Abstract
Recent data reports abiraterone acetate, a specific inhibitor of CYP17 that is key to androgen and estrogen synthesis, improves survival in metastatic castration-resistant prostate cancer (CRPC), confirming the continued dependency of CRPC on the androgen receptor (AR) signaling pathway. MDV3100 is a novel antagonist of AR that is also in Phase III clinical trials. In addition, several other agents targeting the AR axis are undergoing evaluation in early clinical studies. CRPC patients progress on these therapies with a rising PSA, suggesting that repeated therapeutic interventions targeting the AR signaling axis could induce secondary responses and achieve prolonged clinical benefit for a sub-group of patients. These exciting results are good news for patients but introduce a number of treatment paradigm dilemmas for physicians. Clinical studies evaluating the ideal sequence of administration of these new agents, best timing for initiation, combination strategies, discontinuation beyond progression and after commencement of subsequent therapies and coordination with other treatments have not been performed. Predictive biomarkers could allow patient selection for a specific treatment but in their absence most physicians will rely on a trial of treatment with a preferred agent and substitute for an alternative therapy on objective progression. Current data suggests that the response rate to drugs targeting the AR ligand-binding domain decreases with each treatment but we hypothesize that a significant proportion of CRPC remains dependent on the AR axis and therefore novel strategies for disrupting AR signaling merit evaluation.
The mainstay of first-line treatment for patients with metastatic prostate cancer is suppression of gonadal androgens by medical or surgical castration, a strategy that was described seven decades ago by Charles Huggins and colleagues (1). This original observation that prostate cancer is a hormone-dependent cancer remains critically important, especially after recent reports of significant anti-tumor activity with the novel endocrine treatments abiraterone acetate and MDV3100 in castration-resistant prostate cancer (CRPC) patients progressing after multiple prior hormonal manipulations including estrogens, steroids, anti-androgens and the non-specific CYP inhibitor ketoconazole (2-5). Significant anti-tumor activity was also reported in patients previously treated with docetaxel or other chemotherapies (2, 6). The primary end-point utilized to evaluate anti-tumor activity in early clinical studies of abiraterone acetate and MDV3100 was a decline in prostate specific antigen (PSA). Notably, declines in PSA were associated with declines in circulating tumor cell (CTC) count, symptomatic improvements, radiological regression and in the Phase I/II study of MDV3100, inhibition of 18F-fluoro-5alpha-dihydrotestosterone on PET imaging (2, 4-7). These data led to the conduct of pivotal Phase III studies of abiraterone acetate and MDV3100 in both chemotherapy-naïve and chemotherapy-treated CRPC patients; the post-chemotherapy study of abiraterone acetate was recently reported and confirmed that targeting of the androgen receptor (AR) is a valid therapeutic strategy in CRPC imparting overall survival (OS) benefit in advanced prostate cancer. Expression of PSA is predominantly regulated by upstream promoter and enhancer androgen response elements (ARE) (8). A rising PSA implies transcription of genes regulated by an ARE and arguably suggests activation of the AR or other steroid receptor signaling pathways (9). This is an important hypothesis as a rise in PSA appears to be associated with cancer progression in the majority of patients receiving treatment with abiraterone acetate as well as MDV3100, suggesting that the biological mechanisms causing treatment resistance are associated with reactivation of the AR. We therefore hypothesize that targeting of the AR through multiple approaches, for example MDV3100 and abiraterone acetate in combination or sequentially, could improve patient outcome. This review will outline how these results could change the treatment paradigms for CRPC and discuss the challenges now faced in the development of novel therapeutics for this disease.
Suppression of androgens and estrogens that bind the androgen receptor
Gonadal androgens account for up to 80% of serum androgenic steroids (10). Castration therefore does not suppress adrenal androgens and achieves a “hormone-reduced” rather than a “hormone-free” state, hence the recent renaming of this stage of the disease as castration-resistant in preference to hormone-refractory. CRPC cells undergo a number of genomic and expression changes involving the AR and its associated co-activators and co-repressors that could allow continued activation of the AR signaling axis by castrate levels of androgens (11). Moreover, intra-tumoral hormone synthesis associated with over-expression of key enzymes including CYP17 could cause resistance to castration (12-14). Although the latter remains a very challenging phenomenon to unequivocally prove, the body of circumstantial evidence for suggesting tumors synthesize their own androgens is compelling and introduces the interesting possibility of therapeutically directly targeting tumor hormone synthesis. In 2005 we hypothesized that continuous, specific inhibition of CYP17, a key enzyme in androgen and estrogen biosynthesis, could induce secondary responses in progressing CRPC patients (10). Ketoconazole, a non-specific CYP inhibitor that weakly inhibits CYP17 at high doses and has definite anti-tumor activity in CRPC was routinely used in a number of academic centres to treat CRPC as an off-license indication (15). However, the significant toxicities in up to two-thirds of patients limit its widespread use and prevent escalation to doses that irreversibly inhibit CYP17. In fact, resistance to ketoconazole was associated with rebound increases in circulating androgens (15). A number of specific CYP17 inhibitors that could test our hypothesis had been developed: abiraterone acetate had been developed by chemists in our institution a decade earlier (10, 16) but due to concerns regarding drug safety, and an absence of interest in targeting AR signaling, continuous administration was only tested for a maximum of twelve days in non-castrate men (17). We hypothesized that CYP17 blockade would not result in adrenal insufficiency and would have important anti-tumor activity in CRPC. With renewed support from Cougar Biotechnology, we designed the first clinical studies to confirm the safety and anti-tumor activity of continuous, daily, single-agent abiraterone acetate (without concurrent steroids) in chemotherapy-naïve patients (3, 4). The latter patient population was not dependent on steroids to maintain their fitness and as they generally had a better performance status, we hypothesized that they could tolerate the predicted toxicities of secondary mineralocorticoid excess. In keeping with reports of teenagers with familial CYP17 deficiency who present with delayed puberty and are found to be hypertensive (18), single-agent abiraterone acetate was not associated with adrenocortical insufficiency as a result of a compensatory rise in ACTH that drives up levels of the weak glucocorticoids deoxycorticosterone and corticosterone ten to forty-fold, thus maintaining the glucocorticoid requirements of patients (4). However, the mineralocorticoid properties of steroids upstream of CYP17 caused side-effects in two-thirds of patients characterized by hypokalaemia, hypertension and fluid overload (3, 4). As spironolactone was reported to bind and activate wild-type AR, the more specific mineralocorticoid receptor antagonist eplerenone (that was previously shown not to bind wild-type AR) was used to treat these toxicities (19). With prompt and careful use of eplerenone (commencing at 50 mg and dose escalating to 200mg daily), exogenous glucocorticoids were only required to control side-effects associated with mineralocorticoid excess in a minority of patients (20). However, due to the risks associated with hypokalaemia, especially in older men with concurrent heart disease and taking anti-arrhythmic medication, regular monitoring of serum electrolytes and blood pressure is required until the commencement of a mineralocorticoid antagonist or glucocortocoid and may limit the administration of single-agent abiraterone acetate by non-specialist centres.
In Phase I and II clinical studies of abiraterone acetate, 50-60% of chemotherapy-naïve patients had a decline in PSA by ≥50% and the median time to PSA progression (as defined by PSAWG I (21)) was about 230 days (3, 5). Importantly, 20-30% of patients had a ≥90% PSA decline that was associated with a patient sub-group that had near complete radiological responses, normalization of CTC count and PSA progression free survival lasting longer than one year. Anti-tumor activity was reported at all doses from 250mg to 2000mg daily but 1000mg once daily was selected for Phase II development due to a plateau in the feedback-driven increase of steroids upstream of CYP17 at 750mg, 1000mg and 2000mg daily (4). Addition of dexamethasone or prednisone to patients on single-agent abiraterone acetate significantly extends the time on treatment and could also re-induce sensitivity (defined in our study as a decline in PSA ≥50% after commencing steroids) in 25% of patients irrespective of prior treatment with steroids (4, 5). The improved tolerability and efficacy of abiraterone acetate when administered in combination with low dose steroids, that prevent a compensatory ACTH rise, have led to its development in metastatic CRPC in combination with prednisone. We initiated a study of single-agent abiraterone acetate in post-docetaxel patients, confirming single-agent anti-tumor activity in this setting, but due to the long-term prior use of low dose steroids by the majority of these patients prior to receiving abiraterone, we allowed continuation of steroids from the start of study in about half of patients to maintain their general fitness (6). Two separate Phase II studies reported significant anti-tumor activity in chemotherapy-treated patients, with a time to PSA progression of about 170 days, suggesting that docetaxel-treated CRPC remained hormone-dependent (6, 7). Although the rate of PSA decline ≥50% and time to PSA progression is less than in chemotherapy-naïve patients, direct comparisons are not possible due to the significant heterogeneity between the two patient populations accrued to these studies.
These data led to the conduct of two pivotal Phase III trials in metastatic CRPC. Abiraterone acetate has been combined with prednisone 10mg daily (prednisolone 10mg daily in the UK) to minimize toxicity and maximize efficacy. The first study, which was reported recently (22), accrued 1197 CRPC docetaxel-pretreated CRPC patients randomized 2:1 to receive abiraterone acetate and prednisone. As mitoxantrone is not universally used and has not been reported to improve median survival, the control arm utilized prednisone (and placebo). Accrual was initiated in April 2008 and completed in July 2009. Although significant anti-tumor activity has been reported in ketoconazole-treated patients (a significant number of whom would have stopped treatment due to toxicity rather than resistance) (3, 7), the data on cross-resistance between ketoconazole and abiraterone acetate is confounding and prior treatment with ketoconazole was therefore an exclusion criterion. Fifteen percent of patients had received two prior lines of chemotherapy and the median OS of the placebo and prednisone arm was 10.9 months. The median survival of patients treated with abiraterone acetate and prednisone was 14.8 months (HR=0.646 (0.54-0.77), P < 0.0001) (22). Abiraterone acetate in combination with prednisone has also been evaluated in a randomized, placebo-controlled, double-blind Phase III study in metastatic chemotherapy-naïve CRPC patients (NCT00887198) (Table 1). The primary end-points are OS and progression-free survival. Based on the Phase II data, one would expect abiraterone acetate and prednisone to have equivalent or greater efficacy in the pre-docetaxel setting and due to its better tolerability when compared to taxanes, abiraterone acetate may be increasingly used prior to chemotherapy (Table 2). In patients who are asymptomatic from their metastatic prostate cancer, the Cushingoid side-effects of long-term ACTH suppression by prednisone 10mg daily may become problematic. The combination of abiraterone acetate with alternative oral steroid dosing regimens or mineralcocorticoid receptor antagonists merits further evaluation in this patient population.
Table 1.
Agents targeting AR in clinical development for metastatic CRPC
| Mechanism of action |
Drug | Patient population | Phase of development |
Clinicaltrials.gov registration number |
|---|---|---|---|---|
| Rationally designed specific CYP 17 inhibitors |
Abiraterone acetate |
chemotherapy treated | Phase III – reported at ESMO annual meeting 2010 (19) |
NCT00638690 |
| chemotherapy-naive | Phase III | NCT00887198 | ||
| Orteronel (TAK- 700) |
chemotherapy treated | Phase III | NCT01193257 | |
| chemotherapy-naive | Phase III | NCT01193244 | ||
| Novel AR antagonists |
MDV-3100 | chemotherapy treated | Phase III | NCT00974311 |
| chemotherapy-naive | Phase III | NCT01212991 | ||
| AZD3514 | - | Phase I/II | NCT01162395 | |
| ARN-509 | - | Phase I/II | NCT01171898 | |
| Dual CYP 17 inhibitors and AR antagonist |
TOK-001 | - | Phase I/II | NCT00959959 |
Table 2.
Predicted castration-resistant prostate cancer treatment dilemmas for physicians in 2012
| Treatment dilemma | Possible answer |
|---|---|
| • What is the frequency of cross-resistance between abiraterone acetate and MDV3100 and is there a benefit in sequential use? |
• Requires formal evaluation in post-regulatory studies. |
| • How does one decide whether to use abiraterone acetate or MDV3100 first? | • Biomarkers that identify resistance to specific agents are required. Sequence will probably be initially defined by local guidelines. |
| • Are abiraterone acetate or MDV3100 best used before docetaxel and is there a patient sub-group that should be offered chemotherapy before either agent? |
• Better toxicity profile of hormonal treatments may lead to their use before chemotherapy. |
| • How does one select patients for cabazitaxel in preference to abiraterone acetate or MDV3100 after treatment with docetaxel? |
• Better toxicity profile of hormonal treatments may lead to their use before cabazitaxel although most patients will be expected to received all approved treatments through the course of management of their disease. |
| • How does one co-ordinate treatment with Sipuleucel-T with initiation of treatment with abiraterone acetate or MDV3100? |
• No data available. |
| • Should abiraterone acetate be continued or stopped at disease progression? | • No data available. |
The significant anti-tumor activity reported with abiraterone acetate has led to the clinical development of other CYP17 inhibitors (Table 1). Due to the postulated similarity in the CYP17 domain that catalyses the C17,20-lyase and 17α-hdyroxylase functions of this enzyme, therapeutics with hundred-fold specificity for the C17,20-lyase activity have not yet been reported. It is therefore possible that due to intra- and inter-patient pharmacokinetic variation, it may not be feasible to irreversibly inhibit C17,20-lyase whilst avoiding any inhibition of cortisol synthesis. However, novel CYP17 inhibitors with different properties may have slightly different clinical benefits. For example, TOK-001 that was originally identified in a drug screen at the University of Maryland to identify compounds that are dual CYP17 inhibitors and AR antagonists (23) is in Phase I/II development (NCT00959959). Also, a placebo-controlled, randomized Phase III study (primary endpoint: OS) of orteronel (TAK-700), another specific CYP17 inhibitor, in combination with prednisone recently commenced accrual of chemotherapy-treated CRPC patients (NCT01193257).
Persistence of ligands that could activate a promiscuous AR in abiraterone-treated patients
Studies to date suggest that there is no rise in serum androgens at progression on abiraterone acetate (4, 5), although comprehensive evaluation of androgen levels in tumors before treatment and after progression is ongoing. Although resistance does not appear to be a result of pharmacological failure, tumoral changes in CYP17 expression could overcome drug effect. Androgens are the most effective agonists of wild-type AR signaling but point mutations, increased expression of the AR and alterations in the AR-coactivator-repressor complex, occur with increasing frequency in patients after sequential hormone treatments and allow activation of the AR in preclinical models by alternative ligands, such as deoxycorticosterone, corticosterone and cortisol (Figure 1) (24-26). Mutations of AR could be clonally selected for by sequential hormonal treatments conferring a survival advantage on cells with a promiscuous AR, with mutations that allow activation by non-suppressed ligands becoming increasingly prevalent in advanced disease. Current treatment strategies may therefore fail to achieve in vivo a tumor environment that is entirely free of potential AR ligands.
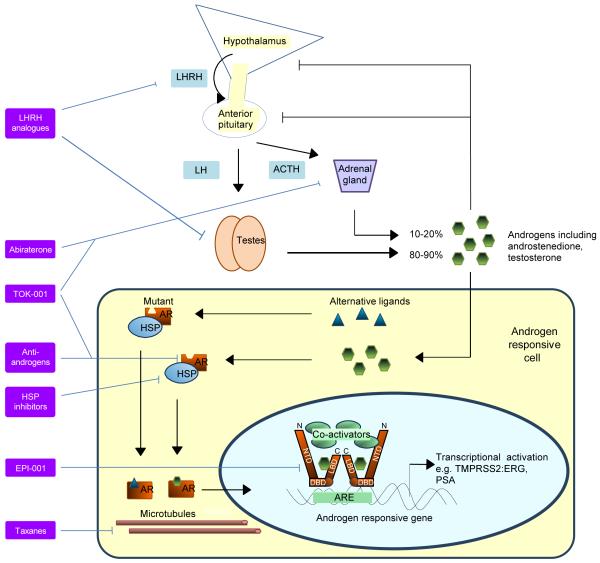
Figure 1. Strategies for therapeutically targeting the androgen receptor (AR).
The hypothalamus-pituitary-gonadal axis controls androgen synthesis as part of a negative feedback loop. Luteinising hormone (LH) is released from the pituitary following stimulation by luteinizing hormone releasing hormone (LHRH) and stimulates testicular androgen production while adrenocorticotropic hormone (ACTH) secreted by the anterior pituitary stimulates the adrenal production. Androgens or alternative ligands bind to wild-type or mutant AR, causing dissociation from heat shock proteins (HSP) and translocation of the AR to the nucleus. The AR binds to androgen response elements on androgen responsive genes including TMPRSS2:ERG and PSA and coregulatory proteins are recruited for transcriptional activation. The 3 main structural components of the AR, the N-terminal domain (NTD), DNA-binding domain (DBD) and the ligand-binding domain (LBD) are shown separately. Strategies with marketing approval or in late stages of clinical development involve targeting of the LBD or androgens; other potential ligands exist. Microtubules may also be essential to AR function and could be disrupted with taxanes. More novel strategies involve targeting of the NTD or chaperone proteins.
Small molecule antagonists of the androgen receptor ligand-binding domain (LBD)
Non-steroidal AR antagonists (most commonly bicalutamide, nilutamide or flutamide) have been standard treatment for advanced prostate cancer for three decades (Figure 1). Several studies have investigated combination of anti-androgens with castration and a meta-analysis of randomized studies suggested a modest survival benefit (27). Also, the duration of response to these anti-androgens is often less than 4 months; their AR binding is reversible and paradoxical agonism of the AR occurs in 10 - 15% of patients (15). The Sawyers lab therefore established a drug discovery programme to screen for novel small molecules that inhibited PSA transcription in a bicalutamide-resistant model of LNCaP cells engineered to over-express wild-type AR (28, 29). The screen started off with a chemical structure known to have exceedingly high affinity for the AR and through iterative structural changes, they identified a number of candidates that potently inhibited PSA in cells over-expressing AR (28). Moreover, this series of candidates also inhibited structurally promiscuous AR (for example with the W751C point mutation) that bicalutamide was agonistic to (29). One of the clinical leads (MDV3100) was licensed to Medivation and underwent evaluation in a 140-patient Phase I/II study conducted by the US Prostate Cancer Clinical Trials Consortium (2). In this study, treatment with MDV3100 at doses ≥60mg resulted in PSA declines ≥50% in 50-60% of chemotherapy-naïve or docetaxel-treated CRPC patients most of whom had previously progressed on treatment with an anti-androgen and multiple other lines of hormone treatments (2). This was associated with other objective end-points of anti-tumor activity, including declines in CTC counts and radiological regression; the median time to PSA progression in these chemotherapy-pretreated patients was 189 days (2). Declines in PSA ≥90% were also reported in 10-30% of patients. Epileptic seizures and severe fatigue were reported at doses ≥340mg that may be a class effect of anti-androgens which cross the blood-brain barrier (30). Nonetheless the significant anti-tumor activity and absence of serious adverse events at doses <240mg led to the initiation of a double-blind, placebo controlled, randomized Phase III study in docetaxel-treated patients of MDV3100 160mg/day in combination with prednisone (NCT00974311). The primary end-point is OS. Comparing different clinical studies is fraught with caveats but the data suggest that the declines in PSA and CTC count, radiological responses, duration of response and time on treatment in Phase I/II studies of MDV3100 are similar to studies with abiraterone acetate. Moreover, MDV3100 is also undergoing evaluation in a large, placebo controlled study in chemotherapy-naïve patients (NCT01212991). Predictably, these data have led to significant investment in the development of novel AR antagonists and several are now undergoing evaluation in early clinical studies, including another clinical candidate from the same screening programme as MDV3100 (ARN-509) (Table 1). A multitude of novel AR antagonists with different pharmacological and pharmacodynamic properties achieving regulatory approval may translate into significant benefits for our patients, with the possibility of sequential, albeit potentially less frequent and of shorter duration, secondary responses. However, in the absence of head-to-head studies, physicians are going to be unable to select the best sequence and type of agent to use.
New treatment paradigms for metastatic prostate cancer
Castration-resistant prostate cancer
The current Phase III studies have been designed to confirm the efficacy of abiraterone acetate and MDV3100 but no attempt has yet been made to develop an evidence-based paradigm for the best treatment schedule. Moreover, the recent FDA approval for metastatic CRPC of Sipuleucel-T (chemotherapy-naïve and treated) and cabazitaxel (docetaxel-treated patients only) based on significant improvements in OS in randomized Phase III studies (31, 32), introduces a number of treatment dilemmas for physicians if both abiraterone acetate and MDV3100 achieve regulatory approval in the pre- and post-chemotherapy space (Table 2). It is possible that most physicians and patients would prefer to use abiraterone acetate or MDV3100 prior to docetaxel due to a perceived better tolerability, although the results of Phase III studies in this setting will ultimately inform on this decision. In the absence of combination or sequential data from randomized studies of abiraterone acetate and MDV3100, most physicians will probably use both agents sequentially with personal preference or local guidelines dictating the order of treatment. A proportion of patients who progress on these treatments with a rise in PSA are likely to benefit from further hormonal manipulations with agents such as estrogens or novel AR targeting therapeutics (multiple novel agents are anticipated to be in clinical trials over the next few years (Table 1)), although it is probable that the response rate will decrease due to cross-resistance. Moreover, the survival benefit from docetaxel or cabazitaxel after treatment with abiraterone acetate or MDV3100 will remain unknown in the absence of the appropriate studies although this may be impacted if the mechanism of action of taxanes are related to their effects on AR signaling (Figure 1) (33). Another critical challenge for physicians that currently occurs when PSA rises is when to discontinue or change treatments (Table 2). Patients may continue to derive benefit from ongoing maximal inhibition of AR as they do from continuous castration and studies are urgently required to evaluate the benefit of continuing treatment with drugs such as abiraterone acetate or MDV3100 beyond progression, including for example after initiation of taxane chemotherapy.
Hormone-naïve prostate cancer and other settings
No clinical studies have yet evaluated the benefit of combining a novel hormonal treatment with castration for hormone-naïve patients. However, it is possible that the improved inhibition of AR will translate into a greater benefit than was observed with flutamide and nilutamide (27). Such Phase III trials are now being planned that will address long-term safety concerns in addition to improved efficacy. Novel hormonal agents may also be critically important in the adjuvant setting to improve the outcome in the setting of high risk locally advanced disease.
Future strategies
Intermediate end-points of treatment effect
PSA is a useful pharmacodynamic biomarker of AR signaling but changes in PSA are not approved by the regulatory authorities as an intermediate endpoint (surrogate) of OS in clinical studies. Although PSA is routinely used in patient practice to identify failure of treatment effect, cases where PSA change is discordant from other end-points of anti-tumor activity are well-described. In particular, drugs targeting AR signaling may have a significant effect on PSA transcription but have minimal cytotoxic effect on resistant tumors. We and others have reported a significant association between CTC count and OS (34-36) and the recently reported abiraterone acetate Phase III study included as a co-primary end-point the evaluation of CTC count as an intermediate endpoint of survival. CTC enumeration has also been included in a number of other Phase III studies and this will allow evaluation of CTC count as an intermediate endpoint using a meta-analysis of several studies of effective agents. The studies to date utilized CellSearch™ for CTC enumeration: this is the only platform with FDA clearance and it is robust with minimal inter-operator variability and uses a well-established protocol for CTC identification. If multiple, prospective, randomized studies confirm a significant association between CTC count and treatment effect, CTC enumeration may become increasingly utilized to inform on early treatment discontinuation.
Utilizing predictive biomarkers to identify patient sub-groups enriched for endocrine sensitive disease
We reported an increased prevalence of patients with hormone-regulated ERG gene rearrangements in the sub-population who had ≥90% PSA declines with abiraterone acetate (37). However, a significant number of patients with an underlying hormone-regulated gene fusion were resistant (37). Moreover, the majority of prostate cancer patients respond by PSA measurements to first-line hormone treatment, suggesting the underlying biology of treatment-naïve disease may be hormone driven (although even in the first-line setting) a fall in PSA does not necessarily equate to anti-tumor activity. There appear to be mechanisms of cross-resistance between different treatments as the response rate to second-line and subsequent hormonal manipulations declines. However, resistance to one treatment may not necessarily denote resistance to other treatments. Predictive biomarkers of resistance will therefore allow patient selection for a specific treatment based on an understanding of the underlying biology, rather than a trial of treatment. As CRPC tissue is often impossible to sequentially acquire, we have utilized CTC to molecularly characterize CRPC (37). Genomic evaluation of loss of PTEN and gain of AR in these studies in a limited number of patients failed to identify an association with response to abiraterone acetate possibly in part due to intrapatient heterogeneity. Other groups have sequenced DNA from CTC for commonly occurring mutations of the AR: these analyses are now required in the context of clinical studies (38). CTC are not reliably identified in all patients and CTC isolation can be costly and time-consuming. The isolation and study of nucleic acids in plasma could therefore be an alternative strategy for characterization of patients using a blood sample (39).
Disrupting the androgen receptor transcription complex
Targeting of chaperones such as HSP90 that include key oncogenes such as HER2 (erbB2) as client proteins is a therapeutic strategy that has been undergoing evaluation in several tumor types for close to a decade. Steroid receptors exist in complexes that include co-activator and co-repressor proteins and chaperones. The understanding of the role of different members of this complex is incomplete. Some studies suggest HSP90 is predominantly cytoplasmic and as activated AR in progressing prostate cancer is predominantly nuclear, it has been proposed that other chaperones, such as HSP27, may be better therapeutic targets (40, 41). We and others have tested several HSP90 inhibitors in early clinical studies and with limited anti-tumor activity reported to date in CRPC, although we have reported a durable response lasting more than a year in a patient treated with 17DMAG (42, 43). It is unclear whether this limited anti-tumor activity is due to poor drug pharmacology, incomplete or transient target inhibition, continued co-existent ligand activation of AR or significant redundancy of chaperone proteins. As HSP27 is not ATP dependent, no specific small molecule inhibitors have been developed to date but an HSP27-targeting locked antisense (OGX-427) in combination with prednisone is currently undergoing evaluation in a randomized Phase II study in CRPC (NCT01120470) (Figure 1). Another strategy that could be employed for disrupting the AR transcriptional complex is the inhibition of histone deacetylases (HDAC) that regulate AR transcriptional activity in vitro (44) although HDAC inhibitors tested in clinical studies to date have failed to reproduce this effect (45).
LBD-independent targeting of the AR
Current hormonal therapies target the AR LBD and predictably most molecular changes that develop with castration resistance predominantly involve aberrations of the AR LBD. The AR amino-terminal domain is responsible for ARE binding and regulation of gene transcription and this introduces the possibility of improving therapeutic efficiency by targeting this domain (Figure 1). A drug discovery screen identified candidate small molecule inhibitors of the AR amino-terminal domain (46) but these data require further preclinical validation prior to consideration for clinical testing. Also as the technologies for developing antisense or RNA silencing therapeutics to silence genes such as AR improve, one could envision the design of species targeting amino-terminal domain sequences.
Targeting steroid-receptor regulated gene fusions
The discovery of chromosomal rearrangements or part-deletions in prostate cancer that result in over-expression of oncogenes by AR or other steroid receptors introduced the possibility of directly targeting gene fusions and avoiding the side effects of and resistance to hormone treatments. The 3′ constituents of gene fusions described to date include members of the ETS family, most commonly ERG and ETV1 and the RAF kinase family, B-RAF and RAF-1 (47, 48). Gene fusions involving ERG, most commonly with the serine protease TMPRSS2 following deletion of the 2.1Mb region between ERG and TMPRSS2 on chromosome 21(q), occur in 30-50% of prostate cancers (48). Several 5′ gene fusion partners have been described (49). A functional ARE sequence has been shown in silico to be in proximity of the majority of promotor genes (50) and expression of the majority of partners has also been confirmed in vitro or in animal models to be regulated by androgens or other steroid ligands (49). These could include estrogens as demonstrated in the AR-negative prostate cancer cell line NCI-H660 where transcription of the TMPRSS2:ERG gene fusion is modulated by estrogen receptor signaling (51). Therapeutics targeting the RAF-MEK axis are undergoing evaluation in non-tumor specific early clinical studies. However rearrangement-dependent over-expression of RAF kinases occurs in <3% of prostate cancers (47) and therefore drug discovery programs to identify therapeutic strategies targeting the commonly rearranged ETS transcription factors are ongoing.
Conclusion
The increase in prostate cancer research funding two decades ago is bearing fruit with new scientific discoveries allowing a better understanding of the biology that underlies the disease and a consequent exponential increase in novel prostate cancer therapeutics entering clinical trials. 2010 saw the unprecedented publication of two positive Phase III trials (Sipuleucel-T and cabazitaxel) (31, 32) with FDA approval and the presentation of a third positive Phase III trial (abiraterone acetate) (22). However, despite seven decades of hormonal treatments for prostate cancer, it is generally accepted that treatments to date fail to achieve indefinite complete inhibition of AR signaling and repeated sequential therapeutic targeting of the AR in metastatic prostate cancer remains necessary to maintain remission.
Acknowledgements
GA, JR and JSdB are employees of the Section of Medicine at The Institute of Cancer Research which is supported by a Cancer Research UK programme grant and an Experimental Cancer Medical Centre (ECMC) grant from Cancer Research UK and the Department of Health (Ref: C51/A7401). GA holds a Prostate Cancer Foundation (Santa Monica, CA) Young Investigator Award. GA and JSdB also acknowledge NHS funding to the NIHR biomedical research centre.
Footnotes
Disclosures
GA, JR and JSdB are employees of The Institute of Cancer Research, which has a commercial interest in the development of abiraterone acetate. JSdB has served as a paid consultant for Johnson & Johnson, Medivation, Astellas, Dendreon and AstraZeneca. GA has served as a paid consultant for Millenium Pharmaceuticals and as an uncompensated advisor for Johnson & Johnson. GA is on The Institute of Cancer Research list of rewards to inventors of abiraterone acetate.
REFERENCES
- 1.Huggins C, stevens RE, Jr, Hodges CV. Arch Surg. 1941;43:209. [Google Scholar]
- 2.Scher HI, Beer TM, Higano CS, Anand A, Taplin M-E, Efstathiou E, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437–46. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaud F, et al. Phase I Clinical Trial of the CYP17 Inhibitor Abiraterone Acetate Demonstrating Clinical Activity in Patients With Castration-Resistant Prostate Cancer Who Received Prior Ketoconazole Therapy. J Clin Oncol. 2010;28:1481–8. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attard G, Reid AHM, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–71. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 5.Attard G, Reid AHM, A’Hern R, Parker C, Oommen NB, Folkerd E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–8. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid AHM, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, et al. Significant and Sustained Antitumor Activity in Post-Docetaxel, Castration-Resistant Prostate Cancer With the CYP17 Inhibitor Abiraterone Acetate. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, et al. Phase II Multicenter Study of Abiraterone Acetate Plus Prednisone Therapy in Patients With Docetaxel-Treated Castration-Resistant Prostate Cancer. J Clin Oncol. 2010;28:1496–501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riegman PH, Vlietstra RJ, van der Korput JA, Brinkmann AO, Trapman J. The promoter of the prostate-specific antigen gene contains a functional androgen responsive element. Mol Endocrinol. 1991;5:1921–30. doi: 10.1210/mend-5-12-1921. [DOI] [PubMed] [Google Scholar]
- 9.Attard G, Cooper CS, de Bono JS. Steroid hormone receptors in prostate cancer: a hard habit to break? Cancer Cell. 2009;16:458–62. doi: 10.1016/j.ccr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Attard G, Belldegrun AS, de Bono JS. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 2005;96:1241–6. doi: 10.1111/j.1464-410X.2005.05821.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 12.Mohler JL, Gregory CW, Ford OH, Kim D, Weaver CM, Petrusz P, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–8. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 13.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Research. 2008;68:6407–15. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025–33. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 16.Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J Med Chem. 1995;38:2463–71. doi: 10.1021/jm00013a022. [DOI] [PubMed] [Google Scholar]
- 17.O’donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, et al. Hormonal impact of the 17 α-hydroxylase/C17,20-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004:1–9. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auchus RJ. The genetics, pathophysiology, and management of human deficiencies of P450c17. Endocrinol Metab Clin North Am. 2001;30:101–19. vii. doi: 10.1016/s0889-8529(08)70021-5. [DOI] [PubMed] [Google Scholar]
- 19.Luthy IA, Begin DJ, Labrie F. Androgenic activity of synthetic progestins and spironolactone in androgen-sensitive mouse mammary carcinoma (Shionogi) cells in culture. Journal of steroid biochemistry. 1988;31:845–52. doi: 10.1016/0022-4731(88)90295-6. [DOI] [PubMed] [Google Scholar]
- 20.Attard G, Reid AHM, de Bono JS. Abiraterone Acetate Is Well Tolerated Without Concomitant Use of Corticosteroids. J Clin Oncol. 2010 doi: 10.1200/JCO.2010.29.5170. [DOI] [PubMed] [Google Scholar]
- 21.Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–7. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 22.de Bono JS, Logothetis C, Fizazi K, North S, Chu L, Chi K, et al. Abiraterone acetate plus low dose prednisone improves survival in patients with metastatic castration-resistant prostate cancer who have progressed after docetaxel-based chemotherapy: Results of COU-AA-301, a randomized double-blind placebo-controlled phase III study; Abstract LBA5 ESMO annual meeting; Milan. 2010. [Google Scholar]
- 23.Handratta VD, Vasaitis TS, Njar VCO, Gediya LK, Kataria R, Chopra P, et al. Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. J Med Chem. 2005;48:2972–84. doi: 10.1021/jm040202w. [DOI] [PubMed] [Google Scholar]
- 24.Chang CY, Walther PJ, McDonnell DP. Glucocorticoids manifest androgenic activity in a cell line derived from a metastatic prostate cancer. Cancer Research. 2001;61:8712–7. [PubMed] [Google Scholar]
- 25.Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703–6. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 26.Veldscholte J, Berrevoets CA, Ris-Stalpers C, Kuiper GG, Jenster G, Trapman J, et al. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. The Journal of steroid biochemistry and molecular biology. 1992;41:665–9. doi: 10.1016/0960-0760(92)90401-4. [DOI] [PubMed] [Google Scholar]
- 27.Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists’ Collaborative Group. Lancet. 2000;355:1491–8. [PubMed] [Google Scholar]
- 28.Jung ME, Ouk S, Yoo D, Sawyers CL, Chen C, Tran C, et al. Structure-activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC) J Med Chem. 2010;53:2779–96. doi: 10.1021/jm901488g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster WR, Car BD, Shi H, Levesque PC, Obermeier MT, Gan J, et al. Drug safety is a barrier to the discovery and development of new androgen receptor antagonists. Prostate. 2010:n/a–n/a. doi: 10.1002/pros.21263. [DOI] [PubMed] [Google Scholar]
- 31.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 32.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels J-P, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 33.Gan L, Chen S, Wang Y, Watahiki A, Bohrer L, Sun Z, et al. Inhibition of the androgen receptor as a novel mechanism of taxol chemotherapy in prostate cancer. Cancer Research. 2009;69:8386–94. doi: 10.1158/0008-5472.CAN-09-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scher HI, Jia X, de Bono JS, Fleisher M, Pienta KJ, Raghavan D, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–9. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 36.Olmos D, Arkenau H-T, Ang JE, Ledaki I, Attard G, Carden CP, et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single-centre experience. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2009;20:27–33. doi: 10.1093/annonc/mdn544. [DOI] [PubMed] [Google Scholar]
- 37.Attard G, Swennenhuis JF, Olmos D, Reid AHM, Vickers E, A’Hern R, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Research. 2009;69:2912–8. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Y, Palma JF, Agus DB, Wang Y, Gross ME. Detection of androgen receptor mutations in circulating tumor cells in castration-resistant prostate cancer. Clin Chem. 2010;56:1492–5. doi: 10.1373/clinchem.2010.143297. [DOI] [PubMed] [Google Scholar]
- 39.Leary RJ, Kinde I, Diehl F, Schmidt K, Clouser C, Duncan C, et al. Development of Personalized Tumor Biomarkers Using Massively Parallel Sequencing. Sci Transl Med. 2010;2:20ra14–20ra14. doi: 10.1126/scitranslmed.3000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foster CS, Dodson AR, Ambroisine L, Fisher G, Møller H, Clark J, et al. Hsp-27 expression at diagnosis predicts poor clinical outcome in prostate cancer independent of ETS-gene rearrangement. Br J Cancer. 2009;101:1137–44. doi: 10.1038/sj.bjc.6605227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zoubeidi A, Zardan A, Beraldi E, Fazli L, Sowery R, Rennie P, et al. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Research. 2007;67:10455–65. doi: 10.1158/0008-5472.CAN-07-2057. [DOI] [PubMed] [Google Scholar]
- 42.de Bono JS, Kristeleit R, Tolcher A, Fong P, Pacey S, Karavasilis V, et al. Phase I pharmacokinetic and pharmacodynamic study of LAQ824, a hydroxamate histone deacetylase inhibitor with a heat shock protein-90 inhibitory profile, in patients with advanced solid tumors. Clin Cancer Res. 2008;14:6663–73. doi: 10.1158/1078-0432.CCR-08-0376. [DOI] [PubMed] [Google Scholar]
- 43.Pacey S, Wilson R, Walton M, Eatock M, Zetterlund H, Arkenau H, et al. A phase I trial of the HSP90 inhibitor, alvespimycin (17-DMAG) administered weekly, intravenously, to patients with advanced, solid tumours. J Clin Oncol. 2009:27. doi: 10.1158/1078-0432.CCR-10-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welsbie DS, Xu J, Chen Y, Borsu L, Scher HI, Rosen N, et al. Histone deacetylases are required for androgen receptor function in hormone-sensitive and castrate-resistant prostate cancer. Cancer Res. 2009;69:958–66. doi: 10.1158/0008-5472.CAN-08-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molife LR, Attard G, Fong PC, Karavasilis V, Reid AHM, Patterson S, et al. Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC) Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2010;21:109–13. doi: 10.1093/annonc/mdp270. [DOI] [PubMed] [Google Scholar]
- 46.Andersen RJ, Mawji NR, Wang J, Wang G, Haile S, Myung J-K, et al. Regression of Castrate-Recurrent Prostate Cancer by a Small-Molecule Inhibitor of the Amino-Terminus Domain of the Androgen Receptor. Cancer Cell. 2010;17:535–46. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 47.Palanisamy N, Ateeq B, Kalyana-Sundaram S, Pflueger D, Ramnarayanan K, Shankar S, et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat Med. 2010;16:793–8. doi: 10.1038/nm.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun X-W, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 49.Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao X, Morris DS, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–9. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]
- 50.Attard G, Clark J, Ambroisine L, Mills IG, Fisher G, Flohr P, et al. Heterogeneity and clinical significance of ETV1 translocations in human prostate cancer. Br J Cancer. 2008;99:314–20. doi: 10.1038/sj.bjc.6604472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Setlur SR, Mertz KD, Hoshida Y, Demichelis F, Lupien M, Perner S, et al. Estrogen-dependent signaling in a molecularly distinct subclass of aggressive prostate cancer. J Natl Cancer Inst. 2008;100:815–25. doi: 10.1093/jnci/djn150. [DOI] [PMC free article] [PubMed] [Google Scholar]