation towards the osteoblastic lineage under basal conditions. These activities are possibly due to targeting either MSCs or progenitor populations within BMSCs. HSCs isolated from animals subjected to an acute stress (e.g. bleeding or 5-FU) were significantly better at inducing osteoblastic differentiation than HSCs obtained from control animals in vitro and in vivo. Importantly, HSC-derived BMP-2 and BMP-6 were responsible for these activities. Yet the BMP-2 response may change over the course an animal’s lifespan and in response in osteoporosis. These observations prove that HSCs are able to direct stromal cell fate and lineage decisions, an activity which heretofore has largely been relegated to the activity of the niche itself.
There are at least two potential mechanisms that we can conceive of whereby HSCs may establish a “paracrine loop” with OBs; (i) HSCs may directly regulate cytokine expression by OBs in response to physiologic demands. Here we have previously demonstrated that the levels of IL-6, LIF, HGF and MIP-1α produced by osteoblasts in the presence of human CD34+ bone marrow cells [33;34]. (ii) HSCs may also influence the pattern of mesenchymal developmental and thereby indirectly modulate cytokine expression in the marrow. The indirect pathway is a realistic modality as there are numerous examples of adaptations in marrow microenvironments associated with a conversion from hematopoietic (e.g., osteoblastic, or red marrow) to non-hematopoietic (e.g., adipose or yellow marrow) supportive phenotypes [35–37]. These include both physiological (e.g., age-related changes) and pathological (e.g., blood loss, aplastic anemia) adaptations. However, the molecular mechanisms for these conversions are unclear. Further studies will be required to determine if the alterations in stem cell function/activities precedes or follows the mesenchymal changes in the marrow.
Our data suggest that HSCs do not rest passively in their niche. The findings reported here suggest that a functional dialogue exists between HSCs and the mesenchymal components of the niche that may involve both direct and indirect actions. As was noted above, previous studies have explored the functional interdependence between these two cell types from the stand point of de novo cytokine secretion [33]. For example, it was observed that when human CD34+ bone marrow cells were co-cultured in direct contact with OBs, a 222 ± 55% (range 153 to 288%) augmentation in IL-6 synthesis was observed. The accumulation of IL-6 protein was most rapid during the initial 24 hour period accounting for nearly 77% of total IL-6 produced by OBs. Cell-to-cell contact did not appear to be required for this activity since culturing the cells separated by a microporous membranes and using conditioned medium derived from human CD34+ bone marrow cells produced similar results as did the direct co-culture [33]. Likewise we have previously demonstrated that the levels of LIF, HGF and MIP-1α produced by osteoblasts in the presence of human CD34+ bone marrow cells [33;38]. To our knowledge, these data represent the first demonstration that normal hematopoietic progenitor cells induce the production molecules required for the production of a normal bone marrow microenvironment by untransformed cells of the OB lineage.
Only in rare circumstances does bone form under the dermis of mammals [26]. Therefore we reasoned that neonatal dermal fibroblasts would provide an appropriate negative control for our studies. We anticipated that the OB stimulating activity would be specific to hematopoietic cells since we have observed coupling of osteoblastic and HSC function before [39–41]. We were surprised however that soluble products derived from dermal fibroblasts suppressed CFU-F or CFU-O formation. The molecular basis for these observations is not clear. One possibility is that the dermal fibrobasts more rapidly exhausted the culture nutrients available to support colony formation than the NABM. Alternatively, the dermal fibroblasts may have either (i) secreted colony formation inhibitors or (ii) failed to secrete factors required to support colony formation. Nutrient depletion seems to be a real possibility as the control cultures appeared significantly more acidic at each time the media was changed. Further studies will be required to distinguish between these possibilities.
From the present study it is not clear if the BMP response by HSCs represents a feedback mechanism to alter HSC pools directly. In fact, several members of the BMP family have been implicated in regulating the proliferation, differentiation and renewal of hematopoietic stem cells (HSCs) [42–44]. Moreover, from our antibody co-culture studies, we were unable to distinguish if the anti-BMP treatments initially target the HSCs which ultimately produce a secondary set of factors that drive MSC differentiation. Genetic studies will be required to address these possibilities. Alternatively, the BMP response may target mesenchmyal populations to increase niche size as mice expressing a conditionally inactivated BMPr1a have both an enhanced pool size of HSCs that correlates with the volume of the trabecular bone [45]. The current findings extend these observations in that HSCs are able to direct stromal cell fate and lineage decisions, an activity which heretofore has largely been relegated to the activity of the niche itself. Yet bone marrow transplant studies have also demonstrated that aspects of the niche are likely to exist independent of the activities produced by HSCs. For if this were not the case, there should be no limit to the number of HSCs that could engraft in the absence of preconditioning [46]. Clearly further studies will be required to sort out these disparities.
Throughout life, bone tissue is in a constant state of turnover. The process of bone remodeling is the result of a combination of ordered removal of bone tissue by osteoclasts and the deposition of new bone by osteoblasts derived from mesenchymal stromal cells. The findings reported herein suggest the possibility that HSCs themselves serve as relevant therapeutic targets for alterations in bone formation. If true, they may in part provide a mechanism as to why defective HSC function is often correlated with functional loss of bone. As a result, therapeutic agents that specifically target HSCs may prove of value in treating bone defects which take advantage of the functional crosstalk between the two tissues. As an example, administration of FLT3 ligand to whole bone marrow cultures increased the recovered number of BMSCs in vitro (McCauley et al., unpublished observations). Similar observations have been made in vivo where bleeding alone [47], or marrow trauma may evoke a systemic osteogenic response [48–53]. Alternatively, the osteopetroses are a heterogeneous group of skeletal disorders characterized by a generalized increase in bone mass caused by decreased bone resorption [54;55]. Conceivably, inappropriate activation of HSCs in a subset of these conditions may result in localized or generalized increases in bone mass and would provide further justification for therapeutic stem cell transplantation.
Marrow transplantation offers a potential curative therapy for many conditions affecting the hematopoietic system, yet its role in the treatment of non-hematopoietic diseases has been limited. Previous reports have demonstrated the therapeutic potential of bone marrow transplant in children with severe osteogenesis imperfecta suggesting that mesenchymal cells can engraft and function in skeletal sites and differentiate into functional OBs [56]. Yet at present it is relatively difficult to achieve durable and high levels of MSC engraftment [57]. In comparison, transplant of HSCs as a therapeutic modality for a number of clinical applications has achieved considerable success. If transplantation of HSCs were able to alter the progression of mesenchymal conditions, the clinical ramifications could be considerable. For example, osteoporosis and osteopenia affect many patients with thalassaemia major (TM). Leung et al reported that HSC transplantation improved bone mineral density scores in TM subjects in a cross sectional study [58]. Likewise, in a subject who was genetically susceptible to ectopic skeletogenesis, transplantation altered the progression of fibrodysplasia ossificans progressive [26]. While other explanations of these cases are clearly possible, activation or targeting HSCs may prove to be a viable therapeutic option to treat skeletal or other mesenchymal abnormalities. Clearly further studies are warranted.
Supplementary Material
Acknowledgments
These investigations were supported in part by the Molecular Biology Core at the University of Michigan Dental School and awards from The National Institutes of Health DE13701 (R.S.T), DE11723(R.T.F), DE13835 (P.H.K, R.S.T) and DK53904 (L.K.M). The authors wish to acknowledge Stephan G. Emerson (University of Pennsylvania) for his helpful discussion and the work is dedicated the work to Jenda Taichman.
Footnotes
Author Contributions:
Younghun Jung: Collection of data, data analysis and interpretation, manuscript writing
Junhui Song: Collection of data, manuscript writing
Yusuke Shiozawa: Collection of data, manuscript writing
Jingcheng Wang: Collection of data
Zhuo Wang: Collection of data
Benjamin Williams: Collection of data
Aaron Havens: Collection of data
Abraham Schneider: Conception and design
Chunxi Ge: Collection of data
Renny T. Franceschi : Conception and design
Laurie K. McCauley : Conception and design
Paul H. Krebsbach : Conception and design
Russell S. Taichman: Conception and design, collection and/or assembly of data, data analysis and interpretation, Manuscript writing
Author Contributions
R.S.T, L.K.M, A.S., P.H.K. conceived the project, and R.S.T. wrote the paper. Y. J., J. S., Y.S., A.H. performed the phlebotomy studies and laboratory analyses. Z.W. performed the animal surgeries and micro-CT analysis. C.G. and R.T.F. provided the knock-in animals and assisted in preparation of BMSC and osteoblasts.
Competing Interests Statement
The authors declare that they have no competing financial interests.
Reference List
- 1.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179:1677–1682. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439(7076):599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 3.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/Angiopoietin-1 Signaling Regulates Hematopoietic Stem Cell Quiescence in the Bone Marrow Niche. Cell. 2004;118(2):149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ, Simmons PJ, Haylock DN. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005:2004–2011. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 6.Beresford JN. Osteogenic stem cells and the stromal system of bone and marrow. Clinical Orthopaedics & Related Research. 1989;(240):270–280. [PubMed] [Google Scholar]
- 7.Dorheim MA, Sullivan M, Dandapani V, Wu X, Hudson J, Segarini PR, Rosen DM, Aulthouse AL, Gimble JM. Osteoblastic gene expression during adiposegenesis in hematopoietic supporting murine bone marrow stromal cells. J Cell Phys. 1993;154:317–328. doi: 10.1002/jcp.1041540215. [DOI] [PubMed] [Google Scholar]
- 8.Gordon MY, Clarke D, Atkinson J, Greaves MF. Hemopoietic progenitor cell binding to the stromal microenvironment in vitro. Exp Hematol. 1990;18(7):837–842. [PubMed] [Google Scholar]
- 9.Quesenberry PJ. Stromal cells in long-term bone marrow cultures. In: Tavassoli M, editor. Handbook of the hematopoietic microenvironment. Clifton, N.J: Humana Press; 1989. pp. 253–285. [Google Scholar]
- 10.Kaigler D, Krebsbach PH, West ER, Horger K, Huang YC, Mooney DJ. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005;19(6):665–667. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- 11.Krebsbach P, Kuznetsov S, Satourma K, Emmons R, Rowe D, Robey P. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–1069. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 12.Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem cell niche. Blood. 2005;1059(7)(7):2631. 2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Li L. Understanding hematopoietic stem-cell microenvironments. Trends in Biochemical Sciences. 2006;31(10):589–95. doi: 10.1016/j.tibs.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Yin T, Li L. The stem cell niches in bone. Journal of Clinical Investigation. 2006;116(5):1195–201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nature Reviews Immunology. 2006;6(2):93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 16.Bloom W, Bloom M, Mclean MCF. Calcification and ossification. Medullary bone changes in the reproductive cycle of female pigeons. American Journal of Anatomy. 1941;81:443–445. [Google Scholar]
- 17.Patt HM, Maloney MA. Bone formation and resorption as a requirement for marrow development. Proceedings of the Society for Experimental Biology & Medicine. 1972;140(1):205–207. doi: 10.3181/00379727-140-36426. [DOI] [PubMed] [Google Scholar]
- 18.Aubin JE. Osteoprogenitor cell frequency in rat bone marrow stromal populations: role for heterotypic cell-cell interactions in osteoblast differentiation. Journal of Cellular Biochemistry. 1999;72(3):396–410. [PubMed] [Google Scholar]
- 19.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, Elson A, Lapidot T. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nature Medicine. 2006;12(6):657–64. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 20.Aronow MA, Gerstenfeld LC, Owne TA, Tassinari, Stein GS, Jane JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Phys. 1990;143:213–221. doi: 10.1002/jcp.1041430203. [DOI] [PubMed] [Google Scholar]
- 21.Taichman RS, Hauschka PV. Effects of interleukin-1 beta and tumor necrosis factor-alpha on osteoblastic expression of osteocalcin and mineralized extracellular matrix in vitro. Inflammation. 1992;16(6):587–601. doi: 10.1007/BF00919342. [DOI] [PubMed] [Google Scholar]
- 22.Franceschi RT, Bhanumathi SI, Yingqi C. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J Bone and Min Res. 1994;9:843–854. doi: 10.1002/jbmr.5650090610. [DOI] [PubMed] [Google Scholar]
- 23.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89(5):765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 24.Bellows CG, Aubin JE, Hersche JNM, Antosz ME. Mineralized nodules formed in vitro from enzymatically released rat calvarial cell populations. Calcif Tiss Intl. 1986;38:143–154. doi: 10.1007/BF02556874. [DOI] [PubMed] [Google Scholar]
- 25.Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human progenitor cells in In Vitro bone marrow cultures. Blood. 1996;87:518–524. [PubMed] [Google Scholar]
- 26.Kaplan FS, Glaser DL, Shore EM, Pignolo RJ, Xu M, Zhang Y, Senitzer D, Forman SJ, Emerson SG. Hematopoietic stem-cell contribution to ectopic skeletogenesis. J Bone Joint Surg Am. 2007;89(2):347–357. doi: 10.2106/JBJS.F.00472. [DOI] [PubMed] [Google Scholar]
- 27.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM Family Receptors Distinguish Hematopoietic Stem and Progenitor Cells and Reveal Endothelial Niches for Stem Cells. Cell. 2005;121(7):1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Pettway GJ, McCauley LK. Methods in Molecular Biology/Molecular Medicine: Osteoporosis. 2007. Ossicle and vossicle implant model systems. In Press. [DOI] [PubMed] [Google Scholar]
- 29.Koh AJ, Demiralp B, Neiva KG, Hooten J, Nohutcu RM, Shim H, Datta NS, Taichman RS, McCauley LK. Cells of the osteoclast lineage as mediators of the anabolic actions of parathyroid hormone in bone. Endocrinology. 2005;146(11):4584–96. doi: 10.1210/en.2005-0333. [DOI] [PubMed] [Google Scholar]
- 30.Kamel HK. Male osteoporosis: new trends in diagnosis and therapy. Drugs & Aging. 2005;22(9):741–8. doi: 10.2165/00002512-200522090-00003. [DOI] [PubMed] [Google Scholar]
- 31.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423(6937):349–55. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 32.Alliston T, Derynck R. Medicine: interfering with bone remodelling. Nature. 2002;416(6882):686–7. doi: 10.1038/416686a. [DOI] [PubMed] [Google Scholar]
- 33.Taichman RS, Reilly MJ, Verma RS, Emerson SG. Augmented production of interleukin-6 (IL-6) by normal human osteoblasts in response to CD34+ hematopoietic bone marrow cells in vitro. Blood. 1997;89:1165–1172. [PubMed] [Google Scholar]
- 34.Taichman RS, Reilly MJ, Matthews LS. Human Osteoblast-Like Cells and Osteosarcoma Cell Lines Synthesize MIP-1α in Response to IL-1β and TNF-α Stimulation In Vitro. Br J Haematol. 2000;108:275–283. doi: 10.1046/j.1365-2141.2000.01873.x. [DOI] [PubMed] [Google Scholar]
- 35.Yamada M, Matsuzaka T, Uetani M, Hayashi, Tsuji Y, Nakamura T. Normal age-related conversion of bone marrow in the mandible: MR imaging findings. AJR 1995; American Journal of Roentgenology. 165(5):1223–1228. doi: 10.2214/ajr.165.5.7572508. [DOI] [PubMed] [Google Scholar]
- 36.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clinical Orthopaedics & Related Research. 1971;80:147–154. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 37.Corral D, Amling M, Priemel M, Loyer E, Fuchs S, Ducy P, Barron R, Karsenty G. Dissociation between bone resorption and bone formation in osteopenic transgenic mice. Proceedings of the National Academy of Science USA. 1998 Nov 1;95:13835–13840. doi: 10.1073/pnas.95.23.13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taichman RS, Reilly MJ, Verma RS, Ehrenman K, Emerson SG. Hepatocyte growth factor is secreted by osteoblasts and cooperatively permits the survival of haematopoietic progenitors. British Journal of Haematology. 2001 Feb;112(2):438–448. doi: 10.1046/j.1365-2141.2001.02568.x. [DOI] [PubMed] [Google Scholar]
- 39.Eipers PG, Kale S, Taichman RS, Pipia GG, Swords NA, Mann KG, Long MW. Bone marrow accessory cells regulate human bone precursor cell development. Experimental Hematology. 2000;28(7):815–25. doi: 10.1016/s0301-472x(00)00183-1. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Song J, Taichman RS, Krebsbach PH. Ablation of proliferating marrow with 5-fluorouracil allows partial purification of mesenchymal stem cells. Stem Cells. 2006;24(6):1573–1582. doi: 10.1634/stemcells.2005-0399. [DOI] [PubMed] [Google Scholar]
- 41.Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, Joe GJ, Hexner E, Choi Y, Taichman RS, Emerson SG. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109(9):3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 42.Detmer K, Walker AN. Bone morphogenetic proteins act synergistically with haematopoietic cytokines in the differentiation of haematopoietic progenitors. Cytokine. 2002;17(1):36–42. doi: 10.1006/cyto.2001.0984. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed N, Sammons J, Carson RJ, Khokher MA, Hassan HT. Effect of bone morphogenetic protein-6 on haemopoietic stem cells and cytokine production in normal human bone marrow stroma. Cell Biology International. 2001;25(5):429–35. doi: 10.1006/cbir.2000.0662. [DOI] [PubMed] [Google Scholar]
- 44.Bhatia M, Bonnet D, Wu D, Murdoch B, Wrana J, Gallacher L, Dick JE. Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. Journal of Experimental Medicine. 1999;189(7):1139–48. doi: 10.1084/jem.189.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang JW, Niu C, Ye L, Huang HY, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li LH. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z, Bunting KD. Hematopoietic stem cell transplant into non-myeloablated W/Wv mice to detect steady-state engraftment defects. Methods Mol Biol. 2008;430:171–181. doi: 10.1007/978-1-59745-182-6_12. [DOI] [PubMed] [Google Scholar]
- 47.Lucas TS, Bab IA, Lian JB, Stein GS, Jazrawi L, Majeska RJ, Attar-Namdar M, Einhorn TA. Stimulation of systemic bone formation induced by experimental blood loss. Clinical Orthopaedics & Related Research. 1997;(340):267–75. doi: 10.1097/00003086-199707000-00034. [DOI] [PubMed] [Google Scholar]
- 48.Bab IA. Postablation bone marrow regeneration: an in vivo model to study differential regulation of bone formation and resorption. Bone. 1995;17(4 Suppl):437S–441S. doi: 10.1016/8756-3282(95)00323-6. [DOI] [PubMed] [Google Scholar]
- 49.Greenberg Z, Chorev M, Muhlrad A, Shteyer A, Namdar-Attar M, Casap N, Tartakovsky A, Vidson M, Bab I. Structural and functional characterization of osteogenic growth peptide from human serum: identity with rat and mouse homologs. Journal of Clinical Endocrinology & Metabolism. 1995;80(8):2330–5. doi: 10.1210/jcem.80.8.7629225. [DOI] [PubMed] [Google Scholar]
- 50.Bab IA, Einhorn TA. Regulatory role of osteogenic growth polypeptides in bone formation and hemopoiesis. Critical Reviews in Eukaryotic Gene Expression. 1993;3(1):31–46. [PubMed] [Google Scholar]
- 51.Bab I, Gazit D, Chorev M, Muhlrad A, Shteyer A, Greenberg Z, Namdar M, Kahn A. Histone H4-related osteogenic growth peptide (OGP): a novel circulating stimulator of osteoblastic activity. EMBO Journal. 1992;11(5):1867–73. doi: 10.1002/j.1460-2075.1992.tb05238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gazit D, Karmish M, Holzman L, Bab I. Regenerating marrow induces systemic increase in osteo- and chondrogenesis. Endocrinology. 1990;126(5):2607–13. doi: 10.1210/endo-126-5-2607. [DOI] [PubMed] [Google Scholar]
- 53.Foldes J, Naparstek E, Statter M, Menczel J, Bab I. Osteogenic response to marrow aspiration: increased serum osteocalcin and alkaline phosphatase in human bone marrow donors. Journal of Bone & Mineral Research. 1989;4(4):643–6. doi: 10.1002/jbmr.5650040424. [DOI] [PubMed] [Google Scholar]
- 54.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 55.Raisz LG. Physiology and pathophysiology of bone remodeling.[erratum appears in Clin Chem 1999 Oct;45(10):1885] Clinical Chemistry. 1999;45(8 Pt 2):1353–8. [PubMed] [Google Scholar]
- 56.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):8932–7. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L, Liu Y, Kalajzic Z, Jiang X, Rowe DW. Heterogeneity of engrafted bone-lining cells after systemic and local transplantation. Blood. 2005;106(10):3650–7. doi: 10.1182/blood-2005-02-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leung TF, Hung ECW, Lam CWK, Li CK, Chu Y, Chik KW, Shing MMK, Lee V, Yuen PMP. Bone mineral density in children with thalassaemia major: determining factors and effects of bone marrow transplantation. Bone Marrow Transplant. 2005;36(4):331–336. doi: 10.1038/sj.bmt.1705053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.