Abstract
One reason for the lack of progress in the treatment of acute graft versus host disease (GVHD) is the lack of reliable biomarkers. GVHD of the gastrointestinal (GI) tract is closely associated with non-relapse mortality (NRM) following hematopoietic cell transplantation (HCT). Using an unbiased, large-scale, quantitative proteomic discovery approach, we identified candidate biomarkers that were increased in plasma from HCT patients with GI GVHD. We then validated the lead candidate, REG3α, by ELISA in samples from more than 1,000 HCT patients from three transplant centers. Plasma REG3α concentrations were 3-fold higher in patients at GI GVHD onset than in all other patients. REG3α concentrations correlated most closely with lower GI GVHD at GVHD onset and predicted response to therapy at 4 weeks, 1-year NRM, and 1-year survival (P≤0.001). Multivariate analysis showed that advanced clinical stage, severe histologic damage, and high REG3α concentrations at the diagnosis of GVHD independently predicted 1-year NRM, which progressively increased with higher numbers of onset risk factors present. We conclude that REG3α is a plasma biomarker of GI GVHD that can be combined with clinical stage and histologic grade to improve risk stratification of patients, perhaps providing a platform for advances in the treatment of high-risk GVHD.
Keywords: biomarker, gastrointestinal (GI), graft versus host disease (GVHD), hematopoietic cell transplantation (HCT), REG3α
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is one of the best curative modalities for patients with intermediate- and high-risk acute leukemia; approximately 3,500 patients receive allo-HCT for acute leukemia annually [1]. The efficacy of this therapy is limited by the development of acute graft-versus-host disease (GVHD), which is measured by dysfunction in three organ systems: the skin, liver and gastrointestinal (GI) tract [2,3]. Acute GVHD of the GI tract affects up to 60% of patients receiving allogeneic HCT [4,5], causing nausea, vomiting, anorexia, secretory diarrhea and, in more severe cases, severe abdominal pain and/or hemorrhage [6]. Acute GVHD is often clinically indistinguishable from other causes of GI dysfunction such as conditioning regimen toxicity, infection, or medication effect. Endoscopic biopsy is often used to confirm the diagnosis [7],7 but histologic severity on biopsy does not consistently correlate with clinical outcome [2,7,8]. Clinical stage two or greater (more than one liter of diarrhea per day) is associated with reduced survival [4,5], but daily stool volume can vary considerably. Lower GI GVHD responds poorly to treatment compared to other target organs [5], and treatment with high-dose systemic steroid therapy carries significant risks, especially infectious complications in profoundly immunosuppressed patients [9,10]. The standard treatment of acute GVHD is higher dose systemic steroids, which has not changed in 40 years. One reason for this lack of progress is the lack of validated biomarkers for acute GVHD. We have recently identified and validated regenerating islet-derived 3-alpha (REG3α), a C-type lectin secreted by Paneth cells [11,12], as a noninvasive, reliable blood biomarker specific for GVHD of the GI tract with diagnostic and prognostic utility that may provide a platform for novel advancements in the treatment of GVHD [13].
Discovery proteomics
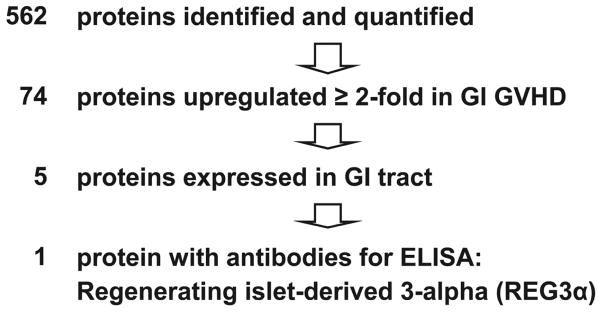
We used the Intact Protein Analysis System proteomics approach to identify candidate biomarkers in a discovery set of pooled plasma samples taken at similar times after HCT from 10 patients with biopsy-proven GI GVHD and 10 patients without GVHD as previously described [14,15]. We identified and quantified 562 proteins of which 74 were increased at least two-fold in patients with GVHD. Of the 5 preferentially expressed in the GI tract, commercially available antibodies suitable for quantification of plasma concentrations by ELISA were available for only 1 of these 5 proteins, thus identifying Regenerating Islet-Derived 3-Alpha as our lead candidate (Figure 1).
Figure 1. Proteomic workflow identifying REG3α as the lead candidate GI GVHD biomarker.
Plasma pooled from 10 patients who never developed GVHD was compared to plasma pooled from 10 patients at the onset of GI GVHD. Of the 562 proteins initially identified, REG3α was chosen as the lead biomarker to validate because it was increased twofold in patients at the onset of GI GVHD, it is preferentially expressed in the GI tract and antibodies suitable for ELISA were commercially available.
Validation studies
We evaluated REG3α plasma concentration as a biomarker of GI GVHD in samples from a large validation set of allogeneic HCT recipients from the University of Michigan. Plasma REG3α concentrations were 3 times higher in patients at the onset of GI GVHD than in all other patients, including those with non-GVHD enteritis (Figure 2A). There was no specific cause of non-GVHD enteritis associated with higher REG3α concentrations (data not shown). Serum REG3α concentrations were also higher in GI GVHD in an independent validation set of 143 HCT patients from Regensburg, Germany, and Kyushu, Japan, although the absolute values were lower (Figure 2B). This difference may be due to a center effect that depends on several factors, including variations in transplant conditioning regimens and supportive care. For example, all patients in Regensburg and Kyushu received oral antibiotics as GVHD prophylaxis, whereas Michigan patients did not and thus increased GI flora might account for greater REG3α secretion.
Figure 2. REG3α concentrations in plasma samples from HCT patients of two independent validation sets.
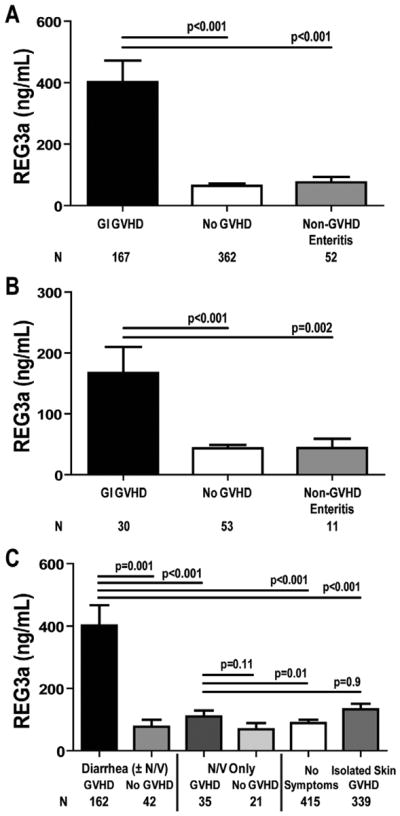
(A) University of Michigan patients (n=581) (B) Regensburg, Germany, and Kyushu, Japan (n= 94). (C) Patients from both validation sets classified by symptoms and etiology (n=1,014).
We combined the two independent validation sets and next analyzed REG3α concentrations according to diagnosis and type of GI symptom. In patients with diarrhea caused by GVHD, REG3α concentrations at the onset of GVHD were 5 times higher than in patients with diarrhea from other causes (Figure 2C). When we categorized patients by the volume of diarrhea, REG3α concentrations at the onset of symptoms continued to distinguish between GVHD and non-GVHD etiologies (P<0.001) but did not correlate with the clinical severity of GVHD.
Prognostic value of REG3α concentrations in patients with lower GI GVHD
The clinical utility of any biomarker increases if it provides prognostic information regarding the future status of a disease and/or patient, eg, the likelihood of response to treatment. We therefore evaluated the prognostic significance of REG3α plasma levels in more than 160 patients taken at the time of diagnosis of lower GI GVHD. REG3α concentrations were 3-fold higher at the time of GVHD diagnosis in patients who had no response to therapy at 4 weeks [16] than in patients who experienced a complete or partial response (635 ± 132 ng/mL vs. 240 ± 61 ng/mL; P<0.001); patients responding to therapy still exhibited REG3α concentrations more than 3 times that of non-GVHD controls (77 ± 22 ng/mL; P<0.001). Because the response to treatment at 4 weeks strongly correlates with nonrelapse mortality (NRM), we hypothesized that the REG3α concentration at GVHD diagnosis would also correlate with NRM. We therefore divided the patients into two equal groups based upon the median REG3α concentration: high (> 151ng/mL, n=81) and low (≤ 151 ng/mL, N=81). NRM was twice as high in patients with high REG3α concentrations, and this difference remained significant after adjusting for known risk factors of donor type, degree of HLA match, conditioning intensity, age, and baseline disease severity (59% [95% CI 48–69%] vs 34% [95% CI 24–46%], P<0.001).
All 4 data elements values for clinical stage, histologic grade, REG3α concentration, and serum albumin level were available in 140 patients. The plasma concentration of REG3α (above versus below the median), the clinical severity of GVHD (stage 1 versus stage 2–4), the histologic severity (stage 1–3 versus stage 4), and serum albumin level (above versus below the median) at GVHD diagnosis independently predicted both lack of response to GVHD therapy 4 weeks following treatment and 1-year NRM after adjustment for the aforementioned risk factors. When lack of response to therapy and NRM were modeled simultaneously on all four parameters, all except albumin remained statistically significant. The inclusion of all three characteristics that remained statistically significant on simultaneous modeling demonstrated that patients with increasing numbers of risk factors present at onset had increasing risk for NRM (Table 2; P<0.001).
Discussion
The etiology of diarrhea following HCT presents a common diagnostic dilemma [17]. We identified REG3α as a candidate biomarker specific for lower GI GVHD through an unbiased, in-depth tandem MS-based discovery approach that can quantify proteins at low concentrations and that we previously used successfully to identify elafin as a plasma biomarker specific for GVHD of the skin [15].
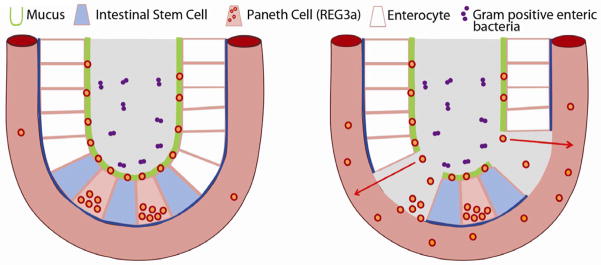
REG proteins act downstream of IL-22 to protect the epithelial barrier function of the intestinal mucosa [18] through the binding of bacterial peptidoglycans [11]. Intestinal stem cells (ISCs) are principal cellular targets of GVHD in the GI tract [2,19], where intestinal flora are critical for amplification of GVHD damage [20]. A leading hypothesis is that ISCs are protected by antibacterial proteins such as REG3α secreted by neighboring Paneth cells into the crypt microenvironment [21]. If death of an intestinal stem cell eventually manifests itself as denudation of the mucosa, the patchy nature of GVHD histologic damage may be explained as the lack of mucosal regeneration following the dropout of individual ISCs [2,19].
REG3α plasma concentrations correlate with disease activity in inflammatory bowel disease, and can distinguish infectious and autoimmune causes of diarrhea [12]. The correlation of mucosal denudation (histologic grade 4) with high REG3α concentrations suggests that microscopic breaches in the mucosal epithelial barrier caused by severe GVHD permit REG3α to traverse into the systemic circulation. The tight proximity of Paneth cells with ISCs concentrates their secretory contents in that vicinity, so that mucosal barrier disruption caused by stem cell dropout may preferentially allow Paneth cell secretions, including REG3α, to traverse into the bloodstream (Figure 3). We hypothesize that plasma levels of REG3α may therefore serve as a surrogate marker for the cumulative area of these breaches to GI mucosal barrier integrity, a parameter impossible to measure by individual tissue biopsies and current endoscopic technology. Such an estimate of total damage to the mucosal barrier may also help explain the prognostic value of REG3α with respect to therapy responsiveness and NRM.
Figure 3. Proposed mechanism for elevated systemic REG3α concentrations in lower GI GVHD.
At homeostasis, REG3α (red/orange dots) is concentrated in the mucous layer lining the luminal surface of the epithelium in intestinal crypts (left panel). In the setting of acute GVHD, apoptosis of enterocytes (white cells), stem cells (blue cells) and REG3α-producing Paneth cells (pink cells) are prominent. The intestinal barrier is breached and the mucous, including REG3α, readily enters systemic circulation (right panel).
In this study, 3 high-risk parameters each independently correlated with lack of response to treatment and to higher NRM: elevated plasma REG3α concentration, higher clinical stage of GVHD at diagnosis, and grade 4 histologic severity. All 3 of these values thus provided important prognostic information prior to the initiation of therapy rather than at the time of maximum grade of GVHD, which by definition includes responsiveness to therapy [4,5,9]. This study confirms earlier reports where higher clinical stage of GI GVHD [4,5] and more severe histology correlated with worse survival [8]. If the prognostic value of REG3α is confirmed in additional patients, we believe the integration of clinical stage, histologic grade, and REG3α plasma concentrations into a single grading system will permit better risk stratification and rapid identification of those patients with severe and extensive GI damage in whom standard treatment is likely to be insufficient.
Table 1.
One year NRM at the onset of Lower GI GVHD based upon number of high-risk factors present at onset (REG3α concentration, clinical stage, histologic severity.
P-values adjusted for donor source, HLA match, conditioning intensity, recipient age, and baseline disease severity according to the Center for International Blood and Marrow Transplant Research (CIBMTR) guidelines.
0 vs. 1 risk factor
1 vs. 2 risk factors
2 vs. 3 risk factors
Footnotes
Conflict of Interest Statement:
No relevant financial relationships with any commercial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR summary slides. 2011 [Google Scholar]
- 2.Mowat A, Socie G. Intestinal graft-vs-host disease. In: Ferrara JLM, Cooke KR, Deeg HJ, editors. Graft-vs-host disease. New York: Marcel Dekker; 2004. pp. 279–327. [Google Scholar]
- 3.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin PJ, McDonald GB, Sanders JE, Anasetti C, Appelbaum FR, Deeg HJ, et al. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10:320–327. doi: 10.1016/j.bbmt.2003.12.304. [DOI] [PubMed] [Google Scholar]
- 5.MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 6.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 7.Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group. Report Biol Blood Marrow Transplant. 2006;12:31–47. doi: 10.1016/j.bbmt.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Ertault-Daneshpouy M, Leboeuf C, Lemann M, Bouhidel F, Ades L, Gluckman E, et al. Pericapillary hemorrhage as criterion of severe human digestive graft-versus-host disease. Blood. 2004;103:4681–4684. doi: 10.1182/blood-2003-05-1548. [DOI] [PubMed] [Google Scholar]
- 9.Weisdorf D, Haake R, Blazar B, Miller W, McGlave P, Ramsay N, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75:1024–1030. [PubMed] [Google Scholar]
- 10.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109:4119–4126. doi: 10.1182/blood-2006-12-041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gironella M, Iovanna JL, Sans M, Gil F, Penalva M, Closa D, et al. Anti-inflammatory effects of pancreatitis associated protein in inflammatory bowel disease. Gut. 2005;54:1244–1253. doi: 10.1136/gut.2004.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrara JL, Harris AC, Greenson JK, Braun TM, Holler E, Teshima T, et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118:6702–6708. doi: 10.1182/blood-2011-08-375006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faca V, Pitteri SJ, Newcomb L, Glukhova V, Phanstiel D, Krasnoselsky A, et al. Contribution of protein fractionation to depth of analysis of the serum and plasma proteomes. J Proteome Res. 2007;6:3558–3565. doi: 10.1021/pr070233q. [DOI] [PubMed] [Google Scholar]
- 15.Paczesny S, Braun TM, Levine JE, Hogan J, Crawford J, Coffing B, et al. Elafin is a biomarker of graft-versus-host disease of the skin. Sci Transl Med. 2010;2:13ra2. doi: 10.1126/scitranslmed.3000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine JE, Logan B, Wu J, Alousi AM, Ho V, Bolanos-Meade J, et al. Graft-versus-host disease treatment: predictors of survival. Biol Blood Marrow Transplant. 2010;16:1693–1699. doi: 10.1016/j.bbmt.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox GJ, Matsui SM, Lo RS, Hinds M, Bowden RA, Hackman RC, et al. Etiology and outcome of diarrhea after marrow transplantation: a prospective study. Gastroenterology. 1994;107:1398–1407. doi: 10.1016/0016-5085(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 18.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 19.Takashima S, Kadowaki M, Aoyama K, Koyama M, Oshima T, Tomizuka K, et al. The Wnt agonist R-spondin1 regulates systemic graft-versus-host disease by protecting intestinal stem cells. J Exp Med. 2011;208:285–294. doi: 10.1084/jem.20101559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerbitz A, Schultz M, Wilke A, Linde HJ, Scholmerich J, Andreesen R, et al. Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood. 2004;103:4365–4367. doi: 10.1182/blood-2003-11-3769. [DOI] [PubMed] [Google Scholar]
- 21.Elphick DA, Mahida YR. Paneth cells: their role in innate immunity and inflammatory disease. Gut. 2005;54:1802–1809. doi: 10.1136/gut.2005.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]