Summary
Background and objectives
Increased risk of mortality in patients with CKD has been attributed to inflammation. However, the association between kidney function, albuminuria, and biomarkers of inflammation has not been examined in a large cohort of CKD patients.
Design, setting, participants, & measurements
This study measured the plasma levels of IL-1β, IL-1 receptor antagonist (IL-1RA), IL-6, TNF-α, TGF-β, high-sensitivity C-reactive protein (hs-CRP), fibrinogen, and serum albumin in 3939 participants enrolled in the Chronic Renal Insufficiency Cohort study between June 2003 and September 2008. An inflammation score was established based on plasma levels of IL-1β, IL-6, TNF-α, hs-CRP, and fibrinogen. Estimated GFR (eGFR) and serum cystatin C were used as measures of kidney function. Albuminuria was quantitated by urine albumin to creatinine ratio (UACR).
Results
Plasma levels of IL-1β, IL-1RA, IL-6, TNF-α, hs-CRP, and fibrinogen were higher among participants with lower levels of eGFR. Inflammation score was higher among those with lower eGFR and higher UACR. In regression analysis adjusted for multiple covariates, eGFR, cystatin C, and UACR were strongly associated with fibrinogen, serum albumin, IL-6, and TNF-α. Each unit increase in eGFR, cystatin C, and UACR was associated with a −1.2% (95% confidence interval, −1.4, −1), 64.9% (56.8, 73.3) and 0.6% (0.4, 0.8) change in IL-6, respectively (P<0.001).
Conclusions
Biomarkers of inflammation were inversely associated with measures of kidney function and positively with albuminuria.
Introduction
A reduced GFR and proteinuria, the hallmarks of CKD, are associated with increased risk of hospitalization, cardiovascular events, and death (1–4). Besides the contribution from the clustering of traditional cardiovascular risk factors in CKD, increased mortality in these patients may also be due to chronic inflammation and its consequences (5–7). Inflammation is part of the complex biologic response of vascular tissue to injury, infection, ischemia, and autoimmune diseases (8). Within physiologic limits, the inflammatory response enables removal of the inciting agent and initiates the healing process. Impaired excretory renal function prolongs plasma t1/2 of several proinflammatory cytokines such as IL-1 (9) and IFNs (10), which may result in enhanced inflammatory load. Unregulated chronic systemic inflammation is associated with increased morbidity and mortality (5). As a consequence of inflammation, a variety of cytokines and acute phase proteins are released in order to augment or attenuate the inflammatory response (11).
An association between kidney function and inflammation has been reported by a number of investigators (12–14), but the association between albuminuria and markers of inflammation has not been studied in a large racially and ethnically diverse cohort of patients with established CKD. Furthermore, the levels of a number of cytokines and acute phase proteins and the utility of a possible measure of intensity of inflammation have not been systematically examined in CKD. We measured plasma levels of selected pro- and anti-inflammatory cytokines as well as acute phase proteins in about 4000 men and women enrolled in the Chronic Renal Insufficiency Cohort (CRIC) study and examined their association with creatinine-based estimates of kidney function (estimated GFR [eGFR]), serum cystatin C level, and albuminuria.
Materials and Methods
The CRIC Study
The CRIC study is a prospective observational cohort study of 3939 participants with established CKD. The organization, design, and methods of the CRIC study have been previously reported (15). Of the study participants, 54.9% were males and the median age at enrollment was 60 years; 41.6% of the participants were non-Hispanic whites, 41.9% were non-Hispanic African Americans, and 12.6% were Hispanics. Age-based eGFR entry criteria were established to limit the proportion of older individuals who were recruited with age-related decrease of GFR. Median values of eGFR and urine albumin to creatinine ratio (UACR) were 42.2 (interquartile range [IQR], 32.6, 51.9) ml/min per 1.73 m2 and 51.9 (IQR, 8.7, 458.8) μg/mg, respectively.
Nearly one-half of the study participants (n=1907) had diabetes, defined as fasting glucose ≥126 mg/dl, random glucose ≥200 mg/dl, or use of insulin or antidiabetic medication. The CRIC study exclusion criteria were monogenetic renal disease, cirrhosis, class III or IV heart failure, HIV, cancer, autoimmune disease, and active immunosuppression. Also excluded were participants with polycystic kidney disease, pregnant women, participants with organ or bone marrow transplant, and persons who had received immunotherapy for primary renal disease or systemic vasculitis within the past 6 months or had received systemic chemotherapy. Upon enrollment, each participant was asked to provide biologic samples including blood and urine. All participants provided written informed consent. The study protocol was approved by the institutional review board at each participating site.
CRIC Data Collection
Demographic characteristics, medical history, smoking status, weight, height, body mass index (BMI), and use of medications including statins, angiotensin converting enzyme inhibitors (ACEIs), and angiotensin receptor blockers (ARBs) were recorded at baseline. Serum creatinine was measured by the Jaffe method on a Beckman Synchron System. Serum cystatin C was measured on a Dade-Behring BNII, with a coefficient of variation of about 1.7%. eGFR was computed using the four-variable Modified Diet in Renal Disease study equation. We also calculated the eGFR using the estimating equation derived from the CRIC cohort (16). The final CRIC GFR estimating equation includes five variables (serum creatinine, cystatin C levels, age, sex, and race) and provides an accurate and unbiased GFR prediction within the study population. This equation may not be applicable to participants with kidney diseases that are not well represented in the CRIC cohort, such as polycystic kidney disease and GN. Proteinuria was estimated as the UACR.
Measurement of Biomarkers of Inflammation
High-sensitivity sandwich ELISAs (Quantikine HS; R&D Systems, Minneapolis, MN) were used to measure plasma IL-1β, IL-6, and TNF-α levels. Standard sandwich ELISAs (Quantikine; R&D Systems) were used to quantify IL-1 receptor antagonist (IL-1RA) and TGF-β levels. The lower detection limits for IL-1β, IL-6, TNF-α, IL-RA, and TGF-β were 0.06 pg/ml, 0.07 pg/ml, 0.11 pg/ml, 6.3 pg/ml, and 4.6 pg/ml, respectively. Integrated performance of IL-1β, IL-1RA, IL-6, and TNF-α ELISAs were implemented using a robotic liquid handling platform (Biomek FXp; Beckman Coulter, Brea, CA). The samples were stored at −80°C and assays performed at the time of initial thawing to prevent degradation (17). All cytokine assays were performed in duplicates. Several blood samples had a concentration of IL-1β below the minimal level for detection; we arbitrarily assigned a very low value for IL-1β (0.00001) to these samples. The coefficient of variation was <13% for all cytokines assays except for TNF-α and TGF-β, for which the estimated imprecision was 15.2% and 21.5%, respectively. High-sensitivity C-reactive protein (hs-CRP) and fibrinogen were quantified in EDTA plasma samples using specific laser-based immunonephelometric methods on the BNII (Siemens Healthcare Diagnostics, Deerfield, IL). The imprecision for hs-CRP and fibrinogen were <5%. Limits of detection for hs-CRP and fibrinogen analyses were 0.16 mg/L and 0.15 g/L, respectively. All tests were performed in a single laboratory.
Calculation of Inflammation Score
An inflammation score has been shown to more accurately predict the phenotype of interest than a single biomarker of inflammation (18–20). In these studies, inflammation was determined to be present in a particular participant if serum level of an inflammatory biomarker exceeded its median value for the whole cohort. We computed a composite score ranging from 0 to 5 based on levels of the following biomarkers and the value at or above which a score of 1 was assigned hs-CRP >3 mg/L (21), fibrinogen >350 mg/dl (22), IL-6 ≥6 pg/ml (23), TNF-α ≥7 pg/ml, and IL-1β ≥0.39 pg/ml (23,24). The cut-off values for individual biomarkers were chosen from published literature.
Statistical Analyses
Selected demographic and clinical characteristics of the study population were summarized by descriptive statistics. The entire population was divided into five groups according to the eGFR (in ml/min per 1.73 m2) as <30, 30–39, 40–49, 50–59, and ≥60. The study participants were also divided into tertiles based on the UACR levels. The numbers of participants with available values for eGFR, UACR, and the computed inflammation score differed from each other due to missing data for that particular variable, which is reflected in the different numbers displayed in the tables and figures. Because study variables were not normally distributed across groups, values are displayed as medians (IQRs). Pearson’s chi-squared test and the nonparametric Wilcoxon rank-sum test (or Kruskal–Wallis test when the number of groups exceeded 2) were used to compare categorical and continuous variables, respectively. Adjustment for multiple comparisons was made using the Bonferroni method. The association of eGFR, cystatin C, and UACR with log-transformed values of biomarkers was examined with multivariable linear regression models, with adjustments for age, sex, race/ethnicity, diabetes, hypertension, ACEI/ARB use, and lipid-lowering drug use. All analyses were done with SAS statistical software (version 9.3; SAS Inc., Cary, NC).
Results
The baseline demographic and clinical characteristics of the study participants, overall and according to the UACR tertiles, are shown in Table 1. UACR measurements were available only for 3791 study participants. The median UACRs were 5.5 μg/mg, 51.9 μg/mg, and 977.0 μg/mg, for the lowest, middle, and highest tertiles, respectively. Patients in the higher tertiles of UACR were more likely to be male, have a higher BMI, smoke, have hypertension and diabetes, and have a lower level of kidney function and elevated white blood cell (WBC) and platelet counts compared with those in the lower tertiles. The plasma levels of inflammatory markers, with the exception of hs-CRP, were higher in participants belonging to upper tertiles of UACR.
Table 1.
Baseline demographic and clinical characteristics of the Chronic Renal Insufficiency Cohort study population overall and by UACR
| Entire Cohort (n=3939) | UACR (μg/mg) (n=3791)a | ||||
|---|---|---|---|---|---|
| Tertile 1 (n=1263) | Tertile 2 (n=1264) | Tertile 3 (n=1264) | P Value | ||
| Male | 2161 (54.9) | 589 (46.6) | 712 (56.3) | 775 (61.3) | <0.001 |
| Ever smoked | 2158 (54.8) | 659 (52.2) | 726 (57.4) | 703 (55.6) | 0.03 |
| Current smoker | 517 (13.1) | 121 (9.6) | 173 (13.7) | 201 (15.9) | <0.001 |
| Hypertensionb | 3391 (86.1) | 982 (77.8) | 1114 (88.1) | 1166 (92.3) | <0.001 |
| Diabetesc | 1907 (48.4) | 400 (31.7) | 605 (47.9) | 824 (65.2) | <0.001 |
| Age (yr) | 60 (52, 66) | 61 (55, 67) | 61.0 (54.0, 67.5) | 57 (48, 64) | <0.001 |
| Body mass index (kg/m2) | 30.9 (26.8, 36.1) | 30.5 (26.7, 35.6) | 30.7 (26.6, 35.6) | 31.6 (27.1, 37.3) | 0.001 |
| Hemoglobin (gm/dl) | 12.5 (11.4, 13.8) | 13.0 (11.9, 14.1) | 12.6 (11.5, 13.8) | 12.1 (11.0, 13.3) | <0.001 |
| WBC (×103/μl) | 6.3 (5.2, 7.7) | 5.9 (4.9, 7.1) | 6.4 (5.2, 7.8) | 6.6 (5.4, 8.0) | <0.001 |
| Platelet count (103/μl) | 237 (195, 284) | 237 (195, 279) | 232 (191, 278) | 241 (200, 292) | <0.001 |
| eGFR (ml/min per 1.73 m2) | 42.2 (32.6, 51.9) | 48.5 (39.9, 57.0) | 42.0 (32.7, 50.7) | 35.8 (28.1, 45.1) | <0.001 |
| Serum creatinine (mg/dl) | 1.7 (1.4, 2.1) | 1.4 (1.2, 1.7) | 1.7 (1.4, 2.1) | 2.0 (1.6, 2.6) | <0.001 |
| Serum cystatin C (mg/L) | 1.4 (1.1, 1.8) | 1.2 (0.9, 1.4) | 1.4 (1.2, 1.8) | 1.7 (1.4, 2.1) | <0.001 |
| BUN (mg/dl) | 26 (20, 36) | 22 (17, 28) | 27 (21, 37) | 32 (24, 42) | <0.001 |
| Acute phase protein | |||||
| Serum albumin (g/dl) | 4.0 (3.7, 4.2) | 4.1 (3.9, 4.3) | 4.1 (3.8, 4.3) | 3.7 (3.4, 4.0) | <0.001 |
| hs-CRP (mg/L) | 2.6 (1.1, 6.5) | 2.2 (1.0, 5.9) | 2.8 (1.1, 7.0) | 2.7 (1.1, 6.5) | 0.002 |
| Fibrinogen (g/L) | 4.0 (3.4, 4.8) | 3.7 (3.2, 4.4) | 4.0 (3.3, 4.6) | 4.5 (3.8, 5.3) | <0.001 |
| Cytokines | |||||
| IL-1β (pg/ml) | 0.2 (0, 1.3) | 0 (0, 0.9) | 0.2 (0, 1.2) | 0.4 (0, 1.8) | <0.001 |
| IL-1RA (pg/ml) | 715.7 (390.0, 1551.0) | 672.7 (360.2, 1404.1) | 676.2 (383.4, 1492.7) | 834.6 (419.4, 1732.6) | <0.001 |
| IL-6 (pg/ml) | 1.9 (1.2, 3.2) | 1.5 (0.9, 2.5) | 2.0 (1.2, 3.3) | 2.2 (1.4, 3.5) | <0.001 |
| TNF-α (pg/ml) | 2.2 (1.5, 3.2) | 1.7 (1.2, 2.6) | 2.2 (1.5, 3.2) | 2.8 (1.9, 3.8) | <0.001 |
| TGF-β (pg/ml) | 11.0 (6.5, 17.9) | 10.4 (5.7, 17.5) | 10.6 (6.3, 17.2) | 11.6 (7.3, 18.7) | <0.001 |
Values are presented as median (interquartile range) or n (%). The chi-squared test was used for a variable presented as n (%), and the Kruskal–Wallis test was used for a variable presented as median (interquartile range). A value of 0 indicates that it was below the detection limit. P values <0.001 were significant after Bonferroni correction for multiple comparisons. UACR, urine albumin to creatinine ratio; IQR, interquartile range; WBC, white cell count; eGFR, estimated GFR; hs-CRP, high sensitivity C-reactive protein; IL-1RA, IL-1 receptor antagonist.
Medians (IQRs) for UACR were as follows: 51.9 (8.7, 458.8) for all three tertiles; 5.5 (3.6, 8.7) for tertile 1; 51.9 (27.1, 113.3) for tertile 2; and 977.0 (458.1, 2239.4) for tertile 3.
Hypertension at entry was defined as either systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or use of antihypertensive medications.
Diabetes was defined as fasting glucose ≥126 mg/dl, random glucose ≥200 mg/dl, or use of insulin or antidiabetic medication.
As shown in Table 2, median values of plasma levels of IL-1β, IL-1RA, IL-6, TNF-α, hs-CRP, and fibrinogen were in most instances higher, and serum albumin levels lower, in participants belonging to the lower categories of eGFR. A nominally significant difference in median plasma levels of TGF-β among eGFR groups was observed. However, this difference was attenuated and failed to retain statistical significance upon adjustment for multiple comparisons.
Table 2.
Plasma levels of inflammatory markers and acute phase proteins by eGFR category
| Biomarker | eGFR (ml/min per 1.73 m2) (n=3939) | P Valuea | ||||
|---|---|---|---|---|---|---|
| >60 (n=412) | 50–59 (n=754) | 40–49 (n=1042) | 30–39 (n=967) | <30 (n=764) | ||
| Acute phase protein | ||||||
| hs-CRP (mg/L) | 1.9 (0.9, 4.2) | 2.5 (1.0, 6.8) | 2.7 (1.1, 6.0) | 2.8 (1.2, 7.1) | 2.8 (1.1, 7.1) | <0.001 |
| Fibrinogen (g/L) | 3.5 (3.0, 4.1) | 3.9 (3.3, 4.5) | 4.0 (3.4, 4.7) | 4.2 (3.5, 5.0) | 4.5 (3.8, 5.4) | <0.001 |
| Albumin (g/dl) | 4.1 (3.8, 4.3) | 4.0 (3.8, 4.3) | 4.0 (3.7, 4.2) | 3.9 (3.6, 4.2) | 3.9 (3.6, 4.2) | <0.001 |
| Cytokines | ||||||
| IL-1β (pg/ml) | 0 (0, 0.7) | 0 (0, 0.9) | 0.2 (0, 1.3) | 0.3 (0, 1.4) | 0.4 (0, 2.0) | <0.001 |
| IL-1RA (pg/ml) | 605.3 (312.6, 1262.8) | 636.6 (350.3, 1340.3) | 698.7 (389.6, 1529.8) | 805.9 (448.8, 1642.6) | 850.5 (426.6, 1771.6) | <0.001 |
| IL-6 (pg/ml) | 1.2 (0.7, 2.1) | 1.6 (1.0, 2.6) | 1.8 (1.2, 2.9) | 2.2 (1.3, 3.5) | 2.4 (1.5, 3.9) | <0.001 |
| TNF-α (pg/ml) | 1.4 (1.0, 2.1) | 1.7 (1.2, 2.6) | 2.1 (1.5, 2.9) | 2.5 (1.8, 3.5) | 3.0 (2.2, 4.1) | <0.001 |
| TGF-β (pg/ml) | 11.3 (6.3, 19.4) | 10.1 (5.8, 16.6) | 11.4 (6.8, 18.2) | 11.2 (6.9, 18.1) | 10.6 (6.4, 17.5) | 0.01 |
Values are presented as the median (interquartile range). A value of 0 indicates that it was below the detection limit. P values <0.001 were significant after Bonferroni correction for multiple comparisons. eGFR, estimated GFR; hs-CRP, high-sensitivity C-reactive protein; IL-1RA, IL-1 receptor antagonist.
Kruskal–Wallis test between categories of eGFR
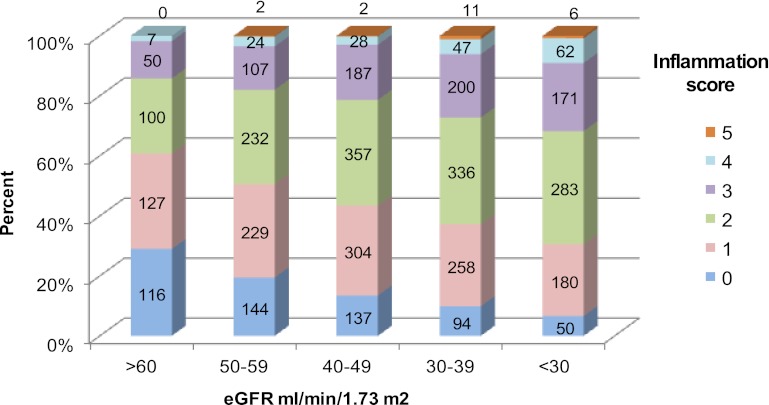
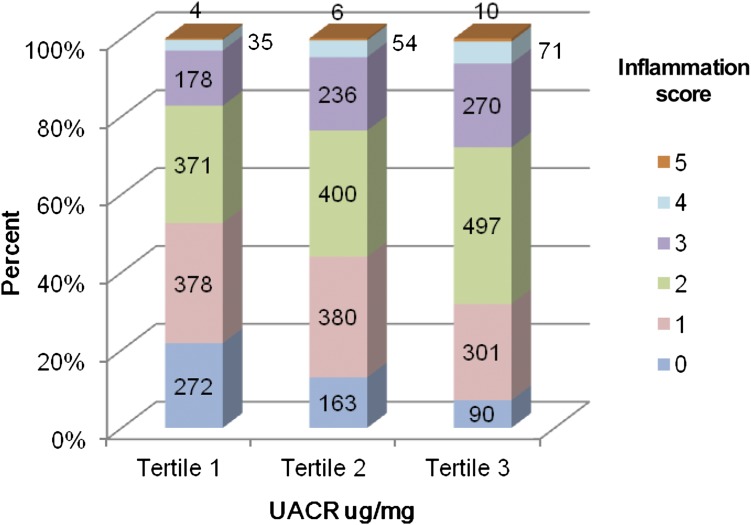
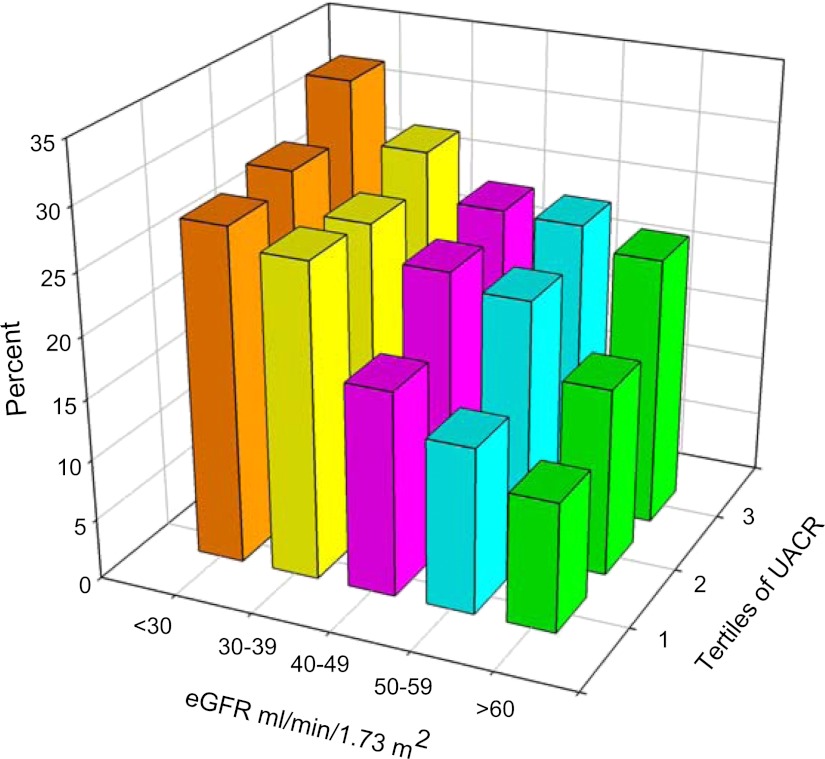
Supplemental Table 1 shows that within each category of eGFR, patients in the highest tertile of UACR had lower serum albumin and elevated levels of TNF-α. An inverse association between the inflammation score and eGFR was seen (P<0.001) (Figure 1). Participants in the highest level of eGFR (≥60 ml/min per 1.73 m2) had the greatest proportion of participants with lower inflammation scores (0 and 1), whereas the proportion of participants with higher inflammation scores (4 and 5) was higher in lower categories of eGFR. Of 752 patients with advanced CKD (eGFR ≤30 ml/min per 1.73 m2), 50 patients had no evidence of inflammation, which was noted. These patients were more likely to be nonsmoking Caucasian males with lower BMI and without diabetes who were receiving therapy with ACEIs/ARBs and lipid-lowering agents compared with those with inflammation (data not shown). Similarly, the inflammation score was higher in upper categories of UACR compared with those in the lower UACR categories (P<0.001) (Figure 2). There were 2852 (76.7%) participants with an inflammation score at or below the median value of 2 and 864 (23.3%) participants with an inflammation score above the median value. The proportion of participants with an inflammation score above the median value of 2 exhibited a tendency to be higher in the patients belonging to the higher tertiles of UACR within each level of eGFR studied (Figure 3). Inflammation score can be calculated based on biomarker levels above the median value in the study population or based on concentrations in the inflammation range, as presented in this study. Analysis of the data using the inflammation score computed with median values as reference points did not change the findings or conclusions significantly (data not shown).
Figure 1.
Distribution of inflammation score by categories of eGFR (n=3850). P value for inflammation score among categories of eGFR <0.001 by Kruskal–Wallis test. eGFR, estimated GFR.
Figure 2.
Distribution of Inflammation score by tertiles of UACR (n=3716). P value for inflammation score among tertiles of UACR <0.001 by Kruskal–Wallis test. UACR, urine albumin to creatinine ratio.
Figure 3.
Participants (%) with inflammation score above median value according to categories of eGFR and tertiles of UACR (n=3716). eGFR, estimated GFR; UACR, urine albumin to creatinine ratio.
In multivariable linear regression, after adjusting for age, sex, race/ethnicity, diabetes, hypertension, ACEI/ARB use, and lipid-lowering drug use, eGFR was inversely related to levels of IL-1β, IL-RA, IL-6, TNF-α, hs-CRP, and fibrinogen, whereas cystatin C was positively associated with them (Tables 3 and 4). The percentage increase in the average value of the outcome variable with each unit increase in the predictor variable is as follows: 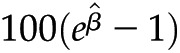 (25). For instance, for each unit increase in eGFR, there was a 0.5% (95% confidence interval, 0.2, 0.8) decrease in the average value of hs-CRP. UACR showed a positive association with fibrinogen, IL-6, and TNF-α and a negative association with serum albumin. We also conducted a sensitivity analysis using the eGFR equation derived from the CRIC cohort (16) and noted that the new estimates and P values did not change significantly (data not shown).
(25). For instance, for each unit increase in eGFR, there was a 0.5% (95% confidence interval, 0.2, 0.8) decrease in the average value of hs-CRP. UACR showed a positive association with fibrinogen, IL-6, and TNF-α and a negative association with serum albumin. We also conducted a sensitivity analysis using the eGFR equation derived from the CRIC cohort (16) and noted that the new estimates and P values did not change significantly (data not shown).
Table 3.
Multivariable regression model showing associations of eGFR, serum cystatin C, and UACR with serum levels of acute phase proteins
| Predictor | Outcome | |||||
|---|---|---|---|---|---|---|
| hs-CRP (mg/L) | Fibrinogen (g/L) | Albumin (g/dl) | ||||
| eGFR (ml/min per 1.73 m2) (n=3939) | −0.005 (−0.008, −0.002) | P<0.001 | −0.004 (−0.005, −0.003) | P<0.001 | 0.001 (0.00098, 0.002) | P<0.001 |
| Serum cystatin C (mg/L) (n=3927) | 0.36 (0.28, 0.43) | P<0.001 | 0.15 (0.13, 0.17) | P<0.001 | −0.046 (−0.053, −0.038) | P<0.001 |
| UACR (μg/100 mg) (n=3791)a | −0.001 (−0.004, 0.001) | P=0.34 | 0.005 (0.004, 0.006) | P<0.001 | −0.0038 (−0.0041, −0.0036) | P<0.001 |
Values are presented as 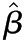 (95% confidence interval). Multivariable linear regression adjusted for age, sex, race/ethnicity, diabetes, hypertension, ACE/ARB use, and lipid-lowering drug use. Outcome variables were log-transformed. P values <0.001 were significant after Bonferroni correction for multiple comparisons. Interpretation:
(95% confidence interval). Multivariable linear regression adjusted for age, sex, race/ethnicity, diabetes, hypertension, ACE/ARB use, and lipid-lowering drug use. Outcome variables were log-transformed. P values <0.001 were significant after Bonferroni correction for multiple comparisons. Interpretation: 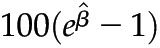 is the percentage increase in the average value of the outcome variable with each unit increase in the predictor variable. As an example, for each unit increase in eGFR, there was a 0.5% decrease in the average value of hs-CRP. eGFR, estimated GFR; UACR, urine albumin to creatinine ratio; hs-CRP, high-sensitivity C-reactive protein; 95% CI, 95% confidence interval; IL-1RA, IL-1 receptor antagonist.
is the percentage increase in the average value of the outcome variable with each unit increase in the predictor variable. As an example, for each unit increase in eGFR, there was a 0.5% decrease in the average value of hs-CRP. eGFR, estimated GFR; UACR, urine albumin to creatinine ratio; hs-CRP, high-sensitivity C-reactive protein; 95% CI, 95% confidence interval; IL-1RA, IL-1 receptor antagonist.
UACR values were converted to μg/100 mg for use in regression analysis.
Table 4.
Multivariable regression model showing associations of eGFR, serum cystatin C, and UACR with inflammatory markers
| Predictor | Outcome | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-1β (pg/ml) | IL-1RA (pg/ml) | IL-6 (pg/ml) | TNF-α (pg/ml) | TGF-β (ng/ml) | |||||||||
| eGFR (ml/min per 1.73 m2) (n=3939) | −0.05 (-0.06, -0.04) | P<0.001 | −0.006 (−0.008, −0.003) | P<0.001 | −0.012 (−0.014, −0.01) | P<0.001 | −0.016 (−0.018, −0.015) | P<0.001 | 0.001 (−0.0007, 0.003) | P=0.20 | |||
| Serum cystatin C (mg/L) (n=3927) | 1.4 (1, 1.7) | P<0.001 | 0.19 (0.13, 0.25) | P<0.001 | 0.5 (0.45, 0.55) | P<0.001 | 0.5 (0.46, 0.54) | P<0.001 | 0.02 (−0.03, 0.07) | P=0.43 | |||
| UACR (μg/100 mg) (n=3791)a | 0.009 (−0.003, 0.02) | P=0.15 | 0.0003 (−0.0018, 0.0023) | P=0.78 | 0.006 (0.004, 0.008) | P<0.001 | 0.006 (0.005, 0.008) | P<0.001 | 0.001 (−0.0005, 0.003) | P=0.17 | |||
Values are presented as 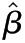 (95% confidence interval). Multivariable linear regression adjusted for age, sex, race/ethnicity, diabetes, hypertension, ACE/ARB use, and lipid-lowering drug use. Outcome variables were log-transformed. P values <0.001 were significant after Bonferroni correction for multiple comparisons. Interpretation:
(95% confidence interval). Multivariable linear regression adjusted for age, sex, race/ethnicity, diabetes, hypertension, ACE/ARB use, and lipid-lowering drug use. Outcome variables were log-transformed. P values <0.001 were significant after Bonferroni correction for multiple comparisons. Interpretation: 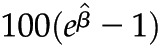 is the percentage increase in the average value of the outcome variable with each unit increase in the predictor variable. As an example, for each unit increase in eGFR, there was a 5% decrease in the average value of IL-1β. eGFR, estimated GFR; UACR, urine albumin to creatinine ratio; hs-CRP, high-sensitivity C-reactive protein; IL-1RA, IL-1 receptor antagonist.
is the percentage increase in the average value of the outcome variable with each unit increase in the predictor variable. As an example, for each unit increase in eGFR, there was a 5% decrease in the average value of IL-1β. eGFR, estimated GFR; UACR, urine albumin to creatinine ratio; hs-CRP, high-sensitivity C-reactive protein; IL-1RA, IL-1 receptor antagonist.
UACR values were converted to μg/100 mg for use in regression analysis.
Discussion
In this study, we examined the association between measures of kidney function (cystatin C and eGFR), albuminuria (UACR), and a panel of cytokines and acute phase proteins in the CRIC study participants. We found that the plasma levels of proinflammatory cytokines and positive acute phase proteins were higher in participants with lower levels of kidney function and higher levels of albuminuria.
Inflammatory response is initiated and sustained by soluble low molecular weight signaling protein including cytokines. These biomolecules act in a highly complex and coordinated network in which they induce or repress their own synthesis as well as that of other cytokines and cytokine receptors (26). We noted that plasma levels of IL-1β, IL-6, and TNF-α were higher in patients with a lower level of kidney function (Table 2). We also measured IL-1RA, the naturally occurring anti-inflammatory soluble receptor of IL-1β (27), which also followed the same trend as IL-1β. The elevated cytokine level in CKD has been attributed to retention of inflammatory mediators as well as increased tissue production of these biomolecules (9,28,29). Among circulating monocytes, subpopulations with proinflammatory characteristics are expanded in patients with kidney disease (30). Several uremic toxins act as ligands in activation of Toll like receptors (TLRs), which are involved in innate immune response and recognition of lipopolysaccharide (31). Feroze et al. and Raj et al. have shown that circulating endotoxin levels and their soluble CD14 receptors are elevated in patients with ESRD (32,33). Sympathetic over activity observed in CKD also could contribute to the augmented inflammatory response to endotoxin (34). In addition, acetylcholine release from vagus nerve stimulation could block cytokine production by cells expressing acetylcholine receptors (35). Thus, multiple factors contribute to the inflammatory state in CKD.
Synthesis of acute phase proteins is regulated by cytokines released from the site of injury (36). We found that the CRP and fibrinogen levels increased across the spectrum of renal function studied. CRP is a member of the pentraxin family of innate immune response proteins and a well recognized risk factor for cardiovascular disease in the general population as well as in patients with CKD (37). In an observational cohort study, Snaedal et al. showed that inflammation assessed by using serial CRP levels is a strong predictor of mortality (38). Fibrinogen is a soluble glycoprotein found in the plasma, with a molecular mass of 340 kD that plays a vital role in a number of physiopathological processes in the body, including inflammation, atherogenesis and thrombogenesis (39). Serum albumin is a marker of visceral protein content as well as a negative marker of inflammation (40). Hypoalbuminemia is a powerful predictor of mortality in the general populationas well as in patients with CKD (41,42). Considerable evidence indicates that protein-energy wasting and inflammation may be linked in accelerating the cardiovascular disease in CKD (43).
Although the concentrations of proinflammatory cytokines and acute phase proteins show a tendency to increase together, there is discordance in the rate and magnitude of surge of individual molecules involved in the inflammation cascade (8). Thus, it is important to integrate information from multiple biomarkers to describe the prevailing inflammatory state (18–20). We computed an inflammation score after integrating the data from all measured proinflammatory markers and found that the intensity of inflammation was positively related to the level of UACR and inversely related to eGFR (Figures 1 and 2).
Inflammation has been shown to predict the long-term risk of developing CKD. In a population-based study of predominantly Caucasians (n=4926) with 15 years of follow-up, Shankar et al. (44) found that hs-CRP, TNF-αR2, WBC count, and IL-6 levels were associated with prevalent CKD at baseline. However, longitudinal analysis showed that only TNF-αR2, WBC count, and IL-6 levels were associated with incident CKD. Recently, Niewczas et al. showed that elevated concentrations of circulating TNF receptors 1 and 2 at baseline are very strong predictors of the subsequent progression to ESRD in participants with type 2 diabetes (45). In this study, multivariable linear regression analysis demonstrated statistically significant associations between indicators of inflammation and eGFR and cystatin C (Tables 3 and 4). The Prevention of Renal and Vascular End Stage Disease (PREVEND) study, which involved 8058 Caucasians aged 28–75 years from the Netherlands, showed that CRP level was associated with cystatin C after adjustment for 24-hour urinary creatinine clearance (46). On the other hand, results from the Heart and Soul study, which recruited 986 adults with stable coronary artery disease from outpatient clinics in the San Francisco Bay Area, did not support an independent association of cystatin C with inflammation (47). The median eGFR was lower and cystatin C concentration higher in CRIC study participants compared with the PREVEND study and Heart and Soul study participants. UACR was positively related to fibrinogen, IL-6, and TNF-α and negatively with serum albumin. While it is possible that proinflammatory cytokines may be pathogenically involved in promoting proteinuria, it is also possible that albuminuria selectively activates cytokines that drives hepatic albumin and fibrinogen synthesis (36).
This study has a number of strengths, which include a large cohort of patients with adequate representation of race/ethnicity, broad range of kidney function, and evaluation of a number of biomarkers of inflammation. However, our findings should be considered within the context of several factors. It is well recognized that cytokine profile and acute phase response exhibit substantial inter- and intra-individual variability over time, being influenced by multiple processes, such as transient infections and comorbidities (48). Thus, biomarker profile at a single time point may not be reflective of the true association with the factors we have considered. IL-1β concentrations were below the detection limit of 0.06 pg/ml in some samples for which we made adjustments in the data analysis. The observed imprecision in some cytokine assays could be due to the highly sensitive signal-amplified ELISAs used in these assays. Finally, this is a cross-sectional study and hence temporal associations and causality cannot be inferred.
Our study demonstrates that the intensity of inflammation is greater in participants with lower kidney function and higher levels of albuminuria; both are well recognized risk factors for cardiovascular disease (49,50). A number of intervention studies targeting established risk factors for mortality in CKD and ESRD have not yielded the anticipated positive results (51,52). Such negative studies could be due to the fact that inflammation in CKD has a competing and possibly overwhelming effect on outcome rendering these interventions ineffective (53). Whether inflammation is an innocent bystander or a culprit that contributes directly to mortality and morbidity is still debated. However, there is some consensus that future studies should be conducted with an aim to understand the mechanisms of inflammation in CKD, so that specific targeted interventions could be considered if causality can be proved.
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to the CRIC study participants and the devoted staff that made this research possible.
This study is supported in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) awarded to D.R. (R01 DK073665-01A1) and M.R. (R01-DK071224). Funding for the CRIC study was obtained under a cooperative agreement from the NIDDK (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the University of Pennsylvania CTRC CTSA (UL1 RR-024134), Johns Hopkins University (UL1 RR-025005), University of Maryland GCRC (M01 RR-16500), Clinical and Translational Science Collaborative of Cleveland (UL1TR000439 from the National Center for Advancing Translational Sciences component of the National Institutes of Health and NIH roadmap for Medical Research), Michigan Institute for Clinical and Health Research (UL1RR024986), University of Illinois at Chicago CTSA (UL1RR029879), The Clinical and Translational Research, Education, and Commercialization Project, and Kaiser NIH/NCRR UCSF-CTSI (UL1RR-024131).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03500412/-/DCSupplemental.
References
- 1.Wison S, Foo K, Cunningham J, Cooper J, Deaner A, Knight C, Ranjadayalan K, Timmis AD: Renal function and risk stratification in acute coronary syndromes. Am J Cardiol 91: 1051–1054, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Reddan DN, Szczech LA: Renal insufficiency and the risk of cardiovascular mortality. Kidney Int 62: 1474–1475, author reply 1474–1475, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE, Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group : Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106: 1777–1782, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Anavekar NS, Gans DJ, Berl T, Rohde RD, Cooper W, Bhaumik A, Hunsicker LG, Rouleau JL, Lewis JB, Rosendorff C, Porush JG, Drury PL, Esmatjes E, Raz I, Vanhille P, Locatelli F, Goldhaber S, Lewis EJ, Pfeffer MA: Predictors of cardiovascular events in patients with type 2 diabetic nephropathy and hypertension: A case for albuminuria. Kidney Int Suppl 92: S50–S55, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Kimmel PL, Phillips TM, Simmens SJ, Peterson RA, Weihs KL, Alleyne S, Cruz I, Yanovski JA, Veis JH: Immunologic function and survival in hemodialysis patients. Kidney Int 54: 236–244, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z: Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 3: 505–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Inflammation and cardiovascular events in individuals with and without chronic kidney disease. Kidney Int 73: 1406–1412, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabay C, Kushner I: Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340: 448–454, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Kudo S, Goto H: Intrarenal handling of recombinant human interleukin-1alpha in rats: Mechanism for proximal tubular protein reabsorption. J Interferon Cytokine Res 19: 1161–1168, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Rostaing L, Chatelut E, Payen JL, Izopet J, Thalamas C, Ton-That H, Pascal JP, Durand D, Canal P: Pharmacokinetics of alphaIFN-2b in chronic hepatitis C virus patients undergoing chronic hemodialysis or with normal renal function: Clinical implications. J Am Soc Nephrol 9: 2344–2348, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Feghali CA, Wright TM: Cytokines in acute and chronic inflammation. Front Biosci 2: d12–d26, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Rao M, Wong C, Kanetsky P, Girndt M, Stenvinkel P, Reilly M, Raj DS: Cytokine gene polymorphism and progression of renal and cardiovascular diseases. Kidney Int 72: 549–556, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, Heimbürger O, Cederholm T, Girndt M: IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int 67: 1216–1233, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM: Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 107: 87–92, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT, Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI, CRIC Study Investigators : Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jager W, Bourcier K, Rijkers GT, Prakken BJ, Seyfert-Margolis V, de JW : Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol 10: 52, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G, Atherosclerosis Risk in Communities Study : Low-grade systemic inflammation and the development of type 2 diabetes: The atherosclerosis risk in communities study. Diabetes 52: 1799–1805, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Recasens M, López-Bermejo A, Ricart W, Vendrell J, Casamitjana R, Fernández-Real JM: An inflammation score is better associated with basal than stimulated surrogate indexes of insulin resistance. J Clin Endocrinol Metab 90: 112–116, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Molnar MZ, Czira ME, Rudas A, Ujszaszi A, Haromszeki B, Kosa JP, Lakatos P, Beko G, Sarvary E, Varga M, Fornadi K, Novak M, Rosivall L, Kiss I, Remport A, Goldsmith DJ, Kovesdy CP, Mucsi I: Association between the malnutrition-inflammation score and post-transplant anaemia. Nephrol Dial Transplant 26: 2000–2006, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, Gallimore JR, Pepys MB: Low grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. BMJ 321: 199–204, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Cutrupi S, Parlongo S, Malatino LS, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Nicocia G, Buemi M: Fibrinogen, inflammation and concentric left ventricular hypertrophy in chronic renal failure. Eur J Clin Invest 33: 561–566, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Tripepi G, Mallamaci F, Zoccali C: Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: Searching for the best risk marker by multivariate modeling. J Am Soc Nephrol 16[Suppl 1]: S83–S88, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Zoccali C, Tripepi G, Mallamaci F: Dissecting inflammation in ESRD: Do cytokines and C-reactive protein have a complementary prognostic value for mortality in dialysis patients? J Am Soc Nephrol 17[Suppl 3]: S169–S173, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE: Regression Methods in Biostatistics, New York, Springer, 2005 [Google Scholar]
- 26.Descamps-Latscha B, Herbelin A, Nguyen AT, Roux-Lombard P, Zingraff J, Moynot A, Verger C, Dahmane D, de Groote D, Jungers P: Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J Immunol 154: 882–892, 1995 [PubMed] [Google Scholar]
- 27.Balakrishnan VS, Schmid CH, Jaber BL, Natov SN, King AJ, Pereira BJ: Interleukin-1 receptor antagonist synthesis by peripheral blood mononuclear cells: A novel predictor of morbidity among hemodialysis patients. J Am Soc Nephrol 11: 2114–2121, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Kimmel PL, Phillips TM, Phillips E, Bosch JP: Effect of renal replacement therapy on cellular cytokine production in patients with renal disease. Kidney Int 38: 129–135, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Pecoits-Filho R, Heimbürger O, Bárány P, Suliman M, Fehrman-Ekholm I, Lindholm B, Stenvinkel P: Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis 41: 1212–1218, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Heine GH, Ulrich C, Seibert E, Seiler S, Marell J, Reichart B, Krause M, Schlitt A, Köhler H, Girndt M: CD14(++)CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int 73: 622–629, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Gollapudi P, Yoon JW, Gollapudi S, Pahl MV, Vaziri ND: Leukocyte toll-like receptor expression in end-stage kidney disease. Am J Nephrol 31: 247–254, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Feroze U, Kalantar-Zadeh K, Sterling KA, Molnar MZ, Noori N, Benner D, Shah V, Dwivedi R, Becker K, Kovesdy CP, Raj DS: Examining associations of circulating endotoxin with nutritional status, inflammation, and mortality in hemodialysis patients. J Ren Nutr 22: 317–326, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raj DS, Carrero JJ, Shah VO, Qureshi AR, Barany P, Heimburger O, Lindholm B, Ferguson J, Moseley PL, Stenvinkel P: Soluble CD14 levels, interleukin 6, and mortality among prevalent hemodialysis patients. Am J Kidney Dis 54: 1072–1080, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safieh-Garabedian B, Poole S, Haddad JJ, Massaad CA, Jabbur SJ, Saadé NE: The role of the sympathetic efferents in endotoxin-induced localized inflammatory hyperalgesia and cytokine upregulation. Neuropharmacology 42: 864–872, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Huston JM, Tracey KJ: The pulse of inflammation: Heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med 269: 45–53, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raj DS, Dominic EA, Wolfe R, Shah VO, Bankhurst A, Zager PG, Ferrando A: Coordinated increase in albumin, fibrinogen, and muscle protein synthesis during hemodialysis: Role of cytokines. Am J Physiol Endocrinol Metab 286: E658–E664, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Ridker PM: High-sensitivity C-reactive protein: Potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 103: 1813–1818, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Snaedal S, Heimbürger O, Qureshi AR, Danielsson A, Wikström B, Fellström B, Fehrman-Ekholm I, Carrero JJ, Alvestrand A, Stenvinkel P, Bárány P: Comorbidity and acute clinical events as determinants of C-reactive protein variation in hemodialysis patients: Implications for patient survival. Am J Kidney Dis 53: 1024–1033, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Wardle EN: Fibrinogen catabolism studies in patients with renal disease. Q J Med 42: 205–219, 1973 [PubMed] [Google Scholar]
- 40.Kaysen GA, Chertow GM, Adhikarla R, Young B, Ronco C, Levin NW: Inflammation and dietary protein intake exert competing effects on serum albumin and creatinine in hemodialysis patients. Kidney Int 60: 333–340, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Goldwasser P, Feldman J: Association of serum albumin and mortality risk. J Clin Epidemiol 50: 693–703, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J Am Soc Nephrol 7: 728–736, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T: Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Shankar A, Sun L, Klein BE, Lee KE, Muntner P, Nieto FJ, Tsai MY, Cruickshanks KJ, Schubert CR, Brazy PC, Coresh J, Klein R: Markers of inflammation predict the long-term risk of developing chronic kidney disease: A population-based cohort study. Kidney Int 80: 1231–1238, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, Cullere X, Eckfeldt JH, Doria A, Mayadas TN, Warram JH, Krolewski AS: Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 23: 507–515, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE: Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 65: 1416–1421, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Singh D, Whooley MA, Ix JH, Ali S, Shlipak MG: Association of cystatin C and estimated GFR with inflammatory biomarkers: The Heart and Soul Study. Nephrol Dial Transplant 22: 1087–1092, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nascimento MM, Pecoits-Filho R, Qureshi AR, Hayashi SY, Manfro RC, Pachaly MA, Renner L, Stenvinkel P, Lindholm B, Riella MC: The prognostic impact of fluctuating levels of C-reactive protein in Brazilian haemodialysis patients: A prospective study. Nephrol Dial Transplant 19: 2803–2809, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Perkovic V, Verdon C, Ninomiya T, Barzi F, Cass A, Patel A, Jardine M, Gallagher M, Turnbull F, Chalmers J, Craig J, Huxley R: The relationship between proteinuria and coronary risk: A systematic review and meta-analysis. PLoS Med 5: e207, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marcus J, Sarnak MJ, Menon V: Homocysteine lowering and cardiovascular disease risk: Lost in translation. Can J Cardiol 23: 707–710, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E, German Diabetes and Dialysis Study Investigators : Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Carrero JJ, Stenvinkel P: Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: A hypothesis proposal. Clin J Am Soc Nephrol 4[Suppl 1]: S49–S55, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.