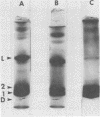
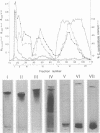
Abstract
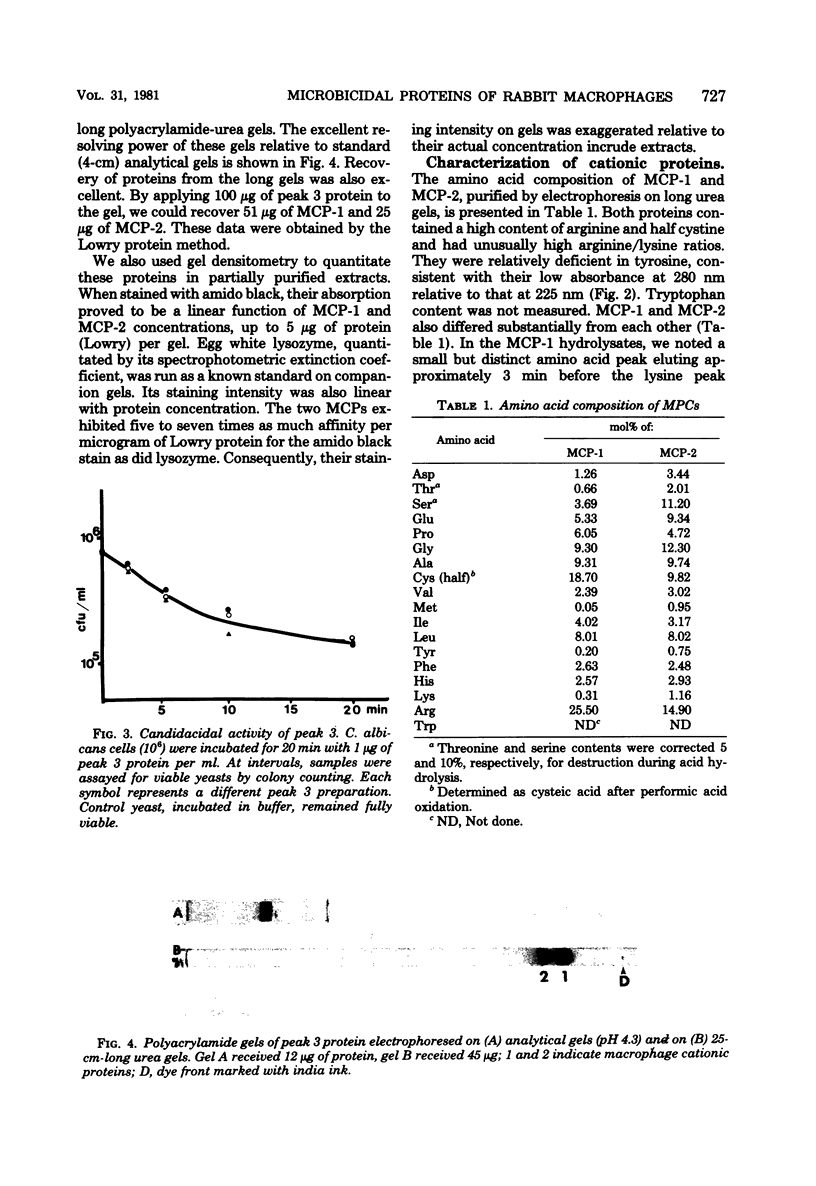
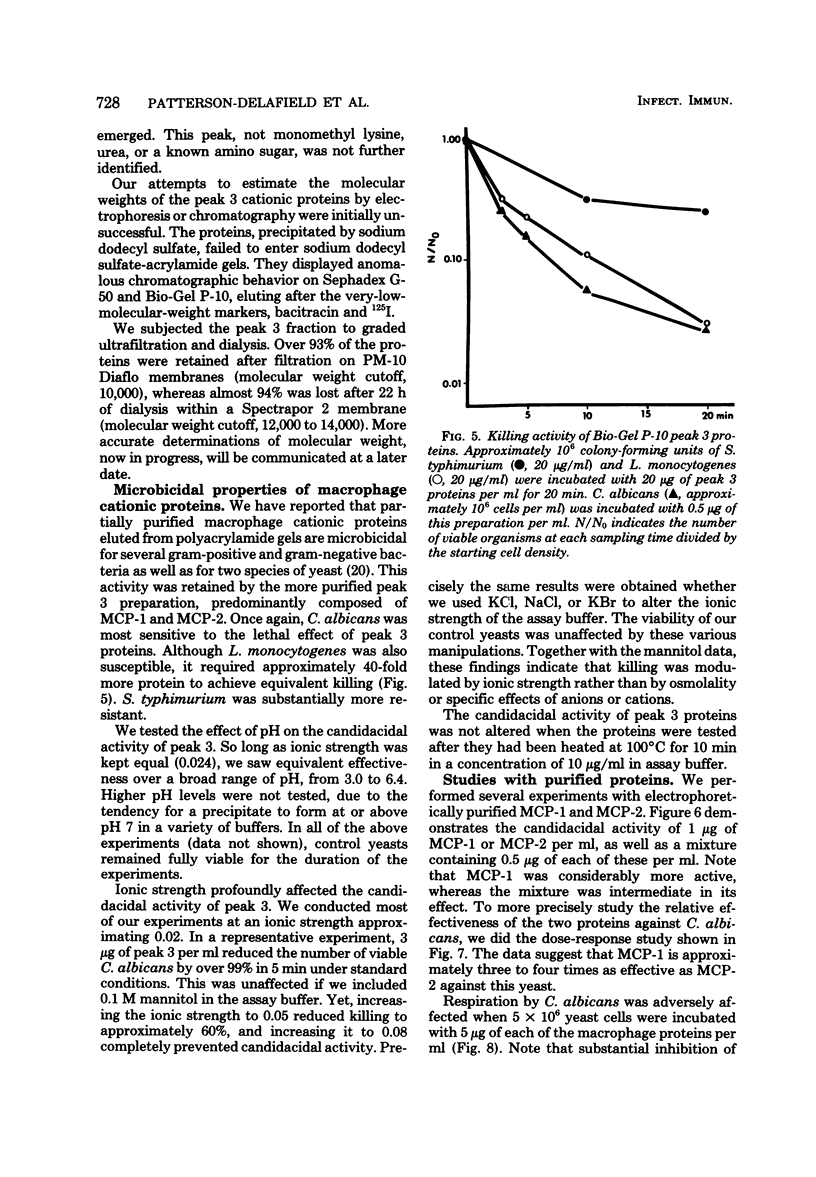
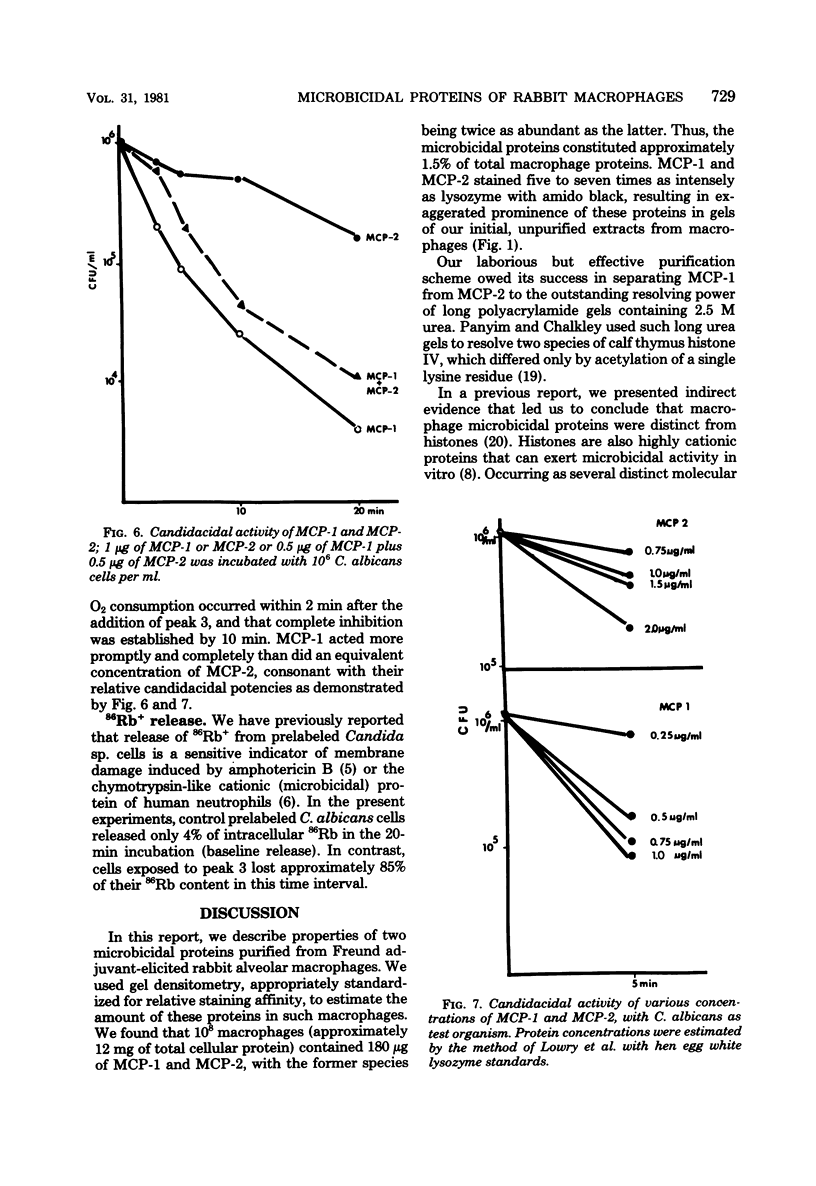
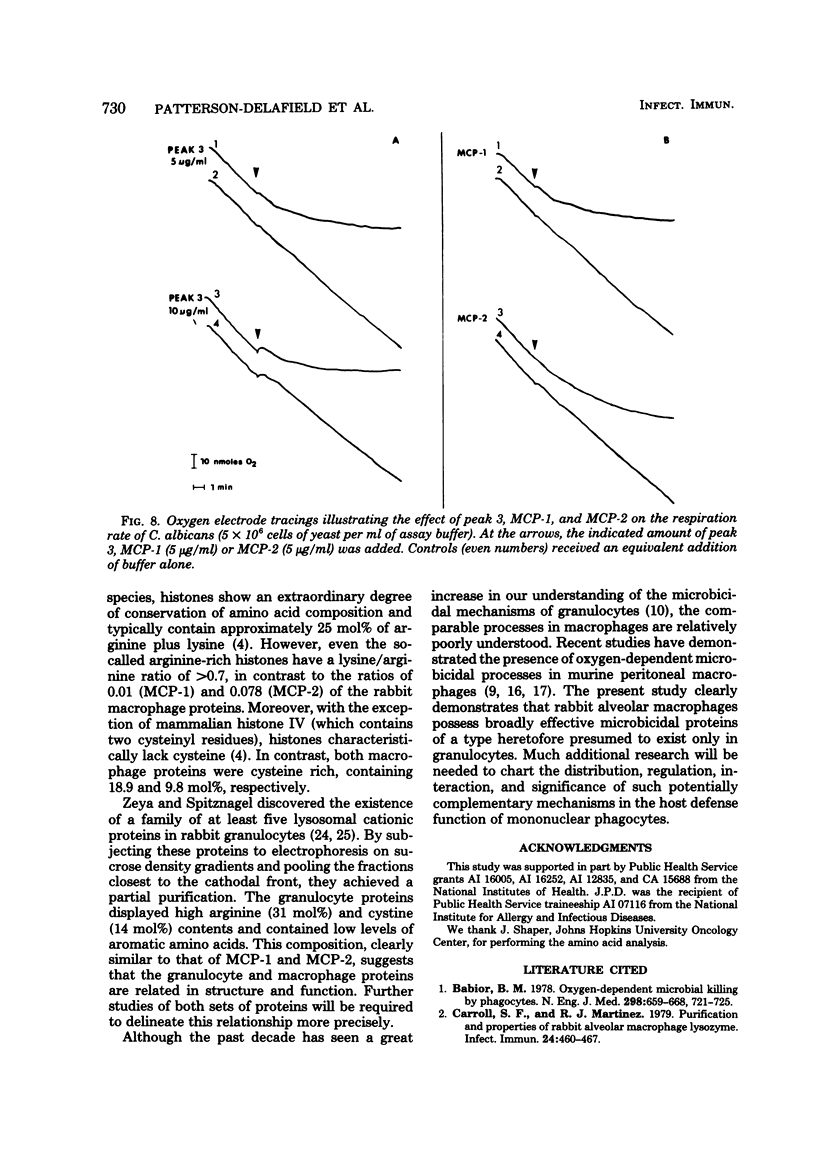
We purified two microbicidal cationic proteins, MCP-1 and MCP-2, from rabbit alveolar macrophages. MCP-1 was remarkably rich in arginine (25.5 mol%) and half cystine (18.7 mol%) residues and constituted approximately 1.5% of the total protein content of Freund adjuvant-elicited alveolar macrophages. MCP-2 was approximately half as abundant as MCP-1 and contained relatively less arginine (14.9 mol%) and half cystine (9.8 mol%). The amino acid compositions of MCP-1 and MCP-2 resembled those reported for the lysosomal cationic proteins of rabbit granulocytes, but were distinct from those of any known histone. MCP-1 (1 microgram/ml) killed 99.6% of Candida albicans in 20 min, whereas MCP-2 killed approximately 80% under similar conditions. Both proteins rapidly suppressed O2 consumption by C. albicans and induced a rapid loss of intracellular 86Rb+. Although more information is needed about the biological origin, distribution, and roles of macrophage microbicidal proteins, it seems likely that MCP-1 and MCP-2 contribute to the microbicidal efficacy of rabbit alveolar macrophages.
Full text
PDF

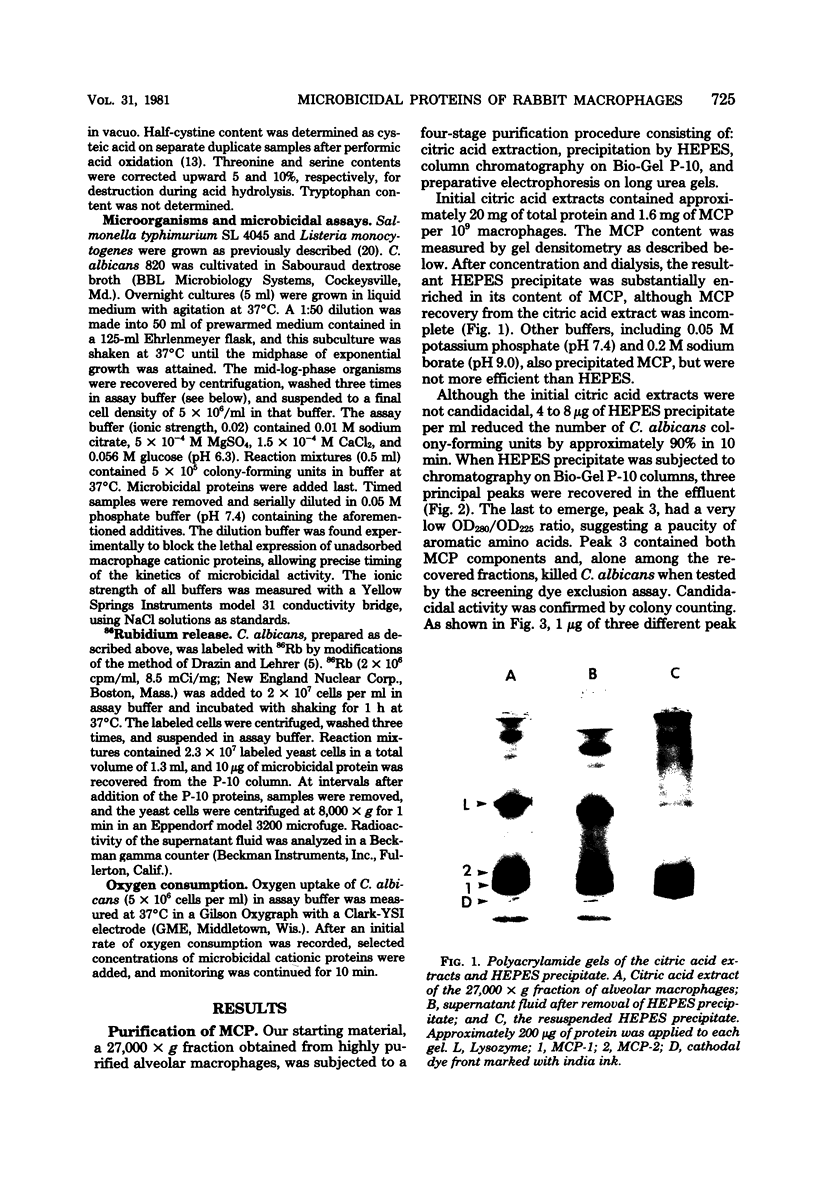
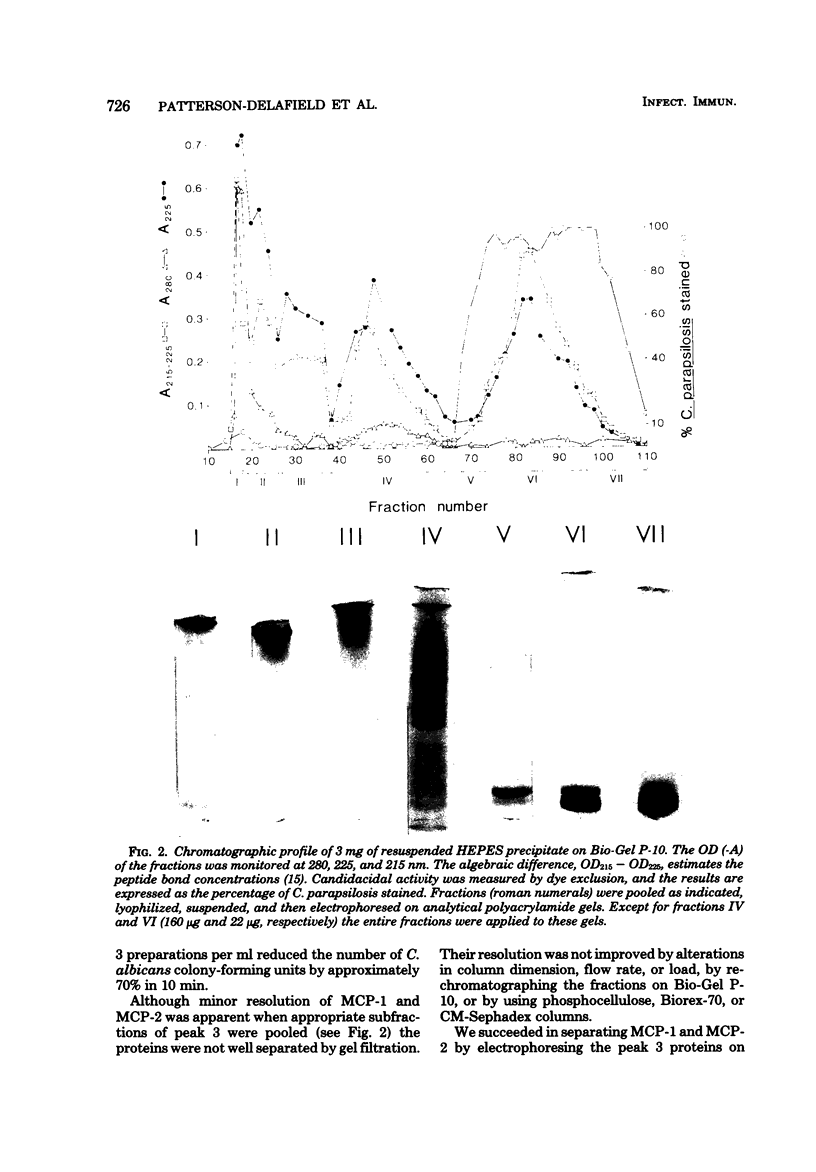

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Carroll S. F., Martinez R. J. Purification and properties of rabbit alveolar macrophage lysozyme. Infect Immun. 1979 May;24(2):460–467. doi: 10.1128/iai.24.2.460-467.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Smith E. L. Histones: structure and function. Annu Rev Biochem. 1971;40:279–314. doi: 10.1146/annurev.bi.40.070171.001431. [DOI] [PubMed] [Google Scholar]
- Drazin R. E., Lehrer R. I. Fungicidal properties of a chymotrypsin-like cationic protein from human neutrophils: adsorption to Candida parapsilosis. Infect Immun. 1977 Aug;17(2):382–388. doi: 10.1128/iai.17.2.382-388.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazin R. E., Lehrer R. I. Rubidium release: a rapid and sensitive assay for amphotericin B. J Infect Dis. 1976 Sep;134(3):238–244. doi: 10.1093/infdis/134.3.238. [DOI] [PubMed] [Google Scholar]
- Fleming A. Lysozyme: President's Address. Proc R Soc Med. 1932 Dec;26(2):71–84. doi: 10.1177/003591573202600201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G. Bactericidal action of histone. J Exp Med. 1958 Dec 1;108(6):925–944. doi: 10.1084/jem.108.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr Oxygen metabolism and the microbicidal activity of macrophages. Fed Proc. 1978 Nov;37(13):2759–2764. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehrer R. I., Ladra K. M., Hake R. B. Nonoxidative fungicidal mechanisms of mammalian granulocytes: demonstration of components with candidacidal activity in human, rabbit, and guinea pig leukocytes. Infect Immun. 1975 Jun;11(6):1226–1234. doi: 10.1128/iai.11.6.1226-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Juangbhanich C. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Root R. K. Hydrogen peroxide release from mouse peritoneal macrophages: dependence on sequential activation and triggering. J Exp Med. 1977 Dec 1;146(6):1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Antibacterial activity of cationic proteins from human granulocytes. J Clin Invest. 1975 Nov;56(5):1118–1124. doi: 10.1172/JCI108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. The heterogeneity of histones. I. A quantitative analysis of calf histones in very long polyacrylamide gels. Biochemistry. 1969 Oct;8(10):3972–3979. doi: 10.1021/bi00838a013. [DOI] [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez R. J., Lehrer R. I. Microbicidal cationic proteins in rabbit alveolar macrophages: a potential host defense mechanism. Infect Immun. 1980 Oct;30(1):180–192. doi: 10.1128/iai.30.1.180-192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Reisner A. H., Nemes P., Bucholtz C. The use of Coomassie Brilliant Blue G250 perchloric acid solution for staining in electrophoresis and isoelectric focusing on polyacrylamide gels. Anal Biochem. 1975 Apr;64(2):509–516. doi: 10.1016/0003-2697(75)90461-3. [DOI] [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978 Apr 25;253(8):2664–2672. [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic protein-bearing granules of polymorphonuclear leukocytes: separation from enzyme-rich granules. Science. 1969 Mar 7;163(3871):1069–1071. doi: 10.1126/science.163.3871.1069. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. I. Resolution of antibacterial and enzymatic activities. J Bacteriol. 1966 Feb;91(2):750–754. doi: 10.1128/jb.91.2.750-754.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]