Summary
Background and objectives
Benefits of dialysis in elderly dependent patients are not clearcut. Some patients forego dialysis, opting for conservative kidney management (CKM). This study prospectively compared quality of life and survival in CKM patients and those opting for dialysis.
Design, setting, participants, & measurements
Quality-of-life assessments (Short-Form 36, Hospital Anxiety and Depression Scale, and Satisfaction with Life Scale) were performed every 3 months for up to 3 years in patients with advanced, progressive CKD (late stage 4 and stage 5).
Results
After 3 years, 80 and 44 of 170 patients had started or were planned for hemodialysis (HD) or peritoneal dialysis, respectively; 30 were undergoing CKM; and 16 remained undecided. Mean baseline estimated GFR ± SD was similar (14.0±4.0 ml/min per 1.73 m2) in all groups but was slightly higher in undecided patients. CKM patients were older, more dependent, and more highly comorbid; had poorer physical health; and had higher anxiety levels than the dialysis patients. Mental health, depression, and life satisfaction scores were similar. Multilevel growth models demonstrated no serial change in quality-of-life measures except life satisfaction, which decreased significantly after dialysis initiation and remained stable in CKM. In Cox models controlling for comorbidity, Karnofsky performance scale score, age, physical health score, and propensity score, median survival from recruitment was 1317 days in HD patients (mean of 326 dialysis sessions) and 913 days in CKM patients.
Conclusions
Patients choosing CKM maintained quality of life. Adjusted median survival from recruitment was 13 months shorter for CKM patients than HD patients.
Introduction
As the dialysis population has expanded in the developed world (1), the proportion of elderly patients undergoing dialysis has increased dramatically (2,3). Many elderly patients with advanced CKD have several comorbid conditions and increased dependency (4). The benefits of dialysis in this setting may not be clearcut (5,6). Dialysis can impose additional burdens, including invasive interventions, time commitment, and what may be considered overmedicalization of dying. Rehabilitation is often unsatisfactory (7), and prognosis is generally poor (4).
Conservative kidney management (CKM) aims to provide a comprehensive package of care to patients choosing to forego dialysis (5). CKM shifts focus from efforts to prolong life to those emphasizing supportive care, maintenance of quality of life (QoL), and control of symptoms. Management of fluid balance, anemia, comorbid conditions, and intercurrent health problems; attempts to preserve residual kidney function where appropriate; and provision of appropriate end-of-life care are important aspects. Use of therapies in a rational and appropriate fashion must be distinguished from rationing (4,8). Rationing limits access to medical interventions to conserve resources, whereas a rational approach might entail foregoing a therapy likely to prove futile or detrimental in other ways to patient well-being.
Most studies of patient survival in CKM imply that in elderly dependent patients with high comorbidity, dialysis confers only a small survival advantage, overall (5,6) or in terms of hospital-free days (9). Women have a better prognosis on CKM than men, and older patients than younger (10). The studies were small, observational, and retrospective. There are few data on QoL in patients receiving CKM (11).
To facilitate patient choice, information on QoL and prognosis in similar patients receiving CKM and those undergoing dialysis is required. We measured self-reported QoL serially for 3 years in a cohort of patients with late stage 4 and stage 5 CKD. This allowed comparison of QoL measures, their trajectories, and survival in patients opting for CKM and those opting for dialysis.
Materials and Methods
Aim
This single-center prospective cohort study compared longitudinal assessments of QoL and survival in patients with advanced CKD who opted for CKM and those who opted for dialysis.
Patients
All patients with progressive CKD (late stage 4 and stage 5) attending our low-clearance clinics were invited to participate. Those lacking capacity or with poor understanding of English were excluded. The Hertfordshire Research Ethics Committee approved the study. All participants provided informed written consent.
Modality Choice
After medical evaluation, patients were assessed at home by a senior nurse and renal counselor/social worker. Assessments were considered by the wider multidisciplinary team, and provisional modality options were recommended. These were discussed with patient and family or caregivers during several clinic visits, and an individualized treatment plan was created. All patients choosing dialysis were offered this if clinically feasible.
Dialysis Program
Dialysis was initiated for persistent uremic symptoms, volume overload, hyperkalemia, or acidosis. HD patients received high-flux treatments, including online hemodiafiltration in 40% of patients. Target two-pool equilibrated Kt/V was 1.2 (12). Target weekly total Kt/V for peritoneal dialysis (PD) was 2.0 (13).
CKM Program
Patients opting for CKM were offered ongoing medical treatment and multidisciplinary support (5) by a team that included nephrologists, specialist nurses, renal counselors, social workers, dietitians, and community and hospice services.
Plan of Study
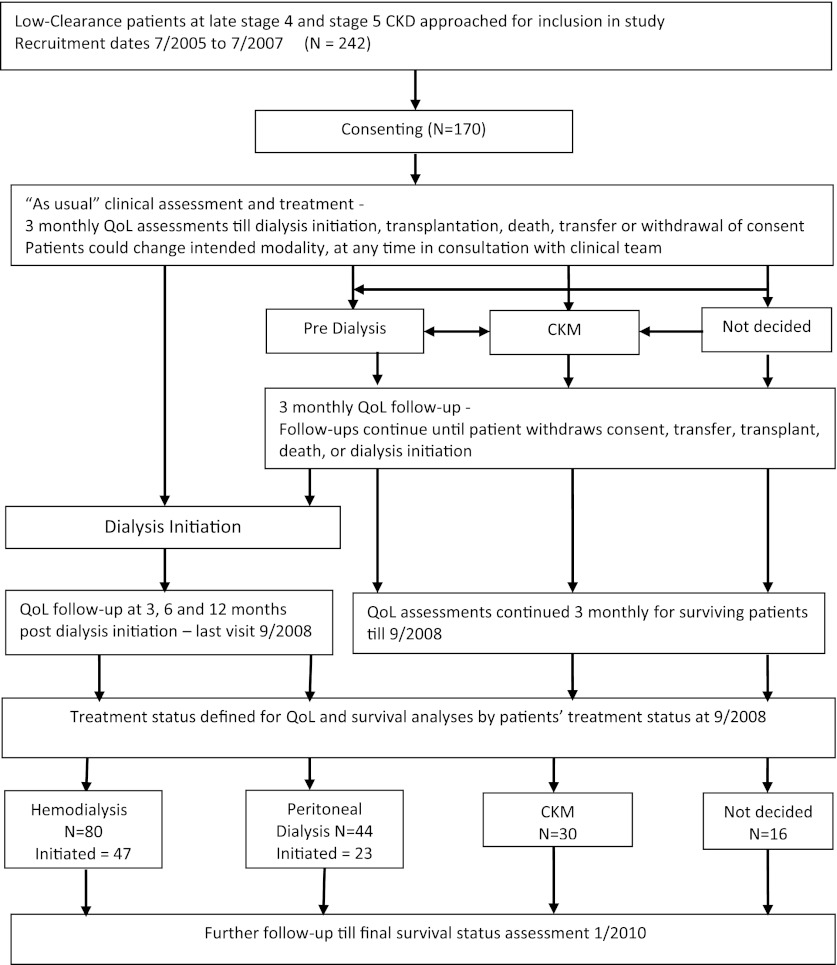
Figure 1 is a schematic representation of the study. Recruitment took place over 24 months. Baseline demographic, clinical, and biochemical data were collected along with assessments of comorbidity, functional status, and QoL. QoL was assessed every 3 months for all patients. Decisions on modality choice took place throughout the recruitment phase and up to an additional 15 months (QoL follow-up period). Group assignment was considered complete at the end of the QoL follow-up period. In the dialysis groups, QoL was assessed until 12 months after dialysis initiation, and in the CKM group it was assessed for a maximum of 3 years or until as near to death as practical. Patients were followed for a further period to ensure a minimum 30 months of follow-up for survival (survival follow-up period). Analyses of QoL and survival data were based on modality group composition at the end of the QoL follow-up period.
Figure 1.
Schematic flow chart depicting study processes. Recruitment took place over 24 months. Quality-of-life (QoL) assessments were continued every 3 months until 12 months after dialysis initiation (dialysis groups) and 3 years or as long as was practical (conservative kidney management [CKM] group) (QoL follow-up period). Patients were followed for an additional period to ensure a minimum of 30 months of follow-up for survival (survival follow-up period). Analysis of QoL and survival data were based on the composition of the modality groups at the end of the QoL follow-up period.
Data Collection
The following baseline data were collected:
Age, sex, body weight.
Primary renal disease.
Presence of diabetes.
Comorbidity score (4) based on disease in the following systems: cardiac, peripheral vascular, central nervous, and respiratory. Severity ranged from 0 (no disease) to 4 (advanced disease). Cancer was graded similarly. Cirrhosis was scored as 4. Patients with summed scores >3, or score of 3 derived from a single system, were considered to have “high comorbidity.” For survival prediction among patients with advanced CKD, including those undergoing dialysis, the method compares well with the Charlson index.
Functional performance, assessed by the Karnofsky performance scale (KPS) (14). Scores <70 were defined as “dependency.”
Serum creatinine, estimated GFR (eGFR, as calculated according to the Modification of Diet in Renal Disease 4 method) (15), hemoglobin level. Estimated GFR (according to the Chronic Kidney Disease-Epidemiology Collaboration) (16) was calculated subsequently.
Patients were visited at home every 3 months by a study team member for QoL assessments, including the Short-Form 36 (SF-36) (17), Hospital Anxiety and Depression Scale (HADS) (18), and Satisfaction with Life Scale (SWLS) (19). The following data were also recorded:
Date and modality at dialysis initiation.
Status at end of QoL follow-up period or at exit from study (defined as PD, HD, CKM, transplanted, not decided, deceased).
Status, as defined, at end of survival follow-up.
Date censored (transplant, transfer) or date of death.
Statistical Analyses
Statistics packages used were Stata software, version 11, and SPSS software, version 19. Groups were compared for continuously distributed variables using ANOVA, unpaired t tests, or Mann-Whitney test as appropriate. Differences in proportions between groups were compared using the Fisher exact test.
Propensity Score.
We controlled for selection bias using a propensity score (20). The score was generated with logistic modeling to predict membership of the CKM group, taking into account such covariates as sex, age, weight, eGFR, comorbidity, and KPS score; in survival models, the physical health component (SF-36) was also included. We explored using propensity models that included HD, PD, CKM, and not-decided groups and those omitting PD or not-decided groups. The models produced similar results; hence the more inclusive models were reported. Overlap in distributions of propensity scores was sufficient to be confident of appropriate adjustment for selection bias. The not-decided group was small, and inclusion of this group in survival models tended to lead to fitting problems. Thus, it was omitted from final models.
QoL Analysis.
We explored how QoL measures changed over time and with dialysis initiation. Multilevel models for change (growth models) were fitted for each QoL measure in turn, allowing for interindividual variation in both baseline level (random intercept) and rate of change over time (random slope; together with random effects). A time-varying predictor for treatment status (pre– and post–dialysis initiation) was included as a fixed and a random effect, allowing estimation of the immediate effect of initiation on the QoL measure under consideration. Baseline values for age, comorbidity (low or high), KPS score, sex, and propensity score were entered into models as fixed effects to adjust for selection bias. Models were fitted using maximum likelihood estimation and unstructured covariances. For each QoL measure, an initial model incorporating a random intercept and slope was evaluated. Further evaluation considered the effect of (1) initiating dialysis, (2) trajectory changes after initiation, (3) differences between treatment groups, (4) adjustment for covariates (age, comorbidity, KPS score, sex, and propensity score), and (5) potential interactions (e.g., between treatment group and change over time). Model estimates were adjusted to ensure the model was representative of a person age 75 years with high comorbidity and a KPS score of 70.
Survival Analysis.
Survival was calculated from the date of recruitment until death, final census date in January 2010, or the date the patient was recorded as censored. Because the differences between groups were marked in terms of clinical and demographic factors (uncontrolled selection bias), Cox proportional hazards regression models were used to estimate adjusted functions, taking into account many of these uncontrolled random variables.
Results
Patient Features: Baseline Comparisons
Of the 242 patients approached to take part during the 24 months from July 2005, 170 (70%) consented. Five cited “not feeling well enough” as a reason for nonparticipation. Patients older than age 75 years were overrepresented in the group that did not consent to participate (36% versus 23% in the study group). Median duration of QoL follow-up was 14.7 (interquartile range, 12.0) months. Median duration of survival follow-up was 31.9 (interquartile range 25.1) months.
At the end of the QoL follow-up period, 80 patients were undergoing or destined for HD, 44 were undergoing or destined for PD, 30 were receiving CKM, and 16 were undecided. Forty-seven (59%) HD patients and 23 (52%) PD patients had initiated dialysis by this time. Diabetes was more prevalent in the HD group than in the CKM and PD groups (35%, 23%, and 16%, respectively; P<0.001 for HD versus PD). The groups differed with respect to age, performance score (KPS score), and comorbidity (Table 1). Compared with the PD group, CKM patients were older, more had high comorbidity, and more were dependent. The HD group was more heterogeneous, having a wider distribution of comorbidity and KPS scores than the other groups. The not-decided group had a higher baseline eGFR (Table 1), but eGFR in the other groups was similar.
Table 1.
Baseline characteristics of modality groups
| Variable | Hemodialysis | Peritoneal Dialysis | Conservative Management | Not Decided | P Value |
|---|---|---|---|---|---|
| Patients (n) | 80 | 44 | 30 | 16 | |
| Men (%) | 76 | 50 | 70 | 56 | 0.02 |
| Age (yr) | 60.6±14.9 | 48.0±15.6 | 77.5±6.5 | 68.3±16.4 | <0.001 |
| Weight (kg) | 83.1±19.7 | 77.8±14.6 | 76.2±18.6 | 78.4±15.2 | No group differences |
| eGFR (ml/min per 1.73 m2) | |||||
| Per MDRD4 | 13.3±3.7 | 14.2±4.4 | 14.3±3.4 | 16.3±4.6 | 0.04 for HD versus not-decided |
| Per CKD-EPI | 12.2±3.7 | 13.7±4.5 | 12.5±3.1 | 14.9±4.8 | No group differences |
| Comorbidity, n (%) | |||||
| Low | 52 (65) | 38 (86) | 8 (26) | 9 (56) | <0.001 |
| High | 28 (35) | 6 (14) | 22 (74) | 7 (44) | |
| KPS score, n (%) | |||||
| >70 | 66 (82.5) | 43 (98) | 10 (33) | 8 (50) | <0.001 |
| <70 | 14 (17.5) | 1 (2) | 20 (66) | 8 (50) | |
| SF-36 score | |||||
| Mental health | 47.6±10.7 | 45.9±10.6 | 49.9±9.9 | 52.0±40.0 | No group differences |
| Physical health | 25.2±8.8 | 30.1±6.5 | 18.0±8.8 | 21.1±24.0 | <0.001 for HD and PD versus CKM 0.01 for HD versus PD |
| HADS score | |||||
| Anxiety | 5.5±3.6 | 4.7±4.0 | 6.9±3.3 | 5.3±1.6 | 0.04 for HD versus CKM 0.02 for PD versus CKM |
| Depression | 6.1±4.0 | 6.4±4.2 | 5.2±3.3 | 4.9±17.6 | No group differences |
| SWLS score | 21.7±8.0 | 22.5±7.3 | 23.2±7.1 | 22.4±35.2 | No group differences |
Unless otherwise noted, values are expressed as mean ± SD. eGFR, estimated GFR; MDRD4, Modification of Diet in Renal Disease 4 equation; CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration; KPS, Karnofsky performance scale; SF-36, quality-of-life assessment using Short-Form 36 questionnaire; HADS, Hospital Anxiety and Depression Scale; SWLS, Satisfaction with Life Scale (see text for explanation).
QoL Assessment
There were 815 QoL assessments: 375 (104 post–dialysis initiation) in HD patients (median, 5 per patient), 220 (42 post–dialysis initiation) in PD patients (median, 5), 164 in CKM patients (median, 5), and 56 in not-decided patients (median, 3.5). Median time between final QoL assessment and death in the CKM group was 4.8 months. Table 2 shows summary results of multilevel growth models for individual QoL measures.
Table 2.
Growth model parameter estimates
| Measure | Fixed-Effects Parameter Estimates | P Value for Model Fit (Wald) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Change over Time | Dialysis Initiation | Age | Comorbidity | KPS Score | Sex | Propensity Score | Constant | ||
| SF-36 | |||||||||
| Mental health | 0.12±0.32a | −0.69±5.8 | 0.21b | −3.09a | −0.43 | 0.68 | 2.68 | 50.7b±6.2 | <0.001 |
| Physical health | 0.04±0.17 | 0.49±1.7 | −0.16b | 1.61 | −1.21b | −0.35 | −4.04 | 21.1b±4.4 | <0.001 |
| SWLS | 0.02±0.11 | −1.84a±4.5 | 0.12b | −2.70a | −0.35 | 0.71 | −0.14 | 23.5b±5.5 | <0.001 |
| HADS | |||||||||
| Depression | −0.03±0.10 | −0.57±1.7 | −0.10b | 1.17 | 0.29b | −0.81 | 0.02 | 5.56b±3.1 | <0.001 |
| Anxiety | −0.004±0.14 | −0.02±2.6 | −0.02 | 0.73 | 0.53b | 0.10 | −0.78 | 5.36b±2.5 | <0.001 |
Unless otherwise noted, values are expressed as mean ± SD. Change over time (growth) for the fixed effect is the estimated change per month in the quality-of-life measure. Similar interpretations can be applied to dialysis initiation and the constant. Age, comorbidity, KPS score, sex, and propensity score were entered into the model as fixed effects only to adjust for variation between groups at baseline. SDs for age, comorbidity, KPS score, sex, and propensity score were not estimated because of constraints on model fitting. KPS, Karnofsky performance scale; SF-36, quality-of-life assessment using Short-Form 36 questionnaire; HADS, Hospital Anxiety and Depression Scale; SWLS, Satisfaction with Life Scale.
P<0.05.
P<0.01.
SF-36: Mental Health Subscale.
There were no baseline group differences in mental health score. For the basic growth model (constant and change over time), there was a small statistically (but not clinically) significant increase in mental health score over time. Adding baseline covariates (age, comorbidity, KPS score, sex, and propensity score) improved model fit considerably. Adding the group had limited influence, indicating that individual baseline differences accounted for group differences. The effect of initiating dialysis varied between individuals (mean change ± SD, −0.69±5.84; P=0.53) and was unrelated to any measured variable (age, comorbidity, KPS score, sex, eGFR).
SF-36: Physical Health Subscale.
Baseline physical health scores were generally low (3–4 SDs below population average). Physical status of CKM patients was significantly lower than that of HD and PD patients and was lower among HD than PD patients. The basic growth model showed little evidence of change in physical status over time. Including baseline covariates (age, comorbidity, KPS score, sex, propensity score) increased model fit and abolished group differences. There was no change in physical health upon dialysis initiation (mean change, 0.49±1.7; P=0.53).
HADS: Depression Scale.
There were no baseline group differences in depression scores. The full growth model showed no evidence that there were group differences, that change in depression scores over time differed between groups, or that trajectory changed after dialysis initiation. Initiation had no consistent effect (mean change, −0.57±1.7; P=0.10).
HADS: Anxiety Scale.
Anxiety scores for CKM patients were higher than those for PD and HD patients. Adjustment for baseline covariates abolished these differences. There was no evidence of group differences in change of anxiety levels over time or of change in slope after dialysis initiation. There was no consistent change on dialysis initiation (mean change, −0.02±2.6; P=0.95).
SWLS.
There was considerable individual variation in scores (range, 5–30) but little evidence of group differences at baseline. Starting dialysis led to a significant decrease in the SWLS score (change, −1.84±4.46; range, −10.8 to 7.1; effect size, 0.41; P=0.01). The magnitude of this differed little over time. There were considerable individual differences in patient response to initiation (SD, 17% of scale range), with 47% experiencing potentially clinically significant reductions >1 SD. No covariate (weight, age, sex, comorbidity, KPS score, or eGFR) was related to change in SWLS score at initiation.
Survival
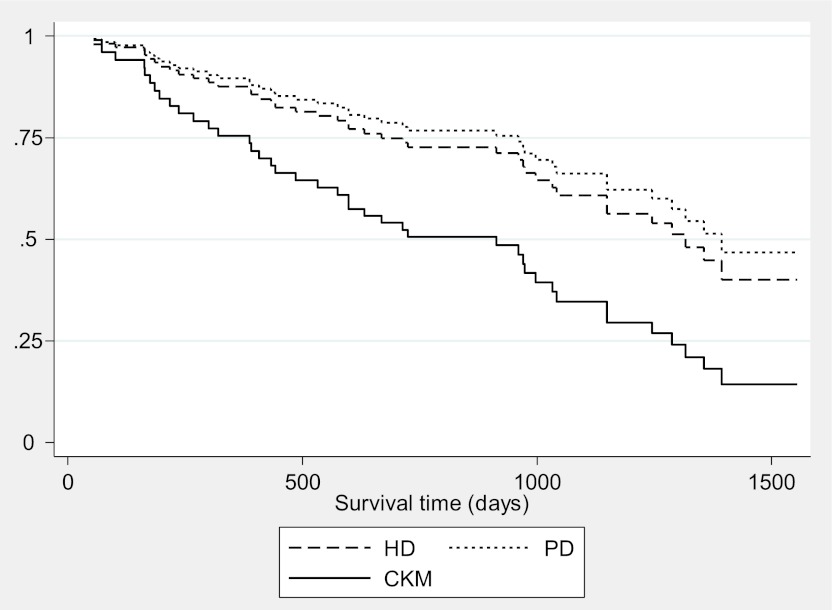
Survival analysis was based on planned modality group composition at the end of the QoL follow-up period. Subsequent movement between groups during follow-up is depicted in Table 3. There were significant group differences in mortality, with 19 deaths (4 before dialysis initiation) in the HD group (24%), 4 (9%) in the PD group, and 20 (67%) in the CKM group (P<0.001). Kaplan-Meier survivor functions demonstrated major differences in unadjusted survival (log-rank test P<0.001). In an adjusted Cox model that included comorbidity, KPS score, age, physical health component of SF-36, and propensity score (Table 4), comorbidity was the major contributor to the survivor function. The mortality risk in the HD group was approximately half that in CKM patients (hazard ratio, 0.47; 95% confidence interval, 0.20–1.10; P=0.08). The difference in estimated median survival from recruitment was 404 days (CKM, 913 days; HD, 1317 days; Figure 2). The difference for PD was slightly higher. The median number of days on which HD patients actually underwent an HD session was 326.
Table 3.
Modality allocation and outcome
| Variable | Status at End of QoL Follow-up (September 2008) | Changed Modality (September 2008–January 2010) | Status at End of Survival Follow-up (January 2010) | |||
|---|---|---|---|---|---|---|
| Planned Modality (Initiated Dialysis) | Died | To PD | To HD | Planned Modality (Initiated Dialysis) | Died | |
| HD | 80 (47) | 13 | 3 | – | 89 (69) | 19 |
| PD | 44 (23) | 2 | – | 9 | 39 (32) | 4 |
| CKM | 30 (—) | 13 | – | 1 | 29 (—) | 20 |
| Undecided | 16 (—) | 8 | 1 | 2 | 13 (—) | 8 |
The table shows the composition of each planned modality group at the end of the quality-of-life follow-up period in September 2008 and at the end of the survival follow-up period in January 2010 (numbers of those who had actually started dialysis at each time are shown in parentheses). The change in modality allocation during the survival follow-up period is also depicted. The numbers of patients who died during the quality-of-life period and throughout the study are also shown. QoL, quality of life; PD, peritoneal dialysis; HD, hemodialysis; CKM, conservative kidney management.
Table 4.
Adjusted Cox model of survival in each of the modalities
| Factor | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Hemodialysis | 0.47 (0.20–1.10) | 0.08 |
| Peritoneal dialysis | 0.39 (0.10–1.48) | 0.17 |
| Age (in yr) | 1.02 (0.97–1.05) | 0.41 |
| Comorbidity (high versus low) | 3.71 (1.63–8.43) | 0.002 |
| KPS score × propensity interaction | 1.18 (0.21–6.64) | 0.85 |
| Physical health score (SF-36) | 0.99 (0.97–1.01) | 0.51 |
Survival time was the time that elapsed from the point at which patients were recruited to the study, to the census date or the date of censoring (date of death, date of transfer to another center, date to transplant, date of requested withdrawal from the study). The table indicates reduced hazard for death for patients undergoing hemodialysis and peritoneal dialysis compared with conservative kidney management, although the differences are of borderline or less statistical reliability. Estimated median survival times were as follows: for hemodialysis, 1317 days; for conservative kidney management, 913 days; and for peritoneal dialysis, 1394 days. High comorbidity was associated with a significantly increased hazard. KPS score × propensity score is the interaction between Karnofsky performance scale score and propensity score, included because of colinearity between these covariates. CI, confidence interval; KPS, Karnofsky performance scale; SF-36, Short-Form 36 questionnaire.
Figure 2.
Adjusted Cox survival functions for patients receiving hemodialysis (HD), peritoneal dialysis (PD), and conservative kidney management (CKM). Adjustments were made for high comorbidity, dependency (Karnofsky performance scale score <70), age older than 75 years, and selection bias. As time progresses, the number of observed events decreases, the modeled events take over, and the curves become more similar.
Discussion
Patients opting for CKM were older, more dependent, and more highly comorbid than those opting for dialysis. At baseline they had poorer physical health (SF-36) and higher levels of anxiety (HADS). Mental health (SF-36), depression symptoms (HADS), and global satisfaction with life (SWLS) were similar in all modality groups. SF-36 and HADS scores changed little during follow-up in any group. SWLS scores, however, decreased significantly after dialysis initiation and did not subsequently recover. SWLS score did not change over time in the CKM group. Not surprisingly, nonadjusted survival from recruitment was markedly lower in CKM patients. However, in Cox models controlling for comorbidity, KPS score, age, physical health component (SF-36), and propensity score, the difference in estimated median survival in the HD and CKM groups narrowed to 13.2 months, 10.7 of which consisted of days on which dialysis sessions took place. The lack of significance (P=0.08) probably reflects lack of power. In elderly dependent patients with significant comorbidity, opting for CKM may allow maintenance of some aspects of QoL, which might otherwise have been compromised by starting dialysis, probably at the expense of some reduction in survival.
There is one previous report of QoL assessment in CKM (11). De Biase et al. found that QoL in 11 elderly, frail patients receiving CKM was similar to that in HD patients. Like us, they found that unadjusted physical health (SF-36) was worse with CKM, although mental health scores were similar. The only change we found in QoL was a reduction in SWLS score after dialysis initiation. SWLS provides an overall assessment of life satisfaction as a cognitive-judgmental process (19,21). SWLS scores have been predictably associated with other measures of subjective well-being in medical outpatients. They are not influenced by sex, age, education level, health insurance status, or social desirability (21). It is also pertinent that no QoL measure improved after dialysis initiation. Our findings are compatible with those of studies demonstrating worsening functional status (22,23) in elderly patients who initiate dialysis. Symptom burden and QoL are similar in patients undergoing long-term dialysis and those receiving palliative care (24). These findings support the notion that for some dependent patients with high comorbid loads, CKM may offer an acceptable alternative to dialysis.
Comparison with previous retrospective survival data are hampered by design issues, particularly differences in methods of matching CKM and dialysis patients (if they were matched) and in reference time points from which survival was assessed. We previously assessed survival from a putative dialysis date (obtained by matching Cockcroft-Gault creatinine clearances). We found similar median survival in CKM and in those recommended for CKM but opting for dialysis (6.3 versus 8.3 months) (5). Carson et al., using similar methods in patients older than 70 years, found median survival durations of 13.9 months in CKM patients and 37.8 months in dialysis patients (9). CKM patients were older but had similar comorbidity. Hospital-free survival was similar in both groups. Median survival duration in dialyzed octogenarians was 28.9 months compared with 8.9 months among those receiving CKM (measured from the date of decision to withhold dialysis) (25). Late referral, diabetes, dependency, and social isolation were more common in CKM. Murtagh et al. found that survival (from eGFR of 15 ml/min) was similar at 22 months in elderly patients (older than 75 years) with high comorbidity, whether receiving CKM or dialysis (6). Recently, we found that median survival duration in CKM patients from entry into stage 5 CKD was 21 months. In those older than age 75 years, the survival advantage from dialysis, corrected for age, comorbidity, and diabetes, was nonsignificant at 4 months (10). Ellam et al. (26) (from CKD stage 5) and Wong et al. (27) (from late stage 4 and stage 5) found similar median survival durations (21 and 23 months, respectively). We now report that estimated median survival durations from recruitment (mean eGFR, 14 ml/min) was 30 months in CKM and 43 months in similar HD patients.
This study has limitations. Sample size was relatively small, and there were major demographic and clinical differences between individuals and modality groups. We used propensity scores to adjust for selection bias, although given the large group differences the potential for residual confounding remains. A multicenter prospective observational study of patients older than 75 years that is powered using effect sizes demonstrated here is needed to confirm these findings. For ethical and technical design reasons, randomized, controlled trials in this area may not be possible. Thirty percent of eligible patients declined to consent. Older patients were overrepresented in this group. This may have influenced modality group composition and perhaps both QoL and survival outcomes. Because of the nature of the study, composition of modality groups changed throughout the study, even after the end of the QoL follow-up period, when group assignment for analysis was considered complete. However, subsequent changes in the CKM group were minimal and considered unlikely to have influenced our interpretations. Finally, in the survival analysis we focused on the difference between CKM and HD groups. The PD group was younger, fitter, and more independent, and few of these patients died. Thus, group comparisons are difficult. In addition, elderly patients with high comorbidity and compromised independence are more likely to receive HD as the most suitable modality (28). Notwithstanding these limitations, the study provides important insights into QoL and survival in CKM, which may be helpful clinically.
Patients opting for CKM tend to maintain QoL, some aspects of which tend to be compromised in patients initiating dialysis. The price may be some reduction in survival. These findings may be helpful in counseling patients on modality choice and aid design of larger prospective studies.
Disclosures
None.
Acknowledgments
The authors acknowledge the help of all the clinical staff of the Lister Renal Service, in particular the predialysis liaison team, without whose help the study would not have been possible. Most of all we wish to acknowledge the huge contribution from our patients.
The authors received a project grant for this work from the British Renal Society.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Deny Dialysis or “D-NI” Dialysis? The Case for “Do Not Initiate; Do Not Ignore” Orders” on pages 1924–1926.
References
- 1.Farrington K, Hodsman A, Casula A, Ansell D, Feehally J: UK Renal Registry 11th Annual Report (December 2008): Chapter 4 ESRD prevalent rates in 2007 in the UK: national and centre-specific analyses. Nephron Clin Pract 111[Suppl 1]: c43–c68, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Jager KJ, van Dijk PC, Dekker FW, Stengel B, Simpson K, Briggs JD, ERA-EDTA Registry Committee : The epidemic of aging in renal replacement therapy: An update on elderly patients and their outcomes. Clin Nephrol 60: 352–360, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Roderick P, Davies R, Jones C, Feest T, Smith S, Farrington K: Simulation model of renal replacement therapy: Predicting future demand in England. Nephrol Dial Transplant 19: 692–701, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Chandna SM, Schulz J, Lawrence C, Greenwood RN, Farrington K: Is there a rationale for rationing chronic dialysis? A hospital based cohort study of factors affecting survival and morbidity. BMJ 318: 217–223, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K: Choosing not to dialyse: Evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract 95: c40–c46, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE: Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 22: 1955–1962, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Kutner NG, Brogan DJ: Assisted survival, aging, and rehabilitation needs: Comparison of older dialysis patients and age-matched peers. Arch Phys Med Rehabil 73: 309–315, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Challah S, Wing AJ, Bauer R, Morris RW, Schroeder SA: Negative selection of patients for dialysis and transplantation in the United Kingdom. Br Med J (Clin Res Ed) 288: 1119–1122, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carson RC, Juszczak M, Davenport A, Burns A: Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 4: 1611–1619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandna SM, Da Silva-Gane M, Marshall C, Warwicker P, Greenwood RN, Farrington K: Survival of elderly patients with stage 5 CKD: Comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant 26: 1608–1614, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Biase V, Tobaldini O, Boaretti C, Abaterusso C, Pertica N, Loschiavo C, Trabucco G, Lupo A, Gambaro G: Prolonged conservative treatment for frail elderly patients with end-stage renal disease: The Verona experience. Nephrol Dial Transplant 23: 1313–1317, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Tattersall JE, DeTakats D, Chamney P, Greenwood RN, Farrington K: The post-hemodialysis rebound: Predicting and quantifying its effect on Kt/V. Kidney Int 50: 2094–2102, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Tattersall JE, Doyle S, Greenwood RN, Farrington K: Maintaining adequacy in CAPD by individualizing the dialysis prescription. Nephrol Dial Transplant 9: 749–752, 1994 [PubMed] [Google Scholar]
- 14.Karnofsky DA, Burchenal JH: The clinical evaluation of chemotherapeutic agents in cancer. In: Evaluation of Chemotherapeutic Agents, edited by Macleod CM, New York, NY, Columbia University Press, 1949, p 196 [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, Westlake L: Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ 305: 160–164, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snaith RP, Zigmond AS: The hospital anxiety and depression scale. Br Med J (Clin Res Ed) 292: 344, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diener E, Emmons RA, Larsen RJ, Griffin S: The Satisfaction With Life Scale. J Pers Assess 49: 71–75, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Newgard CD, Hedges JR, Arthur M, Mullins RJ: Advanced statistics: the propensity score—a method for estimating treatment effect in observational research. Acad Emerg Med 11: 953–961, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Arrindell WA, Meeuwesen L, Huyse FJ: The Satisfaction With Life Scale (SWLS): Psychometric properties in a non-psychiatric medical outpatients sample. Pers Individ Dif 12: 117–123, 1991 [Google Scholar]
- 22.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE: Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 361: 1539–1547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jassal SV, Chiu E, Hladunewich M: Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med 361: 1612–1613, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Yong DS, Kwok AO, Wong DM, Suen MH, Chen WT, Tse DM: Symptom burden and quality of life in end-stage renal disease: A study of 179 patients on dialysis and palliative care. Palliat Med 23: 111–119, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Joly D, Anglicheau D, Alberti C, Nguyen AT, Touam M, Grünfeld JP, Jungers P: Octogenarians reaching end-stage renal disease: Cohort study of decision-making and clinical outcomes. J Am Soc Nephrol 14: 1012–1021, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Ellam T, El-Kossi M, Prasanth KC, El-Nahas M, Khwaja A: Conservatively managed patients with stage 5 chronic kidney disease—outcomes from a single center experience. QJM 102: 547–554, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Wong CF, McCarthy M, Howse ML, Williams PS: Factors affecting survival in advanced chronic kidney disease patients who choose not to receive dialysis. Ren Fail 29: 653–659, 2007 [DOI] [PubMed] [Google Scholar]
- 28.van de Luijtgaarden MW, Noordzij M, Stel VS, Ravani P, Jarraya F, Collart F, Schön S, Leivestad T, Puttinger H, Wanner C, Jager KJ: Effects of comorbid and demographic factors on dialysis modality choice and related patient survival in Europe. Nephrol Dial Transplant 26: 2940–2947, 2011 [DOI] [PubMed] [Google Scholar]