Abstract
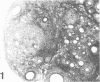
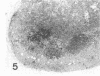
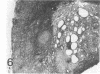
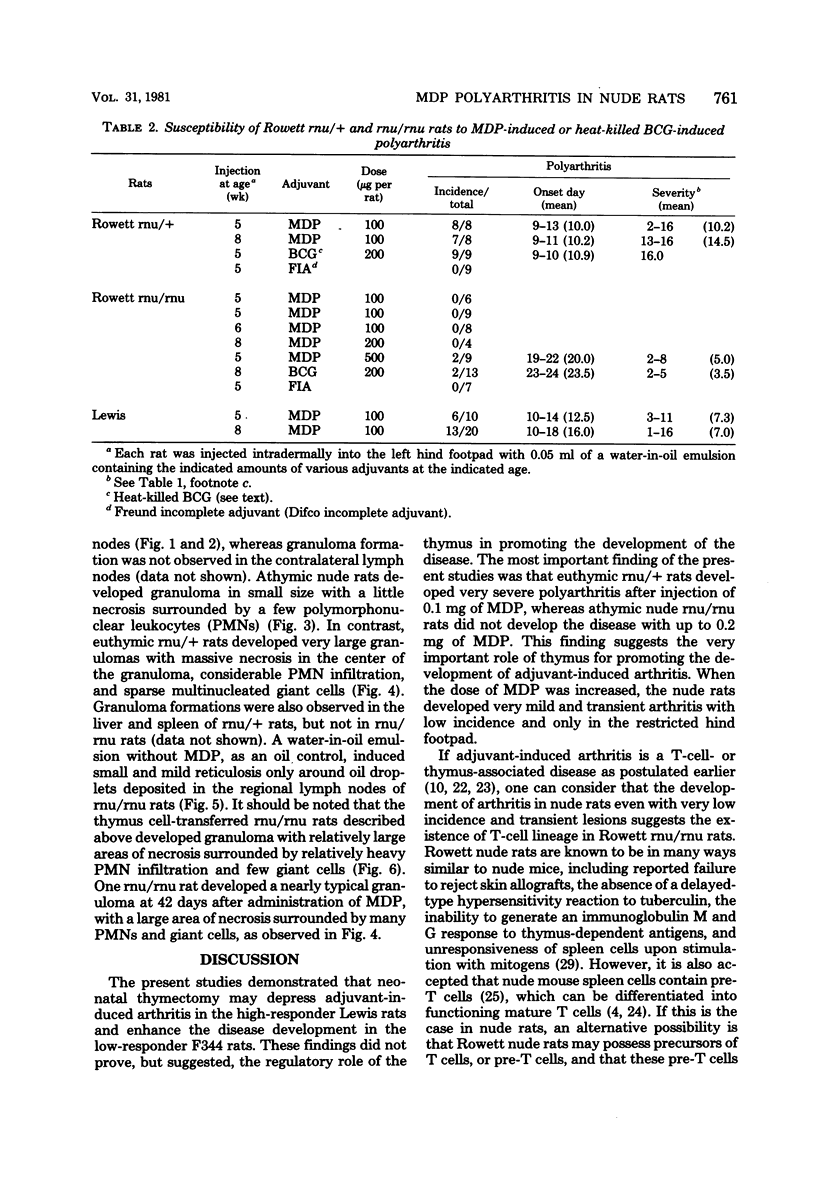
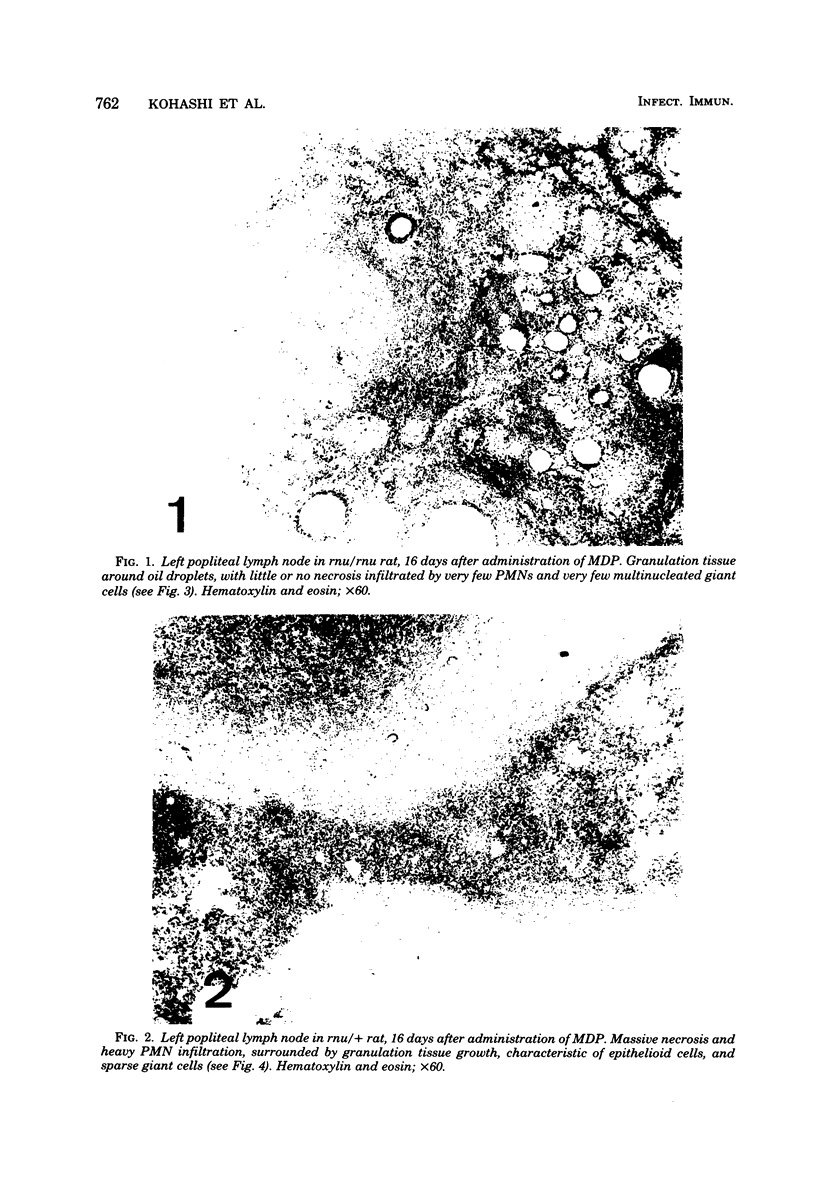
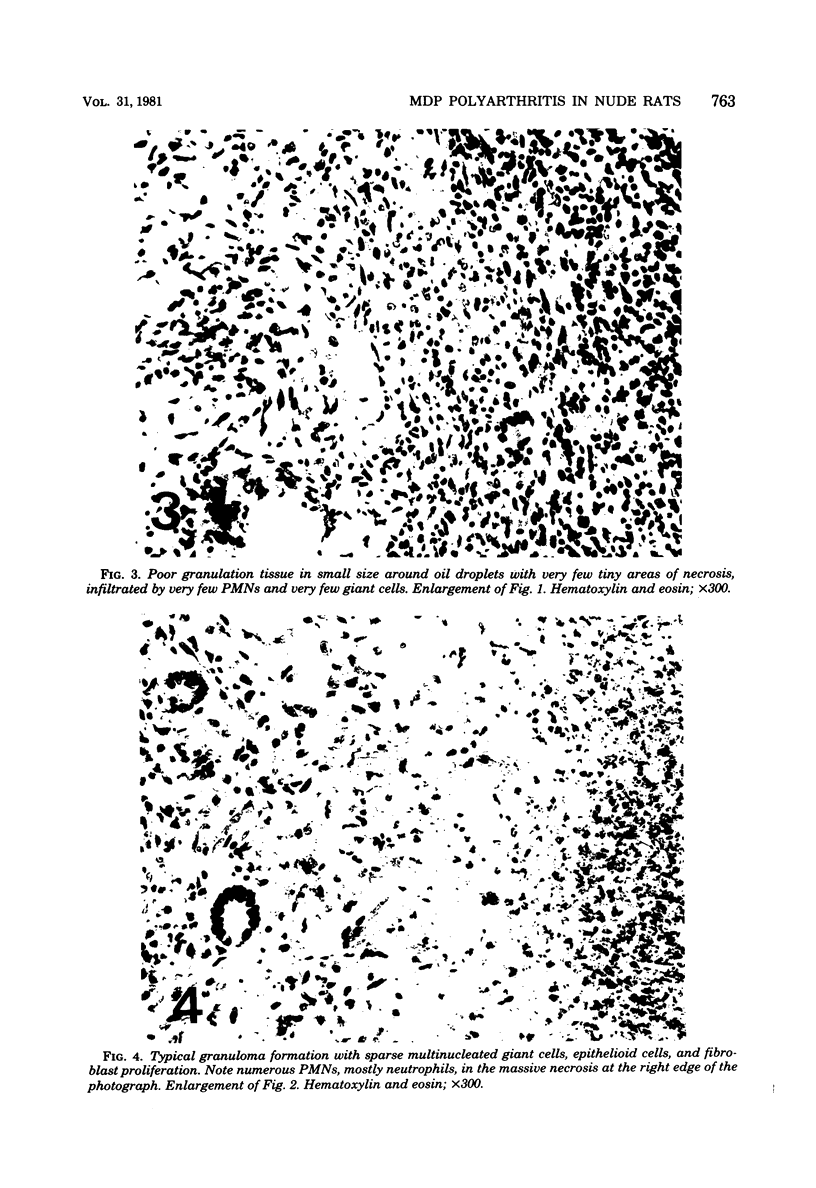
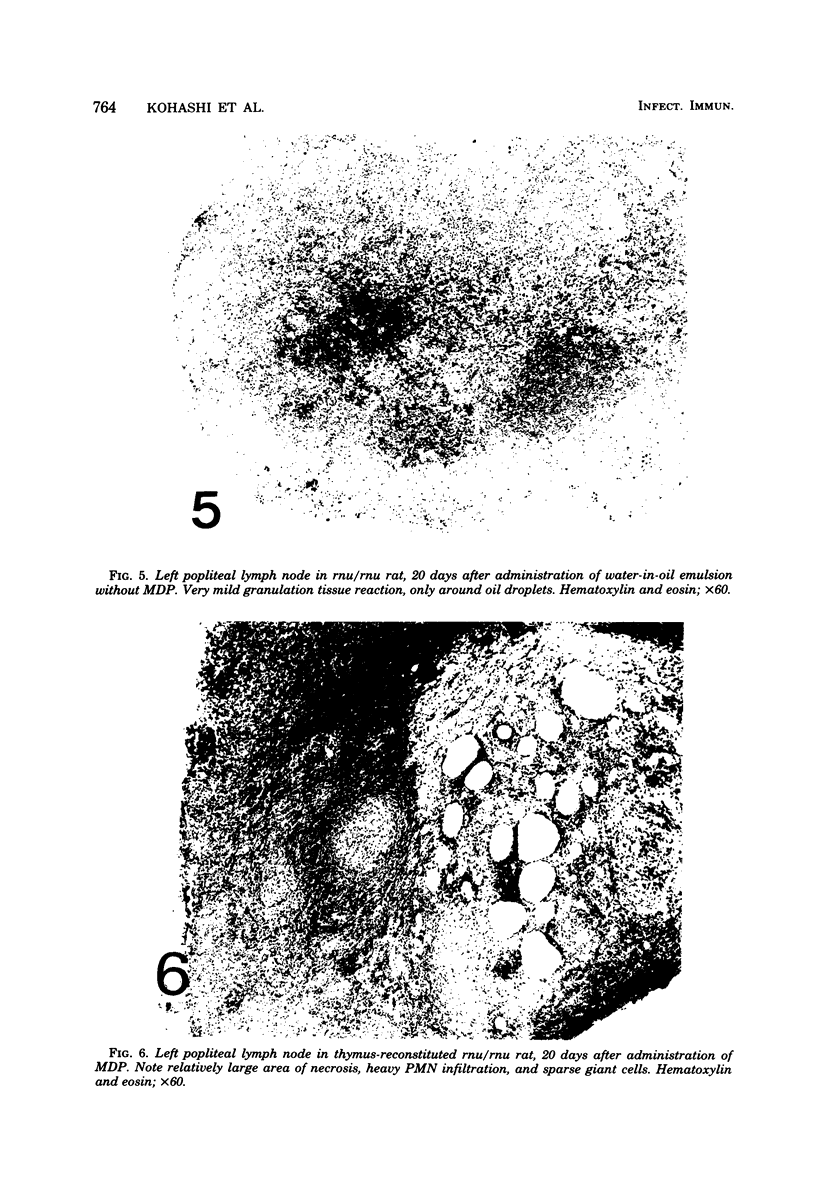
A synthetic adjuvant, N-acetyl muramyl-L-alanyl-D-isoglutamine (MDP), produced extremely severe polyarthritis with almost 100% incidence in Rowett euthymic rnu/+ rats, but the same dose of MDP (100 microgram) did not produce the disease in athymic rnu/rnu rats. Five hundred micrograms of MDP or 0.2 mg of heat-killed Mycobacterium bovis BCG, however, produced mild and transient polyarthritis in nude rats with very low incidence. We have not yet succeeded in reconstituting the disease susceptibility of nude rats by using thymus cells from normal rnu/+ rats. After intradermal inoculation of 100 microgram of MDP, nude rats developed small granulomas with a little necrosis and very few multinucleated giant cells only in the regional lymph nodes, whereas, in addition to the development of polyarthritis, euthymic rnu/+ rats developed typical granuloma with massive necrosis accompanied by numerous polymorphonuclear leukocytes and sparse multinucleated giant cells in the regional lymph nodes. Thymus cell-reconstituted rnu/rnu rats developed granuloma with sparse giant cells, relatively large areas of necrosis, and many polymorphonuclear leukocytes. Neonatal thymectomy may depress adjuvant-induced arthritis in the high-responder Lewis rats and enhance the disease development in the low-responder F344 rats. These findings suggested that (i) thymus plays an important role in promoting the development of MDP-induced arthritis; (ii) MDP-induced granuloma formation does not require thymus functions; (iii) the thymus functions may however be involved in the development of massive necrosis surrounded by considerable polymorphonuclear leukocyte infiltration, the mechanisms of which remain to be determined; and (iv) there is no direct correlation between granuloma formation and development of adjuvant arthritis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNASON B. G., JANKOVIC B. D., WAKSMAN B. H., WENNERSTEN C. Role of the thymus in immune reactions in rats. II. Suppressive effect of thymectomy at birth on reactions of delayed (cellular) hypersensitivity and the circulating small lymphocyte. J Exp Med. 1962 Aug 1;116:177–186. doi: 10.1084/jem.116.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASSELINEAU J., CHOUCROUN N., LEDERER E. Sur la constitution chimique d'un lipo-polysaccharide antigénique extrait de Mycobacterium tuberculosis var. hominis. Biochim Biophys Acta. 1950 Apr;5(2):197–203. doi: 10.1016/0006-3002(50)90164-8. [DOI] [PubMed] [Google Scholar]
- Audibert F., Heymer B., Gros C., Schleifer K. H., Seidl P. H., Chedid L. Absence of binding of MDP, a synthetic immunoadjuvant, to anti-peptidoglycan antibodies. J Immunol. 1978 Oct;121(4):1219–1222. [PubMed] [Google Scholar]
- Chedid L., Audibert F., Lefrancier P., Choay J., Lederer E. Modulation of the immune response by a synthetic adjuvant and analogs. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2472–2475. doi: 10.1073/pnas.73.7.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currey H. L., Ziff M. Suppression of adjuvant disease in the rat by heterologous antilymphocyte globulin. J Exp Med. 1968 Jan 1;127(1):185–203. doi: 10.1084/jem.127.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emori K., Tanaka A. Granuloma formation by synthetic bacterial cell wall fragment: muramyl dipeptide. Infect Immun. 1978 Feb;19(2):613–620. doi: 10.1128/iai.19.2.613-620.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. K., Hsu S. H., Whitney R. A., Jr, Hansen C. T. Immunopathology of schistosomiasis in athymic mice. Nature. 1976 Jul 29;262(5567):397–399. doi: 10.1038/262397a0. [DOI] [PubMed] [Google Scholar]
- JANKOVIC B. D., WAKSMAN B. H., ARNASON B. G. Role of the thymus in immune ractions in rats. I. The immunologic response to bovine serum albumin (antibody formation, Arthus reactivity, and delayed hypersensitivity) in rats thymectomized or splenectomized at various times after birth. J Exp Med. 1962 Aug 1;116:159–176. doi: 10.1084/jem.116.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayashima K., Koga T., Onoue K. Role of T lymphocytes in adjuvant arthritis. I. Evidence for the regulatory function of thymus-derived cells in the induction of the disease. J Immunol. 1976 Nov;117(5 PT2):1878–1882. [PubMed] [Google Scholar]
- Kohashi O., Kotani S., Shiba T., Ozawa A. Synergistic effect of polyriboinosinic acid:polyribocytidylic acid and either bacterial peptidoglycans or synthetic N-acetylmuramyl peptides on production of adjuvant-induced arthritis in rats. Infect Immun. 1979 Nov;26(2):690–697. doi: 10.1128/iai.26.2.690-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M., Watanabe Y., Kotani S., Koga T. Structural requirements for arthritogenicity of peptidoglycans from Staphylococcus aureus and Lactobacillus plant arum and analogous synthetic compounds. J Immunol. 1976 Jun;116(6):1635–1639. [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M., Watanabe Y., Kotani S. Preparation of arthritogenic hydrosoluble peptidoglycans from both arthritogenic and non-arthritogenic bacterial cell walls. Infect Immun. 1977 Jun;16(3):861–866. doi: 10.1128/iai.16.3.861-866.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi O., Tanaka A., Kotani S., Shiba T., Kusumoto S., Yokogawa K., Kawata S., Ozawa A. Arthritis-inducing ability of a synthetic adjuvant, N-acetylmuramyl peptides, and bacterial disaccharide peptides related to different oil vehicles and their composition. Infect Immun. 1980 Jul;29(1):70–75. doi: 10.1128/iai.29.1.70-75.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Shimono T., Harada K., Shiba T. Correlation between the immunoadjuvant activities and pyrogenicities of synthetic N-acetylmuramyl-peptides or -amino acids. Biken J. 1976 Mar;19(1):9–13. [PubMed] [Google Scholar]
- Lennon V. A., Byrd W. J. Letter: Experimental arthritis in thymectomized rats with an impaired humoral immune response. Nature. 1973 Jul 6;244(5410):38–40. doi: 10.1038/244038a0. [DOI] [PubMed] [Google Scholar]
- Merser C., Sinay P., Adam A. Total synthesis and adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1316–1322. doi: 10.1016/0006-291x(75)90503-3. [DOI] [PubMed] [Google Scholar]
- PEARSON C. M. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956 Jan;91(1):95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- PEARSON C. M., WOOD F. D. PASSIVE TRANSFER OF ADJUVANT ARTHRITIS BY LYMPH NODE OR SPLEEN CELLS. J Exp Med. 1964 Oct 1;120:547–560. doi: 10.1084/jem.120.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEARSON C. M., WOOD F. D. Studies of arthritis and other lesions induced in rats by the injection of mycobacterial adjuvant. VII. Pathologic details of the arthritis and spondylitis. Am J Pathol. 1963 Jan;42:73–95. [PMC free article] [PubMed] [Google Scholar]
- Parant M., Damais C., Audibert F., Parant F., Chedid L., Sache E., Lefrancier P., Choay J., Lederer E. In vivo and in vitro stimulation of nonspecific immunity by the beta-D-p-aminophenyl glycoside of N-acetylmuramyl-L-alanyl-D-isoglutamine and an oligomer prepared by cross-linking with glutaraldehyde. J Infect Dis. 1978 Sep;138(3):378–386. doi: 10.1093/infdis/138.3.378. [DOI] [PubMed] [Google Scholar]
- Quagliata F., Phillips-Quagliata J. M. Competence of thoracic duct cells in the transfer of adjuvant disease and delayed hypersensitivity. Evidence that mycobacterial components are required for the successful transfer of the disease. Cell Immunol. 1972 Jan;3(1):78–87. doi: 10.1016/0008-8749(72)90228-6. [DOI] [PubMed] [Google Scholar]
- Sato V. L., Waksal S. D., Herzenberg L. A. Identification and separation of pre T-cells from nu/nu mice: differentiation by preculture with thymic reticuloepithelial cells. Cell Immunol. 1976 Jun 1;24(1):173–185. doi: 10.1016/0008-8749(76)90142-8. [DOI] [PubMed] [Google Scholar]
- Steffen C., Wick G. Delayed hypersensitivity reactions to collagen in rats with adjuvant-induced arthritis. Z Immunitatsforsch Allerg Klin Immunol. 1971;141(2):169–180. [PubMed] [Google Scholar]
- Tanaka A., Nagao S., Saito R., Kotani S., Kusumoto S., Shiba T. Correlation of stereochemically specific structure in muramyl dipeptide between macrophage activation and adjuvant activity. Biochem Biophys Res Commun. 1977 Jul 25;77(2):621–627. doi: 10.1016/s0006-291x(77)80024-7. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Benacerraf B. Immunologic events in experimental hypersensitivity granulomas. Am J Pathol. 1973 Jun;71(3):349–364. [PMC free article] [PubMed] [Google Scholar]
- Vos J. G., Kreeftenberg J. G., Kruijt B. C., Kruizinga W., Steerenberg P. The athymic nude rat. II. Immunological characteristics. Clin Immunol Immunopathol. 1980 Feb;15(2):229–237. doi: 10.1016/0090-1229(80)90033-1. [DOI] [PubMed] [Google Scholar]
- WAKSMAN B. H., PEARSON C. M., SHARP J. T. Studies of arthritis and other lesions induced in rats by injection of mycobacterial adjuvant. II. Evidence that the disease is a disseminated immunologic response to exogenous antigen. J Immunol. 1960 Oct;85:403–417. [PubMed] [Google Scholar]
- Whitehouse D. J., Whitehouse M. W., Pearson C. M. Passive transfer of adjuvant-induced arthritis and allergic encephalomyelitis in rats using thoracic duct lymphocytes. Nature. 1969 Dec 27;224(5226):1322–1322. doi: 10.1038/2241322a0. [DOI] [PubMed] [Google Scholar]