Summary
Background and objectives
Multiple myeloma is responsible for a wide variety of renal pathologies. Urinary protein and monoclonal spike cannot be used to diagnose cast nephropathy (CN). Because albuminuria is a hallmark of glomerular disease, this study evaluated the percentage of urinary albumin excretion (%UAE) as a tool to differentiate CN from Ig light chain amyloidosis (AL), light chain deposition disease (LCDD), and acute tubular necrosis (ATN).
Design, setting, participants, & measurements
Patients were selected from the Renal Biopsy Database and the Dysproteinemia Database. Participants were excluded if laboratory data were missing within 1 week of the renal biopsy. The %UAE was obtained from urine protein electrophoresis.
Results
From 1992 to 2011, 260 patients were biopsied (177 with AL, 28 with LCDD, 43 with CN, and 12 with ATN). The %UAE for CN patients was significantly lower (7%) than for ATN (25%), LCDD (55%), and AL (70%) patients (P<0.001). Significant differences were also found in serum creatinine, serum albumin, free light chain ratio, total urine protein, and urine monoclonal spike; only the %UAE remained independently associated with CN in a logistic regression model (P<0.001). The area under the curve for the receiver operator characteristic curve for %UAE was 0.99. At <25%, the %UAE had a sensitivity of 0.98, specificity of 0.94, positive predictive value of 0.75, and negative predictive value of 0.99.
Conclusions
This study showed that %UAE was significantly less in CN than the other three renal lesions and %UAE may thus be helpful in diagnosis of CN.
Introduction
Renal disease is one of the most common sequelae of monoclonal gammopathy (MG) (1,2). It is influenced by both the hematologic disorder and the monoclonal protein. The incidence of renal impairment in patients with multiple myeloma varies from 18% to 47% and increases with the stage of the myeloma (3–5). Patients with a serum creatinine >2 mg/dl are twice as likely to have stage III Durie-Salmon disease as those with normal serum creatinine (87% versus 44%, respectively) (4). The presence of myeloma also affects the distribution of kidney diseases. In an autopsy series of patients with multiple myeloma, 32% were found to have myeloma cast nephropathy (CN), 11% had Ig light chain amyloidosis (AL), and 5% had light chain deposition disease (LCDD) (5). In contrast, patients with MG who did not meet criteria for myeloma were most likely to have LCDD followed by AL and then CN (2). It is important to recognize that the nephrotoxicity of the monoclonal protein also plays a major role. In animal studies, the type of kidney disease is dictated by the monoclonal protein and not the tumor (6). This is also supported by in vitro data that showed that different light chains (AL versus LCDD) activate different cellular pathways via the same receptor on mesangial cells (7).
Despite the common pathogenic factor, presentation differs depending on the kidney disease. Patients with CN usually present with ARF with varying degrees of proteinuria (8). In contrast, patients with AL are more likely to present with heavy proteinuria but intact renal function (9). The presentation of LCDD is a blend of the two (10). Differences also exist when it comes to treatment response. CN can quickly reverse with treatments that rapidly reduce serum free light chain levels but the renal response in AL can take 12–18 months (10–13). The renal response characteristics of LCDD resemble AL more than that of CN (14,15). These differences are important to keep in mind when designing clinical trials in which renal outcomes are the endpoints. This may explain why different trials in ARF in myeloma patients produced different results (14–18).
The development of ARF significantly affects both morbidity and mortality in patients with myeloma. Patients with ARF are more likely to experience early mortality and worse overall survival (4,19). On the other hand, patients who recover their renal function have the same prognosis as those who never developed ARF (4,19). Therefore, it is important to diagnose the kidney disease correctly so that the proper treatment can be initiated. Rapid reduction of serum free light chains by high cutoff dialyzer or plasmapheresis in combination with bortezomib has produced high rates of renal recovery in patients with CN (11,20,21). Unfortunately, only a kidney biopsy can distinguish CN from other renal lesions. Urinary tests such as proteinuria and urinary monoclonal spike (M-spike) have not been able to reliably distinguish CN from the different renal lesions (22). However, because the injury pattern of AL and LCDD is usually glomerulopathic, whereas the injury in CN is tubulopathic, we hypothesize that the percentage of urinary albumin excretion (%UAE) may be different. This study examines the ability to differentiate CN from AL, LCDD, and acute tubular necrosis (ATN) using %UAE.
Materials and Methods
This study was approved by the Institutional Review Board at the Mayo Foundation in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act guidelines. We performed a retrospective study comparing the %UAE of patients with CN versus patients with AL, LCDD, and ATN. The ability to distinguish CN from the other renal diseases was also compared against other renal tests.
Patients
Patients were selected from the Mayo Clinic Renal Biopsy Database. The database was queried for diagnoses of AL, CN, LCDD, and ATN in the presence of multiple myeloma. The biopsy of each patient was reviewed to confirm the renal disease. These patients were then cross-referenced with the Dysproteinemia Database to extract clinical data including serum protein electrophoresis, serum free light chains, serum albumin, serum creatinine, total urinary protein, urine M-spike, and urine protein electrophoresis. Patients were excluded if they did not have laboratory data obtained within 1 week of the renal biopsy. The %UAE was calculated from the urine protein electrophoretic pattern. The free light chain ratio was calculated by dividing the pathologic light chain by the nonpathologic light chain so that the ratio will always be >1. Urine M-spikes that were under the measurable limit (<0.01 g/24 h) were given a value of 0.
Statistical Analyses
Statistical calculations were performed using the JMP software package (version 9.0.1.; SAS Institute Inc., Cary, NC). Pearson’s chi-squared tests were performed on categorical data. Two-sample t tests and Wilcoxon rank-sum tests were used to compare continuous variables between the two weighted groups. ANOVA was used to compare continuous variables across all groups. Variables with a P value <0.05 were considered significant. Using the receiver operator curve (ROC), best cutoffs were identified as the values with the highest sensitivity (1 − specificity) score that distinguishes CN from the other renal lesions. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated using biopsy results as the gold standard for each parameter identified as being univariately significant. A logistic regression model was used to determine independent association with CN.
Results
Between 1992 and 2011, a total of 279 patients met the study criteria. There were 196 patients with AL, 28 with LCDD, 43 with CN, and 12 with ATN. Nineteen AL patients were excluded for having incomplete laboratory data, leaving 177 patients for analysis. The causes of ATN included the following: zoledronic acid (n=1), nonsteroidal anti-inflammatory drugs (n=4), cyclosporin (n=2), hypercalcemia (n=2), intravenous contrast (n=1), and unknown (n=2). There were no significant differences in age and sex among the four groups (Table 1).
Table 1.
Patient characteristics
| ATN | AL | LCDD | CN | P Value | |
|---|---|---|---|---|---|
| n | 12 | 77 | 28 | 43 | |
| Age (yr) | 64 (51–83) | 61 (36–82) | 56 (38–83) | 62 (29–81) | 0.18 |
| Male sex (%) | 41.7 | 64.9 | 67.9 | 65.1 | 0.44 |
| FLC ratio | 454 (4.1–15333) | 9 (1.1–415) | 53 (1.1–7178) | 430 (3.3–8026) | <0.001 |
| serum creatinine (mg/dl) | 3.5 (1.7–8.0) | 1.4 (0.6–7.1) | 2.8 (1.3–10.2) | 4.9 (1.4–12.5) | <0.001 |
| Serum albumin (g/dl) | 3.9 (2.6–4.6) | 2.2 (0.8–3.5) | 3.3 (2.2–4) | 3.1 (2.1–4.9) | <0.001 |
| Urine protein (g/d) | 1.2 (0.1–12.2) | 5.8 (0.7–70.3) | 2.5 (0.1–10.1) | 2.0 (0.1–19.7) | <0.001 |
| Urine M-spike (g/d) | 0.26 (0.02–1.2) | 0.34 (0.02–2.5) | 0.76 (0.0–2.7) | 1.0 (0.05–17.9) | 0.01 |
| %UAE | 25 (8–62) | 70 (5–81) | 55 (7–78) | 7 (2–26) | <0.001 |
| κ (%) | 70.0 | 19.5 | 80.0 | 57.1 | <0.001 |
Differences were calculated using ANOVA. ATN, acute tubular necrosis; AL, Ig light chain amyloidosis; LCDD, light chain deposition disease; CN, cast nephropathy; FLC, serum free light chain; M-spike, monoclonal spike; %UAE, percentage of urine albumin excretion.
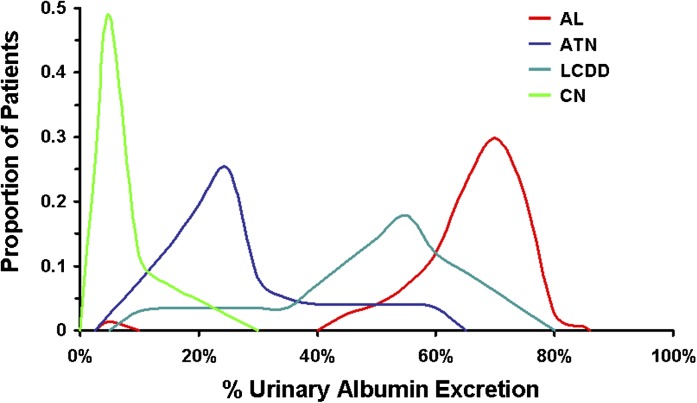
Patients with CN had higher serum creatinine (4.9 mg/dl; range, 1.4–12.5) than patients with AL (1.2 mg/dl; range, 0.6–7.6; P<0.001), and LCDD (2.8 mg/dl; range, 1.3–10.2; P=0.004), but this level was similar to those with ATN (3.5 mg/dl; range, 1.7–8.0; P=0.07). CN patients had significantly less proteinuria than AL patients (2.0 g/d versus 5.8 g/d, respectively; P<0.001) but were similar to those with LCDD (2.5 g/d; P=0.15) and ATN (1.2 g/d; P=0.22). Serum albumin was highest in ATN patients (3.9 g/dl; range, 2.6–4.6) and lowest in AL patients (2.2 g/dl; range, 0.8–4.5). Serum albumin was significantly different for CN patients (3.1 g/dl; range, 2.1–4.9) compared with AL (P<0.001) and ATN patients (P=0.04), but not LCDD patients (P=0.41). The free light chain ratios were highest in the CN patients (430; range, 3.3–8026) and ATN patients (454; range, 4.1–15,333), which were similar (P=0.98). Free light chain ratios were significantly lower in the AL patients (9; range, 1.1–415; P<0.001) and LCDD patients (53; range, 1.1–7178; P=0.01) compared with CN patients. Urine albumin excretion was significantly different among all four groups (P<0.001) (Figure 1). The %UAE was highest among patients with AL (70%; range, 5%–85%), followed by LCDD (55%; range, 7%–78%). ATN patients had 25% (range, 8%–62%) and CN patients had 7% (range, 2%–26%), which was the lowest urinary albumin percentage.
Figure 1.
Distribution curves of percentage of urinary albumin excretion (%UAE) obtained from 24-hour urine collections. The differences in %UAE were significant among all renal lesions (P<0.001) and between each possible combination (P<0.01). The only bimodal pattern is AL, in which one patient had 5% urinary albumin. Renal diagnosis was based on results of the renal biopsy. AL, Ig light chain amyloidosis; ATN, acute tubular necrosis; LCDD, light chain deposition disease; CN, cast nephropathy.
Univariate analysis revealed that serum albumin (P<0.001), serum creatinine (P<0.001), total urine protein (P<0.001), urine M-spike (P<0.001), and %UAE (P<0.001) were all associated with the diagnosis of CN. Using the logistic regression model, a multivariate analysis was performed and only %UAE remained independently associated with CN (P<0.001; odds ratio, 0.83 [0.89–1.20]). No other tests showed independent association with CN (serum albumin (P=0.18), serum creatinine (P=0.27), total urine protein (P=0.55), and urine M-spike (P=0.55). A ROC curve was performed for each of the parameters. The best ROC was that of %UAE, which had an area under the curve of 0.98 with the best cutoff at 25% (Table 2).
Table 2.
Diagnostic capability of serum and urine tests for cast nephropathy
| Sensitivity | Specificity | PPV | NPV | AUC ROC | |
|---|---|---|---|---|---|
| %UAE | 0.99 | ||||
| 22 | 0.98 | 0.94 | 0.78 | 0.99 | 0.96 |
| 25 | 0.98 | 0.94 | 0.75 | 0.99 | 0.98 |
| 27 | 1.0 | 0.92 | 0.71 | 1.0 | 0.96 |
| Urine protein (g/d) | 0.76 | ||||
| 3.0 | 0.72 | 0.70 | 0.32 | 0.93 | 0.71 |
| 4.0 | 0.81 | 0.64 | 0.31 | 0.95 | 0.73 |
| 5.0 | 0.88 | 0.52 | 0.27 | 0.96 | 0.70 |
| Urine M-spike (g/d) | 0.91 | ||||
| 0.02 | 0.98 | 0.80 | 0.49 | 0.99 | 0.85 |
| 0.05 | 0.95 | 0.81 | 0.49 | 0.99 | 0.87 |
| 0.1 | 0.93 | 0.82 | 0.50 | 0.98 | 0.87 |
| Serum albumin (g/dl) | 0.79 | ||||
| 2.6 | 0.88 | 0.56 | 0.31 | 0.91 | 0.71 |
| 2.7 | 0.86 | 0.64 | 0.32 | 0.96 | 0.75 |
| 2.9 | 0.74 | 0.72 | 0.50 | 0.93 | 0.73 |
| Serum creatinine (mg/dl) | 0.87 | ||||
| 2.4 | 0.99 | 0.70 | 0.38 | 0.99 | 0.82 |
| 2.6 | 0.91 | 0.72 | 0.39 | 0.98 | 0.81 |
| 2.8 | 0.84 | 0.77 | 0.46 | 0.95 | 0.79 |
The middle values represent the best cutoff for each test which was calculated by ROC analysis. PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; ROC, receiver operator characteristics; %UAE, percentage of urine albumin excretion; M-spike, monoclonal spike.
Discussion
ARF imposes a significant effect on the morbidity and mortality of patients with multiple myeloma (4). Fortunately, recovery of renal function reverses the negative effects on survival (4,19). Because of the variety of kidney injury that exists in multiple myeloma, it is difficult to distinguish one from another without a kidney biopsy. It is important to recognize that the pattern of recovery varies depending on the type of kidney disease (12,13,22). A mixture of renal pathologies may explain why different rates of renal recovery have been reported from different trials (16–18,21–23).
Unfortunately, a renal biopsy is not always easy to obtain in these patients. Patients treated with immunomodulatory derivatives such as thalidomide, lenalidomide, or pomalidomide require antithrombotic prophylaxis and are often on full-dose aspirin (24). Due to the age of these patients, many may be receiving aspirin for protection or prevention of coronary artery disease. In addition, thrombocytopenia is a common adverse effect of patients treated with bortezomib and other chemotherapy. All of these make obtaining a renal biopsy difficult.
The purpose of this study was to determine if a noninvasive test can be used to distinguish CN from other renal lesions. We looked at three serum tests and three urine tests, all of which were found to be significantly different when evaluating the entire cohort. However, when focused on the ability to distinguish CN, only five were found to be useful. Although they all showed promise in the univariate analysis, only %UAE was independently associated with CN. The area under the curve of the ROC curve for %UAE was excellent at 0.99. In our population, no patient with a diagnosis of CN had a %UAE >26%. The ROC curve analysis suggested that 25% was the best cutoff for CN. The addition of the other tests did not improve its diagnostic ability.
It is important to recognize that because patients in this study were selected from the Renal Biopsy Database, bias was certainly introduced. The true prevalences of different kidney diseases are not represented because biopsy rates differ depending on the clinical situation. For patients with AL and LCDD, renal biopsies were obtained to evaluate the proteinuria, which is a common accepted practice (25). However, renal biopsy has not been universally adopted for evaluation of ARF in multiple myeloma (26). In fact, the largest randomized trial for ARF in multiple myeloma was conducted with few renal biopsies (16). Thus, despite being the most common renal pathology in myeloma patients in autopsy, CN had the fewest number compared with AL and LCDD (5).
Despite our promising results, further studies are needed before %UAE can replace kidney biopsy. First, only four lesions were tested in this study. At least a dozen different renal diseases have been described due to monoclonal plasma cell disorders and more are being discovered (1,27). Even with the near perfect ROC curve for %UAE, there were rare false positives with AL, ATN, and LCDD patients having low %UAE (Figure 1). One particular group is the vascular limited AL (28). These patients usually present with unexplained renal insufficiency with no or little proteinuria, which makes them difficult to distinguish from patients with CN. In addition, more than one form of renal lesion can exist in a single patient (29). It is unclear how %UAE would perform with these patients. Finally, the value that renal biopsies provide cannot be understated. In addition to being the gold standard for diagnosis, they also provide prognostic information that cannot be obtained by other means. It is through renal biopsies that new renal diseases associated with MGs are being discovered. Recent studies show that bleeding is not a greater risk for biopsy in multiple myeloma and AL patients if the usual screening for coagulopathy is performed and should not be an impediment (30,31).
In conclusion, we found the %UAE to be a promising screening test that can help to distinguish CN from other renal lesions in myeloma patients. Using a cutoff of 25%, %UAE has a PPV of 0.75 and a NPV of 0.99. If renal biopsy is not obtainable, a low %UAE strongly suggests CN as the cause of the renal failure. These results need to be confirmed with more patients with additional types of kidney disease. Until then, it should not be generalized to patients with acquired Fanconi syndrome or immunotactoid or proliferative GN who were not included in this study. Renal biopsy remains the gold standard in differentiating the many different types of renal lesions in myeloma patients and is an important tool for diagnostic, prognostic, and research purposes in these patients.
Disclosures
None.
Acknowledgments
We thank the JABBS Foundation and the Predolin Foundation for their generous support.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Leung N, Rajkumar SV: Renal manifestations of plasma cell disorders. Am J Kidney Dis 50: 155–165, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Paueksakon P, Revelo MP, Horn RG, Shappell S, Fogo AB: Monoclonal gammopathy: Significance and possible causality in renal disease. Am J Kidney Dis 42: 87–95, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Alexanian R, Barlogie B, Dixon D: Renal failure in multiple myeloma. Pathogenesis and prognostic implications. Arch Intern Med 150: 1693–1695, 1990 [PubMed] [Google Scholar]
- 4.Bladé J, Fernández-Llama P, Bosch F, Montolíu J, Lens XM, Montoto S, Cases A, Darnell A, Rozman C, Montserrat E: Renal failure in multiple myeloma: Presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med 158: 1889–1893, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Iványi B: Frequency of light chain deposition nephropathy relative to renal amyloidosis and Bence Jones cast nephropathy in a necropsy study of patients with myeloma. Arch Pathol Lab Med 114: 986–987, 1990 [PubMed] [Google Scholar]
- 6.Solomon A, Weiss DT, Kattine AA: Nephrotoxic potential of Bence Jones proteins. N Engl J Med 324: 1845–1851, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Keeling J, Teng J, Herrera GA: AL-amyloidosis and light-chain deposition disease light chains induce divergent phenotypic transformations of human mesangial cells. Lab Invest 84: 1322–1338, 2004 [DOI] [PubMed]
- 8.Winearls CG: Acute myeloma kidney. Kidney Int 48: 1347–1361, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Kyle RA, Gertz MA: Primary systemic amyloidosis: Clinical and laboratory features in 474 cases. Semin Hematol 32: 45–59, 1995 [PubMed] [Google Scholar]
- 10.Pozzi C, D’Amico M, Fogazzi GB, Curioni S, Ferrario F, Pasquali S, Quattrocchio G, Rollino C, Segagni S, Locatelli F: Light chain deposition disease with renal involvement: Clinical characteristics and prognostic factors. Am J Kidney Dis 42: 1154–1163, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Burnette BL, Leung N, Rajkumar SV: Renal improvement in myeloma with bortezomib plus plasma exchange. N Engl J Med 364: 2365–2366, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Leung N, Dispenzieri A, Fervenza FC, Lacy MQ, Villicana R, Cavalcante JL, Gertz MA: Renal response after high-dose melphalan and stem cell transplantation is a favorable marker in patients with primary systemic amyloidosis. Am J Kidney Dis 46: 270–277, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Leung N, Dispenzieri A, Lacy MQ, Kumar SK, Hayman SR, Fervenza FC, Cha SS, Gertz MA: Severity of baseline proteinuria predicts renal response in immunoglobulin light chain-associated amyloidosis after autologous stem cell transplantation. Clin J Am Soc Nephrol 2: 440–444, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Markowitz GS, Valeri AM, Kambham N, Sherman WH, Appel GB, D’Agati VD: Renal monoclonal immunoglobulin deposition disease: The disease spectrum. J Am Soc Nephrol 12: 1482–1492, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Lorenz EC, Gertz MA, Fervenza FC, Dispenzieri A, Lacy MQ, Hayman SR, Gastineau DA, Leung N: Long-term outcome of autologous stem cell transplantation in light chain deposition disease. Nephrol Dial Transplant 23: 2052–2057, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Clark WF, Stewart AK, Rock GA, Sternbach M, Sutton DM, Barrett BJ, Heidenheim AP, Garg AX, Churchill DN, Canadian Apheresis Group : Plasma exchange when myeloma presents as acute renal failure: A randomized, controlled trial. Ann Intern Med 143: 777–784, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Johnson WJ, Kyle RA, Pineda AA, O’Brien PC, Holley KE: Treatment of renal failure associated with multiple myeloma. Plasmapheresis, hemodialysis, and chemotherapy. Arch Intern Med 150: 863–869, 1990 [PubMed] [Google Scholar]
- 18.Zucchelli P, Pasquali S, Cagnoli L, Ferrari G: Controlled plasma exchange trial in acute renal failure due to multiple myeloma. Kidney Int 33: 1175–1180, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Knudsen LM, Hjorth M, Hippe E, Nordic Myeloma Study Group : Renal failure in multiple myeloma: reversibility and impact on the prognosis. Eur J Haematol 65: 175–181, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Hutchison CA, Bradwell AR, Cook M, Basnayake K, Basu S, Harding S, Hattersley J, Evans ND, Chappel MJ, Sampson P, Foggensteiner L, Adu D, Cockwell P: Treatment of acute renal failure secondary to multiple myeloma with chemotherapy and extended high cut-off hemodialysis. Clin J Am Soc Nephrol 4: 745–754, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchison CA, Cockwell P, Stringer S, Bradwell A, Cook M, Gertz MA, Dispenzieri A, Winters JL, Kumar S, Rajkumar SV, Kyle RA, Leung N: Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J Am Soc Nephrol 22: 1129–1136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung N, Gertz MA, Zeldenrust SR, Rajkumar SV, Dispenzieri A, Fervenza FC, Kumar S, Lacy MQ, Lust JA, Greipp PR, Witzig TE, Hayman SR, Russell SJ, Kyle RA, Winters JL: Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chains. Kidney Int 73: 1282–1288, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Ludwig H, Bolejack V, Crowley J, Bladé J, Miguel JS, Kyle RA, Rajkumar SV, Shimizu K, Turesson I, Westin J, Sonneveld P, Cavo M, Boccadoro M, Palumbo A, Tosi P, Harousseau JL, Attal M, Barlogie B, Stewart AK, Durie B: Survival and years of life lost in different age cohorts of patients with multiple myeloma. J Clin Oncol 28: 1599–1605, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Kristinsson SY, Landgren O, Rajkumar VS: Novel therapies in multiple myeloma for newly diagnosed nontransplant candidates. Cancer J 15: 473–478, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San Miguel J, Chanan-Khan A, Ludwig H, Joshua D, Mehta J, Gertz M, Avet-Loiseau H, Beksaç M, Anderson KC, Moreau P, Singhal S, Goldschmidt H, Boccadoro M, Kumar S, Giralt S, Munshi NC, Jagannath S, International Myeloma Workshop Consensus Panel 3 : Consensus recommendations for standard investigative workup: Report of the International Myeloma Workshop Consensus Panel 3. Blood 117: 4701–4705, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Dimopoulos MA, Kastritis E, Rosinol L, Bladé J, Ludwig H: Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia 22: 1485–1493, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Nasr SH, Markowitz GS, Stokes MB, Seshan SV, Valderrama E, Appel GB, Aucouturier P, D’Agati VD: Proliferative glomerulonephritis with monoclonal IgG deposits: A distinct entity mimicking immune-complex glomerulonephritis. Kidney Int 65: 85–96, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Eirin A, Irazabal MV, Gertz MA, Dispenzieri A, Lacy MQ, Kumar S, Sethi S, Nasr SH, Cornell LD, Fidler ME, Fervenza FC, Leung N: Clinical features of patients with immunoglobulin light chain amyloidosis (AL) with vascular-limited deposition in the kidney. Nephrol Dial Transplant 27: 1097–1101, 2011 [DOI] [PubMed]
- 29.Lorenz EC, Sethi S, Poshusta TL, Ramirez-Alvarado M, Kumar S, Lager DJ, Fervenza FC, Leung N: Renal failure due to combined cast nephropathy, amyloidosis and light-chain deposition disease. Nephrol Dial Transplant 25: 1340–1343, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Fish R, Pinney J, Jain P, Addison C, Jones C, Jayawardene S, Booth J, Howie AJ, Ghonemy T, Rajabali S, Roberts D, White L, Khan S, Morgan M, Cockwell P, Hutchison CA: The incidence of major hemorrhagic complications after renal biopsies in patients with monoclonal gammopathies. Clin J Am Soc Nephrol 5: 1977–1980, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soares SM, Fervenza FC, Lager DJ, Gertz MA, Cosio FG, Leung N: Bleeding complications after transcutaneous kidney biopsy in patients with systemic amyloidosis: Single-center experience in 101 patients. Am J Kidney Dis 52: 1079–1083, 2008 [DOI] [PubMed] [Google Scholar]