Summary
Background and objectives
Previous studies have shown that Aboriginals and Caucasians experience similar outcome on dialysis in Canada. Using the Canadian Organ Replacement Registry, this study examined whether dialysis modality (peritoneal or hemodialysis) impacted mortality in Aboriginal patients.
Design, setting, participants, & measurements
This study identified 31,576 adult patients (hemodialysis: Aboriginal=1839, Caucasian=21,430; peritoneal dialysis: Aboriginal=554, Caucasian=6769) who initiated dialysis between January of 2000 and December of 2009. Aboriginal status was identified by self-report. Dialysis modality was determined 90 days after dialysis initiation. Multivariate Cox proportional hazards and competing risk models were constructed to determine the association between race and mortality by dialysis modality.
Results
During the study period, 939 (51.1%) Aboriginals and 12,798 (53.3%) Caucasians initiating hemodialysis died, whereas 166 (30.0%) and 2037 (30.1%), respectively, initiating peritoneal dialysis died. Compared with Caucasians, Aboriginals on hemodialysis had a comparable risk of mortality (adjusted hazards ratio=1.04, 95% confidence interval=0.96–1.11, P=0.37). However, on peritoneal dialysis, Aboriginals experienced a higher risk of mortality (adjusted hazards ratio=1.36, 95% confidence interval=1.13–1.62, P=0.001) and technique failure (adjusted hazards ratio=1.29, 95% confidence interval=1.03–1.60, P=0.03) than Caucasians. The risk of technique failure varied by patient age, with younger Aboriginals (<50 years old) more likely to develop technique failure than Caucasians (adjusted hazards ratio=1.76, 95% confidence interval=1.23–2.52, P=0.002).
Conclusions
Aboriginals on peritoneal dialysis experience higher mortality and technique failure relative to Caucasians. Reasons for this race disparity in peritoneal dialysis outcomes are unclear.
Introduction
The Aboriginal community is one of the fastest-growing segments of the population in Canada, representing a fivefold higher growth rate compared with non-Aboriginals (1). The vast majority of this growth is contained within the younger age demographics; in 2006, the median age of the Aboriginal population was 27 years compared with 40 years for non-Aboriginal people. However, despite this rapid rate of growth, life expectancy among Aboriginals remains significantly reduced because of high rates of medical illnesses, such as hypertension, diabetes, and CKD (2–5). Disproportionately high rates of ESRD in Aboriginal peoples have been described not only in Canada but also among Indigenous populations in the United States, Australia, and New Zealand (3,6–8). As the wave of young Aboriginals in Canada ages, it is likely that rates of ESRD will increase as well.
Canada’s Aboriginal population is geographically diverse, with a larger proportional representation in Western Canada, and they often reside in remote communities with limited access to health services (9). Although home dialysis modalities such as peritoneal dialysis may be considered optimal for the Aboriginal population living in remote locations, concerns regarding potential differences in survival by dialysis modality have been raised (10).
Racial outcomes on different dialysis modalities are conflicting. An earlier study in Canada found no difference in outcomes between Aboriginals and Caucasians irrespective of dialysis modality (11,12). This finding is congruent with studies from Alaska and the United States that also demonstrated similar outcomes for Indigenous peoples on intermittent hemodialysis compared with Caucasians (6,13). However, this finding is in direct contrast to some findings from Australia and New Zealand, where Aboriginals on peritoneal dialysis have worse outcomes with an increased risk of peritonitis, technique failure, hospitalizations, and mortality (14–16). Recently, we have also reported an increase in peritonitis and mortality rates in Aboriginals relative to Caucasians undergoing peritoneal dialysis in Manitoba, Canada (10,17). Whether these differences are caused by differences in patient’s characteristics, methods of care delivery, or study design remains unknown.
We used data from the Canadian Organ Replacement Registry (CORR), a large, contemporary national ESRD database, to investigate whether there are differences in survival among Aboriginal and Caucasian people initiating dialysis in Canada and evaluate potential differences by dialysis modality.
Materials and Methods
Study Design
The study was approved by the Research Ethics Board of the University of Manitoba and the Hospital Ethics Board at St. Boniface Hospital in Winnipeg, Manitoba. We obtained data on all incident dialysis patients from January 1, 2000 to December of 2009 from the CORR. The CORR captures data on all dialysis patients in Canada, excluding Quebec, and it includes demographics, outcomes, dialysis modality, comorbidities, cause of ESRD, and transplantation. Clinical and laboratory data are obtained at the onset of ESRD. The study cohort included 31,576 incident dialysis patients, of which 23,269 were on hemodialysis (Aboriginal=1839, Caucasian=21,430) and 7323 were on peritoneal dialysis (Aboriginal=554, Caucasian=6769) (8).
Cohort Definitions
Racial information in CORR is recorded by healthcare providers based on patient self-report. Modality was taken on day 90 and consisted of either hemodialysis or peritoneal dialysis. For patients on hemodialysis, vascular access (permanent or tunneled catheter or arteriovenous fistula/arteriovenous graft) was determined at dialysis initiation. Comorbid illnesses included a history of angina, myocardial infarction, diabetes mellitus, peripheral vascular disease, malignancy, hypertension medication usage, cigarette smoker, lung disease, and stroke. Causes of ESRD included ischemia, diabetes mellitus, glomerulonephritis, interstitial disease, polycystic kidney disease, obstruction, other, and unknown. Provinces and territories were categorized as geographic regions as follows: Atlantic (New Brunswick, Nova Scotia, Prince Edward Island, and Newfoundland), Central (Ontario), Prairies (Alberta, Saskatchewan, Manitoba, Nunavut, and Northwest Territories), and Pacific (British Columbia and Yukon). Predialysis care was the length of time from when a patient was first seen by a nephrologist to the initiation of dialytic therapy. Distance to center was the spherical distance in kilometers from a patient’s initial dialysis center to a patient’s residence based on postal codes. Patients were classified as rural if they resided greater than 50 km from their initial dialysis center. For peritoneal dialysis patients only, residual renal function was the first available weekly renal Kt/V measurement, dialysis adequacy was the first available total weekly Kt/V, and the peritoneal equilibration test was classified as low, low average, high average, and high according to internationally accepted standard definitions.
Study Outcomes
The primary outcome was all-cause mortality. Competing outcomes used in the sensitivity analysis included transplantation and for peritoneal dialysis only, technique failure. Patients who regained kidney function, were lost to follow-up, transferred out of a participating province, or remained in the study to the end of the study period were censored. Technique failure was defined as transfer from peritoneal dialysis to hemodialysis. In patients with multiple episodes of technique failure, only the first occurrence was counted.
Statistical Analyses
To assess our main study outcome of all-cause mortality, we used the Kaplan–Meier method and Cox proportional hazards models. Models were sequentially adjusted for age, sex, distance from center, era, predialysis care, geographic region, comorbidity, body mass index, cause of ESRD, dialysis access, and initial laboratory investigations (serum albumin, hemoglobin, and serum phosphorous). The primary research question on whether mortality differs based on Aboriginal race and modality was addressed by formally testing the race × modality interaction. The interaction term was highly significant (P=0.002), suggesting that the association between mortality and race differed according to modality. A secondary, exploratory analysis was performed in peritoneal dialysis patients for the outcome of technique failure by race and impact of age. We additionally assessed potential peritoneal dialysis-related confounders, including peritoneal equilibration test, residual renal function, and total delivered dialysis dose. These peritoneal dialysis-specific variables had a high proportion of missing data and thus, were not included in final models.
Because traditional Cox models can be biased by competing outcomes and because Aboriginals have a well-reported differential rate of renal transplantation, we additionally performed a competing outcomes analysis using the modified risks regression according to the work by Fine and Gray (18). In the analysis for patients on hemodialysis, the competing risk was transplantation; in peritoneal dialysis, the competing outcome were transplantation and technique failure. The model by Fine and Gray (18) yields an adjusted subhazard ratio. All models were further adjusted by propensity score. Individual propensity scores were created for each modality by adjusting for the previously mentioned covariates.
Because we assigned dialysis modality as the modality that patients were using by 90 days after the initiation of dialysis, we performed additional sensitivity analyses to examine all patients exposed to peritoneal dialysis.
Multiple imputation was used for missing values, with a random draw from the predictive distribution from an imputation model repeated 10 times. An iterative Markov chain Monte Carlo method was used, and pooled estimates were reported. Missing data imputed included body mass index, serum albumin, and distance from center. Imputed and original data and point estimates were compared, and there were no qualitative differences in any of the outcomes.
Analyses were performed using PASW Version 18, and the analyses using the method in the work by Fine and Gray (18) were performed using R. All hypothesis tests were two-sided, with statistical significance set at a P value<0.05.
Results
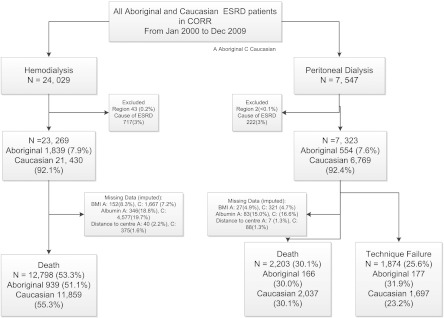
Using the CORR, we identified 31,576 incident dialysis patients between January of 2000 and December of 2009. Among the 23,269 patients on hemodialysis, 12,798 (55.3%) and 939 (51.1%) of the Caucasians and Aboriginals, respectively, died during the study period. Among the 7323 patients on peritoneal dialysis, 2037 (30.1%) and 166 (30.0%), respectively, died. The proportion of missing data was comparable between modalities and races (Figure 1).
Figure 1.
Development of the study cohort.
In general (Tables 1 and 2), Aboriginals starting on dialysis were younger, were more likely female, had a higher body mass index, and were more likely to reside in rural settings. They were less likely to have a history of cardiac disease (angina or coronary artery bypass graft) or malignancy at dialysis initiation. Conversely, they were more likely to have ESRD caused by diabetes mellitus and laboratory derangements, such as anemia, hypoalbuminemia, and hyperphosphatemia. Aboriginals on hemodialysis were more often dialyzed by a central venous catheter, whereas those patients on peritoneal dialysis had lower residual renal function and shorter predialysis care.
Table 1.
Characteristics of the study cohort stratified by race and dialysis modality
| Characteristic | Hemodialysis | P Value | Peritoneal Dialysis | P value | ||
|---|---|---|---|---|---|---|
| Aboriginal | Caucasian | Aboriginal | Caucasian | |||
| N | 1839 | 21,430 | 554 | 6769 | ||
| Age (mean ± SD) | 55.3±14.1 | 66.5±14.5 | <0.001 | 52.7±14.5 | 61.3±15.5 | <0.001 |
| Sex (percent female; N) | 50.4 (926) | 39.6 (8489) | <0.001 | 48.9 (271) | 40.4 (2733) | 0.001 |
| BMI (mean ± SD) | 29.4±6.8 | 27.8±7.1 | <0.001 | 28.8±5.6 | 27.1±5.7 | <0.001 |
| Median distance to center (IQR; km) | 167.5 (217) | 12.0 (47) | <0.001 | 171.6 (380) | 20.2 (70) | <0.001 |
| Rural percent (N) | 61.4 (1130) | 24.1 (5160) | <0.001 | 73.5 (407) | 33.0 (2232) | <0.001 |
| Geographic region percent (N) | ||||||
| Atlantic | 3.4 (64) | 12.6 (2706) | <0.001 | 12 (2.2) | 13.6 (918) | <0.001 |
| Central | 24.8 (456) | 57.0 (12,210) | <0.001 | 28.9 (160) | 51.2 (3468) | <0.001 |
| Prairie | 64.4 (1184) | 20.7 (4431) | <0.001 | 57.8 (320) | 21.4 (1449) | <0.001 |
| Pacific | 7.3 (135) | 9.7 (2083) | 0.01 | 11.2 (62) | 13.8 (933) | 0.09 |
| Comorbidities | ||||||
| Angina | 18.6 (342) | 24.0 (5150) | <0.001 | 13 (72) | 17.3 (1170) | 0.009 |
| Acute coronary syndrome | 18.5 (340) | 23.7 (5085) | <0.001 | 13.5 (75) | 17.1 (1160) | 0.03 |
| Pulmonary edema | 31.1 (572) | 28.3 (6070) | 0.01 | 17.0 (94) | 16.9 (1141) | 0.98 |
| Diabetes mellitus | 73.3 (1390) | 44.6 (9874) | <0.001 | 64.1 (355) | 41.7 (2825) | <0.001 |
| Stroke | 13.7 (252) | 15.4 (3290) | 0.06 | 8.7 (48) | 11.5 (777) | 0.04 |
| Peripheral vascular disease | 24.2 (445) | 21.1 (4512) | 0.002 | 15.5 (86) | 15.7 (1062) | 0.98 |
| Malignancy | 5.0 (92) | 14.8 (3276) | <0.001 | 2.9 (16) | 9.6 (650) | <0.001 |
| Lung disease | 8.8 (161) | 13.5 (2888) | <0.001 | 5.1 (28) | 7.9 (534) | 0.02 |
| Hypertensive medications | 81.7 (1502) | 80.7 (17296) | 0.30 | 82.3 (456) | 85.2 (5764) | 0.07 |
| Current smoker | 23.9 (440) | 13.7 (2930) | <0.001 | 24.2 (134) | 13.6 (920) | <0.001 |
| CABG | 8.8 (162) | 15.0 (3208) | <0.001 | 5.2 (29) | 11.7 (795) | <0.001 |
N, cohort size; BMI, body mass index; IQR, interquartile range; CABG, coronary artery bypass graft.
Table 2.
Dialysis-specific characteristics of the study cohort
| Characteristic | Hemodialysis | P Value | Peritoneal Dialysis | P value | ||
|---|---|---|---|---|---|---|
| Aboriginal | Caucasian | Aboriginal | Caucasian | |||
| Cause of ESRD % (N) | ||||||
| Hypertension | 5.9 (109) | 24.9 (5331) | <0.001 | 8.3 (46) | 21 (1422) | <0.001 |
| Diabetes mellitus | 66.3 (1219) | 34.3 (7349) | <0.001 | 59.2 (328) | 35.5 (2379) | <0.001 |
| Glomerulonephritis | 14.7 (271) | 12.9 (2754) | 0.02 | 20.2 (112) | 19.0 (1285) | 0.48 |
| Obstruction | 1.2 (22) | 3.3 (717) | <0.001 | 1.4 (8) | 2.9(199) | 0.04 |
| Interstitial | 0.8 (15) | 1.1 (227) | 0.34 | 1.3 (7) | 1.3 (86) | 0.96 |
| Polycystic kidney disease | 0.7 (13) | 4.2 (891) | <0.001 | 1.3 (6) | 6.8 (459) | <0.001 |
| Other | 3.5 (72) | 9.1 (1960) | <0.001 | 2.7 (15) | 6.9 (467) | <0.001 |
| Unknown | 6.4 (118) | 10.3 (2210) | <0.001 | 5.8 (32) | 7.0 (471) | 0.32 |
| Albumin (g/L; mean ± SD) | 28.2±6.9 | 31.8±6.8 | <0.001 | 32.0±6.8 | 35.3±6.3 | <0.001 |
| Hemoglobin (g/L; mean ± SD) | 93.5±18.1 | 100.8±17.2 | <0.001 | 103.6±18.1 | 109.3±21.8 | <0.001 |
| Phosphorous (mmol/L; mean ± SD) | 2.21±0.82 | 1.96±0.73 | <0.001 | 2.05±0.61 | 1.82±0.58 | <0.001 |
| Median number of days with predialysis care (IQR) | 108 (588) | 119 (790) | 0.62 | 214 (744) | 340 (1123) | 0.002 |
| Any predialysis care % (N) | 71.9 (1323) | 70.3 (15,071) | 0.22 | 81.2 (450) | 78.1 (5293) | <0.001 |
| AVF/AVG % (N) | 11.2 (206) | 14.4 (3083) | <0.001 | NA | NA | NA |
N, cohort size; IQR, interquartile range; AVF, arteriovenous fistula; AVG, arteriovenous graft; NA, not applicable.
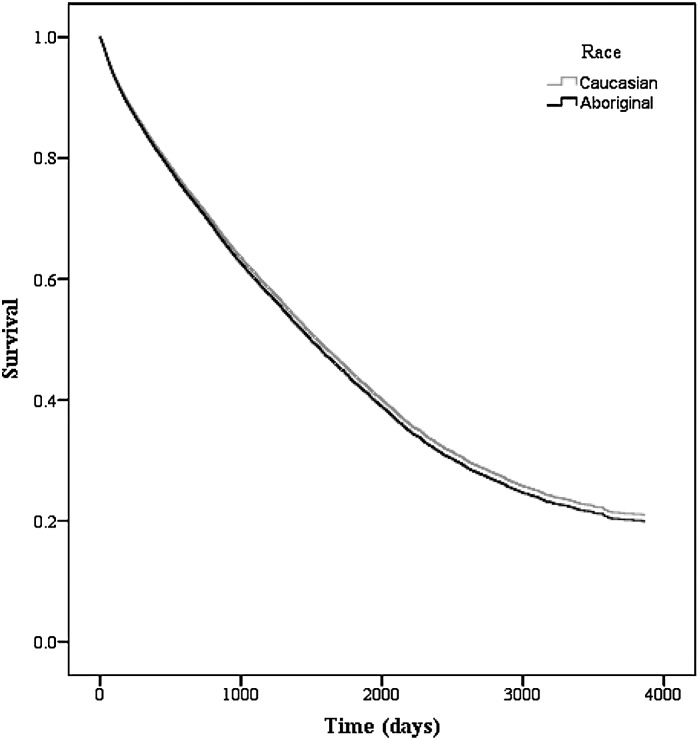
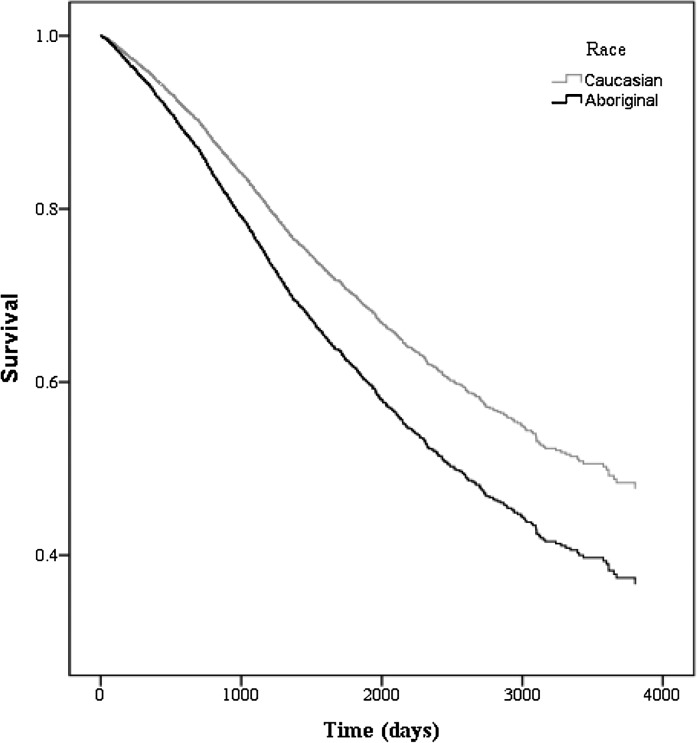
Among patients on hemodialysis, there were 12,798 deaths (Aboriginal=939 [51.1%], Caucasian=11,859 [55.3%]) during the study period. The median survival time was 2051 and 1835 days for Aboriginals and Caucasians, respectively (P<0.001).The unadjusted hazard ratio (HR) suggested a mortality benefit for Aboriginals on hemodialysis (HR=0.82, 95% confidence interval [CI]=0.77–0.87, P<0.001); however, this finding was attenuated after adjustment [HR=1.04, 95% CI=0.96–1.11, P=0.37) (Figure 2 and Table 3). Among patients on peritoneal dialysis, there were 2203 deaths (Aboriginal=166 [30.0%], Caucasian=2037 [30.1%]) and 1874 episodes of technique failure (Aboriginal=177 [31.9%], Caucasian=1697 [23.2%]). The median survival time was 2941 and 3670 days for Aboriginals and Caucasians, respectively (P=0.47). After adjustment, Aboriginal status was independently associated with mortality (adjusted HR=1.36, 95% CI=1.13–1.62, P=0.001) (Figure 3 and Table 4) and technique failure (adjusted HR=1.29, 95% CI=1.05–1.57, P=0.03). In our sensitivity analyses, we used the method by Fine and Gray (18) to account for the competing risk of death and transplantation in hemodialysis and death, transplantation, and technique failure in peritoneal dialysis. The competing risk models were similar to the Cox models, and all models changed minimally after propensity score adjustment (Tables 3 and 4). Additional examination of reclassifying our definition of modality to patients with any exposure to peritoneal dialysis resulted in no qualitative differences in our results.
Figure 2.
Adjusted survival for ESRD patients on hemodialysis by race. Model is adjusted for age, sex, era, comorbidities (angina, acute coronary syndrome, pulmonary edema, lung disease, malignancy, coronary artery bypass graft [CABG], smoker, hypertension medications, stroke, peripheral vascular disease, and diabetes mellitus), geographic region, any predialysis care, distance to center, serum albumin, and body mass index.
Table 3.
Crude and adjusted survival for ESRD patients on hemodialysis by race accounting for competing risks and propensity score
| Cox HR | 95% CI | P Value | Competing Risks HR | 95% CI | P Value | |
|---|---|---|---|---|---|---|
| Unadjusted | 0.82 | 0.77–0.87 | <0.001 | 0.83 | 0.78–0.86 | <0.001 |
| Adjusted | 1.04 | 0.96–1.11 | 0.37 | 1.05 | 0.96–1.11 | 0.22 |
| Propensity score adjusted | 1.01 | 0.94–1.09 | 0.72 | 1.02 | 0.94–1.10 | 0.70 |
Data on 23,269 patients (Aboriginal=1839, Caucasian=21,430) and 12,798 deaths (Aboriginal=939, Caucasian=11,859). Model adjusted for age, sex, era, comorbidities (angina, acute coronary syndrome, pulmonary edema, lung disease, malignancy, coronary artery bypass graft, smoker, hypertension medications, stroke, peripheral vascular disease, and diabetes mellitus), geographic region, any predialysis care, distance to center, serum albumin, and body mass index. HR, hazard ratio; CI, confidence interval.
Figure 3.
Adjusted survival for ESRD patients on peritoneal dialysis by race. Model is adjusted for age, sex, era, comorbidities (angina, acute coronary syndrome, pulmonary edema, lung disease, malignancy, coronary artery bypass graft [CABG], smoker, hypertension medications, stroke, peripheral vascular disease, and diabetes mellitus), geographic region, any predialysis care, distance to center, serum albumin, and body mass index.
Table 4.
Crude and adjusted survival for ESRD patients on peritoneal dialysis by race accounting for competing risks
| Cox HR | 95% CI | P Value | Competing Risks HR | 95% CI | P Value | |
|---|---|---|---|---|---|---|
| Mortality | ||||||
| Unadjusted | 1.05 | 0.91–1.24 | 0.49 | 1.00 | 0.86–1.17 | 0.99 |
| Adjusted | 1.36 | 1.13–1.62 | 0.001 | 1.30 | 1.09–1.56 | 0.004 |
| Propensity score adjusted | 1.35 | 1.12–1.63 | 0.002 | 1.28 | 1.06–1.55 | 0.01 |
| Technique failure | ||||||
| Unadjusted | 1.24 | 1.02–1.50 | 0.03 | 1.20 | 0.99–1.44 | 0.06 |
| Adjusted | 1.29 | 1.05–1.57 | 0.03 | 1.19 | 0.95–1.48 | 0.11 |
| Propensity score adjusted | 1.33 | 1.05–1.68 | 0.02 | 1.13 | 0.89–1.43 | 0.29 |
Data on 7323 patients (Aboriginal=554, Caucasian=6769), 2203 deaths (Aboriginal=166, Caucasian=2037), and 1874 technique failures (Aboriginal=177, Caucasian=1697). Model adjusted for age, sex, era, comorbidities (angina, acute coronary syndrome, pulmonary edema, lung disease, malignancy, coronary artery bypass graft, smoker, hypertension medications, stroke, peripheral vascular disease, and diabetes mellitus), geographic region, any predialysis care, distance to center, serum albumin, and body mass index. HR, hazard ratio; CI, confidence interval.
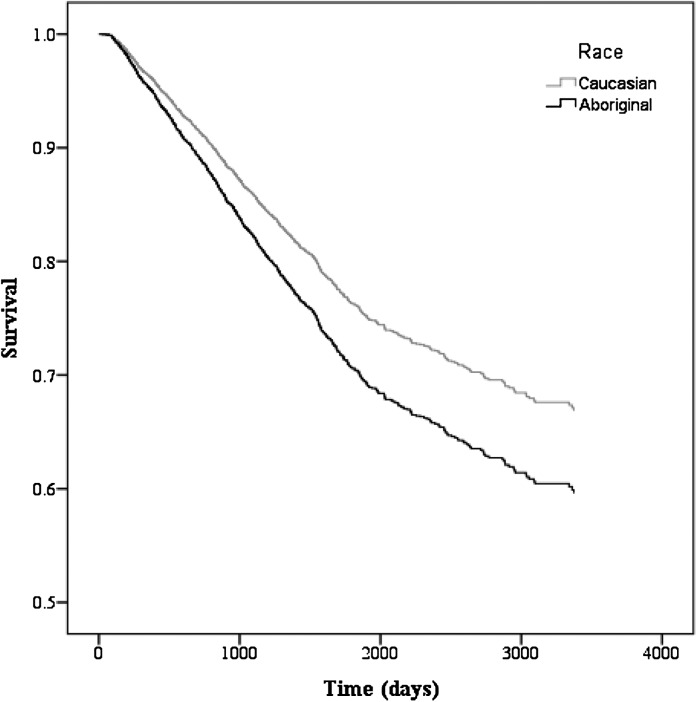
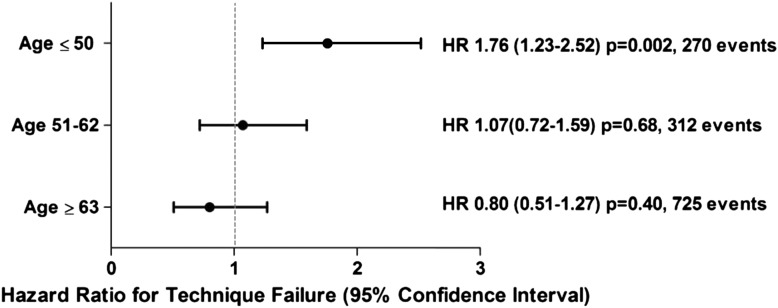
In a secondary analysis, we detected a statistically significant age × race interaction for the outcome of technique failure. After stratification by age (≤50, 51–62, and ≥63 years), the adjusted HR for technique failure among Aboriginals≤50 years old was 1.76 (95% CI=1.23–2.52, P=0.002) compared with Caucasians (Figures 4 and 5), whereas there were no race disparities in the older age groups.
Figure 4.
Adjusted technique survival for patients on peritoneal dialysis by race. Model is adjusted for age, sex, era, comorbidities (angina, acute coronary syndrome, pulmonary edema, lung disease, malignancy, coronary artery bypass graft [CABG], smoker, hypertension medications, stroke, peripheral vascular disease, and diabetes mellitus), geographic region, any predialysis care, distance to center, serum albumin, and body mass index.
Figure 5.
Adjusted technique survival in Aboriginals on peritoneal dialysis stratified by age groups of ≤50, 51–62, and ≥63 years. Caucasian is the referent. Data are on 7323 patients (Aboriginal=554, Caucasian=6769) and 1874 technique failures (Aboriginal=177, Caucasian=1697). Values in parentheses are 95% confidence intervals. HR, hazard ratio.
Discussion
As the epidemic of CKD and ESRD in Canada’s Aboriginal peoples continues, it is imperative that we understand outcomes on dialysis for this patient population. This understanding is coupled with the perceived ideal suitability of peritoneal dialysis in the Aboriginal community, because patients often are younger and reside in remote environments. In the present study, we identified higher mortality and technique failure for Aboriginal patients compared with Caucasians on peritoneal dialysis, a finding not observed for patients on hemodialysis. These race disparities were not accounted for by comprehensive adjustment for demographic, medical, and geographic variables in our analysis and after accounting for competing outcomes. Our findings suggest that a racial ethnic disparity may exist for survival on peritoneal dialysis for Aboriginals in Canada.
Previous literature has shown conflicting results on peritoneal dialysis and mortality in Aboriginals (10,12,14,15,17). Our findings are consistent with a recent report from Manitoba, where we investigated 161 Aboriginal patients compared with 566 non-Aboriginal patients on peritoneal dialysis and found an increase in mortality (adjusted HR=1.48, 95% CI=1.07–2.03) and peritonitis (adjusted HR=1.79, 95% CI=1.35–2.36) among Aboriginals (10,17). An earlier study reported on 101 Aboriginal patients who initiated peritoneal dialysis in the Prairie provinces compared with 734 Caucasian patients from 1990 to 2000 (12). This study used CORR data; however, it found no difference in mortality between the two races and a nonstatistically significant trend to increased technique failure. There are several notable differences between our work and the previous CORR study. We investigated a 10-fold larger, geographically broader, and more contemporary cohort of Aboriginal patients. Furthermore, there have been considerable changes in dialysis care, such as the increased use of automated peritoneal dialysis techniques, increased predialysis care, and incorporation of multidisciplinary care clinics (8,19,20). We were also able to adjust for a number of additional potential confounders, including accounting for residual renal function and serum albumin, both of which are associated with mortality in dialysis patients (21). Finally, we verified our results using a competing risk survival analysis. There was little change in the point estimates using the competing risks analysis, suggesting that the accounting for patients with transplantation and technique failure (for peritoneal dialysis only) had little impact on overall mortality. This finding is important to confirm, because there are recognized disparities in the rates of renal transplantation between the two populations; accounting for patients who were transplanted as an individual outcome would eliminate any theoretical influence of informative censoring (22,23).
Survival on intermittent hemodialysis was comparable between Aboriginals and Caucasians and remained similar after comprehensive adjustment and accounting for competing outcomes. This finding is congruent with previous findings in Canada and the United States. Reports on Native Indians in the United States and Alaska have identified barriers and discrepancies in care delivery; however, these findings did not seem to impact mortality (6,13,24). In Canada, similar outcomes were reported in a cohort limited to the Prairie provinces and recently, children and adolescents (25).
The increase in mortality in Aboriginal peoples on peritoneal dialysis but not hemodialysis is likely multifactorial and involves differences in care delivery and social determinants of health. In-center hemodialysis patients, in contrast to those patients on peritoneal dialysis, will be in more frequent contact with healthcare providers, allowing for encouraged compliance and early detection and treatment of medical illnesses. In contrast, the success and viability of peritoneal dialysis is dependent on an individual’s compliance and local environment. In Canada, Aboriginal status is associated with lower income, lower educational level, and poorer quality housing, and any of these factors could threaten the success of home dialysis (1). In the present analysis, we assessed the impact of remoteness using the distance from one’s initial dialysis center. The lack of availability of patient-level data precluded addition of social determinants of health and compliance into our analysis. Despite this limitation, we favor care delivery and socioeconomic factors over inherent biologic differences for the survival discrepancy, because adjustment for measured biologic differences between Aboriginal and Caucasian populations, such as age, diabetes mellitus, and serum albumin, did not attenuate the hazard for death or technique failure. Furthermore, the mortality difference attributable to race was observed only for patients on peritoneal dialysis, a self-care modality, and not for HD, an in-center modality. To better understand the interplay of compliance, socioeconomic status and determinants of population health on peritoneal dialysis, additional research is urgently required.
Similar to our results, technique failure and the subsequent conversion from peritoneal dialysis to hemodialysis was significantly increased in the Aboriginal population. This finding is consistent with previous reports from Australia/New Zealand (14,15). One of the most common causes for technique failure is peritonitis, and previous work suggests that Aboriginals have a very high risk of peritonitis with differing microbiology profile (17). Of additional concern is that young people are especially prone to technique failure, a population that, theoretically, is better suited for peritoneal dialysis, because it allows one to maintain independence. Whether this finding is because of an increase of peritonitis in this group or other factors, such as differences in treatment compliance, remains unclear.
Our data should not be construed as evidence favoring selection of hemodialysis over peritoneal dialysis in Aboriginal patients. Overall, Aboriginals on peritoneal dialysis had a median survival time of over 8 years compared with 5.6 years for Aboriginals on hemodialysis, and thus, physicians should not be unduly concerned about recommending peritoneal dialysis to Aboriginal patients. Our data do highlight racial disparities in patients on peritoneal dialysis, which requires additional study and intervention.
There are some notable limitations to our work. Residual confounding may have occurred, because we did not account for population health indicators, socioeconomic status, and employment status, all factors that significantly impact health. Among our peritoneal dialysis patients, we did not have information on peritonitis. Our comorbidities and laboratory values were cross-sectional and obtained at the initiation of dialysis versus multiple time-varying values. We did not account for switches in modality, and our analysis was intention to treat. Previous analyses that are time-varying with respect to modality have shown little difference in outcome (26). We used multiple imputation to account for missing data, and although we investigated each of our models sequentially, the inclusion of some of the covariants (albumin) had a significant portion of missing data. Classification of individuals as Aboriginals is inherently problematic, because it is a general term that encompasses numerous races and ethnicities. Although racial differences in technique failure were examined, age as a significant effect modifier was detected on an exploratory analysis and requires confirmation.
Canada’s Aboriginal peoples on peritoneal dialysis experience increased mortality and technique failure relative to Caucasians. Technique failure especially seems to be more common in the younger Aboriginal (under the age of 50 years) population. Additional study and targeted interventions are warranted.
Disclosure
M.M.S. received advisory fees from Roche and Amgen.
Acknowledgments
We thank the Canadian Organ Replacement Registry (CORR), Dr. Louise Moist, Bob Williams, Dolores Friesen, and Frank Ivis for their invaluable help.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Statistics Canada: Canada's Aboriginal population in 2017, 2005. Available at: http://www.statcan.gc.ca Accessed January 2010.
- 2.MacMillan HL, Walsh CA, Jamieson E, Wong MY, Faries EJ, McCue H, MacMillan AB, Offord DD, Technical Advisory Committee of the Chiefs of Ontario : The health of Ontario First Nations people: Results from the Ontario First Nations Regional Health Survey. Can J Public Health 94: 168–172, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao S, Manns BJ, Culleton BF, Tonelli M, Quan H, Crowshoe L, Ghali WA, Svenson LW, Hemmelgarn BR, Alberta Kidney Disease Network : Prevalence of chronic kidney disease and survival among aboriginal people. J Am Soc Nephrol 18: 2953–2959, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Dyck R, Osgood N, Lin TH, Gao A, Stang MR: Epidemiology of diabetes mellitus among First Nations and non-First Nations adults. CMAJ 182: 249–256, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diabetes among Aboriginal people in Canada: The evidence. Health Canada, 2000. Available at: http://www.hc-sc.gc.ca/fniah-spnia/pubs/diseases-maladies/_diabete/2001_evidence_faits/index-eng.php Accessed January, 2012 [Google Scholar]
- 6.Frankenfield DL, Roman SH, Rocco MV, Bedinger MR, McClellan WM: Disparity in outcomes for adult Native American hemodialysis patients? Findings from the ESRD Clinical Performance Measures Project, 1996 to 1999. Kidney Int 65: 1426–1434, 2004 [DOI] [PubMed] [Google Scholar]
- 7.McDonald SP, Russ GR: Current incidence, treatment patterns and outcome of end-stage renal disease among indigenous groups in Australia and New Zealand. Nephrology (Carlton) 8: 42–48, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Canadian Institute for Health Information: Treatment of end-stage organ failure in Canada, 1998 to 2007. Canadian Organ Replacement Register 2009 Annual Report; Ottawa, Ontario, CIHI, 2009
- 9.Martens PJ, Sanderson D, Jebamani L: Health services use of Manitoba First Nations people: Is it related to underlying need? Can J Public Health 96[Suppl 1]: S39–S44, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sood MM, Komenda P, Sood AR, Reslerova M, Verrelli M, Sathianathan C, Eng L, Eng A, Rigatto C: Adverse outcomes among Aboriginal patients receiving peritoneal dialysis. CMAJ 182: 1433–1439, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonelli M, Hemmelgarn B, Manns B, Pylypchuk G, Bohm C, Yeates K, Gourishankar S, Gill JS: Death and renal transplantation among Aboriginal people undergoing dialysis. CMAJ 171: 577–582, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tonelli M, Hemmelgarn B, Manns B, Davison S, Bohm C, Gourishankar S, Pylypchuk G, Yeates K, Gill JS: Use and outcomes of peritoneal dialysis among Aboriginal people in Canada. J Am Soc Nephrol 16: 482–488, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Hall YN, Jolly SE, Xu P, Abrass CK, Buchwald D, Himmelfarb J: Regional differences in dialysis care and mortality among American Indians and Alaska Natives. J Am Soc Nephrol 22: 2287–2295, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim WH, Johnson DW, McDonald SP: Higher rate and earlier peritonitis in Aboriginal patients compared to non-Aboriginal patients with end-stage renal failure maintained on peritoneal dialysis in Australia: Analysis of ANZDATA. Nephrology (Carlton) 10: 192–197, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Lim WH, Boudville N, McDonald SP, Gorham G, Johnson DW, Jose M: Remote indigenous peritoneal dialysis patients have higher risk of peritonitis, technique failure, all-cause and peritonitis-related mortality. Nephrol Dial Transplant 26: 3366–3372, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Miles R, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, Bannister KM, Johnson DW: Predictors and outcomes of fungal peritonitis in peritoneal dialysis patients. Kidney Int 76: 622–628, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Hildebrand A, Komenda P, Miller L, Rigatto C, Verrelli M, Sood AR, Sathianathan C, Reslerova M, Eng L, Eng A, Sood MM: Peritonitis and exit site infections in First Nations patients on peritoneal dialysis. Clin J Am Soc Nephrol 5: 1988–1995, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine PJ, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 19.Verger C, Ryckelynck JP, Duman M, Veniez G, Lobbedez T, Boulanger E, Moranne O: French peritoneal dialysis registry (RDPLF): Outline and main results. Kidney Int Suppl 70[S103]: S12–S20, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Mujais S, Story K: Peritoneal dialysis in the US: Evaluation of outcomes in contemporary cohorts. Kidney Int Suppl 70[S103]: S21–S26, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Bargman JM, Thorpe KE, Churchill DN, CANUSA Peritoneal Dialysis Study Group : Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J Am Soc Nephrol 12: 2158–2162, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Kucirka LM, Grams ME, Lessler J, Hall EC, James N, Massie AB, Montgomery RA, Segev DL: Association of race and age with survival among patients undergoing dialysis. JAMA 306: 620–626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber CLC, Rush DN, Jeffery JR, Cheang M, Karpinski ME: Kidney transplantation outcomes in Canadian aboriginals. Am J Transplant 6: 1875–1881, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Stidley CA, Shah VO, Scavini M, Narva AS, Kessler D, Bobelu A, MacCluer JW, Welty TK, Zager PG: The Zuni kidney project: A collaborative approach to an epidemic of kidney disease. J Am Soc Nephrol 14[Suppl 2]: S139–S143, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Samuel SM, Foster BJ, Tonelli MA, Nettel-Aguirre A, Soo A, Alexander RT, Crowshoe L, Hemmelgarn BR, Pediatric Renal Outcomes Canada Group : Dialysis and transplantation among Aboriginal children with kidney failure. CMAJ 183: E665–E672, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perl J, Wald R, McFarlane P, Bargman JM, Vonesh E, Na Y, Jassal SV, Moist L: Hemodialysis vascular access modifies the association between dialysis modality and survival. J Am Soc Nephrol 22: 1113–1121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]